Submitted:
05 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental methods
2.1. Materials and methods
2.2. Synthesis of Ca3(BTC)2
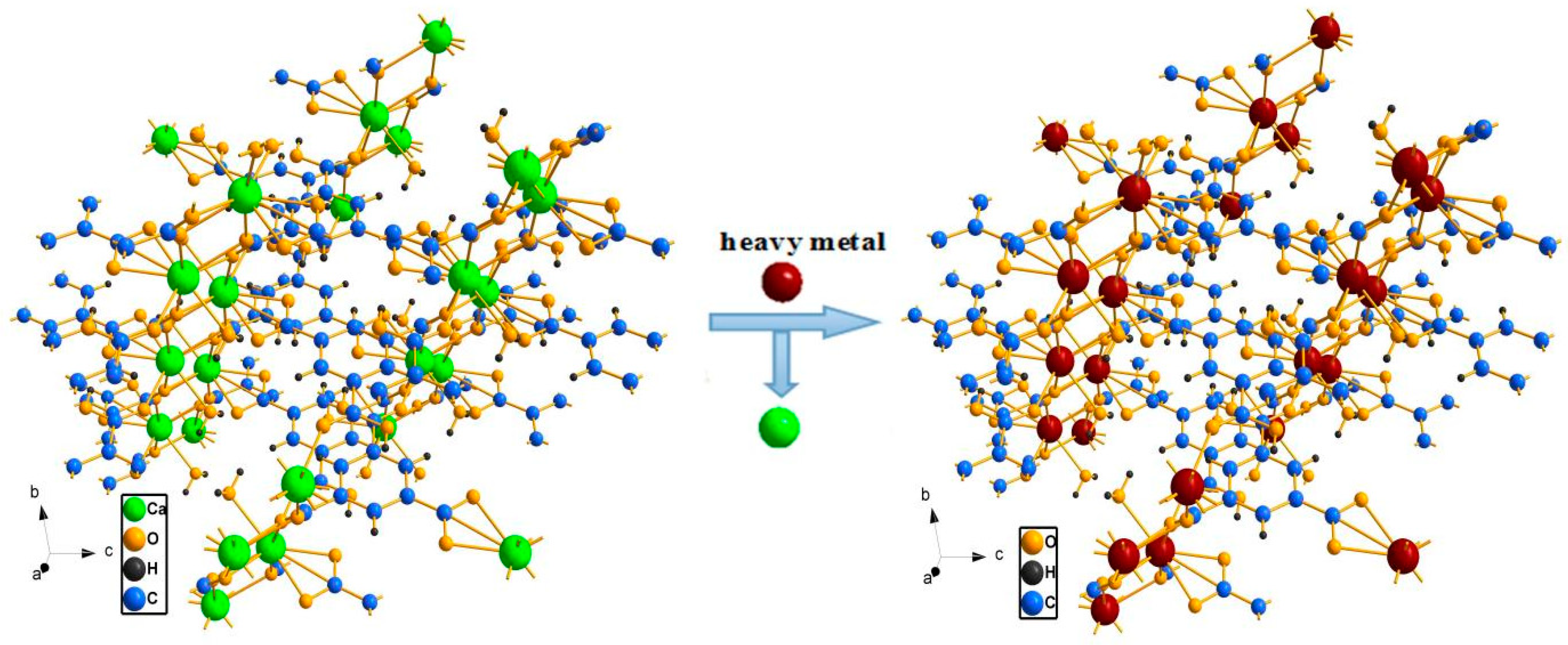
2.3. Capture and selectivity of Ca3(BTC)2 for Pb(II)
3. Results and discussion
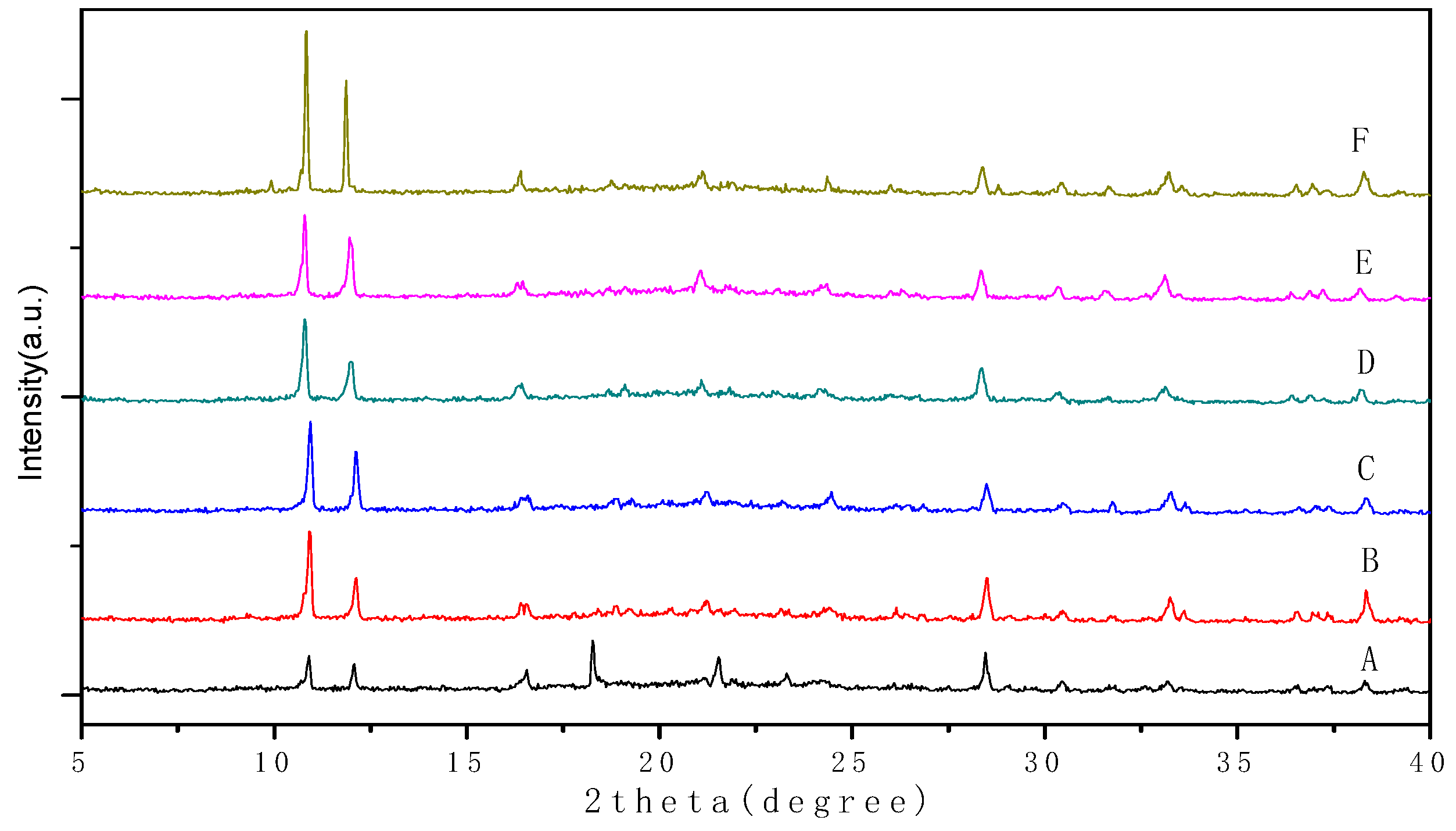
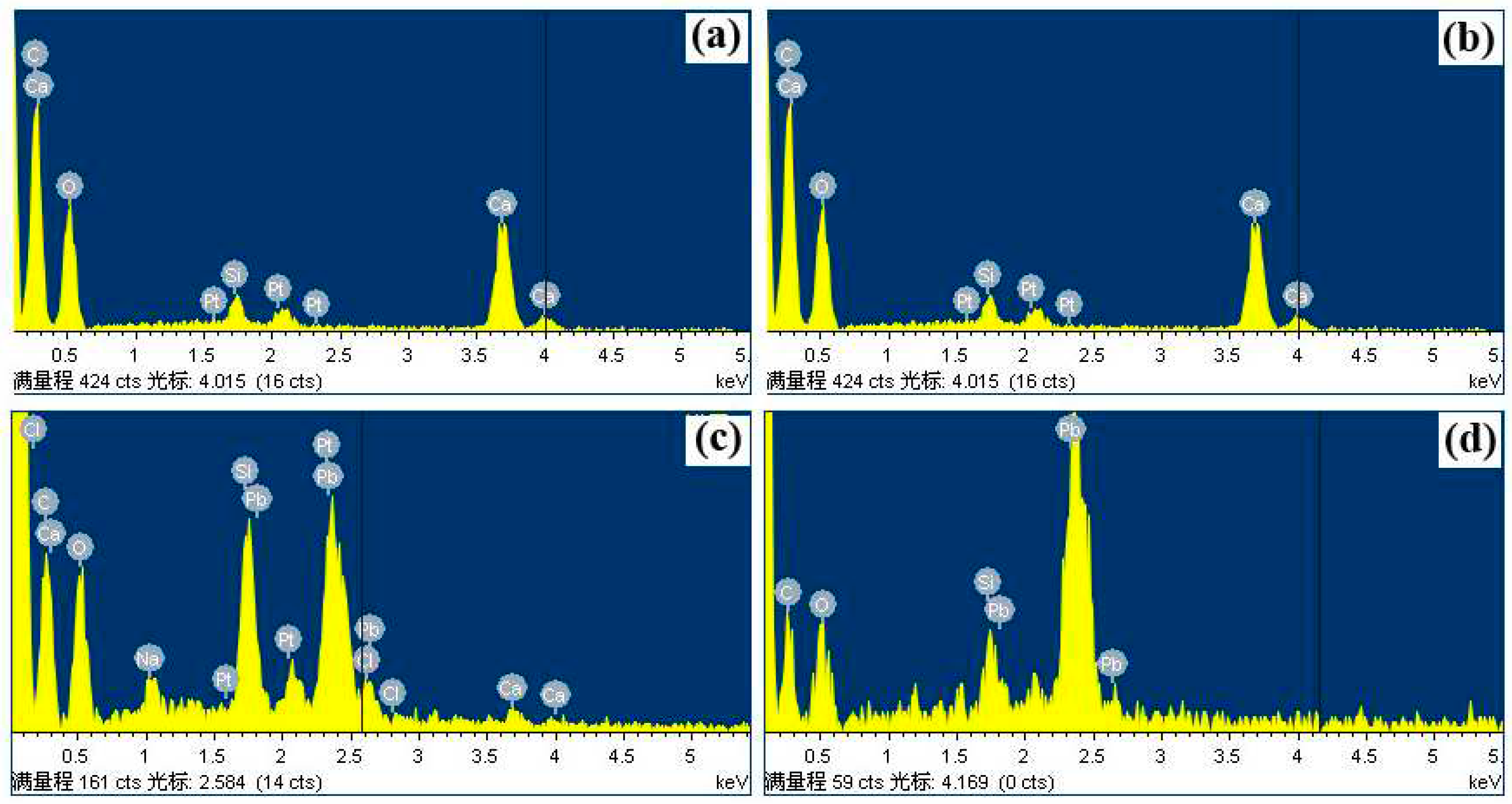
3.1. Measurement of Ca3(BTC)2 samples before and after ion-exchange
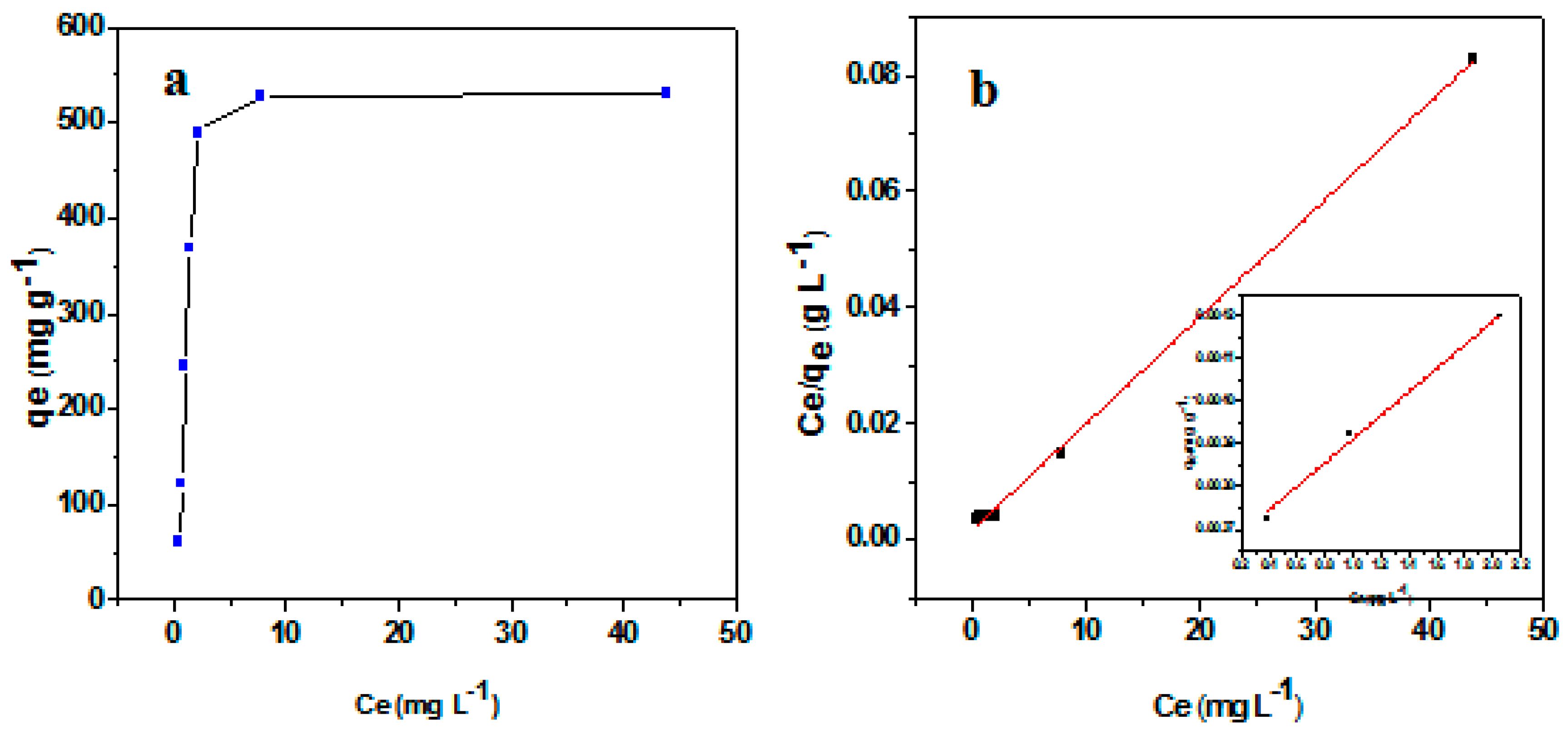
3.2. Adsorption isotherms for Pb(II)
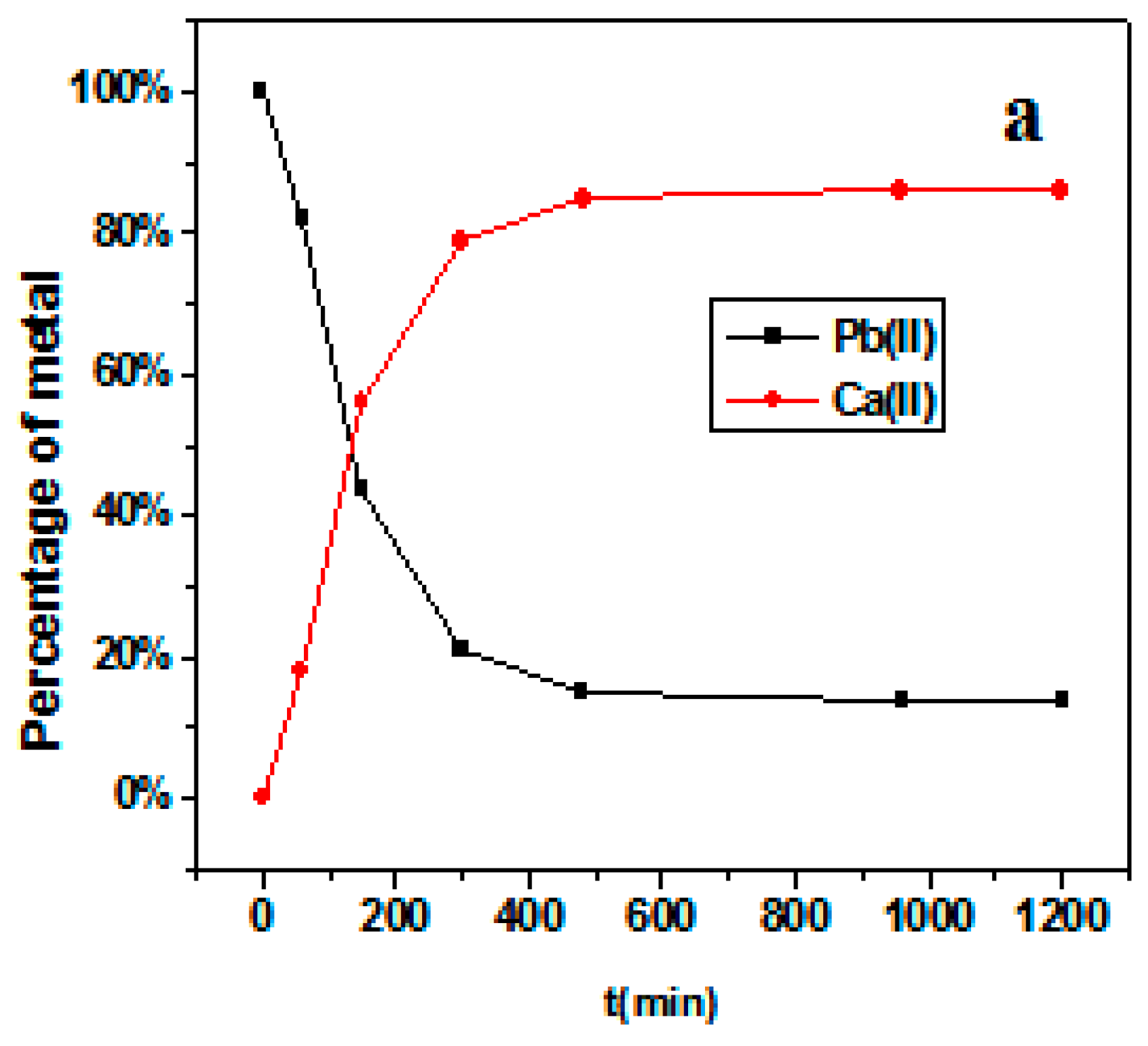
3.3. Adsorption kinetics
4. Conclusions
Acknowledgements
References
- X.Y. Shi, Y.Y. Shan, M. Du, et al. Synthesis and application of metal-organic framework films. Coord. Chem. Rev., 444 (2021) 214060. [CrossRef]
