1. Introduction
Bacterial adhesion and biofilm formation inhibition are increasingly promising topics because of the urgent need for strategies alternative to conventional antimicrobial principles [
1]. In the oral environment, active principles must interact positively with the surfaces of natural and artificial hard tissues, modulating the biofilm behavior instead of eliminating them. In this context, antiadhesion and biofilm inhibition strategies could effectively restore the plaque ecosystem's balance [
2,
3,
4]. From this point of view, one of the most promising strategies is based on using a wide variety of nanoparticles, taking advantage of their ability to interact with both the microorganisms and their colonization surface [
5].
In this sense, cellulose nanocrystals (CNCs) represent a valid possibility. They are cellulose-derived nanomaterials that can be easily obtained [
6] because cellulose is one of the most diffused vegetable wastes produced by circular economies [
7]. CNCs are widely available and easily produced through acidic hydrolysis from many widely available cellulose sources, including agricultural and agro-industrial residues, such as wood, non-wood fibers, and algae [
8,
9,
10].
CNCs show many desirable properties, such as excellent mechanical strength, absence of toxicity, and, above all, biocompatibility [
9,
11]. These characteristics make it worthwhile for several biomedical applications.
As stated above, the most interesting property of CNCs is their antibacterial potential [
12,
13], expressed against bacterial adherence and biofilm formation, without any notable side effects. These characteristics fit the general requirements of an active principle that can be used to prevent biofilm-related oral diseases. In particular, the absence of known side effects and toxicity [
13,
14,
15,
16] seems ideal for oral hygiene products such as toothpaste and mouth rinses. For this reason, CNCs have attracted the curiosity of those in the oral field who are looking for antimicrobial agents.
Such agents target a broad spectrum of oral microorganisms, with particular attention to cariogenic species, such as
Streptococcus mutans, and, for different reasons, related to mucosae infections,
Candida albicans.
S. mutans, in particular, is the species that exhibits intense cariogenicity and biofilm formation capability, being, for this reason, a model microorganism for in vitro caries testing [
17,
18]. Furthermore, growth conditions represent a crucial parameter to provide in vitro data with good translatability to clinical conditions. For these reasons, when testing the antimicrobial properties of new active principles for the oral environment, it is of utmost importance that the two main phases of microbial colonization (adherence and biofilm formation) are considered and tested in an environment with hydrodynamic stress similar to the natural one [
19].
This study aimed to evaluate the ability of a CNC solution to reduce bacterial adherence and biofilm formation using in vitro microbiological models reproducing different aspects of the oral environment. The null hypothesis was that CNC solutions would not reduce microbial adherence and biofilm formation by S. mutans and C. albicans and that such solutions would not reduce biofilm formation by a multispecies oral biofilm.
2. Materials and Methods
2.1. Preparation of CNCs
According to a previously reported procedure [
20], CNCs were obtained from Whatman filter paper by acid hydrolysis in 64% of H
2SO
4 for 1 h at 55 °C. CNCs were recovered in about 40 % yield, giving a suspension at around 1 wt. %. They were characterized by DLS measurements, and we found a ζ-potential of −39.0 ± 1.0 mV, a dimension of 92.4 ± 1.8 nm, while AFM and TEM analyses confirmed the rod-like structure, as previously reported [
21]. Among the different characteristics, particular attention was devoted to the surface of the CNCs. This is characterized by different hydroxyl groups of cellulose and negatively charged sulfate half-ester groups introduced during the extraction, as indicated by the negative ζ-potential [
22]. The CNCs were recovered by centrifugation (12.000 × g, 4 °C, 30 min), and a 12 wt. % stock solution was prepared.
2.2. Procedures
Serial dilutions were prepared from stock solution as 4%, 2%, 1%, 0.5%, 0.25%, 0.125%, and 0.063% by dilution using filter-sterile water, while a control solution was filter-sterile water.
