Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials & Methods
2.1. Chicken Embryos and Tissue Collection
2.2. Tissue Cultures and miRNA Transfections
2.3. PE/ST Migration Assays and Time-Lapse Confocal Image Analyses
2.4. Quantitative Analyses of Time-Lapse Migration
2.5. RNA Isolation and qPCR
2.6. Statistical Analyses
3. Results
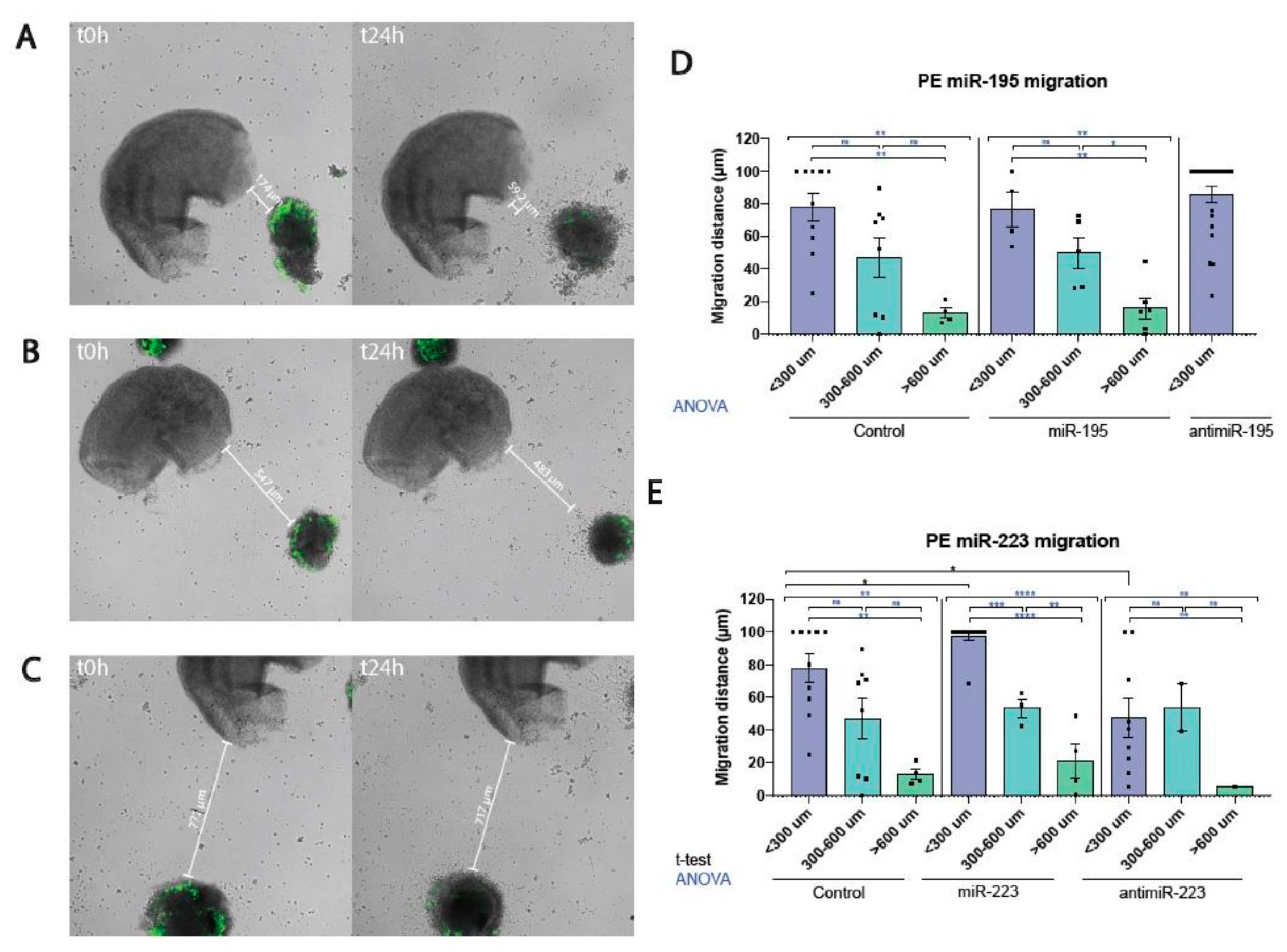
3.1. Establishment of an Ex Vivo Model of Myocardial-Epicardial Cell Migration
3.2. The Role of miR-195 Modulating PE/ST Cell Migration
3.3. The Role of miR-223 Modulating PE/ST Cell Migration
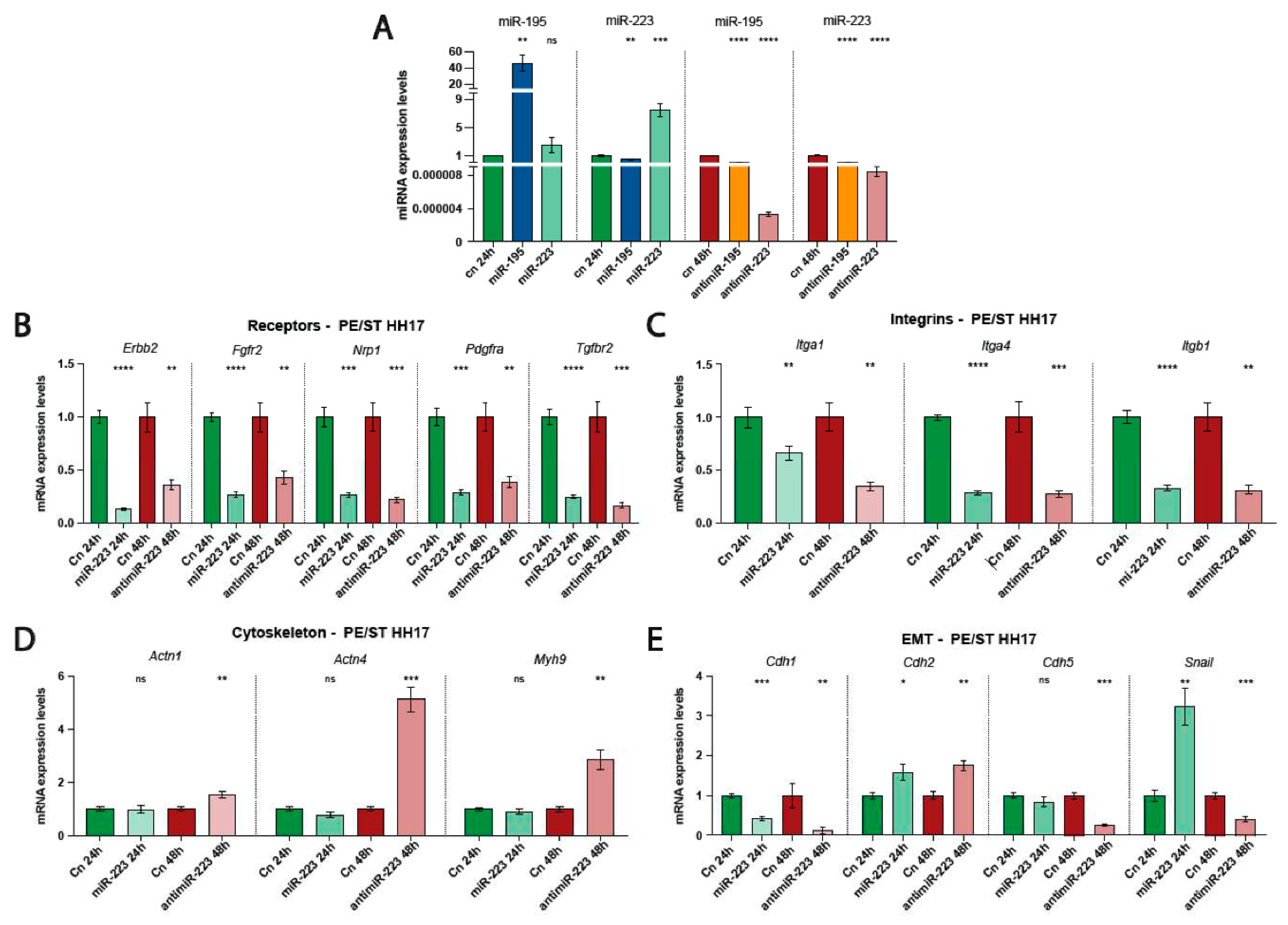
3.4. Co-Regulation of miR-195 and miR-223 Expression in PE/ST Explants
3.5. Dissecting the Molecular Mechanisms Driving miR-223-Mediated PE/ST Cell Migration
4. Discussion
Acknowledgments
References
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003 Oct;83(4):1223-67. [CrossRef] [PubMed]