- P. Hirschle, T. Preiß, F. Auras, et al. Exploration of MOF nanoparticles sizes using various physical characterization methods-is what you measure what you get. Cryst. Eng. Comm., 18 (2016) 4359-4368. [CrossRef]
- B.J. Zhu, X.Y. Yu, Y. Jia. Iron and 1,3,5-Benzenetricarboxylic Metal−Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. Phys. Chem., 116 (2012) 8601-8607. [CrossRef]
- N.A. Khan, Z. Hasan, S.H. Jhung. Adsorptive Removal of Hazardous Materials Using Metal-Organic Frameworks (MOFs): A Review. J. Hazard. Mater., 244-245 (2013) 444-456. [CrossRef]
- F. L.Fu, L.P. Xie, B. Tang, et al. Application of a novel strategy-Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater. Chem. Eng. J., 189-190 (2012) 283-287. [CrossRef]
- G. Sharma, B. Thakur, M. Naushad, et al. Fabrication and characterization of sodium dodecyl sulphate@ironsilicophosphate nanocomposite: Ion exchange properties and selectivity for binary metal ions. Mater. Chem. Phys., 193 (2017) 129-139. [CrossRef]
- J. Song, H. Oh, H. Kong, et al. Polyrhodanine modified anodic aluminum oxide membrane for heavy metal ions removal. J. Hazard. Mater.,187(2011) 311-317. [CrossRef]
- R.T. Brosky, S. Pamukcu. Role of DDL processes during electrolytic reduction of Cu(II) in a low oxygen environment. J. Hazard. Mater.,262 (2013) 878-882. [CrossRef]
