1. Introduction
Numerous studies showed that the mGlu5 receptor has been implicated in the pathophysiology of depression and the mechanism of action of antidepressant drugs (AD) [
1,
2,
3]. In turn, ligands of this receptor, especially the antagonists and NAMs, including 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP), have been widely studied for their potential antidepressant effects in animal models [
1,
3]. Despite promising results, research related to their implementation into the clinic has been limited, mainly due to side effects, including cognitive dysfunctions and psychotomimetic effects. For example, mGlu5 NAM, MPEP, potentiated the phencyclidyne-induced locomotor activity and disrupted pre-puls inhibition [
4]. Furthermore, MPEP impaired working memory and instrumental learning and augmented the detrimental effects of MK-801 on cognition in rats [
5]. Altogether, studies using full mGlu5 NAMs showed a narrow therapeutic index between efficacious doses and those that induce adverse effects. So far, no mGlu5 antagonists or NAMs have been developed commercially as antidepressants (AD). The only approved mGlu receptor ligand is a mGlu5 receptor antagonist, fenobam, registered as an anxiolytic. However, it was approved as a drug before being characterized as a mGlu5 receptor ligand [
6].
An alternative to mGlu5 receptor antagonists and NAMs are compounds that only partially inhibit its response to glutamate. The group of partial mGlu5 receptor NAMs includes M-5MPEP (2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine), which binds to the allosteric MPEP site on mGlu5 receptor and functionally acts as a partial mGlu5 antagonist [
7]. In vitro studies showed that partial mGlu5 NAMs, including M-5MPEP, used at concentrations that fully occupy the allosteric site on the mGlu5 receptor, block less than 100% of the effect compared to full NAMs [
7]. Nevertheless, the effectiveness of these compounds in inducing some therapeutic effects in animals, including anxiolytic-like, antidepressant-like, and anti-cocaine abuse effects, appears to be comparable to the effectiveness of full NAMs, suggesting that partial mGlu5 NAM activity may be sufficient to produce therapeutic effects similar to full mGlu5 NAMs [
8]. On the other hand, studies undertaken to comparatively evaluate the adverse effects induced by partial versus full mGlu5 NAMs in rodents have shown a significantly broader therapeutic window for partial NAMs compared with full NAMs [
8]. Above all, partial mGlu5 NAMs, including M-5MPEP, did not induce psychotomimetic-like effects, which have been one of the significant limitations for the clinical development of full mGlu5 NAMs [
8].
A recent study by Holter et al. [
9] confirmed previous data performed using screening tests, indicating the antidepressant potential of M-5MPEP. It was found that M-5MPEP decreased rapid eye movement (REM) sleep and increased REM sleep latency, recognized as biomarkers of antidepressant-like activity [
9]. Therefore, it seems that M-5MPEP may be considered a potential therapeutic agent in the treatment of depression.
Recently, studies on the search for new antidepressants have been mainly focused on RAADs, such as ketamine [
10], because classic ADs not only have limited effectiveness but, above all, require several weeks of waiting for a therapeutic effect. In contrast, ketamine produces a rapid effect, just a few hours to 24 hours after admi6nistration [
10,
11,
12]. Thanks to this action profile,
(S)-ketamine has been registered and introduced into therapy as an RAAD in 2019 [
13]. Nevertheless, due to the side effects caused by ketamine, other substances that modulate glutamatergic transmission, which act via mechanisms convergent to those of ketamine, are still being sought. Numerous data indicate that antagonists of some metabotropic glutamate receptors, especially mGlu2/3 receptors, share many synaptic and neural mechanisms of antidepressant-like activity with ketamine [
14,
15,
16,
17]. Moreover, they induce rapid and sustained antidepressant-like effects in screening tests and animal models of depression after single or short-term (three-day) administration [
17,
18].
Therefore, we decided to investigate whether partial NAM of the mGlu5 receptor, M-5MPEP, induces a rapid and sustained antidepressant-like effect in mice after single or short-term administration. Furthermore, we studied the possible involvement of the TrkB/BDNF pathway, as well as mTOR, eEF2, and serotonergic system activation in the mechanism of the antidepressant-like effect of M-5MPEP, using behavioral methods, Western blot, and ELISA techniques. Finally, the enhancement of the antidepressant-like effect of (R)-ketamine by M-5MPEP was investigated in the TST.
2. Materials and Methods
2.1. Animals and housing
The experiments were performed on male C57BL/6J mice (Charles River, Germany). Animals were maintained under standard laboratory conditions in terms of temperature (22 ± 2 °C), humidity (55 ± 10%), and lighting (light phase 6:00-18:00), with free access to food and tap water. The mice were eight weeks old at the beginning of the experiments. Each experimental group consisted of seven to eight animals. All subjects were experimentally naive and used only once in each test. Behavioral experiments were performed during the light period (9:00 am to 2:00 pm) by an observer unaware of the treatment. All experimental procedures followed the National Institutes of Health Animal Care and Use Committee guidelines and were approved by the Second Local Ethics Committee in Kraków, Poland. The three Rs principles were applied in the planning and execution of the experiments.