- Campione M, Franco D. Current Perspectives in Cardiac Laterality. J Cardiovasc Dev Dis. 2016 Dec 9;3(4):34. [CrossRef] [PubMed] [PubMed Central]
- Campione M, Ros MA, Icardo JM, Piedra E, Christoffels VM, Schweickert A, Blum M, Franco D, Moorman AF. Pitx2 expression defines a left cardiac lineage of cells: evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev Biol. 2001 Mar 1;231(1):252-64. [CrossRef] [PubMed]
- Männer, J. 1992. The development of pericardial villi in the chick embryo. Anat Embryol (Berl) 186: 379–385. [CrossRef]
- Männer, J. 1993. Experimental study on the formation of the epicardium in chick embryos. Anat Embryol (Berl) 187: 281–289. [CrossRef]
- Männer, J., Perez-Pomares, J.M., Macias, D., and Munoz-Chapuli, R. (2001) The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169, 89–103. [CrossRef]
- Gittenberger-de Groot, A.C., Vrancken Peeters, M.-P.F.M., Mentink, M.M.T., Gourdie, R.G., and Poelmann, R.E. (1998) Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 82, 1043–1052. [CrossRef]
- Pérez-Pomares JM, Macías D, García-Garrido L, Muñoz-Chápuli R. Contribution of the primitive epicardium to the subepicardial mesenchyme in hamster and chick embryos. Dev Dyn. 1997 Oct;210(2):96-105. [CrossRef] [PubMed]
- Pérez-Pomares JM, Macías D, García-Garrido L, Muñoz-Chápuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol. 1998 Aug 1;200(1):57-68. [CrossRef] [PubMed]
- Muñoz-Chápuli R, Macías D, González-Iriarte M, Carmona R, Atencia G, Pérez-Pomares JM. The epicardium and epicardial-derived cells: multiple functions in cardiac development. Rev Esp Cardiol. 2002 Oct;55(10):1070-82. Spanish. [CrossRef]
- Pérez-Pomares JM, Carmona R, González-Iriarte M, Atencia G, Wessels A, Muñoz-Chápuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002 Dec;46(8):1005-13.
