Submitted:
12 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
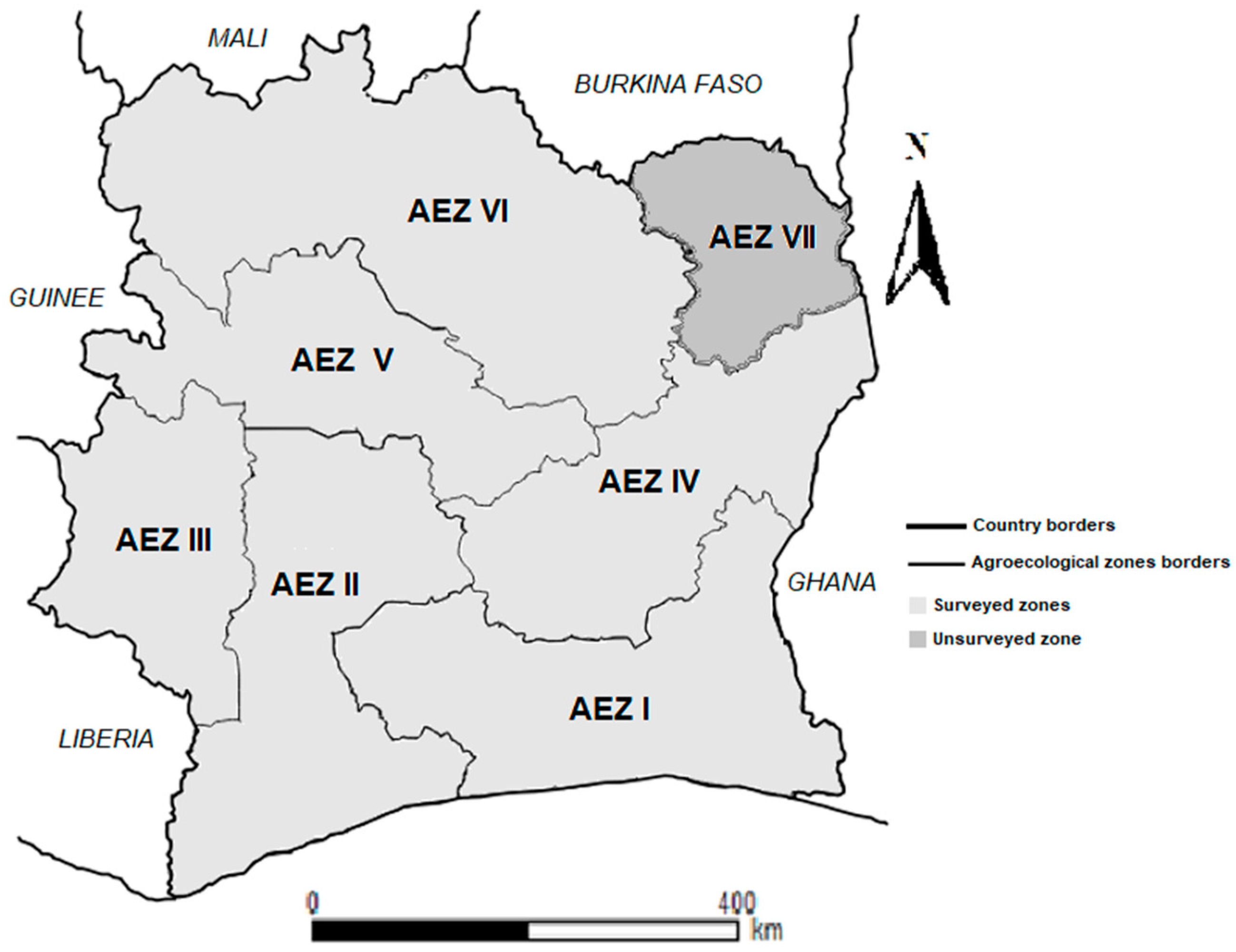
2.1. Study area
2.2. CMD epidemiological assessment
2.2.1. Survey data collection
2.2.2. Statistical analysis
2.3. PCR diagnostic of cassava mosaic begomoviruses