2.3. Preparation of enamel disks
Anterior adult bovine teeth were used to prepare a total of 256 round enamel-dentin slabs with a diameter of 6.0 mm and a thickness of 2.0 mm. Disks were cut from the labial surfaces using a water-cooled trephine diamond bur (INDIAM, Carrara, Italy). Dentin bottoms were removed, and the enamel surfaces were subjected to a standardized polishing protocol, including polishing with 1000/4000-grit grinding paper (Buehler, Lake Bluff, IL, USA) using a polishing machine (Motopol 8; Buehler, Düsseldorf, Germany). All disks were sterilized before the experiments using a chemiclave with hydrogen peroxide gas plasma technology (Sterrad; ASP, Irvine, CA, USA). Limiting the maximum temperature to 45 °C prevented heat-related damage to the specimens [
23].
2.3.1. Saliva preparation
Stimulated whole saliva was collected by expectoration from the experimenters (ACI, EB, BM). They refrained from oral hygiene for 24 h, had no active dental disease, and did not have antibiotic therapy for at least 3 mo before the experiment. Procedures followed the protocol published by Guggenheim et al. 2001 [
24]. Saliva was collected in chilled tubes, pooled, heated to 60 °C for 30 min to inactivate endogenous enzymes, and centrifuged (12.000 × g, 4 °C, 15 min). The supernatant was transferred into sterile tubes, stored at -20 °C, and thawed at 37 °C for 1 h before beginning the experiments. To obtain the artificial oral microcosm inoculum, whole human saliva was additionally collected from the same experimenters after 24 h, pooled, and immediately used to inoculate the plates [
25].
2.3.2. Bacteria
Streptococcus mutans ATCC 35668 was cultured according to a previously published protocol [
26]. Briefly,
Mitis Salivarius Bacitracin agar was inoculated with
S. mutans and incubated at 37 °C in a 5% supplemented CO
2 environment for 48 h. A pure suspension of the microorganism in Brain Heart Infusion additioned with 1 wt.% sucrose was obtained from these plates after incubating at 37 °C in a 5% supplemented CO
2 environment for 12 h (early log phase).
S. mutans cells were harvested by centrifugation (2.200 × g, 19° C, 5 min), washed twice with sterile phosphate-buffered saline (PBS), and resuspended in BHI 1 wt. % sucrose. The suspension was subsequently subjected to low-intensity ultrasonic energy (Sonifier model B-150; Branson; Danbury, CT, USA; operating at 7-W energy output for 30s) to disperse bacterial chains. The suspension was then adjusted to a 1.0 on the McFarland scale, corresponding to a microbial concentration of approximately 3.0 × 10
8 cells/mL.
A pure culture of Candida albicans strain ATCC 90028 in BHI + 1 wt. % sucrose was obtained at 37 °C in a 5% supplemented CO2 environment after 24h of incubation. Cells were harvested by centrifugation (2200 × g, 19° C, 5 min), washed twice with sterile PBS, and resuspended in BHI + 1 wt. % sucrose, the suspension adjusted to a turbidity equivalent to a 1.0 McFarland standard.
2.3.3. Adherence
Enamel disks were placed with sterile tweezers, one into each well of 48-well plates (Nunc, Kastrup, Denmark), and 50 L of sterile thawed saliva was added on the surface of each disk, and plates were incubated at 37 °C in a 5% supplemented CO2 environment for 24 h to allow for the development of the acquired salivary pellicle. After that, excess saliva was discarded, and the surface of the disks was washed with sterile PBS. A total of 250 µL of each concentration of CNCs was added to each of n=8 wells. Then, 250 µL of either S. mutans or C. albicans microbial suspension was added to each well. Thus, the final concentration of CNCs in each well was 2.00%, 1.00%, 0.50%, 0.25%, 0.12%, 0.06%, 0.03%, and 0% (negative control). A total of 64 disks for each strain were used. After 2 h of incubation, the enamel disks were washed with sterile PBS, and biomass viability was assessed.