2.2. Compounds and treatment
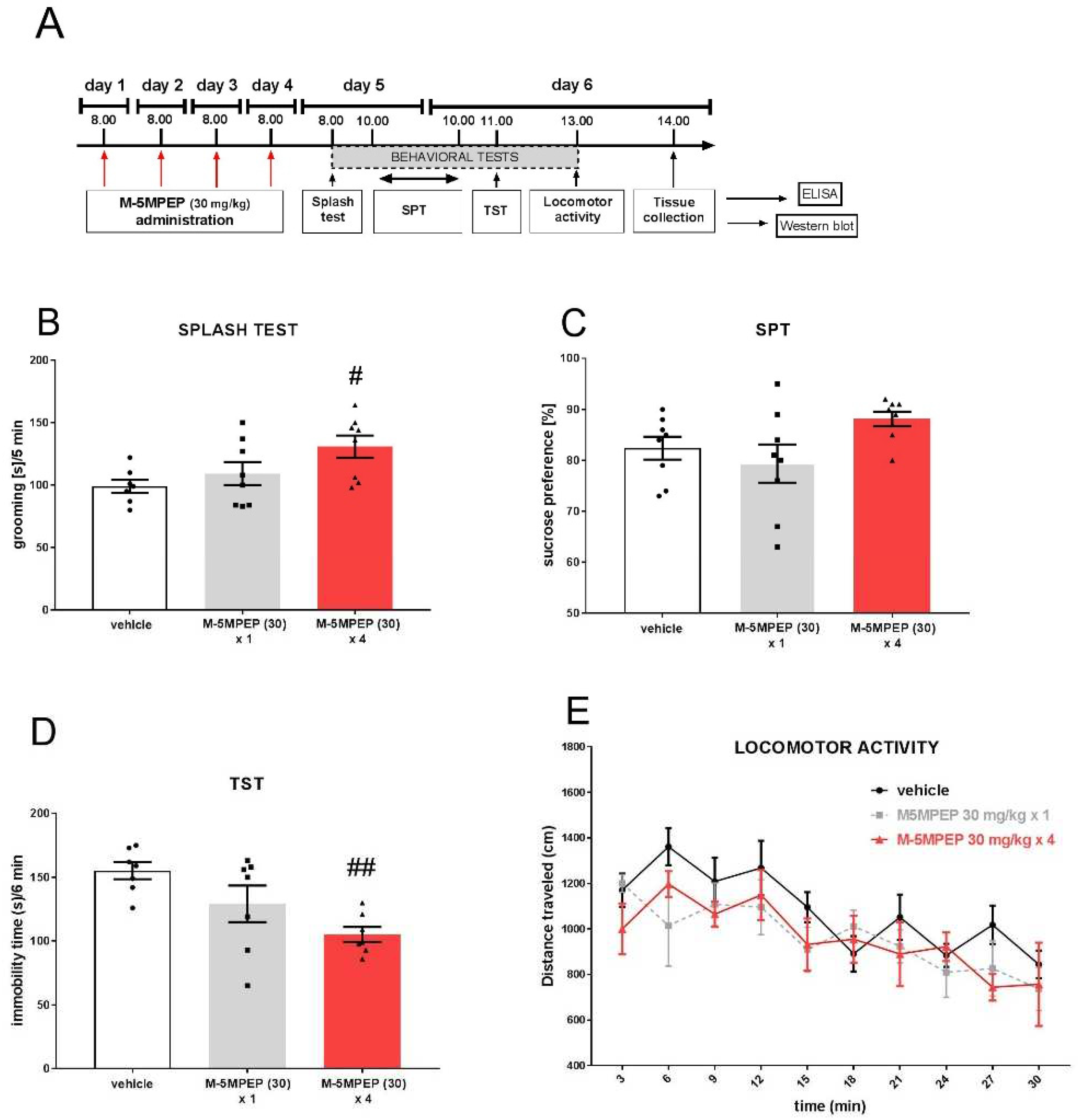
M-5MPEP (2-[2-(3-methoxyphenyl)ethynyl]-5-methylpyridine, synthesized in the Department of Medicinal Chemistry, Maj Institute of Pharmacology Polish Academy of Sciences, by K.K.), (S)-(+)-ketamine hydrochloride (Tocris Cookson, Ltd., Bristol, UK), (R)-ketamine hydrochloride (Cayman Chemicals, Ann Arbor, USA), NBQX disodium salt (Tocris Cookson Ltd.), ritanserin (Tocris Cookson Ltd., Bristol, UK) and citalopram (Ascent Scientific Ltd., Bristol, UK) were diluted in a suspension of 0.5% methylcellulose/0.9% NaCl, which was used as a vehicle. ANA-12 (Tocris Cookson Ltd.) was dissolved in 2% DMSO suspended in 0.5% methylcellulose. In Experiment 1 and 3, M-5MPEP was injected separately or in coadministration 60 minutes before the test. NBQX and WAY100635 were given 10 minutes before M-5MPEP, and ANA-12 and ritanserin were administered 30 minutes before M-5MPEP injection. (R)-ketamine and (S)-ketamine were given jointly with M-5MPEP. Citalopram was given 60 min before the test. In Experiment 2, behavioral tests started 24 hours after a single or fourfold administration of M-5MPEP. ANA-12 was given 30 min before M-5MPEP administration. A detailed schedule for Experiment 2 is provided in Figure 3A and Figure 7A. All compounds and vehicles were injected intraperitoneally (i.p.) at a constant volume of 10 ml/kg. Doses and times of drug administration were determined based on our own previous research and literature data.
2.3. Tail Suspension Test (TST)
The TST was carried out following the procedure described by Steru et al. [
19] and standardly used in our laboratory. Before the experiment, the animals were habituated to the experimental room for 30 min. Then, the mice were individually attached by their tails to a table with adhesive tape (placed approximately 1 cm from the distal end of the tail). A trained observer assessed the total duration of immobility for 6 minutes. The subjects were considered immobile when they hung down passively with no limb, body, or head movements.
2.4. Splash Test
With minor modifications, the splash test was performed as described [
20]. The experimental room was illuminated with dim lighting. Before the experiment, the mice were habituated to the experimental conditions for 30 min. Then, the splash test was carried out, during which the animal's behavior was observed in its home cage after a high viscosity 10% sucrose solution was sprayed on the mouse's back, thus stimulating self-grooming behavior. The sprayer allowed the delivery of a fixed volume (approximately 0.2 ml) of sucrose solution, and each subject received five sprays. The duration of grooming was recorded for five minutes by a trained observer.
2.5. Sucrose Preference Test (SPT)
The SPT was performed as previously described [
18], with some necessary modifications. The test lasted 24 hours. During this time, each home cage was equipped with two identical bottles: the first contained a 1% sucrose solution, and the second contained tap water. The position of the bottles was switched 12 hours after the start of the experiment. No previous food or water deprivation was applied before the test. At the beginning and end of the test, the bottles were weighed, and the liquid consumption was calculated. Twenty-four hours before the SPT, mice were allowed to consume a 2.5% sucrose solution for two hours to diminish the effect of neophobia. The preference for sucrose was calculated as a percentage of consumed sucrose solution in terms of the total amount of liquid drunk.
2.6. Locomotor Activity
The locomotor activity of the mice was measured in Plexiglas locomotor activity chambers (40 × 40 × 40 cm) in a 12-station photobeam activity system (Opto Varimex 4, Auto Track System 4.41, Columbus Instruments, Columbus, OH, USA). The animals were placed individually in locomotor activity chambers. Then, the total distance traveled during a 30-minute experimental session was measured and stored every 3 min.