2.4. Sequencing and phylogenetic analysis
3. Results
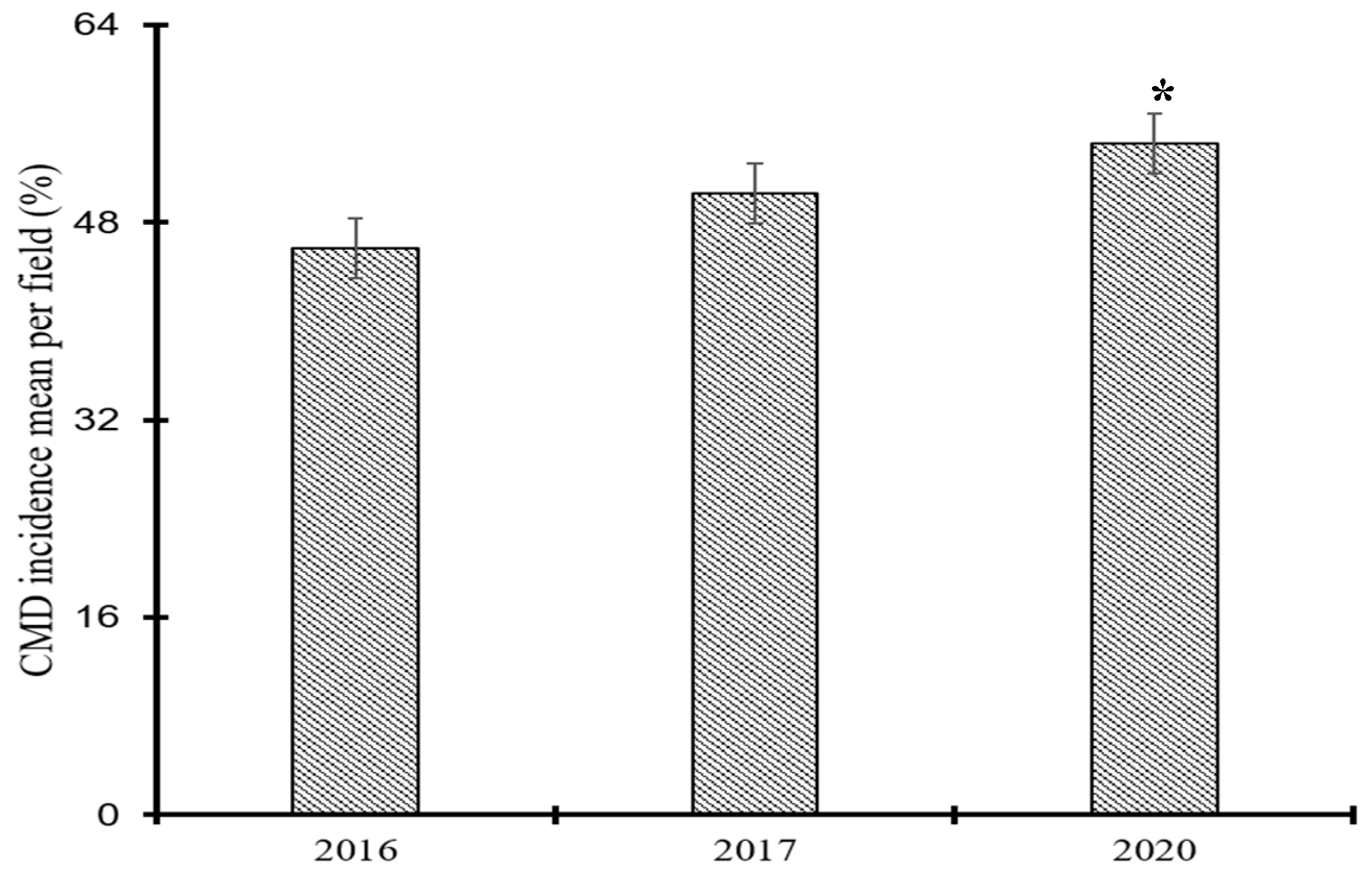
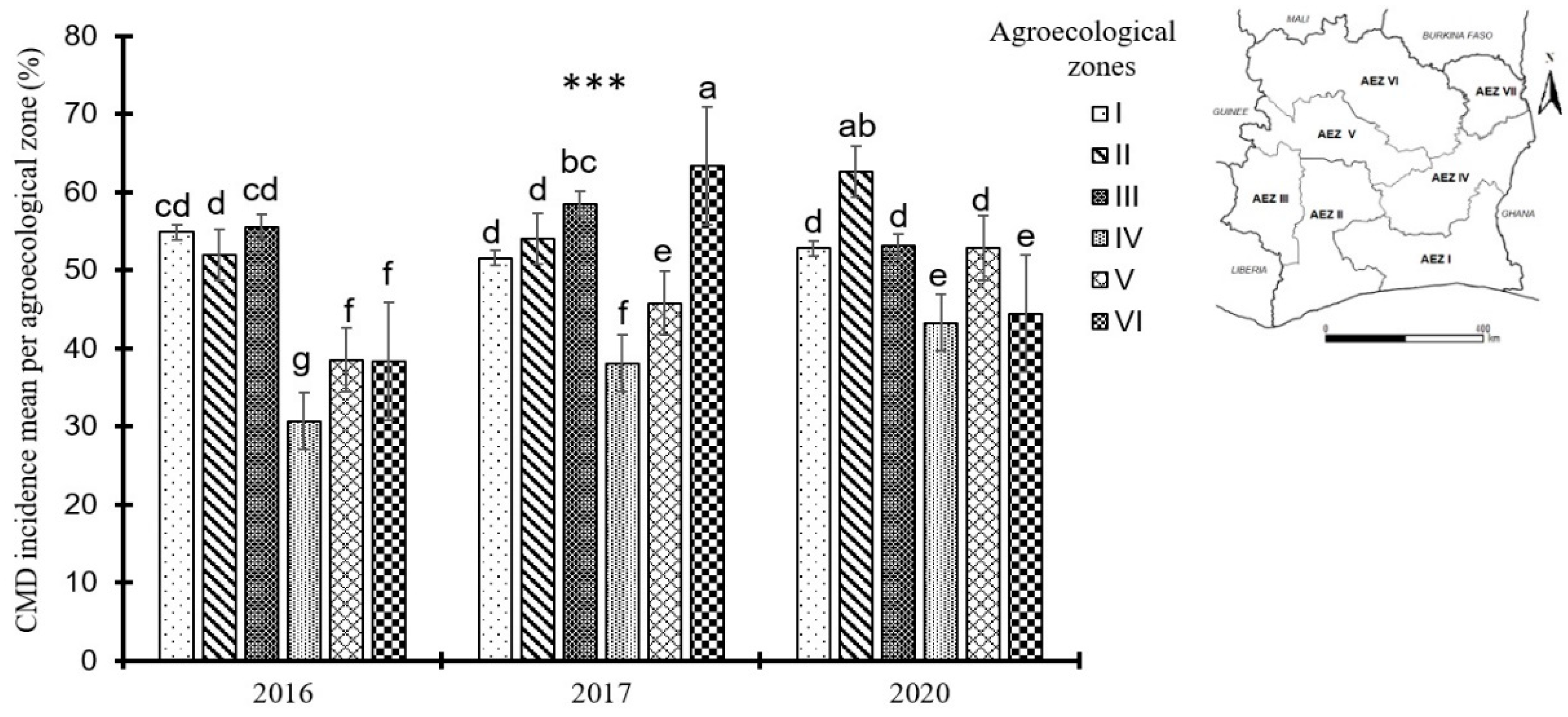
3.1. CMD incidence in 2016, 2017 and 2020
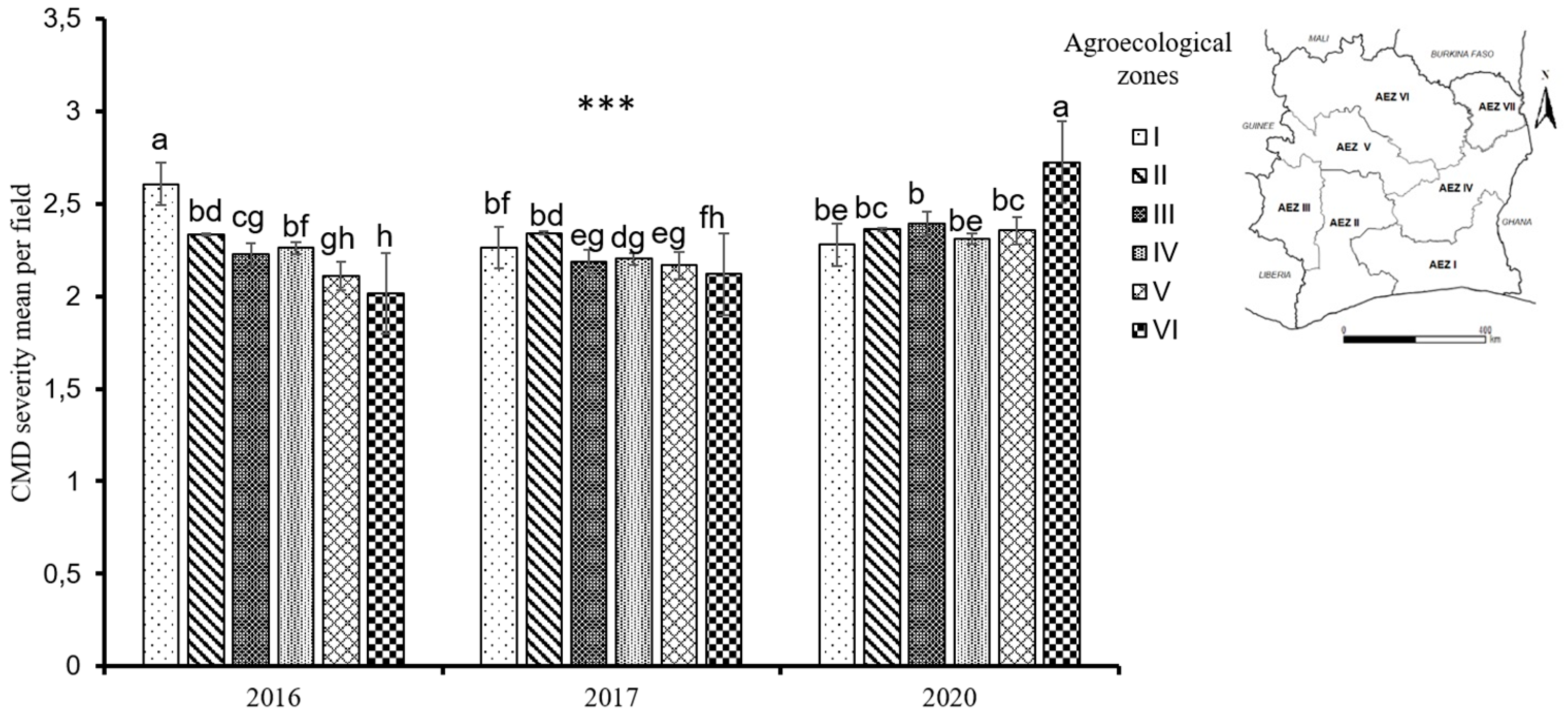
3.2. CMD severity and mode of infection
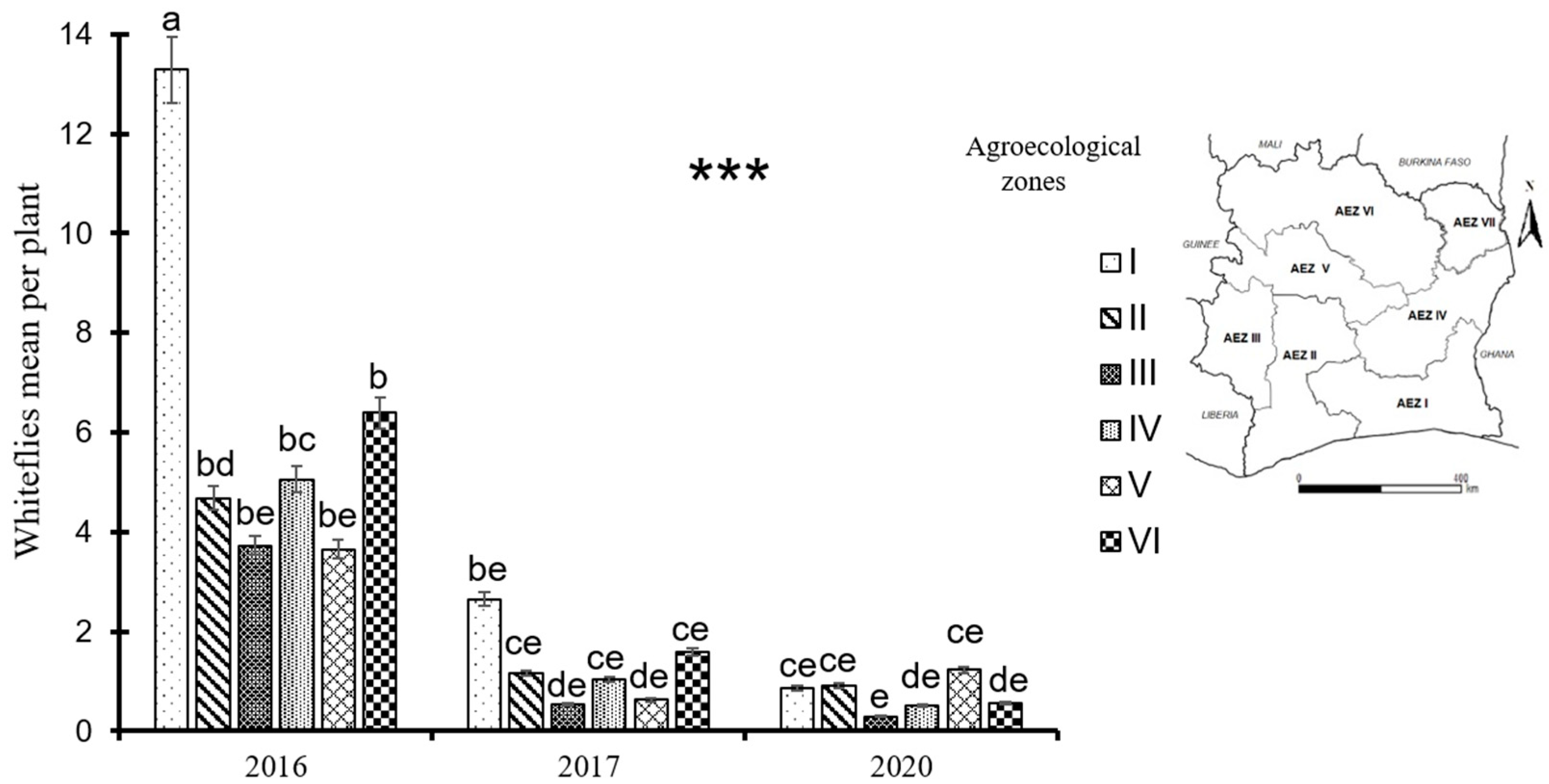
