Introduction
This article presents a patient with a 16-year history of HER2 exon 20 insertion (HER2 exon20ins) lung adenocarcinoma. After the administration of various TKIs, including Gefitinib, Poziotinib, Cabozantinib, and Lenvatinib, all of them resulted in drug resistance. Eventually, the patient developed respiratory distress and confusion.After initiating treatment with Sunvozertinib at a dose of 300mg Qd, the patient's respiratory condition improved and mental clarity was restored after 3 days. However, after 3 months, the patient developed resistance to Sunvozertinib with a progression-free survival (PFS) of 87 days. Ultimately, the patient died due to respiratory distress 2 months after discontinuing Sunvozertinib.
Case Report
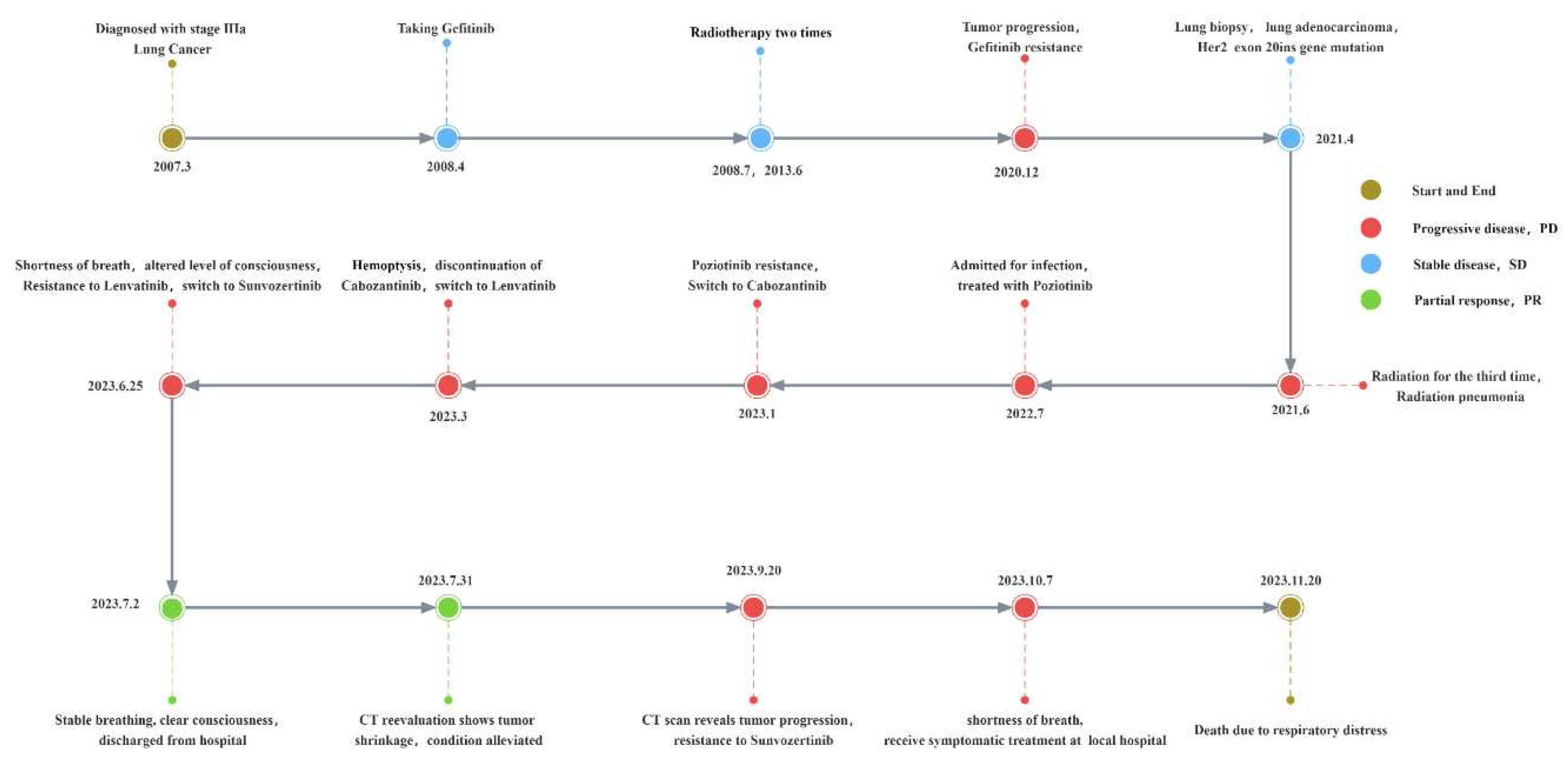
Patient, female, 67 years old, non-smoker, diagnosed with Lung Adenocarcinoma 16 years ago, admitted to hospital due to sudden onset of "shortness of breath and altered consciousness". The patient has no family history of cancer or infectious diseases and has normal psychological and mental status. In 2007, the patient's routine examination revealed lung adenocarcinoma, Stage IIIa, T2N2M0. From May 2008 to December 2020, the patient was treated with Gefitinib 250mg/Qd for 12 years, with two rounds of adjuvant radiotherapy during this period, and the tumor progressed slowly. The patient experienced tumor progression and developed resistance to Gefitinib in December 2020.Subsequent lung tissue biopsy revealed HER2 gene mutation, p.Y772_A775dup exon 20 non-frame shift insertion mutation, with plasma abundance of 0.4% and tissue abundance of 18.1%. On October 15, 2021, the patient underwent the third round of radiotherapy, which resulted in radiation pneumonitis. Relief