2.3.4. Biofilm formation
Biofilm formation under shear stress conditions was evaluated using a modified-drip flow reactor (M-DFR) according to Ionescu et al. 2019 [
26]. The modified design allowed the placement of customized specimen trays on the bottom of the flow cells and the complete immersion of the surfaces of the enamel disks into the surrounding flowing medium. All tubing and specimen-containing trays were sterilized before the experiments using the Sterrad chemiclave. The M-DFR was assembled inside a sterile hood and transferred into an incubator to operate at 37 °C. To simulate salivary pellicle formation, the enamel disk surfaces in each flow cell were exposed to the thawed sterile saliva for 24 h. Subsequently, excess saliva was discarded. Biofilm formation was obtained on the surfaces of the enamel disks by inoculating 10 mL of either
S. mutans or pooled whole saliva suspension into each flow cell. A total of 64 disks for each strain were used. After 4 h, a multichannel, computer-controlled peristaltic pump (RP-1; Rainin, Emeryville, CA, USA) was turned on to provide a constant flow of nutrient broth through the flow cells. The sterile nutrient broth was enriched with 10.0 g/L sucrose and consisted of 2.5 g/L mucin (type II, porcine gastric), 2.0 g/L bacteriological peptone, 2.0 g/L tryptone, 1.0 g/L yeast extract, 0.35 g/L NaCl, 0.2 g/L KCl, 0.2 g/L CaCl
2, 0.1 g/L cysteine hydrochloride, 1 mg/L hemin, and 0.2 mg/L vitamin K
1. The flow rate was set to 9.6 mL/h. After 24 h, the flow of nutrient broth was stopped, the flow cells were opened, and the trays containing the specimens were carefully removed and immediately placed in dishes containing sterile PBS at 37 °C. The specimens were gently removed from the tray and transferred into 48-well plates. Each specimen was exposed to 500
L of CNC solution previously diluted (2%, 1.00 %, 0.50%, 0.25%, 0.12%, 0.06%, 0.03%, and 0%, n = 8 disks for each dilution). Plates were incubated at 37 °C in a 5% supplemented CO
2 environment for 2 h. Then, the solutions were discarded, the wells were washed with sterile PBS, and biomass viability was assessed on the surface of the enamel disks.
2.4. Biomass viability assay
The viable biomass was conducted as previously described [
26]. Briefly, two stock solutions were prepared by dissolving 5 mg/mL 3-(4,5)-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) and 0.3 mg/mL of N-methylphenazinium methyl sulfate (PMS) in sterile PBS. The solutions were stored at 2 °C in light-proof vials until the day of the experiment, when a fresh measurement solution (FMS) was prepared by diluting 1:1:8 the MTT stock solution, PMS stock solution, and sterile PBS, respectively. A lysing solution (LS) was prepared by dissolving 10 vol % of sodium dodecyl sulfate and 50 vol % dimethylformamide in deionized water.
A total of 300 µL of FMS solution was added to each well, and the plates were incubated at 37 °C under light-proof conditions for 1 h. During incubation, electrons transported across the microbial plasma membrane, and, to a lesser extent, microbial redox systems convert the yellow salt to insoluble purple formazan crystals. The conversion at the cell membrane level was facilitated by the intermediate electron acceptor (PMS). The unreacted FMS solution was gently removed, and the formazan crystals were dissolved by adding 300 µL of LS to each well. The plates were stored under light-proof conditions at 37 °C for an additional 1 h, and 100 µL of the solution from each well was then transferred into 96-well plates. The absorbance of the solution was measured using a spectrophotometer (Genesys 10-S, Thermo Spectronic, Rochester, NY, USA) at a wavelength of 550 nm. The results were expressed as relative absorbance in optical density (OD) units corresponding to the amount of adherent, viable, and metabolically active biomass. The results were converted to % viability, considering negative control (filter-sterile water) as 100% and a 1 wt. % chlorhexidine digluconate solution in filtered sterile water (positive control) as 0%.
2.5. Statistical analysis
Analyses were performed using JMP 17.0 software (SAS Institute, Cary, NC, USA). The normal distribution of data was checked using Shapiro-Wilk’s test (p < 0.05), and the homogeneity of variances was verified using Levene’s test (p < 0.05). Means and standard errors were calculated from the raw data. One-way analysis of variance (ANOVA) was performed considering α value of 0.05. Tukey’s HSD was employed as a post-hoc test (p < 0.05).