- Männer J, Schlueter J, Brand T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev Dyn. 2005 Aug;233(4):1454-63. [CrossRef] [PubMed]
- Plavicki JS, Hofsteen P, Yue MS, Lanham KA, Peterson RE, Heideman W. Multiple modes of proepicardial cell migration require heartbeat. BMC Dev Biol. 2014 May 15;14:18. [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Chinchilla A, Lozano E, Daimi H, Esteban FJ, Crist C, Aranega AE, Franco D. MicroRNA profiling during mouse ventricular maturation: a role for miR-27 modulating Mef2c expression. Cardiovasc Res. 2011 Jan 1;89(1):98-108. Epub 2010 Aug 24. [CrossRef] [PubMed]
- Garcia-Padilla C, Dueñas A, Franco D, Garcia-Lopez V, Aranega A, Garcia-Martinez V, Lopez-Sanchez C. Dynamic MicroRNA Expression Profiles During Embryonic Development Provide Novel Insights Into Cardiac Sinus Venosus/Inflow Tract Differentiation. Front Cell Dev Biol. 2022 Jan 11;9:767954. [CrossRef] [PubMed] [PubMed Central]
- Torrado M, Franco D, Lozano-Velasco E, Hernández-Torres F, Calviño R, Aldama G, Centeno A, Castro-Beiras A, Mikhailov A. A MicroRNA-Transcription Factor Blueprint for Early Atrial Arrhythmogenic Remodeling. Biomed Res Int. 2015;2015:263151. Epub 2015 Jun 28. [CrossRef] [PubMed] [PubMed Central]
- Alzein M, Lozano-Velasco E, Hernández-Torres F, García-Padilla C, Domínguez JN, Aránega A, Franco D. Differential Spatio-Temporal Regulation of T-Box Gene Expression by microRNAs during Cardiac Development. J Cardiovasc Dev Dis. 2021 May 14;8(5):56. [CrossRef] [PubMed] [PubMed Central]
- Toro R, Pérez-Serra A, Mangas A, Campuzano O, Sarquella-Brugada G, Quezada-Feijoo M, Ramos M, Alcalá M, Carrera E, García-Padilla C, Franco D, Bonet F. miR-16-5p Suppression Protects Human Cardiomyocytes against Endoplasmic Reticulum and Oxidative Stress-Induced Injury. Int J Mol Sci. 2022 Jan 18;23(3):1036. [CrossRef] [PubMed] [PubMed Central]
- Bonet F, Dueñas Á, López-Sánchez C, García-Martínez V, Aránega AE, Franco D. MiR-23b and miR-199a impair epithelial-to-mesenchymal transition during atrioventricular endocardial cushion formation. Dev Dyn. 2015 Oct;244(10):1259-75. Epub 2015 Aug 26. [CrossRef] [PubMed]
- Garcia-Padilla C, Garcia-Lopez V, Aranega A, Franco D, Garcia-Martinez V, Lopez-Sanchez C. Inhibition of RhoA and Cdc42 by miR-133a Modulates Retinoic Acid Signalling during Early Development of Posterior Cardiac Tube Segment. Int J Mol Sci. 2022 Apr 10;23(8):4179. [CrossRef] [PubMed] [PubMed Central]
- Lopez-Sanchez C, Franco D, Bonet F, Garcia-Lopez V, Aranega A, Garcia-Martinez V. Negative Fgf8-Bmp2 feed-back is regulated by miR-130 during early cardiac specification. Dev Biol. 2015 Oct 1;406(1):63-73. Epub 2015 Jul 10. [CrossRef] [PubMed]
- Singh MK, Lu MM, Massera D, Epstein JA. MicroRNA-processing enzyme Dicer is required in epicardium for coronary vasculature development. J Biol Chem. 2011 Nov 25;286(47):41036-45. Epub 2011 Oct 3. [CrossRef] [PubMed] [PubMed Central]
- Brønnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, Kalluri R, Sheikh SP. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PLoS One. 2013;8(2):e56280. Epub 2013 Feb 18. [CrossRef] [PubMed] [PubMed Central]
- Pontemezzo E, Foglio E, Vernucci E, Magenta A, D'Agostino M, Sileno S, Astanina E, Bussolino F, Pellegrini L, Germani A, Russo MA, Limana F. miR-200c-3p Regulates Epitelial-to-Mesenchymal Transition in Epicardial Mesothelial Cells by Targeting Epicardial Follistatin-Related Protein 1. Int J Mol Sci. 2021 May 7;22(9):4971. [CrossRef] [PubMed] [PubMed Central]
- Dueñas A, Expósito A, Muñoz MDM, de Manuel MJ, Cámara-Morales A, Serrano-Osorio F, García-Padilla C, Hernández-Torres F, Domínguez JN, Aránega A, Franco D. MiR-195 enhances cardiomyogenic differentiation of the proepicardium/septum transversum by Smurf1 and Foxp1 modulation. Sci Rep. 2020 Jun 9;10(1):9334. [CrossRef] [PubMed] [PubMed Central]
- Hamburger,V. Hamilton,H.L.,1951. A series of normal stages in the development of the chicken embryo. J. Morphol. 88,49–92.