3.3. Abundance of whitefly in cassava fields surveyed
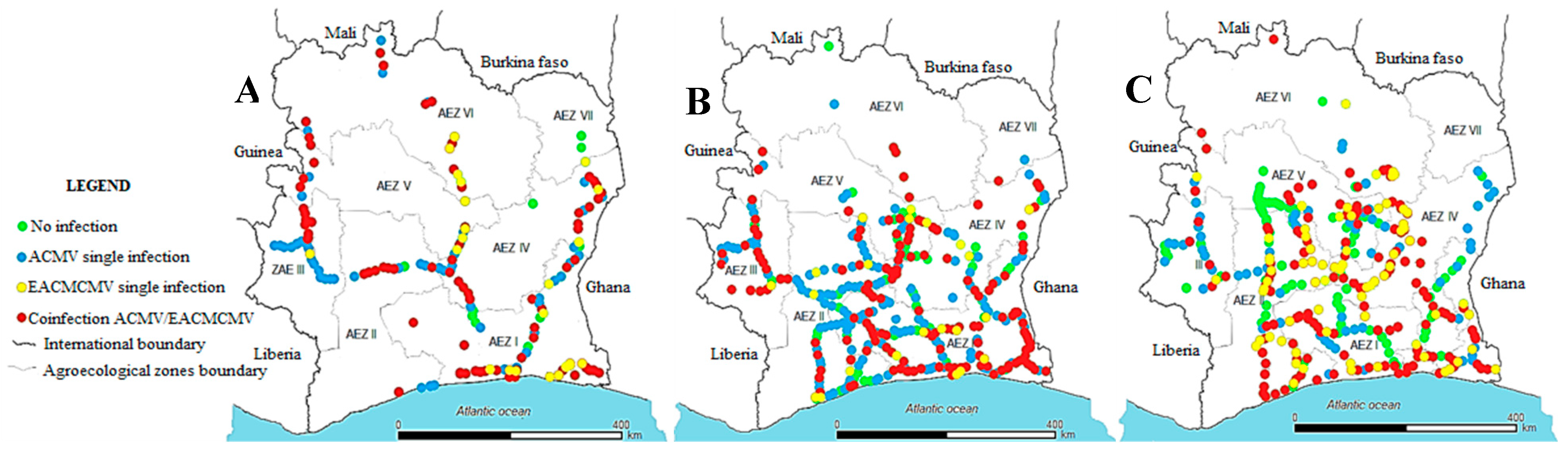
3.4. Evolution and distribution of cassava mosaic begomoviruses
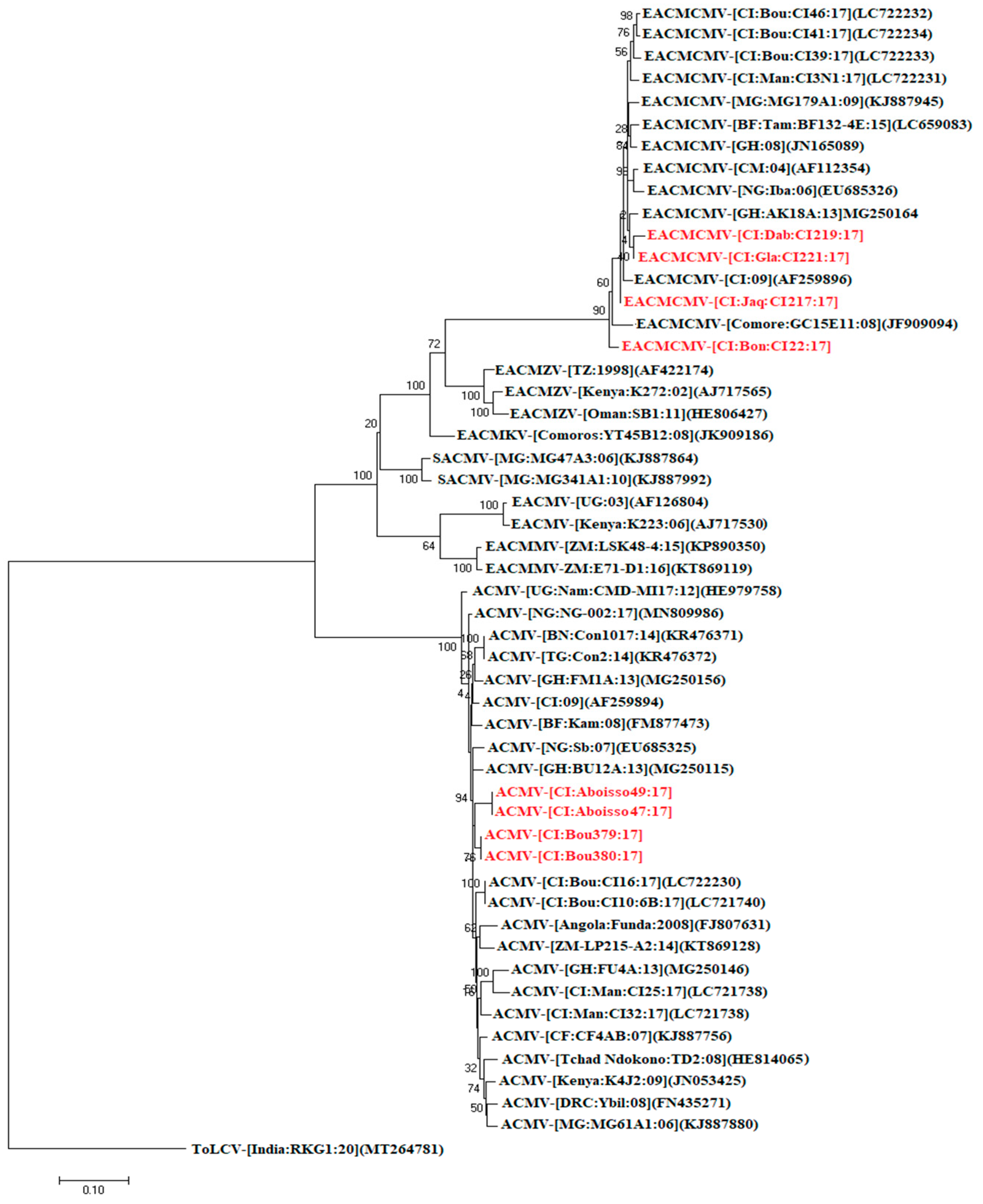
3.5. Phylogenetic analysis of ACMV and EACMCMV Coat Protein genes
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amelework, A.B.; Bairu, M.W.; Marx, R.; Owoeye, L.; Laing, M.; Venter, S.L. On-Farm Multi-Environment Evaluation of Selected Cassava. Plants 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Jarvis, A.; Ramirez-Villegas, J.; Campo, B.V.H.; Navarro-Racines, C. Is Cassava the Answer to African Climate Change Adaptation? Trop. Plant Biol. 2012, 5, 9–29. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics. 2021, Available online:. Available online: https://www.fao.org/faostat/fr/#data/QCL (accessed on 15 February 2023).