2.7. Synaptosome preparation and Western blotting
Tissue samples were dissected from the prefrontal cortex (PFC) and hippocampus and homogenized in ice-cold lysis buffer [0.32 M sucrose, 20 mM HEPES (pH 7.4); 1 mM EDTA; 1 × protease inhibitor cocktail, 5 mM NaF, and 1 mM NaVO3]. Homogenates were then centrifuged at 2800 rpm for 10 min at 4 °C. The resulting supernatant was centrifuged at 12,000 rpm for 10 min at 4 °C. The resulting pellets were sonicated in protein lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF, and protease inhibitor cocktail. Protein concentrations were measured using a BCA kit (Thermo Scientific, USA). Next, the proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes, and blocked for 1 h in 1% of blocking solution [BM Chemiluminescence Western Blotting Kit (Mouse/Rabbit) made by Roche, Switzerland]. After blocking, the membranes were incubated overnight at 4 °C with the primary antibodies. The following antibodies were used: Anti-mTOR (mTOR 1:1000; Cell Signaling Technology, USA), Anti-phospho-mTOR (pmTOR, S2481, 1:1000; Abcam, USA), Anti-eEF2 (eEF2 1:1000; Abcam, USA), Anti- phospho-eEF2 (pheEF2 (phospho T56) 1:1000; Abcam, USA), Anti-SERT (SERT 1:1000; Sigma-Aldrich, Germany), Anti-TrkB ( TrkB 1:1000; Cell signaling Technology, USA). On the following day, the membranes were washed three times for 10 min in Tris-buffered saline with Tween (TBS-T) and incubated for 60 min with secondary antibodies (anti-mouse or anti-rabbit-IgG-peroxidase-conjugated antibodies Vector Laboratories, USA). After incubation, the membranes were washed thrice for 10 min with TBS-T. The blots were incubated with a detection reagent (Bio-Rad, USA) in the last step. The signal from the tested proteins was visualized and measured using a Fuji-Las 1000 system and Fuji Image Gauge v.4.0 software. A primary monoclonal antibody, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:500; Millipore, Germany), was indicated on each blot to check the transfer and loading. The final result is the ratio of particular proteins' optical density to GAPDH's optical density.
2.8. Measurement of BDNF concentration
According to the manufacturer's recommendations, the total BDNF concentrations were measured with an ELISA kit purchased from R&D Systems, Inc. Minneapolis, MN, USA (Catalog Number SBNT00). The total BDNF concentrations were determined by comparing the samples to the standard curve. All samples were thawed on ice and diluted (2-fold for plasma and 100-fold for tissue lysates in Calibrator Diluent RD5K). Fifty microliters of Assay Diluent RD1-123 was added, and 50 µl of each sample or standard was added to a 96-well microtiter plate in triplicate. The plate was incubated for two hours at room temperature with vigorous mixing (550 rpm, ThermoMixer C, Eppendorf, Hamburg, Germany). Each well was rinsed four times with 400 µl of wash buffer. An enzyme-linked monoclonal antibody specific for BDNF was added (200 µl) to the wells and incubated at room temperature for one hour with vigorous mixing (550 rpm, ThermoMixer C). Next, the wells were washed four times with 400 µl of wash buffer to remove any unbound antibody enzyme reagent. Two hundred microliters of substrate solution were added and incubated in the dark for one hour. Then, the plates were read at 450 nm and 540 nm using a spectrophotometer (Synergy HTX multimode reader machine; BioTek Instruments Inc., Winooski, VT, USA). The optical density was corrected by subtracting the readings at 540 nm from the readings at 450 nm. A four-parameter logistic-curve fit generated a standard curve. The data were linearized by plotting the total BDNF concentrations log versus the O.D log. The best-fit line was determined by regression analysis. Finally, the total BDNF concentration was normalized to the protein concentration of each sample. For standard curve fitting and sample-data interpolation, GraphPad Prism version 9.2.0 for Windows was used (GraphPad Software, San Diego, CA, USA).
2.9. Statistical analysis
All the results obtained are expressed as the mean ± standard error of the mean (SEM). Behavioral data statistical analyses were performed using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, USA). One-way ANOVA, followed by Dunnet`s test, was used to analyze the dose-response effect of M-5MPEP. Two-way ANOVA was used to analyze the effects of drug combinations in the splash test, SPT, and TST. Locomotor activity data were evaluated by repeated-measures ANOVA, followed by Bonferroni’s multiple comparisons test. The results obtained using the ELISA method and Western blot were analyzed by one-way ANOVA followed by Tukey's multiple comparisons test using GraphPad Prism version 9.2.0 for Windows (GraphPad Software, San Diego, CA, USA). The results were considered significant if the p-values were below 0.05.
4. Discussion
So far, only one RAAD, namely
(S)-ketamine, has been introduced into therapy for depression [
13]. This compound has an advantage over classic ADs in terms of speed of action, but unfortunately, it has many undesirable effects, including psychostimulant actions, dissociations, and abuse potential [
21]. Therefore, research is still being conducted to find an effective and safe alternative to
(S)-ketamine in the therapy of depression. A potential target that meets these requirements may be one of the mGlu receptors. This is indicated, among others, by research on the rapid and sustained antidepressant-like effects of mGlu2/3 receptor antagonists, showing that the mechanism of their antidepressant-like activity is similar to that of ketamine [
16,
17,
18]. Moreover, mGlu5 receptor NAM, MPEP, showed ketamine-like antidepressant-like effects in the novelty-suppressed feeding (NSF) test [
22]. However, the question of the sustained, ketamine-like antidepressant effect of mGlu5 NAMs has not been resolved because, for example, MTEP did not show such an effect in any of the tested parameters in the FST in rats [
23].
Here, we decided to investigate if the partial mGlu5 receptor NAM, M-5MPEP, shares rapid and sustained ketamine-like antidepressant effects in mice. This compound was chosen for the study because of promising results showing antidepressant-like actions and a favorable safety profile that distinguishes partial NAMs from full NAMs or mGlu5 receptor antagonists.
