Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physiologic Features of Eosinophils and Basophils
2.1. Physiologic Features of Eosinophils
2.1.1. Charcot-Leyden Crystals
2.2. Physiologic Features of Basophils
2.2.1. Metachromatic Granules
3. AML with Increased Eosinophils (Table 1)
3.1. AML with inv(16) or t(16;16)
| AML subtype | Epidemiology | Clinical features | Diagnostic methods | Treatment | Prognosis |
|---|---|---|---|---|---|
| AML with inv(16)(p13.1q22)/ t(16;16)(p13.1;q22); CBFB‒MYH11 | 5-8% of AML cases; more common in young patients | Myelomonocytic (M4) differentiation; abnormal eosinophil morphology (basophilic granules, hypolobated); peripheral eosinophilia uncommon |
RT-PCR Karyotype FISH |
Standard intensive regimen plus gemtuzumab ozogamicin | Favorable |
| AML with t(8;21)(q22;q22); RUNX1‒RUNX1T1 | 1-5% of AML cases; more common in young patients |
Myeloblastic differentiation (M2 or M1); normal eosinophil morphology; excess basophils; peripheral eosinophilia uncommon | RT-PCR Karyotype FISH | Standard intensive regimen plus gemtuzumab ozogamicin | Favorable |
| AML with t(9;12)(q34;p13); ETV6‒ABL1 | Rare; most common in young men | Typically accompanied by peripheral-blood eosinophilia; abnormal eosinophil morphology (coarse eosinophilic and/or basophilic granules) | FISH RT-PCR Karyotype |
Standard chemotherapy plus second generation TKI | Poor prognosis; aggressive disease |