- Ogbe, F.O.; Thottappilly, G.; Dixon, A.G.O.; Atiri, G.I.; Mignouna, H.D. Variants of East African Cassava Mosaic Virus and Its Distribution in Double Infections with African Cassava Mosaic Virus in Nigeria. Plant Dis. 2003, 87, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.; Ndalahwa, M.; Yabeja, J.; Ndyetabula, I.; Bouwmeester, H.; Shirima, R.; Mtunda, K. Community Phytosanitation to Manage Cassava Brown Streak Disease. Virus Res. 2017, 241, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Chikoti, P.C.; Mulenga, R.M.; Tembo, M.; Sseruwagi, P. Cassava Mosaic Disease: A Review of a Threat to Cassava Production in Zambia. J. Plant Pathol. 2019, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Mbanzibwa, D.R.; Tian, Y.P.; Tugume, A.K.; Patil, B.L.; Yadav, J.S.; Bagewadi, B.; Abarshi, M.M.; Alicai, T.; Changadeya, W.; Mkumbira, J.; et al. Evolution of Cassava Brown Streak Disease- Associated Viruses. 2011, 974–987. [CrossRef]
- Munguti, F.M.; Nyaboga, E.N.; Kilalo, D.C.; Yegon, H.K.; Macharia, I.; Mwango’mbe, A.W. Survey of Cassava Brown Streak Disease and Association of Factors Influencing Its Epidemics in Smallholder Cassava Cropping Systems of Coastal Kenya. Front. Sustain. Food Syst. 2023, 6. [Google Scholar] [CrossRef]
- Legg, J.P.; Kumar, P.L.; Makeshkumar, T.; Cuellar, W.J.; Tripathi, L.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P. Cassava Virus Diseases: Biology, Epidemiology, and Management Cassava Virus Diseases : Biology, Epidemiology, and Management. Control Plant Virus Dis. 2015, 85–142. [Google Scholar]
- Sseruwagi, P.; Rey, M.E.C.; Brown, J.K.; Legg, J.P. The Cassava Mosaic Geminiviruses Occurring in Uganda Following the 1990s Epidemic of Severe Cassava Mosaic Disease. Ann. Appl. Biol. 2004, 145, 113–121. [Google Scholar] [CrossRef]
- Thresh, J.M.; Otim-Nape, G.W.; Thankappan, M.; Muniyappa, V. The Mosaic Diseases of Cassava in Africa and India Caused by Whitefly-Borne Geminiviruses. Rev. Plant Pathol. 1998, 77, 935–945. [Google Scholar]
- Zhou, X.; Liu, Y.; Calvert, L.; Munoz, C.; Otim-Nape, G.W.; Robinson, D.J.; Harrison, B.D. Evidence That DNA-A of a Geminivirus Associated with Severe Cassava Mosaic Disease in Uganda Has Arisen by Interspecific Recombination. J. Gen. Virol. 1997, 78, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Pita, J.S.; Fondong, V.N.; Sangaré, A.; Kokora, R.N.N.; Fauquet, C.M. Genomic and Biological Diversity of the African Cassava Geminiviruses. Euphytica 2001, 120, 115–125. [Google Scholar] [CrossRef]
- Szyniszewska, A.M.; Busungu, C.; Boni, S.B.; Shirima, R.; Bouwmeester, H.; Legg, J.P. Spatial Analysis of Temporal Changes in the Pandemic of Severe Cassava Mosaic Disease in Northwestern Tanzania. Phytopathology 2017, 107, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Salim, N.; Malathi, V.G.; Briddon, R.W.; Markham, P.G.; Stanley, J. Characterisation of Sri Lankan Cassava Mosaic Virus and Indian Cassava Mosaic Virus: Evidence for Acquisition of a DNA B Component by a Monopartite Begomovirus. Virology 2002, 293, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.G.; Robinson, D.J.; Harrison, B.D. Nucleotide Sequence Evidence for the Occurrence of Three Distinct Whitefly-Transmitted Geminiviruses in Cassava. J. Gen. Virol. 1993, 74, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Pita, J.S.; Fondong, V.N.; Sangaré, A.; Otim-Nape, G.W.; Ogwal, S.; Fauquet, C.M. Recombination, Pseudorecombination and Synergism of Geminiviruses Are Determinant Keys to the Epidemic of Severe Cassava Mosaic Disease in Uganda. J. Gen. Virol. 2001, 82, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Toualy, M.N.Y.; Akinbade, S.A.; Koutoua, S.; Diallo, H.A.; Lava, P. Incidence and Distribution of Cassava Mosaic Begomoviruses in Côte d’Ivoire International Institute of Tropical Agriculture (IITA), PMB 5320, Ibadan, Nigeria. 2014, 4, 131–139.