- Bustin, S. A., Benes,V., Garson, J.A., Hellemans, J.,Huggett, J.,Kubista,M., et al. (2001). The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin C(T)) Method. Methods 25 (4), 402–408. [CrossRef]
- Lozano-Velasco E, Vallejo D, Esteban FJ, Doherty C, Hernández-Torres F, Franco D, Aránega AE. A Pitx2-MicroRNA Pathway Modulates Cell Proliferation in Myoblasts and Skeletal-Muscle Satellite Cells and Promotes Their Commitment to a Myogenic Cell Fate. Mol Cell Biol. 2015 Sep 1;35(17):2892-909. Epub 2015 Jun 8. [CrossRef] [PubMed] [PubMed Central]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402-8. [CrossRef] [PubMed]
- Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015 Aug 12;4:e05005. [CrossRef] [PubMed] [PubMed Central]
- Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev Dyn. 2003 Aug;227(4):511-23. [CrossRef] [PubMed]
- Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM, Mikawa T, Basson CT. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiol Genomics. 2004 Jul 8;18(2):129-40. [CrossRef] [PubMed]
- Peralta M, Steed E, Harlepp S, González-Rosa JM, Monduc F, Ariza-Cosano A, Cortés A, Rayón T, Gómez-Skarmeta JL, Zapata A, Vermot J, Mercader N. Heartbeat- driven pericardiac fluid forces contribute to epicardium morphogenesis. Curr Biol. 2013 Sep 23;23(18):1726-35. Epub 2013 Aug 15. [CrossRef] [PubMed]
- Hofsteen P, Plavicki J, Johnson SD, Peterson RE, Heideman W. Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol Pharmacol. 2013 Sep;84(3):353-60. Epub 2013 Jun 17. [CrossRef] [PubMed] [PubMed Central]
- Li J, Miao L, Zhao C, Shaikh Qureshi WM, Shieh D, Guo H, Lu Y, Hu S, Huang A, Zhang L, Cai CL, Wan LQ, Xin H, Vincent P, Singer HA, Zheng Y, Cleaver O, Fan ZC, Wu M. CDC42 is required for epicardial and pro-epicardial development by mediating FGF receptor trafficking to the plasma membrane. Development. 2017 May 1;144(9):1635-1647. [CrossRef] [PubMed] [PubMed Central]
- Andrés-Delgado L, Ernst A, Galardi-Castilla M, Bazaga D, Peralta M, Münch J, González-Rosa JM, Marques I, Tessadori F, de la Pompa JL, Vermot J, Mercader N. Actin dynamics and the Bmp pathway drive apical extrusion of proepicardial cells. Development. 2019 Jul 4;146(13):dev174961. [CrossRef] [PubMed] [PubMed Central]
- Zheng D, Ma J, Yu Y, Li M, Ni R, Wang G, Chen R, Li J, Fan GC, Lacefield JC, Peng T. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia. 2015 Aug;58(8):1949-58. Epub 2015 May 21. [CrossRef] [PubMed] [PubMed Central]
- Chen C, Jia KY, Zhang HL, Fu J. MiR-195 enhances cardiomyocyte apoptosis induced by hypoxia/reoxygenation injury via downregulating c-myb. Eur Rev Med Pharmacol Sci. 2016 Aug;20(16):3410-6. [PubMed]
- Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011 Oct 1;92(1):75-84. Epub 2011 May 27. [CrossRef] [PubMed]
- He X, Ji J, Wang T, Wang MB, Chen XL. Upregulation of Circulating miR-195-3p in Heart Failure. Cardiology. 2017;138(2):107-114. Epub 2017 Jun 16. [CrossRef] [PubMed]
- Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, Franz M, Möbius-Winkler S, Drosatos K, George I, Chen EI, Colombo PC, Schulze PC. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation. 2018 May 8;137(19):2052-2067. Epub 2018 Jan 12. [CrossRef] [PubMed] [PubMed Central]
- Wang DM, Jin JJ, Tian LM, Zhang Z. MiR-195 promotes myocardial fibrosis in MI rats via targeting TGF-β1/Smad. J Biol Regul Homeost Agents. 2020 Jul-Aug;34(4):1325-1332. [CrossRef] [PubMed]
- Carvalho A, Ji Z, Zhang R, Zuo W, Qu Y, Chen X, Tao Z, Ji J, Yao Y, Ma G. Inhibition of miR-195-3p protects against cardiac dysfunction and fibrosis after myocardial infarction. Int J Cardiol. 2023 Sep 15;387:131128. Epub 2023 Jun 24. [CrossRef] [PubMed]
- Wang L, Qin D, Shi H, Zhang Y, Li H, Han Q. MiR-195-5p Promotes Cardiomyocyte Hypertrophy by Targeting MFN2 and FBXW7. Biomed Res Int. 2019 Jun 25;2019:1580982. [CrossRef] [PubMed] [PubMed Central]
- Zhu H, Chen Z, Yu J, Wu J, Zhuo X, Chen Q, Liang Y, Li G, Wan Y. MiR-195-5p suppresses the proliferation, migration, and invasion of gallbladder cancer cells by targeting FOSL1 and regulating the Wnt/β-catenin pathway. Ann Transl Med. 2022 Aug;10(16):893. [CrossRef] [PubMed] [PubMed Central]
- Chen LP, Zhang NN, Ren XQ, He J, Li Y. miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma. Molecules. 2018 Nov 10;23(11):2938. [CrossRef] [PubMed] [PubMed Central]
- Pan SS, Zhou HE, Yu HY, Xu LH. MiR-195-5p inhibits the cell migration and invasion of cervical carcinoma through suppressing ARL2. Eur Rev Med Pharmacol Sci. 2019 Dec;23(24):10664-10671. [CrossRef] [PubMed]
- Long Z, Wang Y. miR-195-5p Suppresses Lung Cancer Cell Proliferation, Migration, and Invasion Via FOXK1. Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820922587. [CrossRef] [PubMed] [PubMed Central]
- Zhao X, Dai L, Yue Q, Wang H, Wang XU, Li Y, Chen R. MiR-195 inhibits migration, invasion and epithelial-mesenchymal transition (EMT) of endometrial carcinoma cells by targeting SOX4. J Biosci. 2019 Dec;44(6):146. [PubMed]
- Zhou D, Xu X, Liu Y, Liu H, Cheng X, Gu Y, Xu Y, Zhu L. MiR-195-5p facilitates the proliferation, migration, and invasion of human trophoblast cells by targeting FGF2. J Obstet Gynaecol Res. 2022 Aug;48(8):2122-2133. Epub 2022 Jun 18. [CrossRef] [PubMed]
- Gu YL, Rong XX, Wen LT, Zhu GX, Qian MQ. miR-195 inhibits the proliferation and migration of chondrocytes by targeting GIT1. Mol Med Rep. 2017 Jan;15(1):194-200. Epub 2016 Dec 5. [CrossRef] [PubMed]
- Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016 Sep 1;37(33):2602-11. Epub 2016 Jan 21. [CrossRef] [PubMed]
- Zhang L, Yang J, Guo M, Hao M. MiR-223-3p affects myocardial inflammation and apoptosis following myocardial infarction via targeting FBXW7. J Thorac Dis. 2022 Apr;14(4):1146-1156. [CrossRef] [PubMed] [PubMed Central]
- Wang Y, Wang H, Zhang L, Zhang J, Liu N, Zhao P. A novel identified circular RNA, circSnap47, promotes heart failure progression via regulation of miR-223-3p/MAPK axis. Mol Cell Biochem. 2023 Mar;478(3):459-469. Epub 2022 Jul 28. [CrossRef] [PubMed]