3. Results
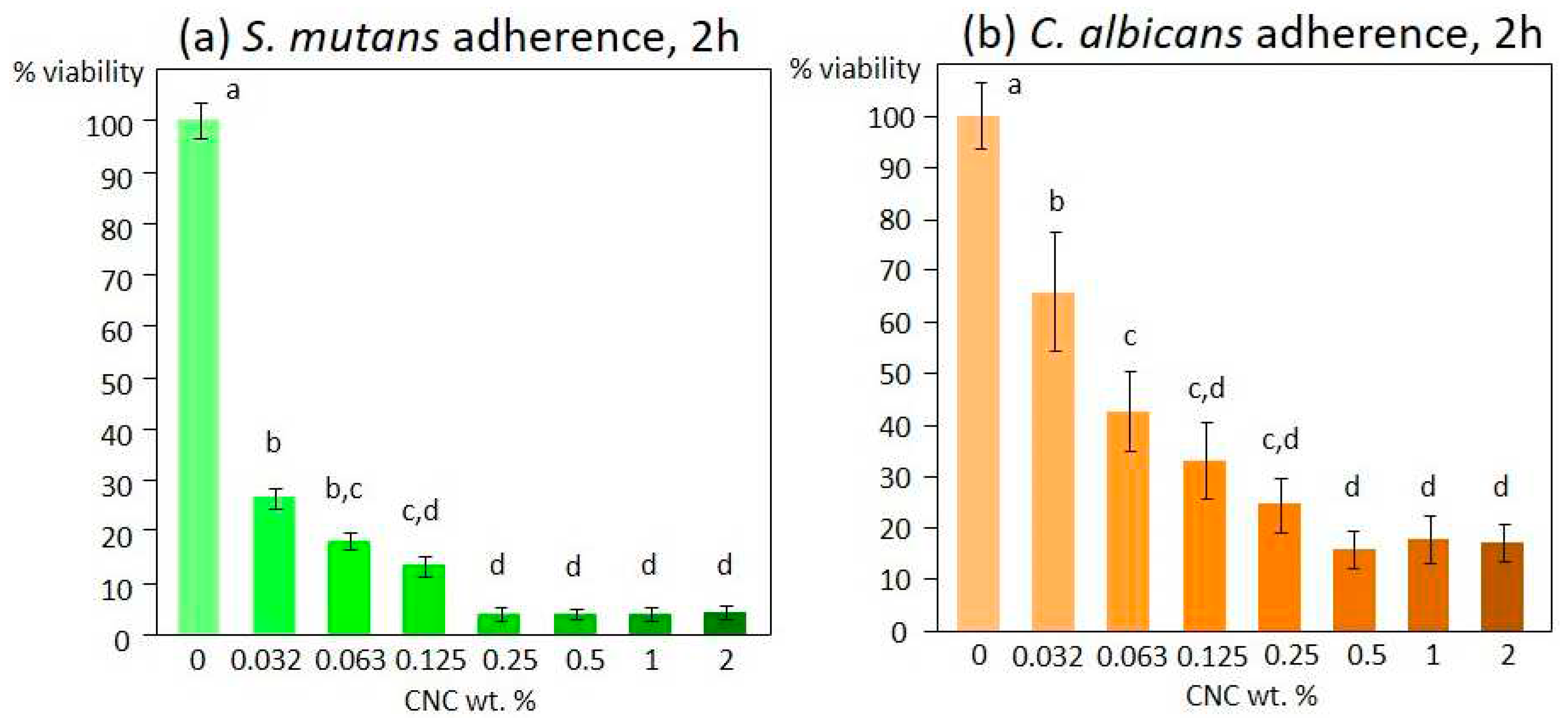
S. mutans and C. albicans adherence in response to the CNC solution tested at different concentrations showed a similar trend. The viable mass results indicated a significant inhibition of adherence on both S. mutans and C. albicans, starting from the lowest concentration tested (0.03 %, p < 0.0001 and p=0.008, respectively). This anti-adherent effect was much more pronounced on S. mutans (- 70 %) than on C. albicans (- 30 %). The effect reached maximum values at 0.25 % and 0.5 % concentrations for S. mutans and C. albicans, respectively. Complete inhibition was obtained for S. mutans, while a maximum of 80 % inhibition was reached for C. albicans (
Figure 1).
S. mutans biofilm formation (
Figure 2a) was significantly reduced by CNCs when in a concentration of at least 0.5 % (p=0.01), reaching about –15 %. Increasing concentrations did not produce additional reductions. Only the highest concentration of CNC tested (2 %, p=0.01) reduced the artificial oral microcosm biofilm by about 25 % (
Figure 2b).