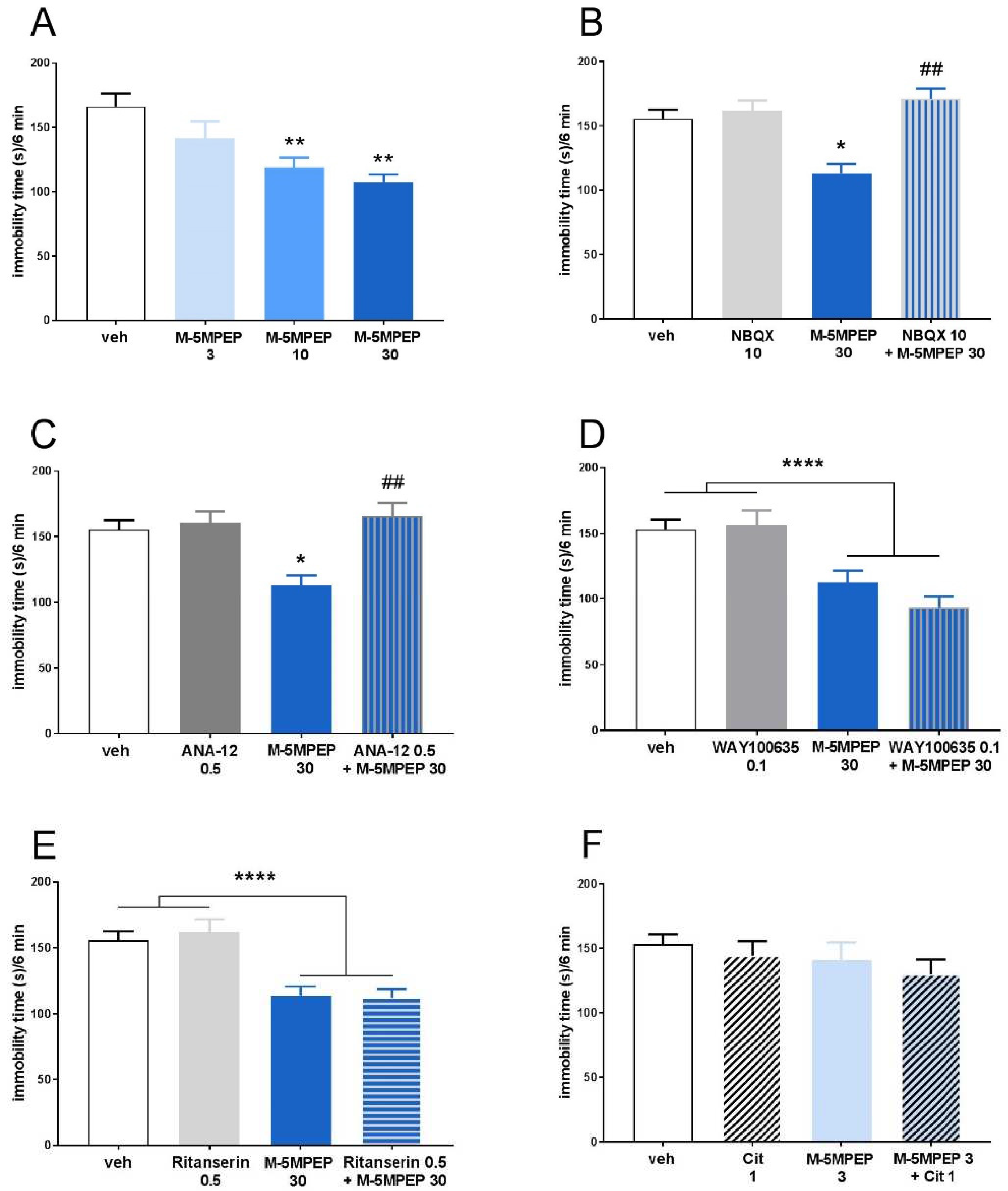
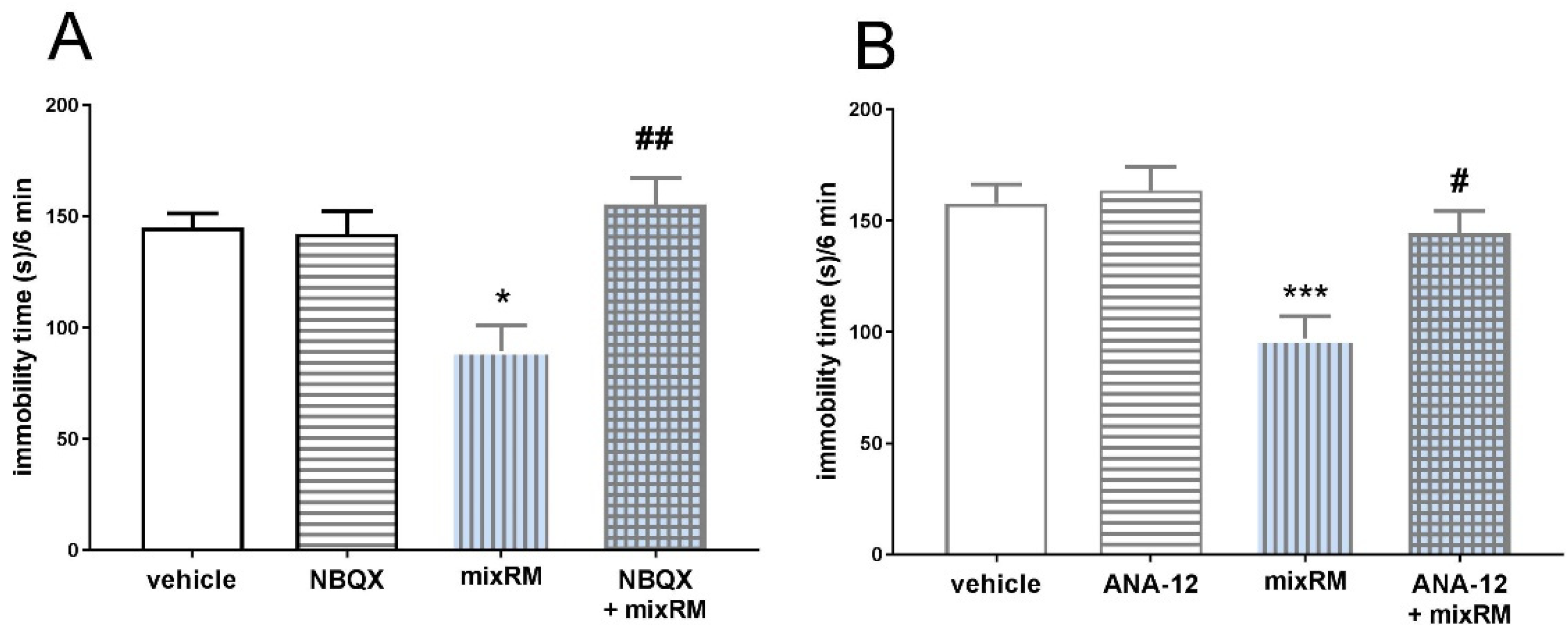
First, the effects of M-5MPEP were examined in the TST, a screening test used for estimating the antidepressant-like activity of new potential ADs or pharmacological and genetic manipulations involved in depression [
24]. M-5MPEP (3 – 30 mg/kg) dose-dependently decreased immobility time in this test 60 min after injection, indicating the antidepressant-like effect. An AMPA receptor antagonist, NBQX, and the TrkB receptor antagonist, ANA-12, reversed the action of M-5MPEP (30 mg/kg), suggesting the involvement of these receptors in the mechanism of M-5MPEP activity in the TST. A crucial role for AMPA receptor activation in the rapid antidepressant-like effects of RAADs, including ketamine, has been widely described based on the results showing that NBQX blocked the behavioral effects and some rapid biochemical alterations induced by ketamine [
14,
15,
25,
26,
27]. Therefore, M-5MPEP shares the mechanism with RAAD in this respect. Notably, previous studies showed that the rapid antidepressant-like effect of a full mGlu5 NAM, MTEP, was not antagonized by NBQX in the FST both in rats and mice, suggesting that the activation of the AMPA receptor was not essential in the production of the antidepressant-like effect of this compound [
23,
28]. Therefore, regarding AMPA receptor involvement in the rapid antidepressant-like effects, the mechanism of M-5MPEP action differs from that of full mGlu5 receptor NAMs.