- Wang PP, Zhang YJ, Xie T, Sun J, Wang XD. MiR-223 promotes cardiomyocyte apoptosis by inhibiting Foxo3a expression. Eur Rev Med Pharmacol Sci. 2018 Sep;22(18):6119-6126. [CrossRef] [PubMed]
- Liu X, Zhang Y, Du W, Liang H, He H, Zhang L, Pan Z, Li X, Xu C, Zhou Y, Wang L, Qian M, Liu T, Yin H, Lu Y, Yang B, Shan H. MiR-223-3p as a Novel MicroRNA Regulator of Expression of Voltage-Gated K+ Channel Kv4.2 in Acute Myocardial Infarction. Cell Physiol Biochem. 2016;39(1):102-14. Epub 2016 Jun 20. [CrossRef] [PubMed]
- Yang L, Li Y, Wang X, Mu X, Qin D, Huang W, Alshahrani S, Nieman M, Peng J, Essandoh K, Peng T, Wang Y, Lorenz J, Soleimani M, Zhao ZQ, Fan GC. Overexpression of miR-223 Tips the Balance of Pro- and Anti-hypertrophic Signaling Cascades toward Physiologic Cardiac Hypertrophy. J Biol Chem. 2016 Jul 22;291(30):15700-13. Epub 2016 May 20. [CrossRef] [PubMed] [PubMed Central]
- Xiaoyu L, Wei Z, Ming Z, Guowei J. Anti-apoptotic Effect of MiR-223-3p Suppressing PIK3C2A in Cardiomyocytes from Myocardial Infarction Rat Through Regulating PI3K/Akt Signaling Pathway. Cardiovasc Toxicol. 2021 Aug;21(8):669-682. Epub 2021 May 17. [CrossRef] [PubMed]
- Wei Y, Peng J, He S, Huang H, Lin L, Zhu Q, Ye L, Li T, Zhang X, Gao Y, Zheng X. miR-223-5p targeting ERG inhibits prostate cancer cell proliferation and migration. J Cancer. 2020 May 18;11(15):4453-4463. [CrossRef] [PubMed] [PubMed Central]
- Jiang L, Lv L, Liu X, Jiang X, Yin Q, Hao Y, Xiao L. MiR-223 promotes oral squamous cell carcinoma proliferation and migration by regulating FBXW7. Cancer Biomark. 2019;24(3):325-334. [CrossRef] [PubMed] [PubMed Central]
- Li S, Feng Y, Huang Y, Liu Y, Wang Y, Liang Y, Zeng H, Qu H, Wei L. MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB. Open Life Sci. 2020 Jun 11;15(1):389-399. [CrossRef] [PubMed] [PubMed Central]
- Ding Q, Shen L, Nie X, Lu B, Pan X, Su Z, Yan A, Yan R, Zhou Y, Li L, Xu J. MiR-223-3p overexpression inhibits cell proliferation and migration by regulating inflammation-associated cytokines in glioblastomas. Pathol Res Pract. 2018 Sep;214(9):1330-1339. Epub 2018 May 17. [CrossRef] [PubMed]
- Wang X, Tong Z, Liu H. MiR-223-3p targeting epithelial cell transforming sequence 2 oncogene inhibits the activity, apoptosis, invasion and migration of MDA-MB-468 breast cancer cells. Onco Targets Ther. 2019 Sep 18;12:7675-7684. [CrossRef] [PubMed] [PubMed Central]
- Han LL, Zhou XJ, Li FJ, Hao XW, Jiang Z, Dong Q, Chen X. MiR-223-3p promotes the growth and invasion of neuroblastoma cell via targeting FOXO1. Eur Rev Med Pharmacol Sci. 2019 Oct;23(20):8984-8990. [CrossRef] [PubMed]
- Britsch, S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1-65. [PubMed]
- Safa RN, Peng XY, Pentassuglia L, Lim CC, Lamparter M, Silverstein C, Walker J, Chen B, Geisberg C, Hatzopoulos AK, Sawyer DB. Neuregulin-1β regulation of embryonic endothelial progenitor cell survival. Am J Physiol Heart Circ Physiol. 2011 Apr;300(4):H1311-9. Epub 2011 Jan 14. [CrossRef] [PubMed] [PubMed Central]
- Rudat C, Norden J, Taketo MM, Kispert A. Epicardial function of canonical Wnt-, Hedgehog-, Fgfr1/2-, and Pdgfra-signalling. Cardiovasc Res. 2013 Dec 1;100(3):411-21. Epub 2013 Sep 2. [CrossRef] [PubMed]