was achieved after using Pirfenidone. The patient was treated with the dual-targeted therapy against EGFR/HER2, Poziotinib, in October 2022. However, after three months, the tumor progressed and developed resistance, resulting in the discontinuation of the treatment. The patient was switched to the multi-targeted therapy Cabozantinib. After one month of use, the treatment was discontinued due to the occurrence of hemoptysis. Subsequently, the patient was switched to Lenvatinib, an anti-tumor angiogenesis inhibitor and proliferation inhibitor.
After using Lenvatinib for two months, on June 25, 2023, the patient experienced sudden onset of dyspnea, worsened wheezing, altered consciousness, bedridden and unable to move, with SPO
2 94%, BP 162/88mmHg, respiratory rate 22 breaths /min, and white blood cell count of 10.12*10
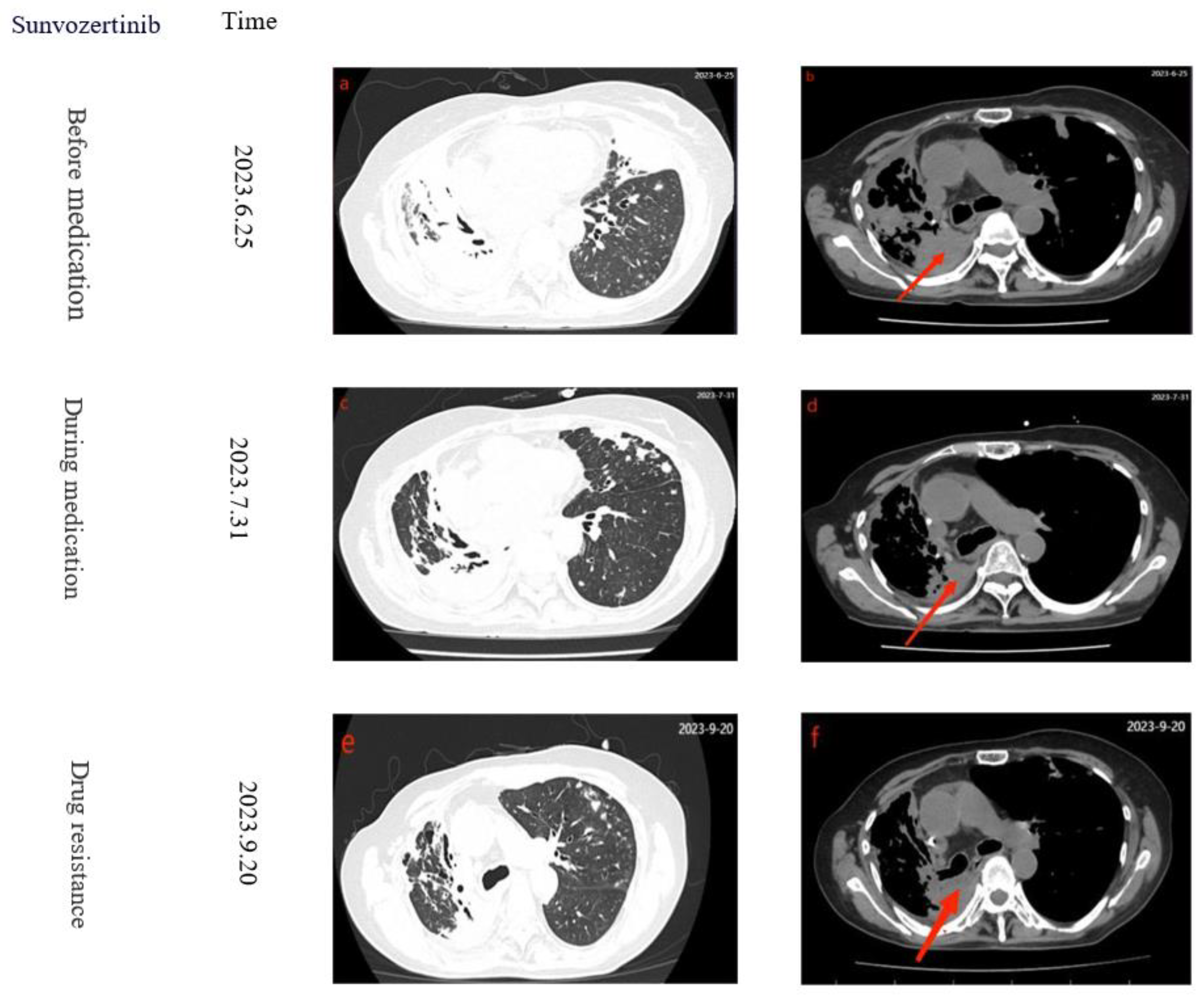
9/L. Chest CT showed multiple patchy high-density lesions in both lungs (
Figure 2 a-b), indicating resistance to the treatment. Lenvatinib was discontinued and switched to Sunvozertinib 300mg/Qd. After three days of Sunvozertinib, the patient's wheezing and dyspnea improved, mental status became clear, SPO
2 99%, BP 129/76mmHg, and respiratory rate was 16 breaths/min. After continuing the treatment for one week, the patient was able to walk and was discharged with continued use of Sunvozertinib. One month later, the patient returned to the hospital for a follow-up examination. The patient had stable breathing and a clear consciousness. A chest CT scan revealed a reduction in the size of the cancerous lesions (
Figure 2 c-d). After 50 days, the patient was readmitted due to chest tightness, and CT revealed tumor progression (