| AML with FIP1L1‒PDGFRA | Most common in young men | Typically accompanied by peripheral-blood eosinophilia; dysplastic eosinophils; frequent mast cells and reticulin fibrosis | FISH RT-PCR |
Standard chemotherapy plus imatinib | High rate of complete remission; increased risk of relapse |
| AML with PDGFRB (5q31-q33) rearrangement | Most common in men | Typically accompanied by peripheral-blood eosinophilia (but less prominent than FIP1L1‒PDGFRA); dysplastic eosinophils | FISH Karyotype | Standard chemotherapy plus imatinib | High rate of complete remission; increased risk of relapse |
| AML with FGFR1 (8p11) rearrangement | Wide range of ages (3-84 years) | Frequent hepatosplenomegaly | FISH Karyotype | TKI, midostaurin, pemigatinib | Poor prognosis |
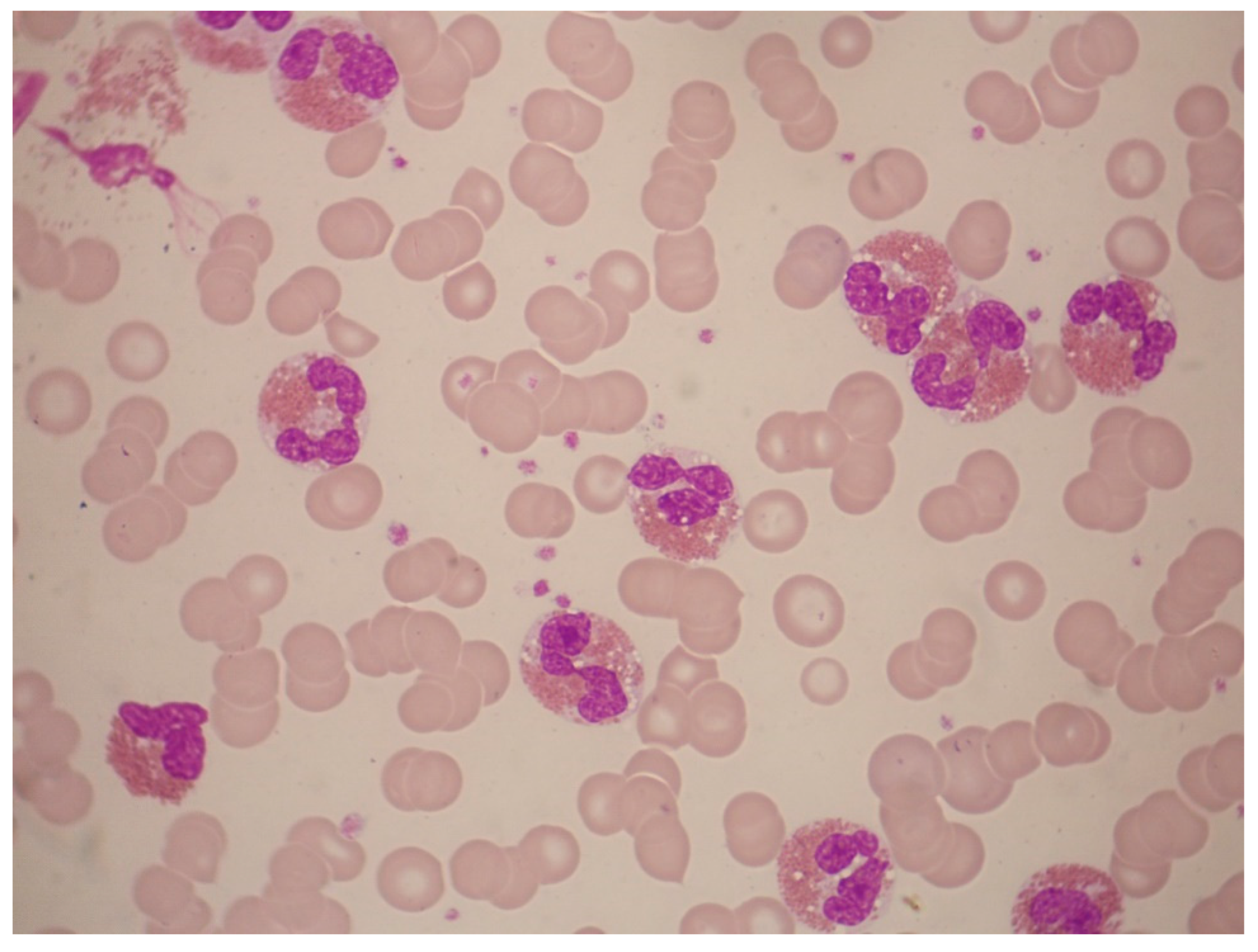
3.1.1. Morphology
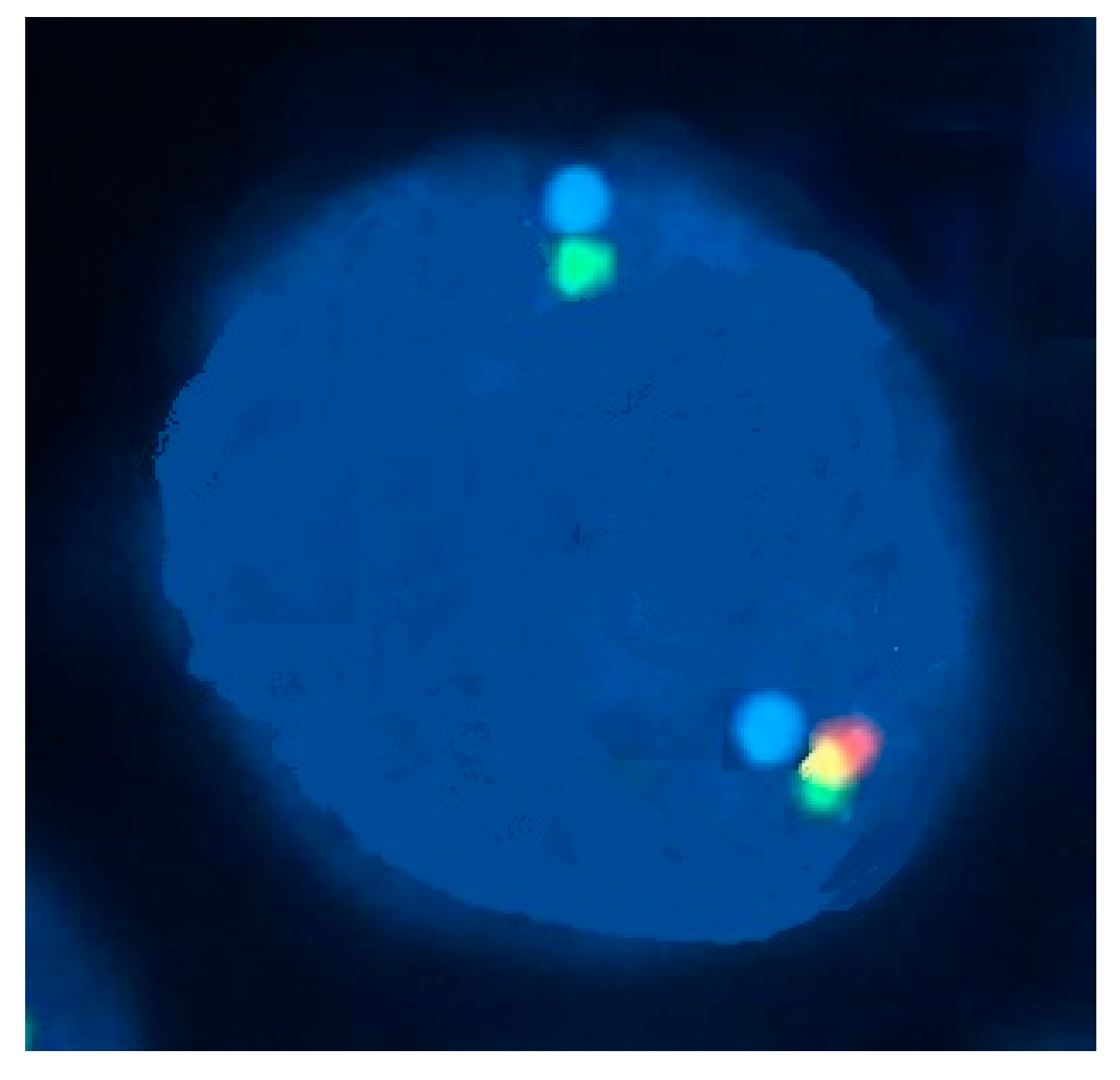
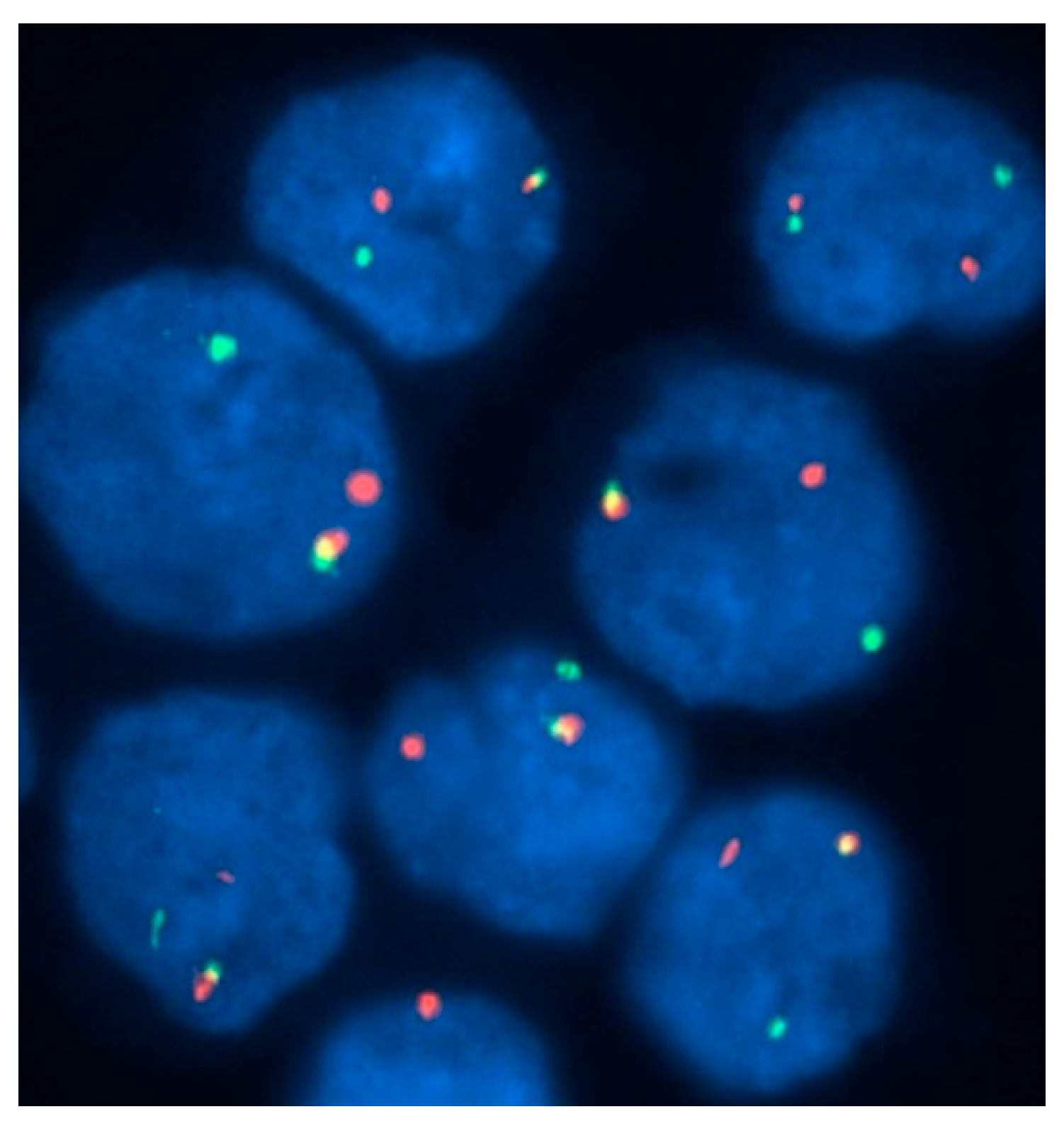
3.1.2. Confirmation of Diagnosis
3.2. AML with t(8;21)
3.2.1. Morphology
3.2.2. Confirmation of Diagnosis
3.3. AML with ETV6‒ABL1 (ΤΕL‒ABL1)
3.3.1. Morphology
3.3.2. Confirmation of Diagnosis
3.4. AML with PDGFRA, PDGFRΒ, and FGFR1 Rearrangements
3.4.1. AML with PDGFRA Rearrangements
- eosinophils with a trilobed nucleus or hypersegmented eosinophils;
- unilobed eosinophils (nuclear hyposegmentation);
- eosinophils with reduced or sparse granulation (abnormal granulation);
- eosinophils with smaller than normal granules (microgranulation);
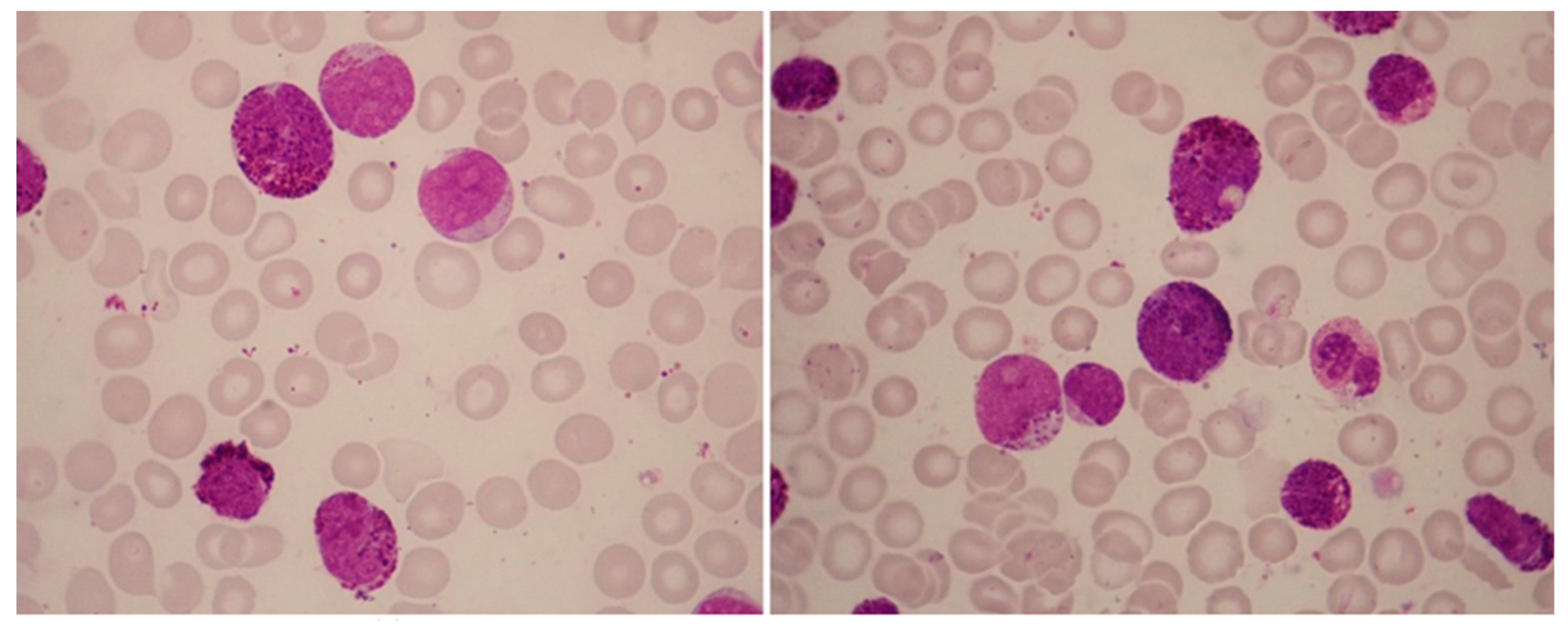
- eosinophils with many cytoplasmic vacuoles due to degranulation (Figure 6).