BDNF and its specific receptor, tropomyosin-related kinase (TrkB), also have a role in the rapid antidepressant-like activity of ketamine. For example, Sun et al. [
29] showed that a rapid (30 min) antidepressant effect of ketamine in the forced swim test (FST) in rats was blocked by co-administration of ANA-12 [
29]. On the other hand, the lack of effect of the TrkB antagonist on the rapid (30 min) antidepressant-like effect of ketamine in mice was demonstrated in the TST [
30]. However, different experimental conditions, including a different way of administration (i.c.v) and a different TrkB antagonist (K252a), might contribute to discrepancies in the results [
30]. However, several other data indicated the critical role of BDNF in the rapid antidepressant-like effect. Autry et al. [
25] showed that ketamine did not produce fast antidepressant-like effects in the FST in BDNF-knockouts (KO). However, it was active in ketamine-treated wild-type littermates (30 min after administration). A lack of ketamine-induced rapid antidepressant-like effects in the TrkB KO mice was also shown, both in the FST and the NSF test. Furthermore, Western blot and ELISA analyses showed that BDNF protein levels were markedly increased at 30 min after ketamine treatment [
25]. Thus, the dependence of the rapid antidepressant-like action M-5MPEP on the activation of the TrkB receptor, observed in our study, resembles RAAD's mechanism of action.
Next, we investigated the possible role of the serotonergic system in the rapid antidepressant-like activity of M-5MPEP since the involvement of this neurotransmitter system in the action of RAADs has been proposed [
31,
32]. For example, it was shown that the rapid antidepressant-like effect of ketamine in NSF test in mice was entirely blocked by prior administration of the 5HT
1A antagonist, WAY100635, but not by pretreatment with 5HT
2A/2C antagonist, ritanserin [
14]. The authors concluded that activation of serotonergic neurons in the dorsal raphe may be involved in the rapid antidepressant-like ketamine effect [
15]. Here, we show that the rapid antidepressant-like action of M-5MPEP in the TST was not antagonized by WAY100635 or ritanserin. Moreover, the subthreshold dose of an SSRI, citalopram, was not potentiated by the subthreshold dose of M-5MPEP, which additionally indicates a lack of involvement of the serotonergic system in the mechanism of action of the tested mGlu5 NAM. These results contrast with those regarding the mechanism of antidepressant action of full mGlu5 NAM, MTEP in the same screening test (TST) and in the same mouse strain (C57BL/6J) [
33]. We have previously shown that the mechanism of action of MTEP depended on the activation of the serotonergic system and that prior administration of ritanserin, yet not WAY100635, blocked the MTEP-induced antidepressant-like effect [
33]. Moreover, a sub-effective dose of MTEP coadministered with a subeffective dose of citalopram induced an antidepressant-like effect in the TST [
33]. Similar results were obtained with another full mGlu5 antagonist, MPEP, in the NSF test, where the MPEP-induced antidepressant-like effects were dose-dependently blocked by ritanserin but not by WAY100635 [
34]. Thus, the serotonergic system may play a different role in the mechanism of action of full mGlu5 NAMs vs. partial mGlu5 NAMs. However, convergence with the mechanism of action of RAADs cannot be indicated, as data regarding the acute antidepressant-like effect of ketamine are inconsistent and show both dependence and independence from the 5HT system activity [
31,
32,
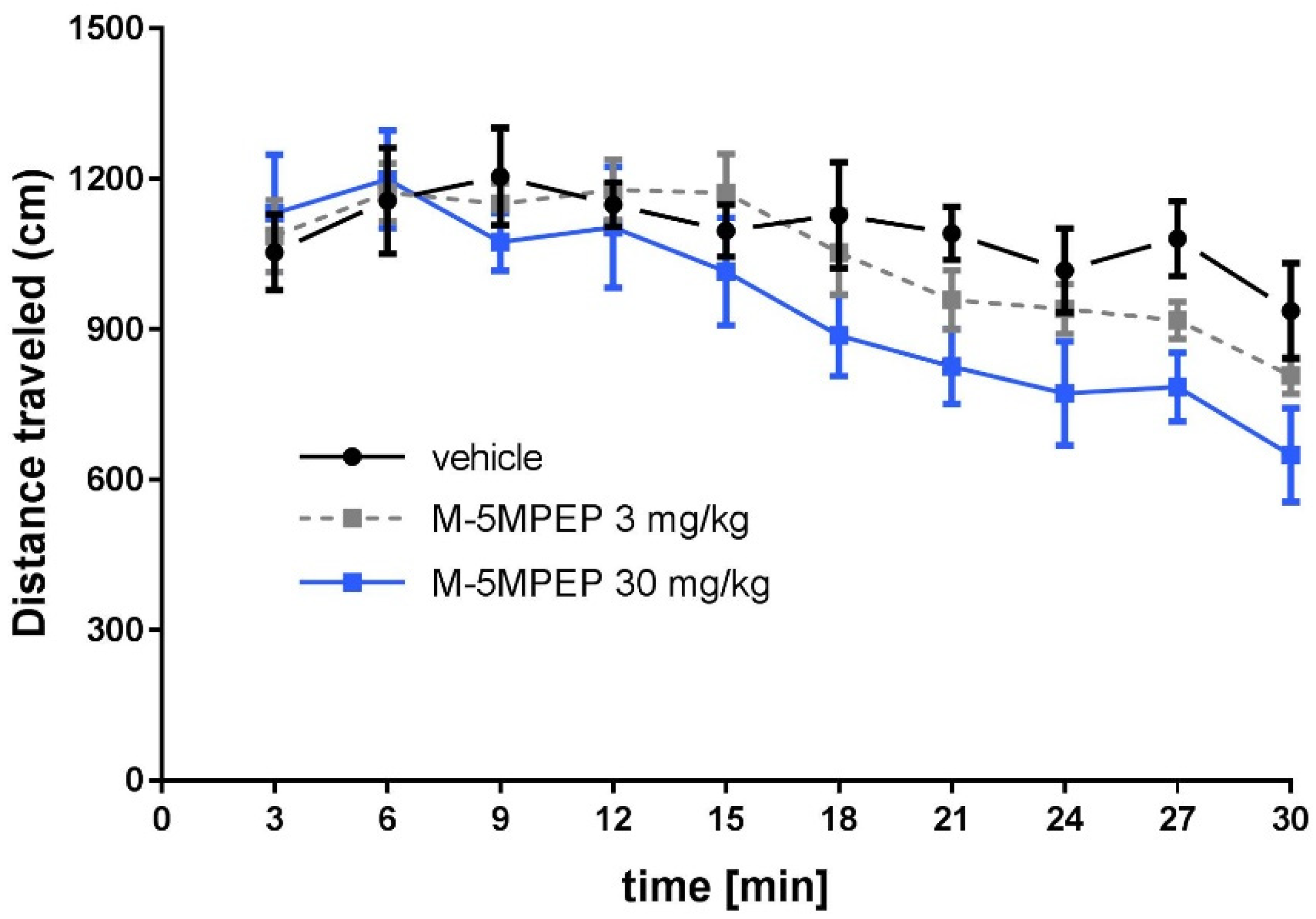
35]. Therefore, the involvement of the 5HT system in the mechanism of action of acute M-5MPEP administration requires further research, including pharmacological serotonin depletion. Finally, a locomotor activity test was performed since compounds that increase locomotor activity can provide false positive results in the TST. M-5MPEP at doses of 3 and 30 mg/kg did not affect the locomotor activity of mice, thus adding validity to the antidepressant-like effect of the drug in the TST.