Figure 2 e-f). After developing resistance to Sunvozertinib, patients discontinued the use of the drug, the PFS with Sunvozertinib was 87 days. After a 17-day cessation of the medication, on October 7, 2023, the patient was admitted to a local hospital for palliative care due to respiratory distress. However, the treatment had limited efficacy, and while on a ventilator, the patient's blood oxygen saturation ranged from 90% to 93%. Eventually, on November 20, the patient died due to respiratory distress. Finally, considering the patient's 16-year history of lung cancer and multiple drug resistances, the patient's family expressed understanding regarding the patient's death .The specific treatment timeline can be seen in (
Figure 1)
This article is a single-patient case report, and therefore, the efficacy of Sunvozertinib on HER2 exon 20ins has certain limitations. However, it also potentially suggests that Sunvozertinib may have some effectiveness for patients with HER2 exon 20ins mutations.
Discussion
HER2 (ERBB2) mutation is one of the common driver gene mutations in lung cancer, detectable in approximately 2% of lung cancer cases, with exon 20 insertion mutation (A775 G776insYVMA) being the most common [
1]. Epidemiologically, it primarily occurs in young, non-smoking patients [
2]. Currently, promising treatment options come from phase II clinical trials of two antibody-drug conjugates (ADCs): trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) [
3,
4]. Based on the favorable data from the DESTINY-Lung02 trial, T-DXd has received accelerated FDA approval as the first targeted therapy for treating HER2-mutant NSCLC [
5]. However, these two drugs are not yet approved for use in some regions. Therefore, an alternative treatment option is needed for patients with HER2 mutations.