- Yao, F.; Koffi, M.; Abe, I.; Djetchi, M.N.; Konan, T.; Sanogo, T.A. Characterization of Cassava Mosaic Viruses and Current Mosaic Disease Concern in Three Major Cassava Production Areas in Côte D ’ Ivoire. Int. J. Plant Pathol. Mol. 2021, 12, 12–20. [Google Scholar] [CrossRef]
- Legg, J.P.; Jeremiah, S.C.; Obiero, H.M.; Maruthi, M.N.; Ndyetabula, I.; Okao-Okuja, G.; Bouwmeester, H.; Bigirimana, S.; Tata-Hangy, W.; Gashaka, G.; et al. Comparing the Regional Epidemiology of the Cassava Mosaic and Cassava Brown Streak Virus Pandemics in Africa. Virus Res. 2011, 159, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.P.; Ogwal, S. Changes in the Incidence of African Cassava Mosaic Virus Disease and the Abundance of Its Whitefly Vector along South–North Transects in Uganda. J. Appl. Entomol. 1998, 122, 169–178. [Google Scholar] [CrossRef]
- Neuenschwander, P.; Hughes, J.D.A.; Ogbe, F.; Ngatse, J.M.; Legg, J.P. Occurrence of the Uganda Variant of East African Cassava Mosaic Virus (EACMV-Ug) in Western Democratic Republic of Congo and the Congo Republic Defines the Westernmost Extent of the CMD Pandemic in East/Central Africa. Plant Pathol. 2002, 51, 385. [Google Scholar] [CrossRef]
- Tiendrébéogo, F.; Lefeuvre, P.; Hoareau, M.; Traoré, V.S.E.; Barro, N.; Reynaud, B.; Traoré, A.S.; Konaté, G.; Traoré, O.; Lett, J.M. Occurrence of East African Cassava Mosaic Virus-Uganda (EACMV-UG) in Burkina Faso. Plant Pathol. 2009, 58, 783. [Google Scholar] [CrossRef]
- Halle, B.; Bruzon, V. Commission Europeenne Offre de Service Dans le Secteur de la Cooperation Relatif au: Profil Environnemental de La Côte d ’ Ivoire; 2006.
- Soro, M.; Tiendrébéogo, F.; Pita, J.S.; Traoré, E.T.; Somé, K.; Tibiri, E.B.; Néya, J.B.; Mutuku, J.M.; Simporé, J.; Koné, D. Epidemiological Assessment of Cassava Mosaic Disease in Burkina Faso. Plant Pathol. 2021, 70, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Drobnik, J. Modern Techniques of Herbarium Protection. Environ. Chang. Biol. Assess. 2008, 243–246. [Google Scholar]
- Eni, A.O.; Efekemo, O.P.; Onile-ere, O.A.; Pita, J.S. South West and North Central Nigeria: Assessment of Cassava Mosaic Disease and Field Status of African Cassava Mosaic Virus and East African Cassava Mosaic Virus Survey Area. 2020, 178, 466–479. [CrossRef]
- Hahn, S.K.; Terry, E.R.; Leuschner, K. Cassava for cassava resistance disease. Euphytica 1980, 29, 673–683. [Google Scholar] [CrossRef]
- Mouketou, A.; Koumba, A.A.; Gnacadja, C.; Zinga-koumba, C.R.; ABESSOLO MEYE, C. Cassava mosaic disease incidence and severity and whitefly. Afr. Crop Sci. J. 2022, 30, 167–183. [Google Scholar] [CrossRef]
- Amoakon, W.J.-L.; Naté, Y.A.A.; Pita, J.S.; Mutuku, J.M.; N’Zué, B.; Combala, M.; Otron, D.H.; Koné, M.; Kouassi, N.K.; Sié, R. Occurrence of Cassava Mosaic Begomoviruses in National Cassava Germplasm Preserved in Two Agroecological Zones of Côte d’Ivoire. Plant Pathol. 2023, 1–11. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Matic, S.; Pais da Cunha, A.T.; Thompson, J.R.; Tepfer, M. Short Communication an Analysis of Viruses Associated with Cassava Mosaic Disease in Three Angolan Provinces. J. Plant Pathol. 2012, 94, 443–450. [Google Scholar]