3.4.2. AML with PDGFRB Rearrangements
3.4.3. AML with FGFR1 Rearrangements
3.5. Rare Translocations Involved in AML with Eosinophilia
3.6. The Authors’ Recommendation for a Practical Approach to AML with Increased Eosinophils
4. AML with Increased Basophils
4.1. Differential Diagnosis of Leukemias with Basophilic Granules (Table 2)
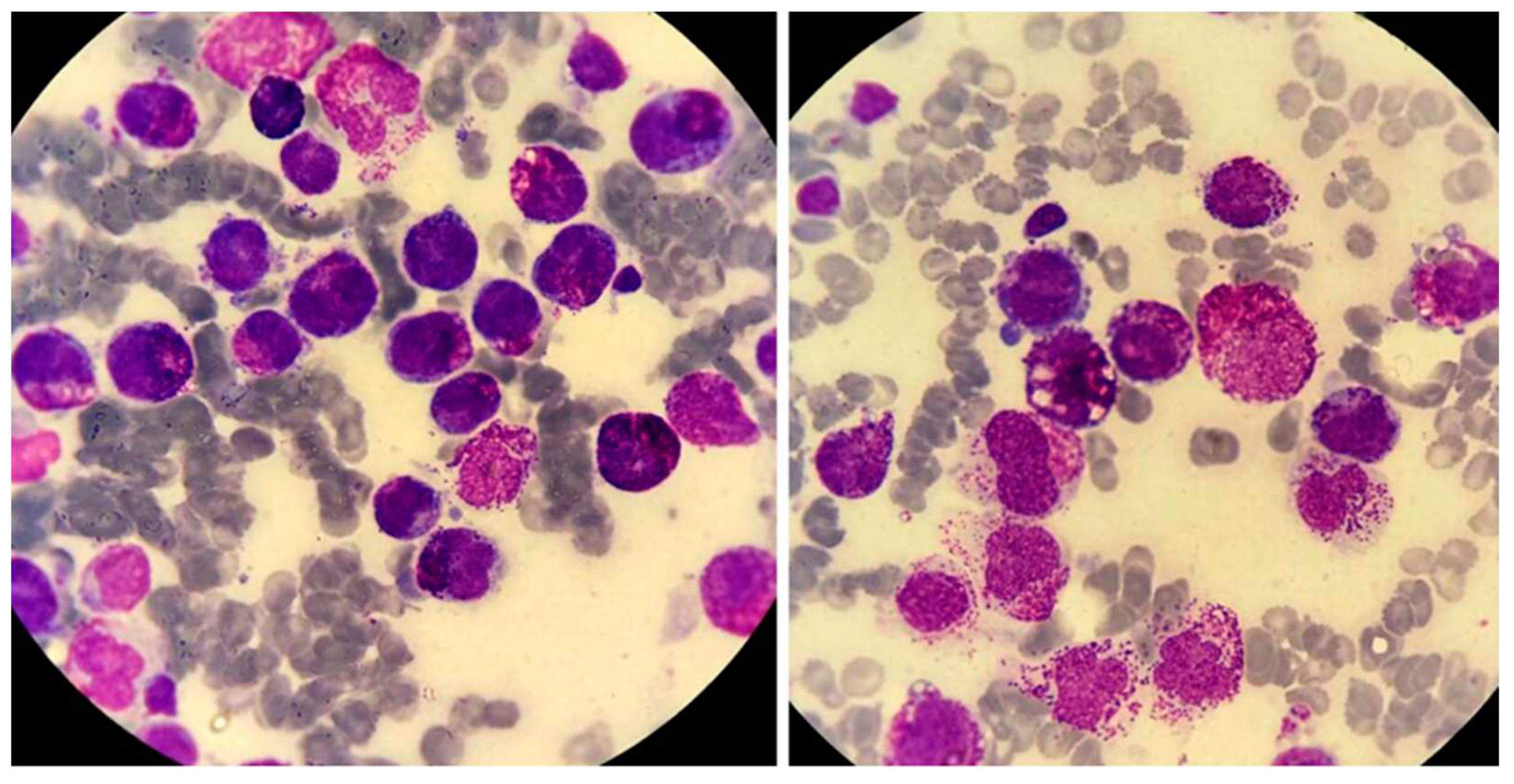
4.1.1. Basophilic Blast Phase of CML
4.1.2. AML with t(6;9)(p23;q34.1); DEK‒NUP214
4.1.3. AML with t(8;21)(q22;q22.1); RUNX1‒RUNX1T1
4.1.4. Acute Mast-Cell Leukemia
4.1.5. Basophilic Variant of APL; PML‒RARA
4.1.6. Acute Basophilic Leukemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993, 82, 3515-3523.
- Mack E.A.; Pear W.S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr Opin Hematol. 2020, 27, 27-33. [CrossRef]
- Tenen D.G. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003, 3, 89-101. [CrossRef]
- Kato M.; Kephart G.M.; Talley N.J.; Wagner J.M.; Sarr M.G.; Bonno M.; McGovern T.W.; Gleich G.J. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998, 252, 418-425. [CrossRef]
- Hoffman R.; Benz E.; Silberstein L.; Heslop H.; Weitz J.; Anastasi J.; Salama M. Hematology: Basic Principles and Practice. Elsevier: Philadelphia, United States, 2018; pp. 330-331. [CrossRef]
- Hoffbrand V.; Higgs R.; Keeling D.; Mehta A. Postgraduate haematology. John Wiley & Sons: Chichester, England, 2016; pp. 260-262.
- Acharya K.R.; Ackerman S.J. Eosinophil granule proteins: form and function. J Biol Chem. 2014, 289, 17406-17415. [CrossRef]
- Valent P.; Klion A.D.; Horny HP.; Roufosse F.; Gotlib J.; Weller P.F.; Hellmann A.; Metzgeroth G.; Leiferman K.M.; Arock M.; Butterfield J.H.; Sperr W.R.; Sotlar K.; Vandenberghe P.; Haferlach T.; Simon H.U.; Reiter A.; Gleich G.J. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012, 130, 607-612. [CrossRef]
- Shomali W.; Gotlib J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022, 97, 129-148. [CrossRef]
- Liapis K. Approach to eosinophilia. Haema. 2019, 10, 118‒128.