This patient initially received first-generation targeted therapy with Gefitinib in 2008 and continued treatment for 12 years. After developing resistance, they were subsequently switched to Poziotinib, Cabozantinib, and Lenvatinib Poziotinib is a novel EGFR-TKI that irreversibly blocks the signaling pathways of EGFR, HER2, and HER4, inhibiting tumor cell proliferation [
6]. Cabozantinib is a multi-kinase inhibitor targeting 9 different targets [
6], including MET, VEGFR1, VEGFR2, VEGFR3, ROS1, RET, AXL, NTRK, and KITd. Lenvatinib, on the other hand, is a multi-targeted TKI that acts on vascular endothelial growth factor (VEGF) receptors VEGFR1, VEGFR2, and VEGFR3 [
7,
8].After developing resistance to the four mentioned drugs, the patient's condition had progressed severely, with impaired blood oxygen saturation and consciousness, leading to a comatose state. However, within three days of using Sunvozertinib, the symptoms were alleviated. Resistance to Sunvozertinib developed after three months, resulting in PFS of 87 days. Following resistance to Sunvozertinib, no suitable medication targeting HER2 exon 20 insertion mutation was found, and the patient died after two months. From this case, it can be observed that Sunvozertinib demonstrates a certain level of effectiveness in patients with HER2 exon 20 insertion mutation who have failed TKI treatment.
From July 9, 2019, to April 3, 2021, two Phase 1 clinical trials of Sunvozertinib were completed.These trials were named WU-KONG1 and WU-KONG2 (WU-KONG1, ClinicalTrials.gov identifier: NCT03974022, and WU-KONG2, Chinese Clinical Trial Registry identifier: CTR20192097). However, these studies primarily focused on patients with EGFR mutations, with only a subset of patients having HER2 mutations. A total of 102 patients were enrolled, with 74 having EGFR mutations and 28 having HER2 exon 20 insertion mutations [
9]. Sunvozertinib is ultimately reported as an EGFR exon20ins inhibitor that demonstrates good pharmacokinetic/pharmacodynamic correlation in xenograft models and exhibits weak activity against wild-type EGFR. In 56 patients with EGFR exon20ins who were evaluable for efficacy assessment, partial response (PR) was observed at the doses of ≥ 100 mg. Across all dose levels, the best objective response rate (ORR) was 41.1%, and confirmed ORR was 37.5%。Among the 26 HER2 exon20ins patients, the results showed PR in 3 cases and stable disease (SD) in 7 cases, but ORR and disease control rate (DCR) were not provided. Subsequently, the clinical trials WU-KONG6 and WU-KONG15 for Sunvozertinib also only included patients with EGFR exon20ins mutations and did not include patients with HER2 exon20ins mutations. Correct, in the current clinical trial efficacy evaluation of Sunvozertinib, only prognostic efficacy indicators for EGFR mutations have been provided. However, for HER2 exon 20 ins mutations, although this drug targets that specific mutation and includes the recruitment of some patients with this mutation, the evaluation of its efficacy for this specific mutation is still unclear.
Conclusion
Sunvozertinib may have certain efficacy in patients with HER2 exon 20 insertion mutations. Currently, clinical trials have not further explored its efficacy in this specific patient population, and further investigation is needed to delve deeper into its potential.
Acknowledgments
This work was supported by a National Key R&D Program of China (2021YFC2301101), Guangzhou Science and Technology Major Clinical Project (2023C-DZ06), Beijing Xisike Clinical Oncology Research Foundation (Y-HS202102-0118).
Conflicts of Interest
The author reports no conflicts of interest in this work.
References
- Vranić, S.; Bešlija, S.; Gatalica, Z. Targeting HER2 expression in cancer: New drugs and new indications. Bosn J Basic Med Sci 2021, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, J.; Ying, J.; et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Buonocore, D.; et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; et al. Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell 2022, 40, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Sentana-Lledo, D.; Academia, E.; Viray, H.; et al. EGFR exon 20 insertion mutations and ERBB2 mutations in lung cancer: a narrative review on approved targeted therapies from oral kinase inhibitors to antibody-drug conjugates. Transl Lung Cancer Res 2023, 12, 1590–1610. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e302–e310. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.C.; Mitchell, P.L.; et al. Sunvozertinib, a Selective EGFR Inhibitor for Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. Cancer Discov 2022, 12, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).