Numerous behavioral and structural studies on animals have shown that the characteristic effect of ketamine, which distinguishes it from classic ADs, is the induction of the prolonged effects observed 24 hours after a single administration [
25,
27]. A similar relationship was observed with other RAADs, although the induction of prolonged effects sometimes required several injections, as in the case of scopolamine and other anti-muscarinic receptor antagonists [
36]. Also, the mGlu2/3 antagonist, LY341495, required longer (three-day) administration to reverse CUMS-induced behavioral effects, which were evident 24 hours after the last injection [
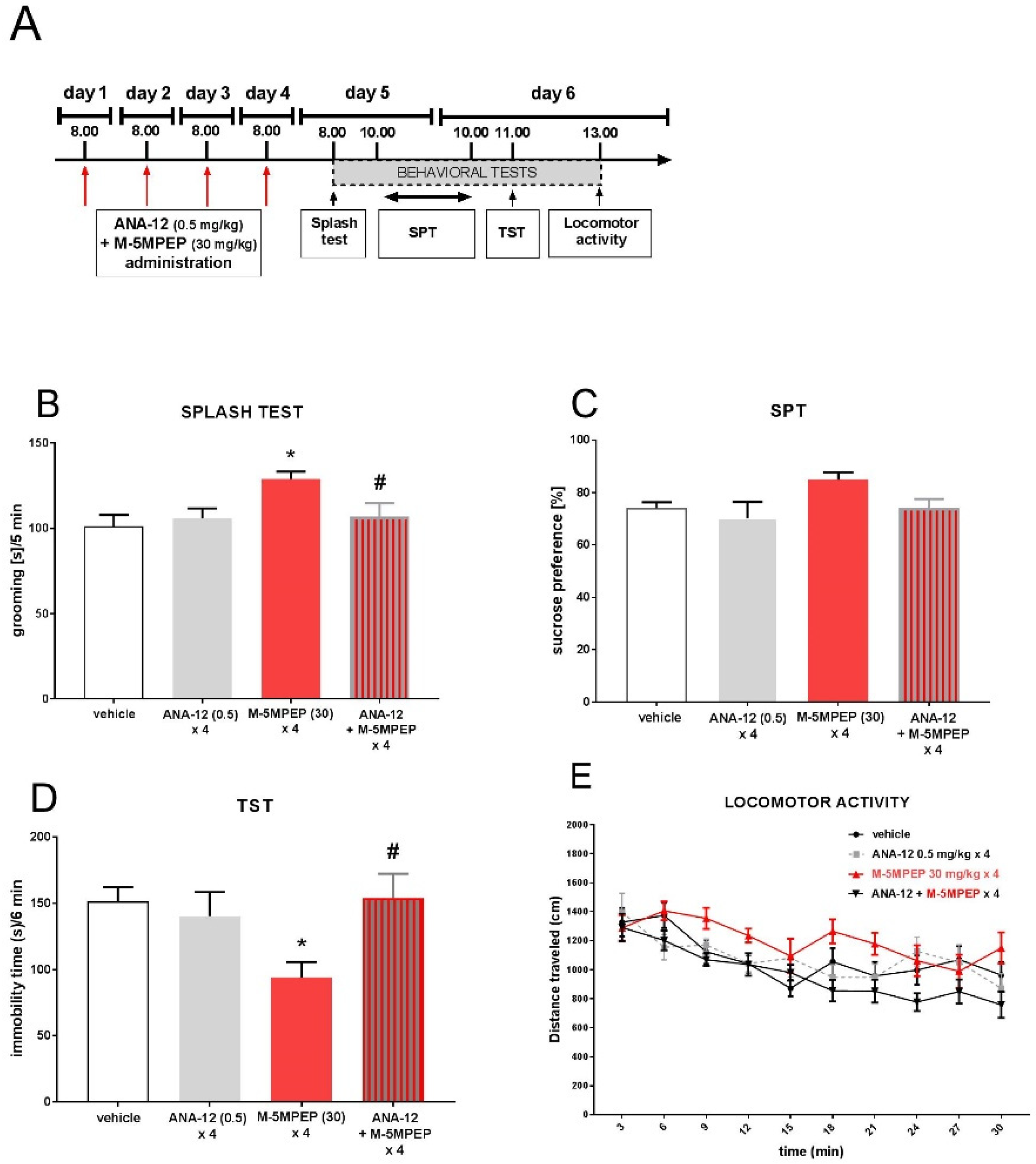
18]. Therefore, we examined the prolonged effect of an effective dose of M-5MPEP (30 mg/kg) 24 h after a single or four-day administration in mice using the splash test, the SPT, and the TST. We have found that a four-day application of M-5MPEP increased self-grooming in the splash test 24 hours after injection, indicating anti-apathetic efficacy. However, a single dose of M-5MPEP did not change the behavior of the mice in this test, inducing only an insignificant increased trend in grooming time. Similar results were obtained in the TST, where only four, but not single, administration of M-5MPEP effectively reduced the animals' immobility time, indicating a sustained antidepressant-like effect. On the other hand, no significant changes were observed in the SPT, which is considered a measure of anhedonia-like behavior. However, it should be noted that the preference for sucrose consumption in control animals was so high that it created a ceiling effect (over 80%), which was almost impossible to overcome from a statistical point of view. Importantly, no significant effect of M-5MPEP on the locomotor activity of mice was observed in either injection regimen. The statistically insignificant tendency to decrease activity after four administrations only confirms the credibility of the effect observed in the TST. Altogether, these results indicate that short-term, four-day administration of M-5MPEP can produce the prolonged antidepressant-like effects characteristic of RAAD.