- Valent P.; Sotlar K.; Blatt K.; Hartmann K.; Reiter A.; Sadovnik I.; Sperr W.R.; Bettelheim P.; Akin C.; Bauer K.; George T.I.; Hadzijusufovic E.; Wolf D.; Gotlib J.; Mahon F.X.; Metcalfe D.D.; Horny HP.; Arock M. Proposed diagnostic criteria and classification of basophilic leukemias and related disorders. Leukemia. 2017, 31, 788-797. [CrossRef]
- Sasaki H.; Kurotaki D.; Tamura T. Regulation of basophil and mast cell development by transcription factors. Allergol Int. 2016, 65, 127-134. [CrossRef]
- Nei Y.; Obata-Ninomiya K.; Tsutsui H.; Ishiwata K.; Miyasaka M.; Matsumoto K.; Nakae S.; Kanuka H.; Inase N.; Karasuyama H. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A. 2013, 110, 18620-18625. [CrossRef]
- Sasaki H.; Kurotaki D.; Osato N.; Sato H.; Sasaki I.; Koizumi S.; Wang H.; Kaneda C.; Nishiyama A.; Kaisho T.; Aburatani H.; Morse H.C. 3rd.; Ozato K.; Tamura T. Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood. 2015, 125, 358-69. [CrossRef]
- Mukai K.; BenBarak M.J.; Tachibana M.; Nishida K.; Karasuyama H.; Taniuchi I.; Galli SJ. Critical role of P1-Runx1 in mouse basophil development. Blood. 2012, 120, 76-85. [CrossRef]
- Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood Transfus. 2012, 10, 148-164. [CrossRef]
- Lichtman M.A.; Segel G.B. Uncommon phenotypes of acute myelogenous leukemia: basophilic, mast cell, eosinophilic, and myeloid dendritic cell subtypes: a review. Blood Cells Mol Dis. 2005, 35, 370-383. [CrossRef]
- Tallman M.S.; Hakimian D.; Snower D.; Rubin C.M.; Reisel H.; Variakojis D. Basophilic differentiation in acute promyelocytic leukemia. Leukemia. 1993, 7, 521-526.
- Adya N.; Stacy T.; Speck N.A.; Liu P.P. The leukemic protein core binding factor beta (CBFbeta)-smooth-muscle myosin heavy chain sequesters CBFalpha2 into cytoskeletal filaments and aggregates. Mol Cell Biol. 1998, 18, 7432-7443. [CrossRef]
- Kanno Y.; Kanno T.; Sakakura C.; Bae S.C.; Ito Y. Cytoplasmic sequestration of the polyomavirus enhancer binding protein 2 (PEBP2)/core binding factor alpha (CBFalpha) subunit by the leukemia-related PEBP2/CBFbeta-SMMHC fusion protein inhibits PEBP2/CBF-mediated transactivation. Mol Cell Biol. 1998, 18, 4252-4261. [CrossRef]
- Haferlach T.; Winkemann M.; Löffler H.; Schoch R.; Gassmann W.; Fonatsch C.; Schoch C.; Poetsch M.; Weber-Matthiesen K.; Schlegelberger B. The abnormal eosinophils are part of the leukemic cell population in acute myelomonocytic leukemia with abnormal eosinophils (AML M4Eo) and carry the pericentric inversion 16: a combination of May-Grünwald-Giemsa staining and fluorescence in situ hybridization. Blood. 1996, 87, 2459-2463. [CrossRef]
- Xiao W.; Yabe M.; Offin M.; Khattar P.; Baik J.; Daley R.J.; Pappacena J.J.; Roshal M.; Zhang Y.; Tallman M.S.; Cai SF. Evolution of a chemosensitive core-binding factor AML into an aggressive leukemia with eosinophilic differentiation. Blood Adv. 2018, 2, 1517-1521. [CrossRef]
- La Starza R.; Trubia M.; Testoni N.; Ottaviani E.; Belloni E.; Crescenzi B.; Martelli M.; Flandrin G.; Pelicci P.G.; Mecucci C. Clonal eosinophils are a morphologic hallmark of ETV6/ABL1 positive acute myeloid leukemia. Haematologica. 2002, 87, 789-794.
- Zaliova M.; Moorman A.V.; Cazzaniga G.; Stanulla M.; Harvey R.C.; Roberts K.G.; Heatley S.L.; Loh M.L.; Konopleva M.; Chen I.M.; Zimmermannova O, Schwab C.; Smith O.; Mozziconacci M.J.; Chabannon C.; Kim M.; Frederik Falkenburg J.H.; Norton A.; Marshall K.; Haas O.A.; Starkova J.;Stuchly J.; Hunger SP.; White D.; Mullighan C.G.; Willman CL.; Stary J.; Trka J.; Zuna J. Characterization of leukemias with ETV6-ABL1 fusion. Haematologica. 2016, 101, 1082-1093. [CrossRef]
- Tirado C.A.; Siangchin K.; Shabsovich D.S.; Sharifian M.; Schiller G. A novel three-way rearrangement involving ETV6 (12p13) and ABL1 (9q34) with an unknown partner on 3p25 resulting in a possible ETV6-ABL1 fusion in a patient with acute myeloid leukemia: a case report and a review of the literature. Biomark Res. 2016, 4, 16. [CrossRef]
- Park J.; Kim M.; Lim J.; Kim Y.; Han K.; Kim J.S.; Lee S.; Kim H.J.; Min W.S. Variant of ETV6/ABL1 gene is associated with leukemia phenotype. Acta Haematol. 2013, 129, 78-82. [CrossRef]
- Yao J.; Xu L.; Aypar U.; Meyerson H.J.; Londono D.; Gao Q.; Baik J.; Dietz J.; Benayed R.; Sigler A.; Yabe M.; Dogan A.; Arcila M.E.; Roshal M.; Zhang Y.; Mauro M.J.; Xiao W. Myeloid/lymphoid neoplasms with eosinophilia/ basophilia and ETV6-ABL1 fusion: cell-of-origin and response to tyrosine kinase inhibition. Haematologica. 2021, 106, 614-618. [CrossRef]
- Schwaab J.; Naumann N.; Luebke J.; Jawhar M.; Somervaille TCP.; Williams M.S.; Frewin R.; Jost P.J.; Lichtenegger F.S.; La Rosée P.; Storch N.; Haferlach T.; Horny HP.; Fabarius A.; Haferlach C.; Burchert A.; Hofmann W.K.; Cross NCP.; Hochhaus A.; Reiter A.; Metzgeroth G. Response to tyrosine kinase inhibitors in myeloid neoplasms associated with PCM1-JAK2, BCR-JAK2 and ETV6-ABL1 fusion genes. Am J Hematol. 2020, 95, 824-833. [CrossRef]
- Golub T.R.; Goga A.; Barker G.F.; Afar D.E.; McLaughlin J.; Bohlander S.K.; Rowley J.D.; Witte O.N.; Gilliland D.G. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol. 1996, 16, 4107-4116. [CrossRef]
- Odero M.D.; Carlson K.; Calasanz M.J.; Lahortiga I.; Chinwalla V.; Rowley J.D. Identification of new translocations involving ETV6 in hematologic malignancies by fluorescence in situ hybridization and spectral karyotyping. Genes Chromosomes Cancer. 2001, 31, 134-142. [CrossRef]
- Odero M.D.; Carlson K.M.; Calasanz M.J.; Rowley J.D. Further characterization of complex chromosomal rearrangements in myeloid malignancies: spectral karyotyping adds precision in defining abnormalities associated with poor prognosis. Leukemia. 2001, 15, 1133-1136. [CrossRef]
- Cools J.; DeAngelo D.J.; Gotlib J.; Stover E.H.; Legare R.D.; Cortes J.; Kutok J.; Clark J.; Galinsky I.; Griffin J.D.; Cross N.C.; Tefferi A.; Malone J.; Alam R.; Schrier S.L.; Schmid J.; Rose M.; Vandenberghe P.; Verhoef G.; Boogaerts M.; Wlodarska I.; Kantarjian H.; Marynen P.; Coutre S.E.; Stone R.; Gilliland D.G. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003, 348, 1201-1214. [CrossRef]
- Gotlib J.; Cools J. Five years since the discovery of FIP1L1-PDGFRA: what we have learned about the fusion and other molecularly defined eosinophilias. Leukemia. 2008, 22, 1999-2010. [CrossRef]
- Buitenhuis M.; Verhagen L.P.; Cools J.; Coffer P.J. Molecular mechanisms underlying FIP1L1-PDGFRA-mediated myeloproliferation. Cancer Res. 2007, 67, 3759-3766. [CrossRef]
- Maccaferri M.; Pierini V.; Di Giacomo D.; Zucchini P.; Forghieri F.; Bonacorsi G.; Paolini A.; Quadrelli C.; Giacobbi F.; Fontana F.; Cappelli G.; Potenza L.; Marasca R.; Luppi M.; Mecucci C. The importance of cytogenetic and molecular analyses in eosinophilia-associated myeloproliferative neoplasms: an unusual case with normal karyotype and TNIP1- PDGFRB rearrangement and overview of PDGFRB partner genes. Leuk Lymphoma. 2017, 58, 489-493. [CrossRef]
- Montano-Almendras C.P.; Essaghir A.; Schoemans H.; Varis I.; Noël L.A.; Velghe A.I.; Latinne D.; Knoops L.; Demoulin JB. ETV6-PDGFRB and FIP1L1-PDGFRA stimulate human hematopoietic progenitor cell proliferation and differentiation into eosinophils: the role of nuclear factor-κB. Haematologica. 2012, 97, 1064-1072. [CrossRef]
- Goasguen J.E.; Bennett J.M.; Bain B.J.; Brunning R.; Zini G.; Vallespi M.T.; Tomonaga M.; Locher C. International Working Group on Morphology of MDS. The role of eosinophil morphology in distinguishing between reactive eosinophilia and eosinophilia as a feature of a myeloid neoplasm. Br J Haematol. 2020, 191, 497-504. [CrossRef]
- Savage N.; George T.I.; Gotlib J. Myeloid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, and FGFR1: a review. Int J Lab Hematol. 2013, 35, 491-500. [CrossRef]
- Abruzzo L.V.; Jaffe E.S.; Cotelingam J.D.; Whang-Peng J.; Del Duca V. Jr.; Medeiros L.J. T-cell lymphoblastic lymphoma with eosinophilia associated with subsequent myeloid malignancy. Am J Surg Pathol. 1992, 16, 236-245. [CrossRef]
- Bain J.; Horny HP.; Arber A.; Tefferi A.; Hasserjian P. Myeloid/Lymphoid Neoplasms with Eosinophilia and Rearrangement of PDGFRA, PDGFRB or FGFR1, or with PCM1-JAK2. In book WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Rev. 4th ed.; Swerdlow S.; Campo E.; Harris NL.; Jaffe E.; Pileri S.; Stein H.; Thiele J, Eds.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 2, pp 72-79.