4. Discussion
There is currently an overwhelming interest in medical science regarding nanoparticles showing antimicrobial properties [
5,
28]. However, many of the possibilities entail concerns about their biocompatibility and toxicity [
10,
11,
12]. In this sense, cellulose nanocrystals have received much attention for their biocompatible and non-toxic properties in addition to promising antimicrobial properties. [
12,
13,
29,
30,
31].
In this study, we evaluated in vitro the anti-adherent and anti-biofilm effect of CNCs against microbiological models of oral interest, including S. mutans, C. albicans, and the oral artificial microcosm made by mixed flora. Our results showed that the tested CNCs were already highly effective as an anti-adherent principle from the lowest tested concentration (0.03 wt. %). This activity was twice as high against S. mutans compared to C. albicans.
This finding is of utmost interest for an active principle in the oral environment. Adherence is an essential step for microbial colonization in the oral cavity [
32]. Dental hard tissues are covered by an acquired salivary pellicle, which, from a microbial colonization point of view, makes them unique substrates in the human body. For this reason, the biofilm that permanently colonizes such an ecological niche possesses peculiar features [
33]. Pioneer microorganisms that first adhere are equipped with adhesins that specifically bind to the acquired salivary pellicle, thus resisting shear stresses [
34]. Colonization by other taxa allows biofilm formation and maturation, including extracellular matrix production and an increase in its complexity to harbor at least 2,000 taxa, with ~700 species residing in an individual's mouth at a lifetime [
20,
35]. Some species are typically commensal and spend their time symbiosis with the human host. Then again, some can cause oral diseases such as dental caries, gingivitis, and periodontitis [
2,
17,
36]. Thus, control of microbial adherence in the oral cavity is essential for the control of biofilm and, ultimately, for the development of the above diseases [
37]. For these reasons, our results open a very promising possibility to control and modulate the interactions of oral microorganisms with the host and its substrata. Furthermore, the fact that the anti-adherent activity was higher for a pathogenic microorganism such as
S. mutans than the other tested strain suggests the promising possibility of a selective activity that could be directed mainly towards pathogenic species. Such a hypothesis will be the core of future studies on the activity of CNCs in the oral environment.
The literature regarding the anti-adherent properties of CNCs is still poor. [
29] In 2017, it was demonstrated that 0.1 to 1 wt. % solutions of CNCs produced a very significant reduction of the adherence by
Escherichia coli to the cell surfaces of an intestinal cell line. This effect was caused by a direct interaction of CNCs with microbial cells rather than a biocidal effect. In fact, a biocidal activity could be conferred to cellulose-based materials only by further functionalization with antimicrobial compounds [
15,
38]. Interestingly, Noronha et al. demonstrated a significant biocidal activity of CNCs against
E. coli due to a disruption of the integrity of lipid bilayer vesicles, assuming, therefore, that CNCs penetrated the cells and inflicted irreversible damage to their membrane [
39]. This result is in contrast with the findings of the present study. It must be noted that oral biofilms develop a thick layer of extracellular polysaccharides that protects microbial cells from the surrounding environment. For this reason, we never saw complete inactivation of microbial cells organized in biofilms to concentrations up to 2 wt. % CNC and only a slight reduction in viability was observed, possibly due to the inactivation of the outer layer of cells. This consideration highlights the importance of the experimental conditions to replicate the physiological conditions as closely as possible. The latter include, for the oral environment, testing microbial cell colonization to natural hard surfaces in the presence of acquired salivary pellicle and shear stresses that force adherent cells to present a biofilm structure and characteristics that resemble clinical ones [
40]. In this sense, using a bioreactor allows the replication of most, if not all, of the clinically relevant biofilm behavior.
5. Conclusions
The results of the present study show that the tested CNCs exhibited excellent anti-adhesive activity against S. mutans and C. albicans. Such activity was significantly higher against S. mutans than C. albicans, suggesting a selective anti-adherent activity against oral pathogenic strains. At the same time, there was a minimal albeit significant anti-biofilm activity against both monospecies S. mutans biofilm and oral microcosm multispecies biofilm. This makes CNCs particularly interesting as anticaries agents, encouraging their use in the oral field.