- Alabi, O.J.; Ogbe, F.O.; Bandyopadhyay, R.; Lava Kumar, P.; Dixon, A.G.O.; D’A. Hughes, J.; Naidu, R.A. Alternate Hosts of African Cassava Mosaic Virus and East African Cassava Mosaic Cameroon Virus in Nigeria. Arch. Virol. 2008, 153, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Fondong, V.N.; Pita, J.S.; Rey, M.E.C.; De Kochko, A.; Beachy, R.N.; Fauquet, C.M. Evidence of Synergism between African Cassava Mosaic Virus and a New Double-Recombinant Geminivirus Infecting Cassava in Cameroon. J. Gen. Virol. 2000, 81, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Chikoti, P.C.; Mulenga, R.M.; Tembo, M.; Sseruwagi, P. Cassava Mosaic Disease: A Review of a Threat to Cassava Production in Zambia. J. Plant Pathol. 2019, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Torkpo, S.K.; Gafni, Y.; Danquah, E.Y.; Offei, S.K. Incidence and Severity of Cassava Mosaic Disease in Farmer’s Fields in Ghana. Ghana J. Agric. Sci. 2018, 53, 61. [Google Scholar] [CrossRef]
- Houngue, J.A.; Pita, J.S.; Cacaï, G.H.T.; Zandjanakou-Tachin, M.; Abidjo, E.A.E.; Ahanhanzo, C. Survey of Farmers’ Knowledge of Cassava Mosaic Disease and Their Preferences for Cassava Cultivars in Three Agro-Ecological Zones in Benin. J. Ethnobiol. Ethnomed. 2018, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Modeste, K.K.; Adolphe, M.; Boni, N.; Edmond, K.; Camille, K. Status of Cassava (Manihot Esculenta Crantz) in Côte d’Ivoire: From Production to Consumption and Evaluation of Technology Adoption. Eur. Sci. J. ESJ 2018, 14, 285. [Google Scholar] [CrossRef]
- Assiri, K.P.; Djaratche, B.; Seka, K.; Yao, K.F.; Toualy, M.N.; Kra, K.D.; Fofana, F.; Atta, D.H. Incidence of Viral Diseases on the Yield of Six Varieties of Cassava (Manihot Esculenta Crantz) Grown in the Localities of Adzope and Yamoussoukro (Côte d’Ivoire). 2017, 6, 502–508.
- N’zue, B.; Zohouri, P.; Sangare, A. Performances agronomiques de quelques varietes de manioc ( manihot esculenta crantz ) dans trois zones agroclimatiques de la cote d ’ ivoire. Agron. Afr. 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Torkpo, S.K.; Offei, K.; Danquah, E.Y.; Gafni, Y. Status of Cassava Mosaic Begomoviruses in Farmers’ Fields in Ghana. AIMS Agric. Food 2017, 2, 279–289. [Google Scholar] [CrossRef]
- Thresh, J.M.; Cooter, R.J. Strategies for Controlling Cassava Mosaic Virus Disease in Africa. Plant Pathol. 2005, 54, 587–614. [Google Scholar] [CrossRef]
- Ariyo, O.A.; Koerbler, M.; Dixon, A.G.O.; Atiri, G.I.; Winter, S. Molecular Variability and Distribution of Cassava Mosaic Begomoviruses in Nigeria. 2005, 231, 226–231.
- Harimalala, M.; Chiroleu, F.; Giraud-carrier, C.; Hoareau, M.; Zinga, I. Molecular Epidemiology of Cassava Mosaic Disease in Madagascar. 2014, 1–7. [CrossRef]
| Primers names | Sequences (5’-3') | Target region | Size | References |
|---|---|---|---|---|
| JSP 001 | ATGTCGAAGCGACCAGGAGAT | ACMV DNA-A (CP)1 | 783 bp | [17] |
| JSP 002 | TGTTTATTAATTGCCAATACT | |||
| ACMVBF | TCGGGAGTGATACATGCGAAGGC | ACMV DNA-B (BV1/BC1) | 628bp | [32] |
| ACMVBR | GGCTACACCAGCTACCTGAAGCT | |||
| JSP 001 | ATGTCGAAGCGACCAGGAGAT | EACMV DNA-A (CP) | 780bp | [17] |
| JSP 003 | CCTTTATTAATTTGTCACTGC | |||
| CMBRepF | CRT CAA TGA CGT TGT ACC A | EACMV DNA-A (AC1) | 650 bp | [33] |
| EACMVRepR | GGT TTG CAG AGA ACT ACA TC | |||
| VNF031F | GGATACAGATAGGGTTCCCAC | EACMV-CM DNA-A (AC2/AC3) | ≈ 560bp | [34] |
| VNF032R | GACGAGGACAAGAATTCCAAT |
| Survey years | Viruses detected | ||||
|---|---|---|---|---|---|
| Number of samples tested | ACMV | EACMCMV | ACMV/EACMCMV | Negative | |
| 2016 | 438 (100%) | 157 (35.84%) | 26 (5.94%) | 139 (31.74%) | 116 (26.48%) |
| 2017 | 806 (100%) | 273 (33.87%) | 29 (3.60%) | 196(24.32%) | 308 (38.21%) |
| 2020 | 844 (100%) | 143 (16.94%) | 78 (9.24%) | 279 (33.06%) | 344 (40.76%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