- Vega-Hernández M, Kovacs A, De Langhe S, Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011 Aug;138(15):3331-40. [CrossRef] [PubMed] [PubMed Central]
- Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011 Aug 1;22(15):2766-76. Epub 2011 Jun 8. [CrossRef] [PubMed] [PubMed Central]
- Zachary, I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy. 2014;99:37-70. Epub 2013 Oct 17. [CrossRef] [PubMed]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the TyPE/STII TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996 Mar 15;174(2):248-57. [CrossRef] [PubMed]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of tyPE/STIII TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999 Mar 26;283(5410):2080-2. [CrossRef] [PubMed]
- Plotnikov SV, Waterman CM. Guiding cell migration by tugging. Curr Opin Cell Biol. 2013 Oct;25(5):619-26. Epub 2013 Jul 3. [CrossRef] [PubMed] [PubMed Central]
- Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001 Jun 8;88(11):1112-9. [CrossRef] [PubMed]
- Lal H, Verma SK, Foster DM, Golden HB, Reneau JC, Watson LE, Singh H, Dostal DE. Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2307-34. [CrossRef] [PubMed]
- Seetharaman S, Etienne-Manneville S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020 Sep;30(9):720-735. Epub 2020 Jul 13. [CrossRef] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014 Mar;15(3):178-96. [CrossRef] [PubMed] [PubMed Central]
- Debnath P, Huirem RS, Dutta P, Palchaudhuri S. Epithelial-mesenchymal transition and its transcription factors. Biosci Rep. 2022 Jan 28;42(1):BSR20211754. [CrossRef] [PubMed] [PubMed Central]
- Chui, MH. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer. 2013 Apr 1;132(7):1487-95. Epub 2012 Sep 28. [CrossRef] [PubMed]
- Gan L, Denecke B. Co-regulation of microRNAs and transcription factors in cardiomyocyte specific differentiation of murine embryonic stem cells: An aspect from transcriptome analysis. Biochim Biophys Acta Gene Regul Mech. 2017 Sep;1860(9):983-1001. Epub 2017 Aug 4. [CrossRef] [PubMed]
- Li S, Yan B, Wu B, Su J, Lu J, Lam TW, Boheler KR, Poon EN, Luo R. Integrated modeling framework reveals co-regulation of transcription factors, miRNAs and lncRNAs on cardiac developmental dynamics. Stem Cell Res Ther. 2023 Sep 13;14(1):247. [CrossRef] [PubMed] [PubMed Central]
- Yamamura S, Imai-Sumida M, Tanaka Y, Dahiya R. Interaction and cross-talk between non-coding RNAs. Cell Mol Life Sci. 2018 Feb;75(3):467-484. Epub 2017 Aug 24. [CrossRef] [PubMed] [PubMed Central]
- Le TD, Liu L, Zhang J, Liu B, Li J. From miRNA regulation to miRNA-TF co-regulation: computational approaches and challenges. Brief Bioinform. 2015 May;16(3):475-96. Epub 2014 Jul 12. [CrossRef] [PubMed]
- Song R, Catchpoole DR, Kennedy PJ, Li J. Identification of lung cancer miRNA-miRNA co-regulation networks through a progressive data refining approach. J Theor Biol. 2015 Sep 7;380:271-9. Epub 2015 May 28. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