- Jackson C.C.; Medeiros L.J.; Miranda R.N. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010, 41, 461-476. [CrossRef]
- Baer C.; Muehlbacher V.; Kern W.; Haferlach C.; Haferlach T. Molecular genetic characterization of myeloid/lymphoid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2. Haematologica. 2018, 103, 348-350. [CrossRef]
- Lee H.; Kim M.; Lim J.; Kim Y.; Han K.; Cho B.S.; Kim H.J. Acute myeloid leukemia associated with FGFR1 abnormalities. Int J Hematol. 2013, 97, 808-812. [CrossRef]
- McKeague S.J.; O'Rourke K.; Fanning S.; Joy C.; Throp D.; Adams R.; Harvey Y.; Keng T.B. Acute leukemia with cytogenetically cryptic FGFR1 rearrangement and lineage switch during therapy: A case report and literature review. Am J Clin Pathol. 2023, 19, 135. [CrossRef]
- Liapis K.; Kousiafes D.; Papanikolaou A.; Pagratis P.; Kokkini G. The 8p11 myeloid and lymphoid neoplasm. Eur J Haematol. 2011, 87, 471-472. [CrossRef]
- Metzgeroth G.; Walz C.; Score J.; Siebert R.; Schnittger S.; Haferlach C.; Popp H.; Haferlach T.; Erben P.; Mix J.; Müller M.C.; Beneke H.; Müller L.; Del Valle F.; Aulitzky W.E.; Wittkowsky G.; Schmitz N.; Schulte C.; Müller-Hermelink K.; Hodges E.; Whittaker S.J.; Diecker F.; Döhner H.; Schuld P.; Hehlmann R.; Hochhaus A.; Cross NC.; Reiter A. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia. 2007, 21, 1183-1188. [CrossRef]
- Sorour Y.; Dalley C.D.; Snowden J.A.; Cross N.C.; Reilly J.T. Acute myeloid leukaemia with associated eosinophilia: justification for FIP1L1-PDGFRA screening in cases lacking the CBFB-MYH11 fusion gene. Br J Haematol. 2009, 146, 225-227. [CrossRef]
- Lierman E.; Michaux L.; Beullens E.; Pierre P.; Marynen P.; Cools J.; Vandenberghe P. FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib. Leukemia. 2009, 23, 845-851. [CrossRef]
- Jawhar M.; Naumann N.; Schwaab J.; Baurmann H.; Casper J.; Dang T.A.; Dietze L.; Döhner K.; Hänel A.; Lathan B.; Link H.; Lotfi S.; Maywald O.; Mielke S.; Müller L.; Platzbecker U.; Prümmer O.; Thomssen H.; Töpelt K.; Panse J.; Vieler T.; Hofmann WK.; Haferlach T.; Haferlach C.; Fabarius A.; Hochhaus A.; Cross NCP.; Reiter A.; Metzgeroth G. Imatinib in myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRB in chronic or blast phase. Ann Hematol. 2017, 96, 1463-1470. [CrossRef]
- Arber D.A.; Orazi A.; Hasserjian R.P.; Borowitz M.J.; Calvo K.R.; Kvasnicka H.M.; Wang S.A.; Bagg A.; Barbui T.; Branford S.; Bueso-Ramos C.E.; Cortes J.E.; Dal Cin P.; DiNardo C.D.; Dombret H.; Duncavage E.J.; Ebert B.L.; Estey E.H.; Facchetti F.; Foucar K.; Gangat N.; Gianelli U.; Godley L.A.; Gökbuget N.; Gotlib J.; Hellström-Lindberg E.; Hobbs G.S.; Hoffman R.; Jabbour E.J.; Kiladjian J.J.; Larson R.A.; Le Beau M.M.; Loh M.L.; Löwenberg B.; Macintyre E.; Malcovati L.; Mullighan C.G.; Niemeyer C.; Odenike O.M.; Ogawa S.; Orfao A.; Papaemmanuil E.; Passamonti F.; Porkka K.; Pui C.H.; Radich J.P.; Reiter A.; Rozman M.; Rudelius M.; Savona M.R.; Schiffer C.A.; Schmitt-Graeff A.; Shimamura A.; Sierra J.; Stock W.A.; Stone R.M.; Tallman M.S.; Thiele J.; Tien H.F.; Tzankov A.; Vannucchi A.M.; Vyas P.; Wei A.H.; Weinberg O.K.; Wierzbowska A.; Cazzola M.; Döhner H.; Tefferi A. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022, 140, 1200-1228. [CrossRef]
- Khoury J.D.; Solary E.; Abla O.; Akkari Y.; Alaggio R.; Apperley J.F.; Bejar R.; Berti E.; Busque L.; Chan JKC.; Chen W.; Chen X.; Chng W.J.; Choi J.K.; Colmenero I.; Coupland S.E.; Cross NCP.; De Jong D.; Elghetany M.T.; Takahashi E.; Emile J.F.; Ferry J.; Fogelstrand L.; Fontenay M.; Germing U.; Gujral S.; Haferlach T.; Harrison C.; Hodge JC.; Hu S.; Jansen J.H.; Kanagal-Shamanna R.; Kantarjian H.M.; Kratz C.P.; Li X.Q.; Lim M.S.; Loeb K.; Loghavi S.; Marcogliese A.; Meshinchi S.; Michaels P.; Naresh K.N.; Natkunam Y.; Nejati R.; Ott G.; Padron E.; Patel K.P.; Patkar N.; Picarsic J.; Platzbecker U.; Roberts I.; Schuh A.; Sewell W.; Siebert R.; Tembhare P.; Tyner J.; Verstovsek S.; Wang W.; Wood B.; Xiao W.; Yeung C.; Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022, 36, 1703-1719. [CrossRef]
- Metzgeroth G.; Schwaab J.; Naumann N.; Jawhar M.; Haferlach T.; Fabarius A.; Hochhaus A.; Hofmann W.K.; Cross NCP.; Reiter A. Treatment-free remission in FIP1L1-PDGFRA-positive myeloid/lymphoid neoplasms with eosinophilia after imatinib discontinuation. Blood Adv. 2020, 4, 440-443. [CrossRef]
- Valent P.; Horny HP.; Arock M. The underestimated role of basophils in Ph+ chronic myeloid leukaemia. Eur J Clin Invest. 2018, 48, e13000. [CrossRef]
- Samorapoompichit P.; Kiener H.P.; Schernthaner G.H.; Jordan J.H.; Agis H.; Wimazal F.; Baghestanian M.; Rezaie-Majd A.; Sperr W.R.; Lechner K.; Valent P. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood. 2001, 98, 2580-2583. [CrossRef]
- Nakayama H.; Ishimaru F.; Avitahl N.; Sezaki N.; Fujii N.; Nakase K.; Ninomiya Y.; Harashima A.; Minowada J.; Tsuchiyama J.; Imajoh K.; Tsubota T.; Fukuda S.; Sezaki T.; Kojima K.; Hara M.; Takimoto H.; Yorimitsu S.; Takahashi I.; Miyata A.; Taniguchi S.; Tokunaga Y.; Gondo H.; Niho Y.; Harada M.; et al. Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukemia. Cancer Res. 1999, 59, 3931-3934.