- N. Hilal, M. Al-Abri, A. Moran, et al. Effects of heavy metals and polyelectrolytes in humic substance coagulation under saline conditions. Desalination 220 (2008) 85-95. [CrossRef]
- L.W. Mi, H.W. Hou, Z.Y. Song, et al. Polymeric Zinc Ferrocenyl Sulfonate as a Molecular Aspirator for the Removal of Toxic Metal Ions, Chem. Eur. J., 14(2008)1814-1821. [CrossRef]
- S. Das, H. Kim, K. Kim. Metathesis in single crystal: complete and reversible exchange of metal ions constituting the frameworks of metal-organic frameworks, J. Am. Chem. Soc., 131 (2009) 3814-3815. [CrossRef]
- N. Dizge, B. Keskinler, H. Barlas. Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin[J]. J. Hazard. Mater., 167 (2009) 167915-167926. [CrossRef]
- C.H. Xiong, Y.J. Feng, C.P. Cai. Adsorption of Pb2+ on macroporous weak acid adsorbent resin from aqueous solutions: Batch and column studies. J. Cent. South Univ. Technol., 16 (2009): 569-574. [CrossRef]
- S.B. Wang, Y..L. Peng, Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J., 156 (2010) 11-24. [CrossRef]
- E. Erdem, N. Karapinar, R. Donat. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci., 280 (2004) 309-314. [CrossRef]
- R.F. Balderas-Valadez, M. Weiler, V. Agarwal, et al. Optical characterization of porous silicon monolayers decorated with hydrogel microspheres. Nano. Res. Lett., 9 (2014) 425-431. [CrossRef]
- E. Mery, S.A. Alekseev, V.N. Zaitsev, et al. Covalent grafting of ion-exchanging groups on porous silicon for microsystem applications. Sens. Actuators, B: Chem., 126 (2007) 120-125. [CrossRef]
- M. Plabst, L.B. McCusker, T. Bein. Exceptional Ion-Exchange Selectivity in a Flexible Open Framework Lanthanum(III)tetrakisphosphonate. J. Am. Chem. Soc., 131 (2009) 18112-18118. [CrossRef]
- H.H. Fei, J.F. Cahill, K.A. Prather, et al.Tandem Postsynthetic Metal Ion and Ligand Exchange in Zeolitic Imidazolate Frameworks. Inorg. Chem., 52 (2013) 4011-4016. [CrossRef]
- J. Canivet, A. Fateeva, Y. Guo, et al. Water adsorption in MOFs: fundamentals and Applications Farrusseng. Chem. Soc. Rev. 43 (2014) 5594-5617.