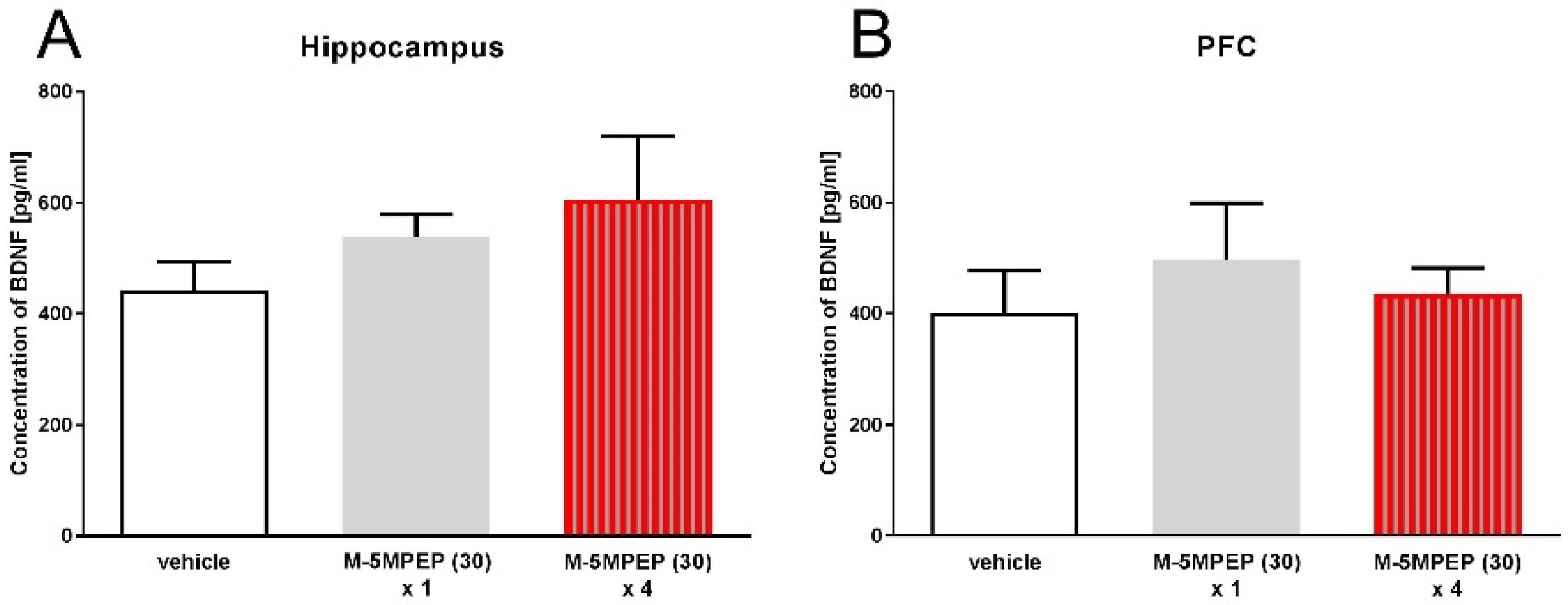
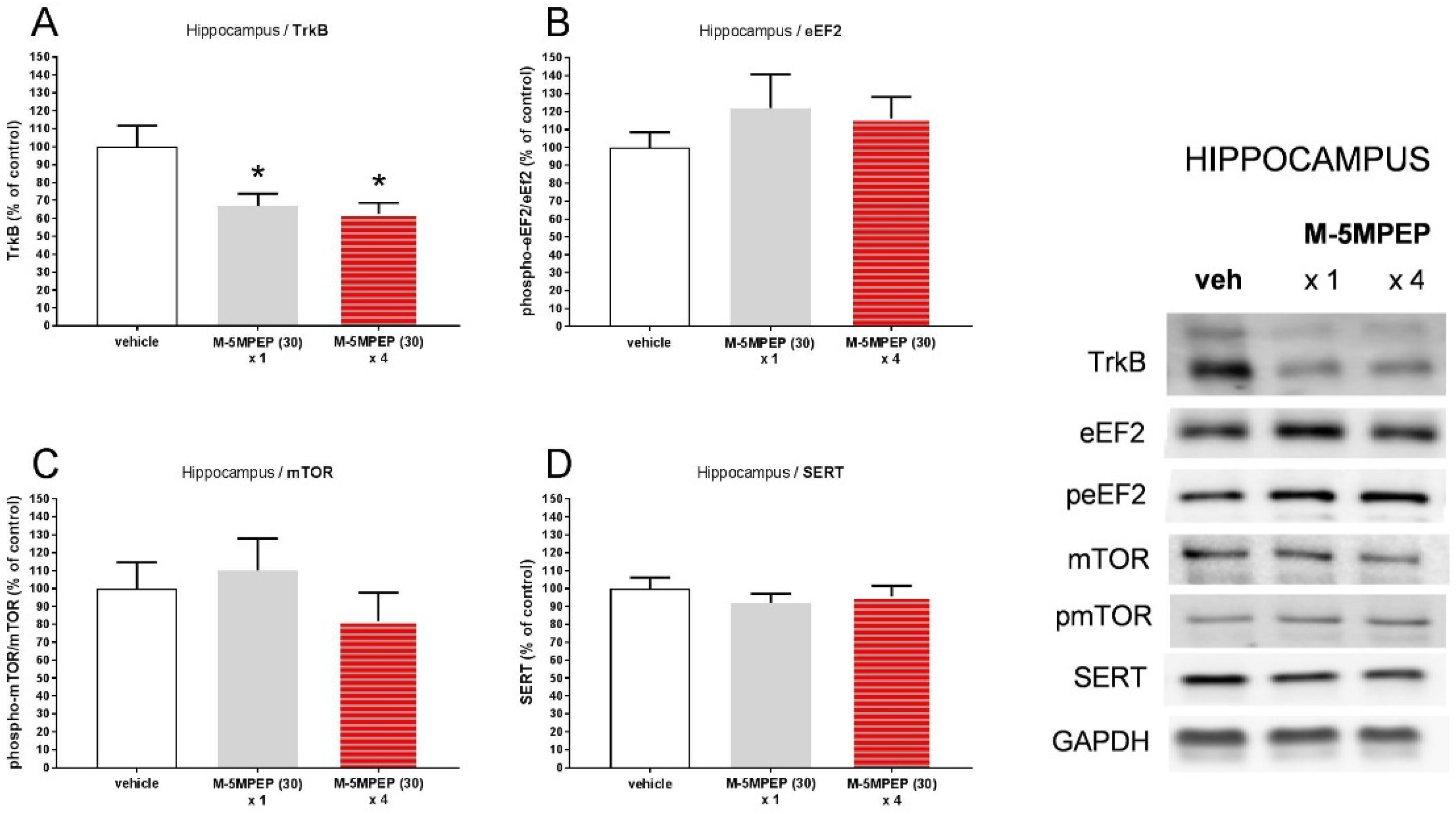
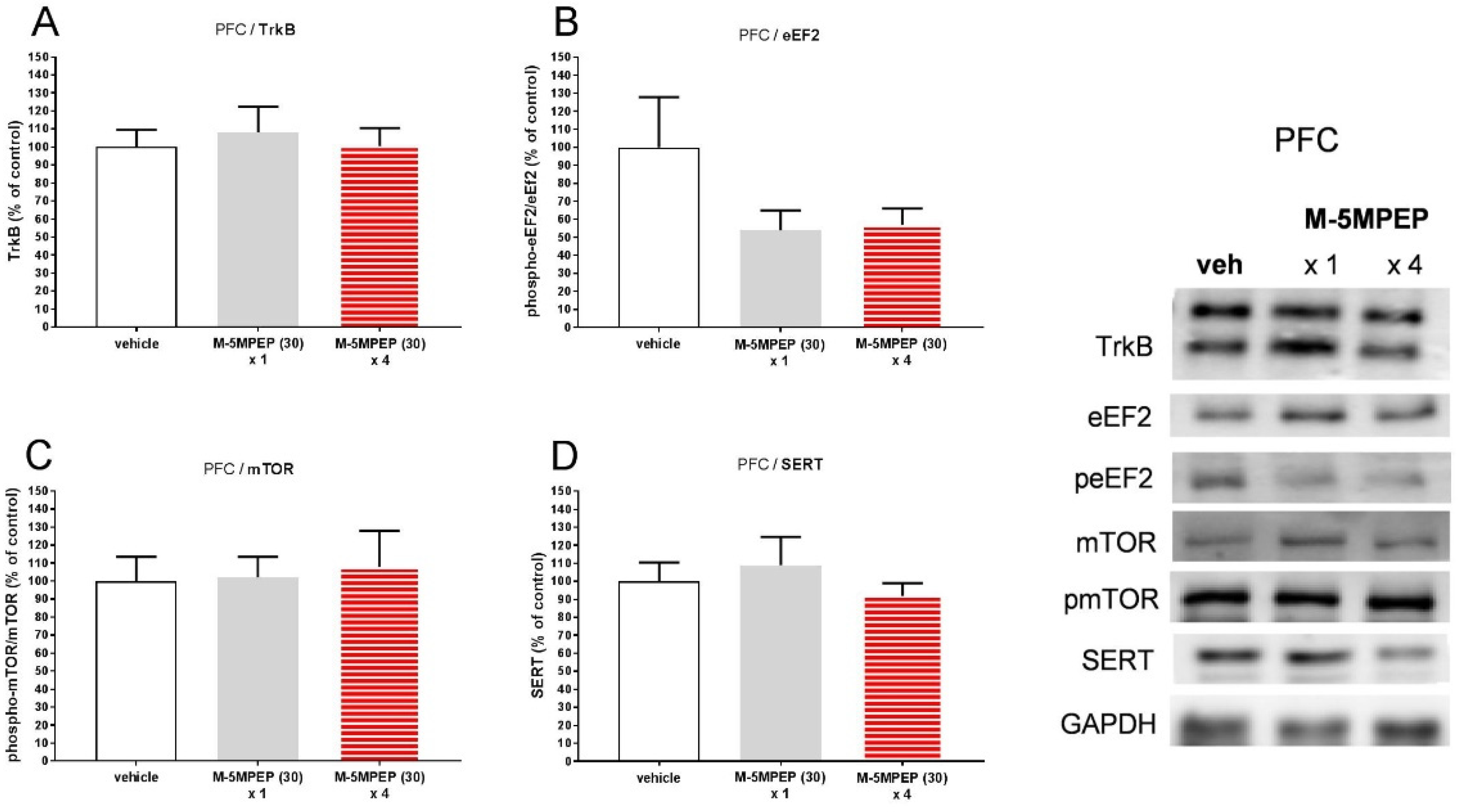
Considering the well-established role of BDNF in the mechanism of action of RAAD [
25], we decided to investigate its possible role in the observed processes. We found that in hippocampi obtained from mice after four days of M-5MPEP administration, the level of BDNF was higher than in control mice. However, this change did not reach statistical significance due to the low N value. Moreover, we did not observe any changes in BDNF levels between the experimental groups in the PFC. Western blot analyses showed that M-5MPEP-decreased TrkB levels in the hippocampus but not in the PFC, which suggests that the TrkB/BDNF pathway in the hippocampus may play a role in the mechanism of the prolonged antidepressant-like effect of M-5MPEP. Notably, the remaining Western blot analyses showed no differences in phosphorylated mTOR, eEF2, and SERT, both in the hippocampus and PFC, although a robust non-statistical tendency to reduce the activity of the phosphorylated form of eEF2 was observed in the PFC, which is consistent with the hypothesis regarding the role of this factor in the mechanism of the antidepressant effect of RAAD [
25]. Lack of M-5MPEP-induced changes in the expression of the above factors does not resolve the issue of their involvement in the mechanism of sustained antidepressant-like effects. It should be remembered that the measurements were carried out on the tissue taken 54 hours after injection. So, it is difficult to compare them with the results obtained by other authors who used various experimental schemes [
25,
27].