- Mullighan C.G.; Miller C.B.; Radtke I.; Phillips L.A.; Dalton J.; Ma J.; White D.; Hughes T.P.; Le Beau M.M.; Pui C.H.; Relling M.V.; Shurtleff S.A.; Downing J.R. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008, 453, 110-114. [CrossRef]
- Beer PA.; Knapp D.J.; Miller P.H.; Kannan N.; Sloma I.; Heel K.; Babovic S.; Bulaeva E.; Rabu G.; Terry J.; Druker B.J.; Loriaux M.M.; Loeb K.R.; Radich J.P.; Erber W.N.; Eaves C.J. Disruption of IKAROS activity in primitive chronic-phase CML cells mimics myeloid disease progression. Blood. 2015, 125, 504-515. [CrossRef]
- Arber A.; Brunning D.; Orazi A.; Porwit A.; Peterson C.; Thiele J.; Le Beau M.; Hasserjian P. Acute myeloid leukaemia, NOS. In book WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Rev. 4th ed.; Swerdlow S.; Campo E.; Harris NL.; Jaffe E.; Pileri S.; Stein H.; Thiele J, Eds.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 2, pp 164-165.
- Drexler H.G .; Sperling C .; Ludwig W.D. Terminal deoxynucleotidyl transferase (TdT) expression in acute myeloid leukemia. Leukemia. 1993, 7, 1142-1150.
- Thiede C.; Steudel C.; Mohr B.; Schaich M.; Schäkel U.; Platzbecker U.; Wermke M.; Bornhäuser M.; Ritter M.; Neubauer A.; Ehninger G.; Illmer T. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002, 99, 4326-4335. [CrossRef]
- Seth T.; Vora A.; Bhutani M.; Ganessan K.; Jain P.; Kochupillai V. Acute basophilic leukemia with t(8;21). Leuk Lymphoma. 2004, 45, 605-608. [CrossRef]
- Georgin-Lavialle S.; Lhermitte L.; Dubreuil P.; Chandesris M.O.; Hermine O.; Damaj G. Mast cell leukemia. Blood. 2013, 121, 1285-1295. [CrossRef]
- McKenna R.W.; Parkin J.; Bloomfield C.D.; Sundberg R.D.; Brunning R.D. Acute promyelocytic leukaemia: a study of 39 cases with identification of a hyperbasophilic microgranular variant. Br J Haematol. 1982, 50, 201-214. [CrossRef]
- Ghimire A.; Liesveld J.;Wallace D.; Zhao J.; Burack R.; Bennett J. Case of acute promyelocytic leukemia with basophilic differentiation and an ETV6 mutation. J. Hematop. 2021, 14, 333–336.
- Joachim, G. Uber Mastzellenleukamien. Dtsch. Arch. Kiln. Med. 1906, 87, 437.
- Horny HP.; Sotlar K.; Reiter A.; Valent P. Myelomastocytic leukemia: histopathological features, diagnostic criteria and differential diagnosis. Expert Rev Hematol. 2014, 7, 431-437. [CrossRef]
- Duchayne E.; Demur C.; Rubie H.; Robert A.; Dastugue N. Diagnosis of acute basophilic leukemia. Leuk Lymphoma. 1999, 32, 269-278. [CrossRef]
- Staal-Viliare A.; Latger-Cannard V.; Didion J.; Grégoire M.J.; Lecompte T.; Jonveaux P.; Rio Y. CD203c /CD117-, an useful phenotype profile for acute basophilic leukaemia diagnosis in cases of undifferentiated blasts. Leuk Lymphoma. 2007, 48, 439-441. [CrossRef]
- Giagounidis A.A.; Hildebrandt B.; Heinsch M.; Germing U.; Aivado M.; Aul C. Acute basophilic leukemia. Eur J Haematol. 2001, 67, 72-76. [CrossRef]
- Scolyer R.A.; Brun M.; D'Rozario J.; Webb M. Acute basophilic leukemia presenting with abnormal liver function tests and the absence of blast cells in the peripheral blood. Pathology. 2000, 32, 52-55. [CrossRef]
- Liso V.; Troccoli G.; Specchia G. Mast cell leukemia and acute basophilic leukemia. Cytochemical studies. Bibl Haematol. 1978, 45, 142-146. [CrossRef]
- Quelen C.; Lippert E.; Struski S.; Demur C.; Soler G.; Prade N.; Delabesse E.; Broccardo C.; Dastugue N.; Mahon FX.; Brousset P. Identification of a transforming MYB-GATA1 fusion gene in acute basophilic leukemia: a new entity in male infants. Blood. 2011, 117, 5719-5722. [CrossRef]
- Toda Y.; Nagai Y.; Shimomura D.; Kishimori C.; Tsuda K.; Fukutsuka K.; Hayashida M.; Ohno H. Acute basophilic leukemia associated with the t(16;21)(p11;q22)/FUS-ERG fusion gene. Clin Case Rep. 2017, 5, 1938-1944. [CrossRef]
- Kritharis A.; Brody J.; Koduru P.; Teichberg S.; Allen SL. Acute basophilic leukemia associated with loss of gene ETV6 and protean complications. J Clin Oncol. 2011, 29, 623-626. [CrossRef]
- Shimizu T.; Kondo T.; Nannya Y.; Watanabe M.; Kitawaki T.; Shindo T.; Hishizawa M.; Yamashita K.; Ogawa S.; Takaori-Kondo A. Next-generation sequencing in two cases of de novo acute basophilic leukaemia. J Cell Mol Med. 2021, 25, 7095-7099. [CrossRef]
- Greene L.W.; Asadipooya K.; Corradi P.F.; Akin C. Endocrine manifestations of systemic mastocytosis in bone. Rev Endocr Metab Disord. 2016, 17, 419-431. [CrossRef]
- Shah I.; Lewkow L.M.; Koppitch F. Acute basophilic leukemia. Am J Med. 1984, 76, 1097-1099. [CrossRef]
- Bernini J.C.; Timmons C.F.; Sandler E.S. Acute basophilic leukemia in a child. Anaphylactoid reaction and coagulopathy secondary to vincristine-mediated degranulation. Cancer. 1995, 75, 110-114.