Author Contributions
Conceptualization, A.C.I., B.L.F., L.Z., and E.B.; methodology, A.C.I., A.P., and P.S.; software, P.S. and A.P.; validation, G.M.T., E.B., and B.L.F.; investigation, A.C.I., A.P., and P.S.; resources, B.L.F., L.Z., A.C.I.; writing—original draft preparation, A.P., A.C.I., E.B.; writing—review and editing, B.L.F., L.Z., P.S., and G.M.T.; project administration, E.B., G.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are made available by the Corresponding Author (ACI) upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, F.; Hung, H.C.; Yan, W.; Wu, K.; Shimchuk, A.A.; Gray, S.D.; He, W.; Huang, X.; Zhang, H. Inhibition of oral biofilm formation by zwitterionic nonfouling coating. J Biomed Mater Res B Appl Biomater 2021, 109, 1418–1425. [Google Scholar] [CrossRef]
- Zaura, E., Keijser, B., J. F., Huse, S.,M., Crielaard, W. Defining the healthy ”core microbiome” of oral microbial communities. BMC Microbiol 2009, 9, 259. [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome - an update for oral healthcare professionals. Br Dent J 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol 2017, 44 Suppl 18, S12–S22. [Google Scholar] [CrossRef]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J Dent Res 2010, 89, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y., Qin, Z-Y., Yan, C-F., Yao, J-M. Green nanocomposites based on functionalized cellulose nanocrystals: a study on the relationship between interfacial interaction and property enhancement. ACS Sustain Chem Eng 2014, 2(4), 875–886. [CrossRef]
- Iakovlev, M., You, X., van Heiningen, A., Sixta, H. SO2-ethanol-water (SEW) fractionation process: production of dissolving pulp from spruce. Cellulose 2014, 21(3), 1419–1429. [CrossRef]
- Zhang, S., Keshwani, D., R., Xu, Y., Hanna, M.A. Alkali combined extrusion pretreatment of corn stover to enhance enzyme saccharification. Industrial Crops Prod 2012, 37, 352–357. [CrossRef]
- Yu, H., Qin, Z., Liang, B., Liu, N., Zhou, Z., Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A 2013, 1, 3938–3944. [CrossRef]
- Reddy, N., Yang, Y. Completely biodegradable soyprotein-jute biocomposites developed using water without any chemicals as plasticizer. Industrial Crops Prod 2011, 33, 35–41. [CrossRef]
- Li, Y., Liu, Y., Chen, W., Wang, Q., Liu, Y., Li, J., Yu, H. Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chem 2016, 18, 1010–1018. [CrossRef]
- Mali, P., Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydrate Pol 2022, 275, 118668. [CrossRef]
- Taheri, A., Mohammadi, M. The Use of Cellulose Nanocrystals for Potential Application in Topical Delivery of Hydroquinone. Chem biol drug design 2015, 86(1), 102–106. [CrossRef] [PubMed]
- Sunasee, R., Hemraz, U.D., Ckless, K. Cellulose nanocrystals: a versatile nanoplatform for emerging biomedical applications. Expert opin drug deliv 2016, 13(9), 1243–1256. [CrossRef]
- Shojaeiarani, J., Bajwa, D.S., Chanda, S. Cellulose Nanocrystals Based Composites: A review. Open Access 2021. [CrossRef]
- Roman, M., Dong, S., Hirani, A., Lee, Y.W. Cellulose nanocrystals for drug delivery. ACS Symp Ser Am Chem Soc 2009, 1017, 81–91. [CrossRef]
- Xiao, J., Grier, A., Faustoferri, R.C., Alzoubi, S., Gill, A.L., Feng, C., Liu, Y., Quivey, R.G., Kopycka-Kedzierawski, D.T., Koo, H., et al. Association between Oral Candida and Bacteriome in Children with Severe ECC. J Dent Res 2018, 97(13), 1468-1476. 1468. [CrossRef]
- Xiao, J., Moon, Y., Li, L., Rustchenko, E., Wakabayashi, H., Zhao, X., Feng, C., Gill, S. R., McLaren, S., Malmstrom, H., et al. Candida albicans Carriage in Children with Severe Early Childhood Caries (S-ECC) and Maternal Relatedness. PloS one 2016, 11(10), e0164242. [CrossRef]