- M. Kim, J.F. Cahill, H. Fei, et al. Postsynthetic Ligand and Cation Exchange in Robust Metal–Organic Frameworks. J. Am. Chem. Soc., 134 (2012) 18082-18088. [CrossRef]
- M. Shu, C.L. Tu, W.D. Xu, et al. Reversible Anion Exchanges of Porous Metal-Organic Frameworks: Syntheses and Structures of Silver Complexes with Novel Rigid Tripodal Nitrogen Ligands. Cryst. Growth Des., 6 (2006) 1890-1896. [CrossRef]
- P. S. Prasad, B. C. G. Marupalli, S. Das, et al. Surfactant-assisted synthesis of hydroxyapatite particles: a comprehensive review. J. Mater. Sci., 58 (2023): 6076-6105. [CrossRef]
- F. Ke, L.G. Qiu, Y.P. Yuan, et al. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater., 196 (2011) 36-43. [CrossRef]
- R.K. Vakiti, B.D. Garabato, N.P. Schiebe, et al. Synthesis and Characterization of Two- and Three-Dimensional Calcium Coordination Polymers Built with Benzene-1,3,5-tricarboxylate and/or Pyrazine-2-carboxylate, Cryst. Growth Des., 12 (2012) 3937-3943. [CrossRef]
- Y.Y. Yang, Z.Q. Huang, L. Szeto, et al. A two-dimensional layer structure containing calcium: [Ca3(1,3,5-benzenetricarboxylate)2(H2O)12]n. Appl. Organomet. Chem., 18 (2004) 97-98.
- M.J. Platers, R. A. Howie, A.J. Roberts. Hydrothermal synthesis and X-ray structural characterisation of calcium benzene-1,3,5-tricarboxylate, Chem. Commun. 7 (1997) 893-894. [CrossRef]
- C.W. Abney, J.C. Gilhula, K. Lu, et al. Metal-Organic Framework Templated Inorganic Sorbents for Rapid and Effi cient Extraction of Heavy Metals. Adv. Mater., 26 (2014) 7993-7997.
- C.M. Sun, F. Ma, G.H. Zhang, R.J. Qu, et al. Removal of Mercury Ions from Ethanol Solution Using Silica Gel Functionalized with Amino-Terminated Dendrimer-Like Polyamidoamine Polymers: Kinetics and Equilibrium Studies, J. Chem. Eng. Data, 56 (2011) 4407-4415. [CrossRef]
- J.D. Xiao, L.G. Qiu, X. Jiang, et al. Magnetic porous carbons with high adsorption capacity synthesized by a microwave-enhanced high temperature ionothermal method from a Fe-based metal-organic framework. Carbon, 59 (2013) 372-382. [CrossRef]
- M.A. Farajzadeh, A.B. Monji. Adsorption characteristics of wheat bran towards heavy metal cations. Sep. Purif. Technol., 38 (2004) 197-207. [CrossRef]
- M. Kumar, B.P. Tripathi, V.K. Shahi, Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater., 172 (2009) 1041-1048. [CrossRef]
- A. Ozer, H.B. Pirincci, The adsorption of Cd(II) ions on sulphuric acid-treated wheat bran. J. Hazard. Mater., 137 (2006) 849-855. [CrossRef]
- V.N. Tirtom, A. Dinçer, S. Becerik, et al. Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan-clay composite beads in aqueous solution, Chem. Eng. J., 197 (2012) 379-386. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