- Anderson W.; Helman C.A.; Hirschowitz B.I. Basophilic leukemia and the hypersecretion of gastric acid and pepsin. Gastroenterology. 1988, 95, 195-198. [CrossRef]
- Luo X.H.; Zhu Y.; Tang X.Q. Acute basophilic leukemia presenting with maculopapular rashes and a gastric ulcer: A case report. Oncol Lett. 2014, 8, 2513-2516. [CrossRef]
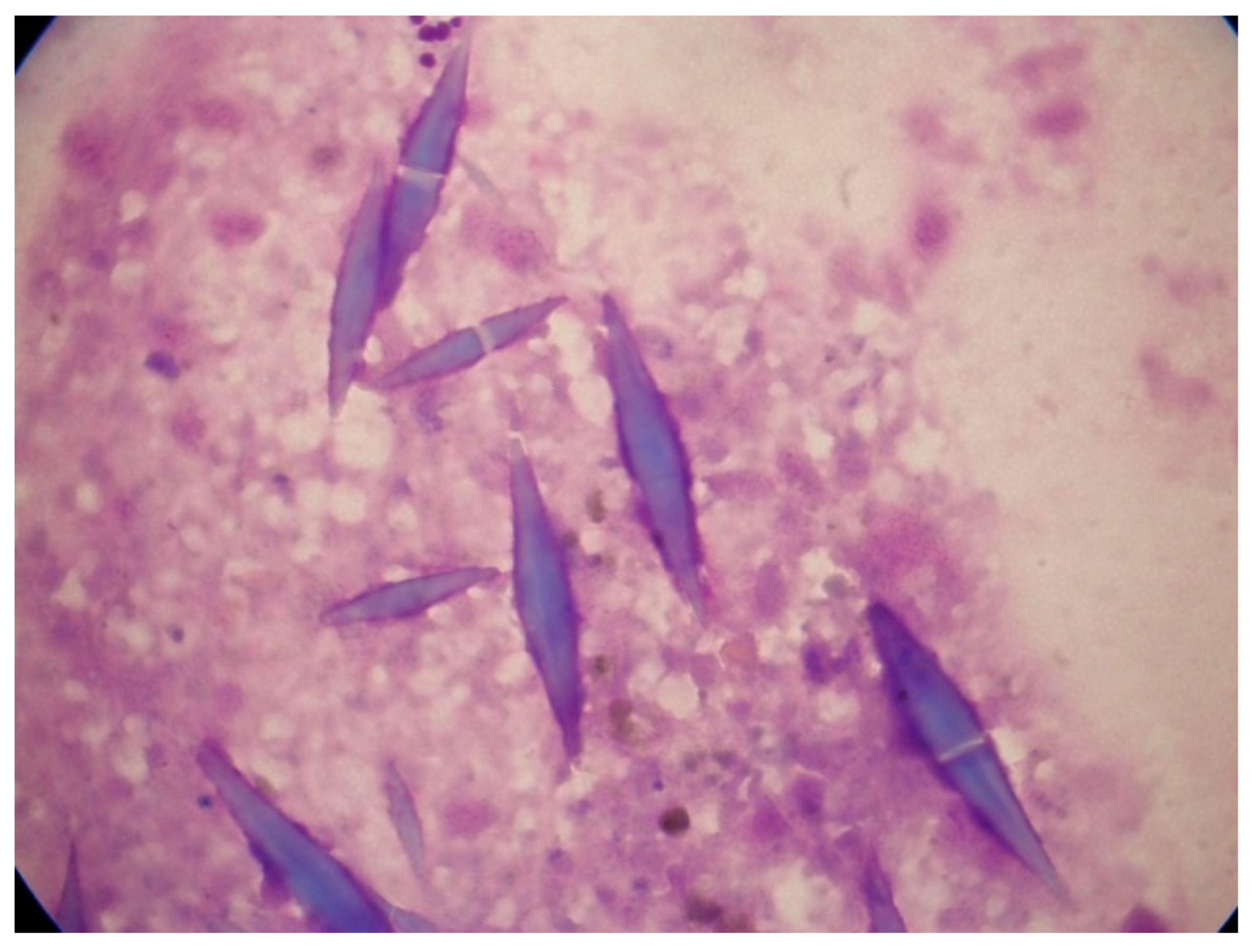
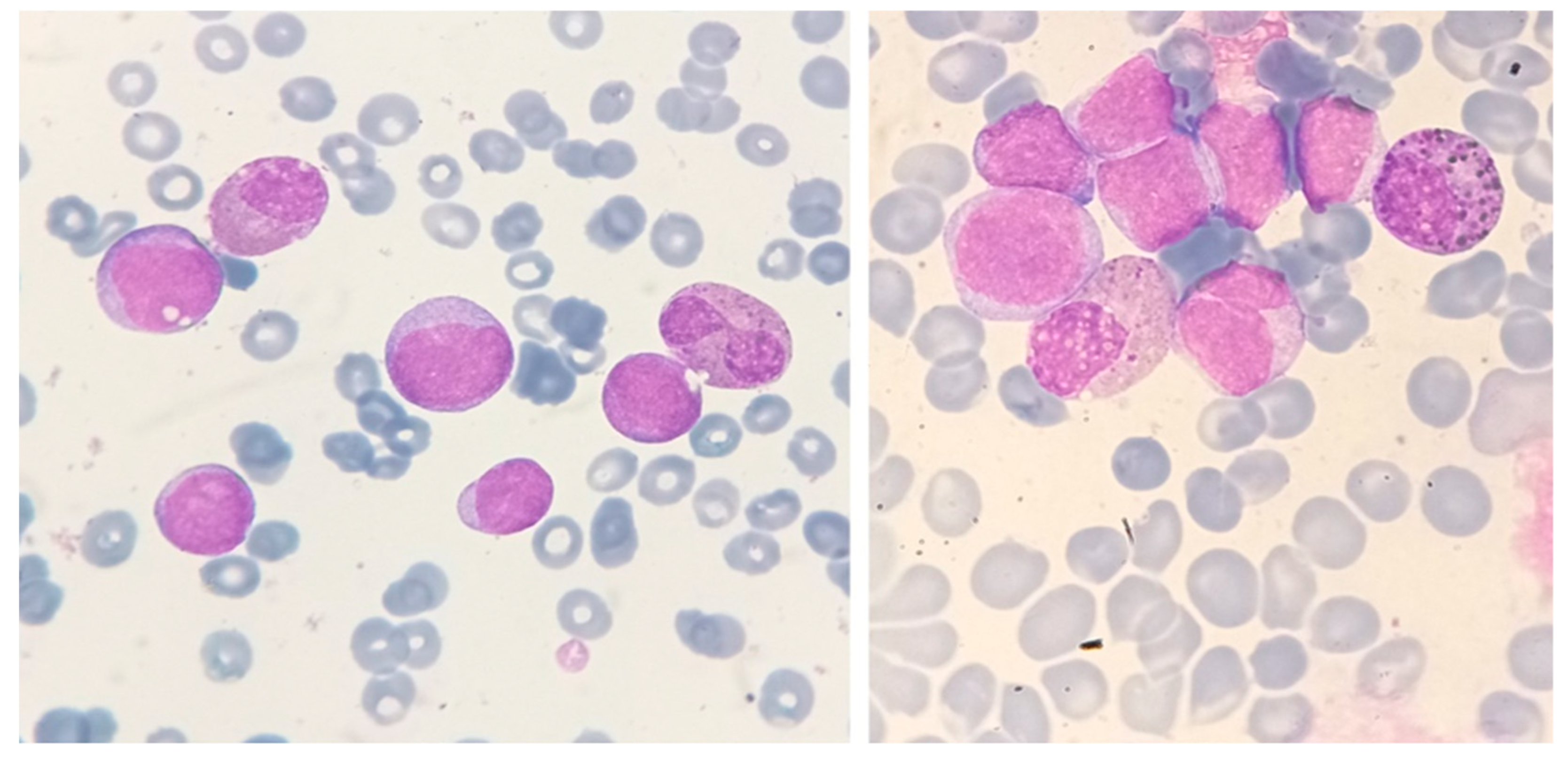
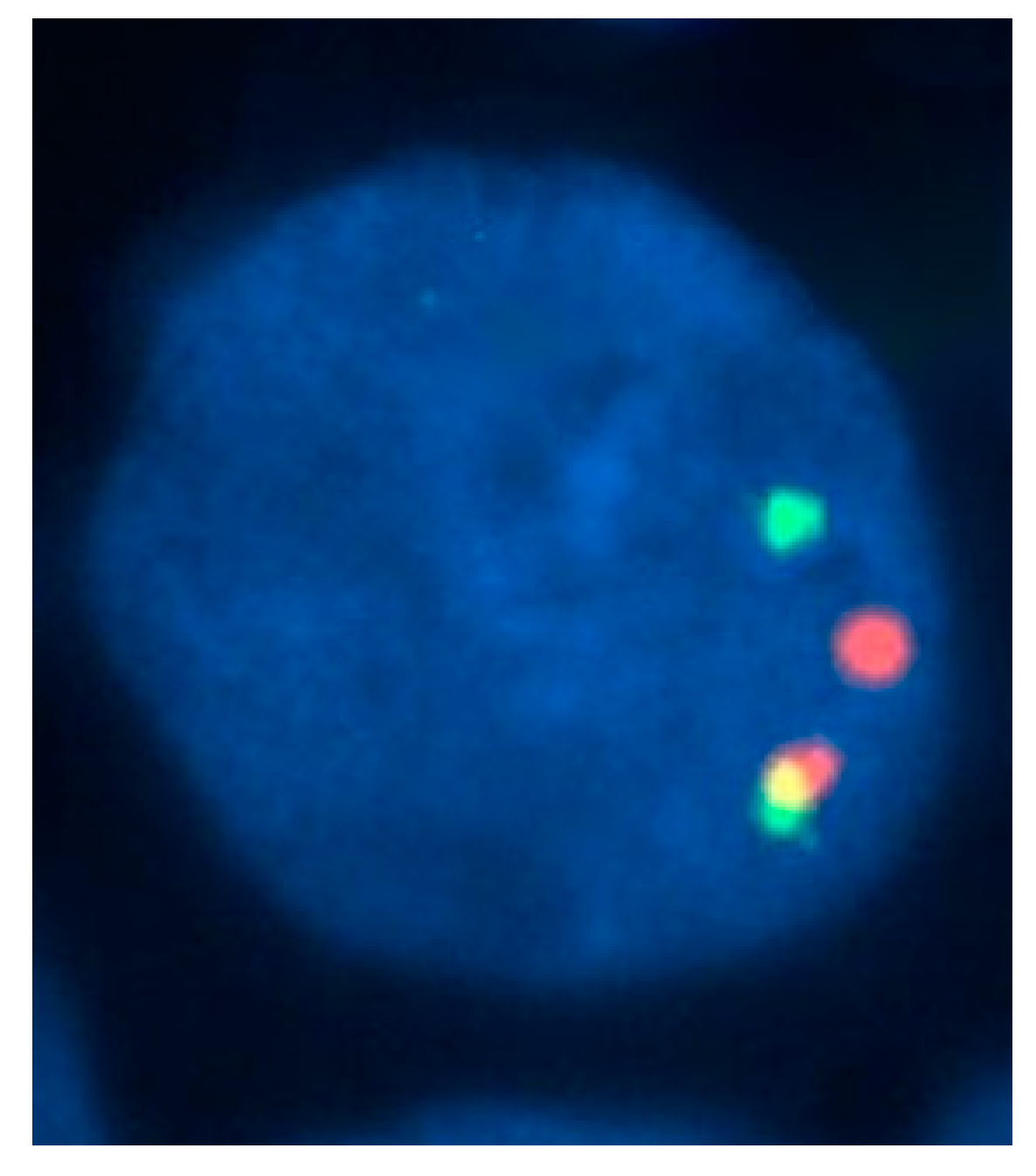
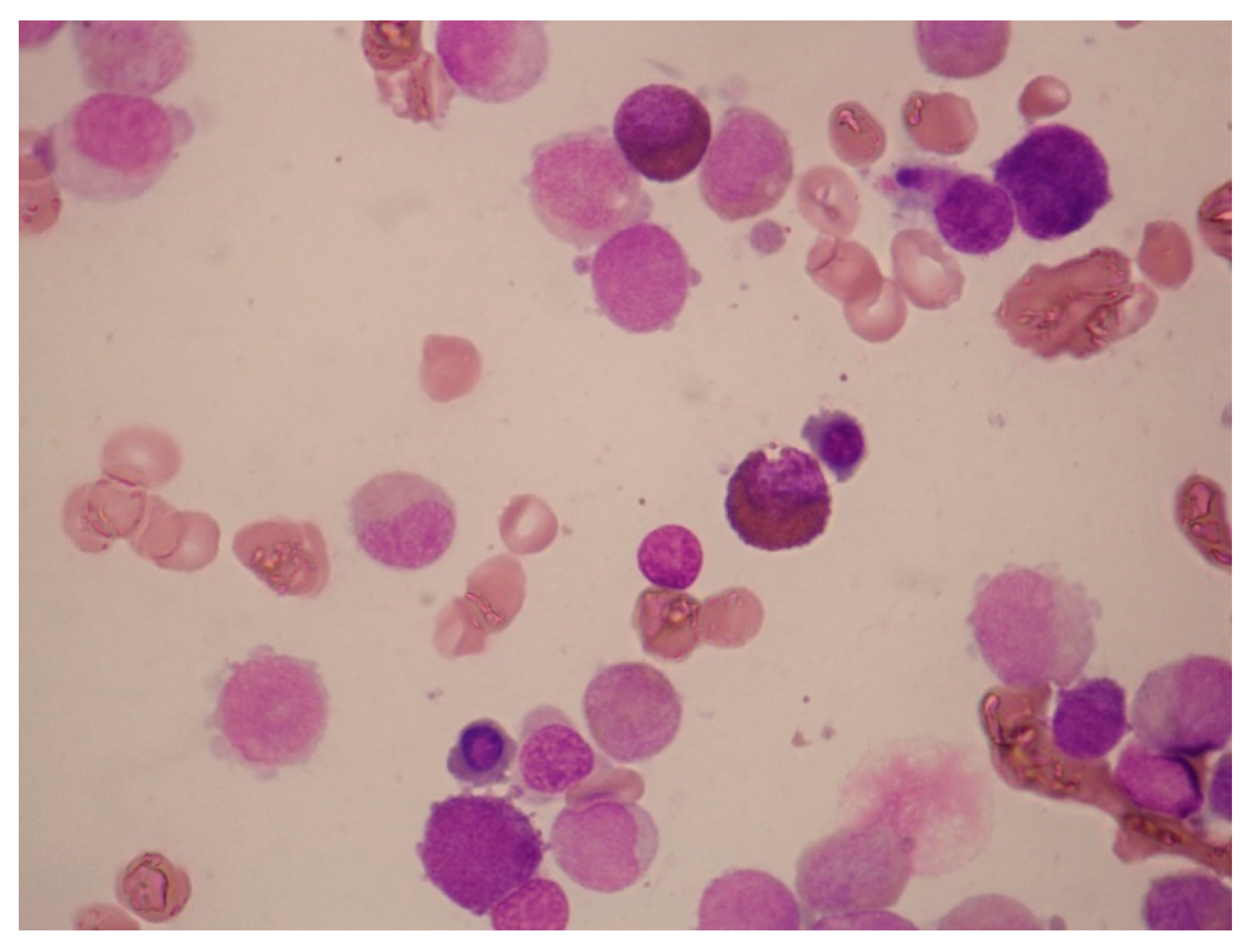
|
| Acute basophilic leukemia | Acute mast-cell leukemia | |
|---|---|---|
| Clinical features | ||
| Hyperhistaminemia Skin involvement Hepatosplenomegaly Lymphadenopathy |
Common Common Common Uncommon |
Common Uncommon Common Common |
| Special stains | ||
| Tolouidine Blue | Positive | Positive |
| Periodic-acid Schiff (P.A.S) | Positive | Negative or weak |
| Chloracetate esterase (ChorE) | Negative | Positive |
| Tryptase | Negative or weak | Positive |
| Myeloperoxidase (MPO) | Negative | Negative |
| Immunophenotypic studies | ||
| CD34 | Negative or weakly positive | Negative or weakly positive |
| CD45weak/CD13/CD33 expression | Positive | Positive |
| CD14/CD15/CD64 expression | Negative | Negative |
| CD11b | Positive | Negative |
| CD17 | Positive | Negative |
| CD123 | Positive | Negative |
| CD203c | Positive | Negative or weakly positive |
| CD2 | Negative | Positive or negative |
| CD25 | Positive | Positive or negative |
| CD117 | Negative | Strongly positive |
| Cytogenetic and molecular studies | ||
| t(X;6)(p11;q23) | C-KIT mutations (e.g. C-KIT D816V) | |
| t(16;21)(p11;q22) | ||
| del(12p) | TET2 mutations | |
| TP53, TET2 and NPM1 mutations | SRSF2, ASXL1, RUNX1 (“S/A/R”) mutations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