- Yawata, Y.; Nguyen, J.; Stocker, R.; Rusconi, R. Microfluidic Studies of Biofilm Formation in Dynamic Environments. J Bacteriol 2016, 198, 2589–2595. [Google Scholar] [CrossRef]
- Barana, D., Salanti, A., Orlandi, M., Ali, D.S., Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Industrial Crops Prod 2016, 86, 31-39. -39. [CrossRef]
- Colombo, L., Zoia, L., Violatto, M. B., Previdi, S., Talamini, L., Sitia, L., Nicotra, F., Orlandi, M., Salmona, M., Recordati, C., et al. Organ Distribution and Bone Tropism of Cellulose Nanocrystals in Living Mice. Biomacromol 2015, 16(9), 2862–2871. [CrossRef]
- Abitbol, T., Kloser, E, Gray, D., G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [CrossRef]
- Ionescu, A.C., Cazzaniga, G., Ottobelli, M., Garcia-Godoy, F., Brambilla, E. Substituted nano-hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces J Funct Biomater, 2020, 11(2), 36. [CrossRef]
- Guggenheim, B., Giertsen, E., Schüpbach, P., Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res 2001, 80(1), 363–370. [CrossRef]
- Ionescu, A.C., Cazzaniga, G., Ottobelli, M., Ferracane, J. L., Paolone, G., Brambilla, E. In vitro biofilm formation on resin-based composites cured under different surface conditions. J Dent 2018, 77, 78-86. [CrossRef]
- Brambilla, E., Ionescu, A.C., Cazzaniga, G., Ottobelli, M., Samaranayake, L.P. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation J Basic Microbiol 2016, 56(5), 480–492. 5. [CrossRef]
- Ionescu, A., Brambilla, E., Hahnel, S. Does recharging dental restorative materials with fluoride influence biofilm formation? Dent Mater 2019, 35, 1450–1463. [CrossRef]
- Auria-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Vinuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomater 2019, 9. [Google Scholar] [CrossRef]
- D'Orazio, G., Munizza, L., Zampolli, J., Forcella, M., Zoia, L., Fusi, P., Di Gennaro, P., La Ferla, B. Cellulose nanocrystals are effective in inhibiting host cell bacterial adhesion. J Mater Chem B 2017, 5(34), 7018–7020. [CrossRef] [PubMed]
- Baldelli, A., Etayash, H., Oguzlu, H., Mandal, R., Jiang, F., Hancock R.E.W., Pratap-Singh, A. Antimicrobial properties of spray-dried cellulose nanocrystals and metal oxide-based nanoparticles-in-microspheres. Chem Engineer J Adv 2022, 10(100273). [CrossRef]
- Rashid, A.B.; Hoque, M.E.; Kabir, N.; Rifat, F.F.; Ishrak, H.; Alqahtani, A.; Chowdhury, M.E.H. Synthesis, Properties, Applications, and Future Prospective of Cellulose Nanocrystals. Polymers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Burns, L.H.; Jack, A.A.; Back, C.R.; Dutton, L.C.; Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Microbial interactions in building of communities. Mol Oral Microbiol 2013, 28, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Gunaratnam, G.; Dudek, J.; Jung, P.; Becker, S.L.; Jacobs, K.; Bischoff, M.; Hannig, M. Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Berger, D., Rakhamimova, A., Pollack, A., Loewy, Z. . Oral Biofilms: Development, Control, and Analysis. High-throughput 2018, 7(3), 24. [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv Dent Res 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Bechinger, B., Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J Dent Res 2017, 96(3), 254–260. [CrossRef]
- Nemeş, N.S., Ardean, C., Davidescu C.M., Negrea A., Ciopec M., Duteanu N., Negrea P., Paul C., Duda-Seiman D., Muntean D. Antimicrobial Activity of Cellulose Based Materials. Polymers 2022, 14, 735. [CrossRef]
- Noronha, V.T., Camargos, C.H.M., Jackson, J.C., Souza Filho, A.G., Paula, A. J., Rezende, C.A., Faria, A.F. Physical Membrane-Stress-Mediated Antimicrobial Properties of Cellulose Nanocrystals. ACS Sustain Chem Eng 2021, 9, 3203–3212. [CrossRef]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 2007, 71, 653–670. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).