Since ELISA and Western blot results indicated the possible involvement of BDNF and its receptor, TrkB, in the mechanisms of the prolonged antidepressant-like effect of M-5MPEP in mice, we decided to use behavioral methods to investigate whether M-5MPEP effects were dependent on the activation of the TrkB receptor. For this purpose, we used its antagonist, ANA-12, which actively penetrates the brain after peripheral administration. We found that ANA-12 administered 30 min before each M-5MPEP injection, fully reversed both the anti-apathetic effect in the splash test and the decreased immobility time in the TST, indicating the TrkB-dependent mechanism of the sustained antidepressant-like effects of a partial mGlu5 NAM, M-5MPEP.
The dependence of prolonged antidepressant effects on TrkB receptor activation is a hallmark of RAAD and was demonstrated in various animal models of depression [
25,
29,
30,
37,
38]. Similarly, a prolonged antidepressant-like effect of the mGlu2/3 receptor antagonist, LY341495, seems to depend on BDNF since its action was blocked by the TrkB receptor antagonist, K252a, in the TST and the NSF tests [
30]. Interestingly, previous data showed that the prolonged effects of the full mGlu5 antagonist, MPEP, were not blocked by prior administration of the TrkB receptor antagonist, K252a, which may suggest differences in the mechanisms of full and partial mGlu5 antagonists [
22]. However, the studies used other antagonists, which may influence different results.
Our recent studies have shown that one of the mGlu ligands, LY341495, which itself induces an antidepressant-like effect, enhances the antidepressant-like action of
(R)-ketamine (but not
(S)-ketamine) in a mechanism related to the activation of BDNF/TrkB pathway [
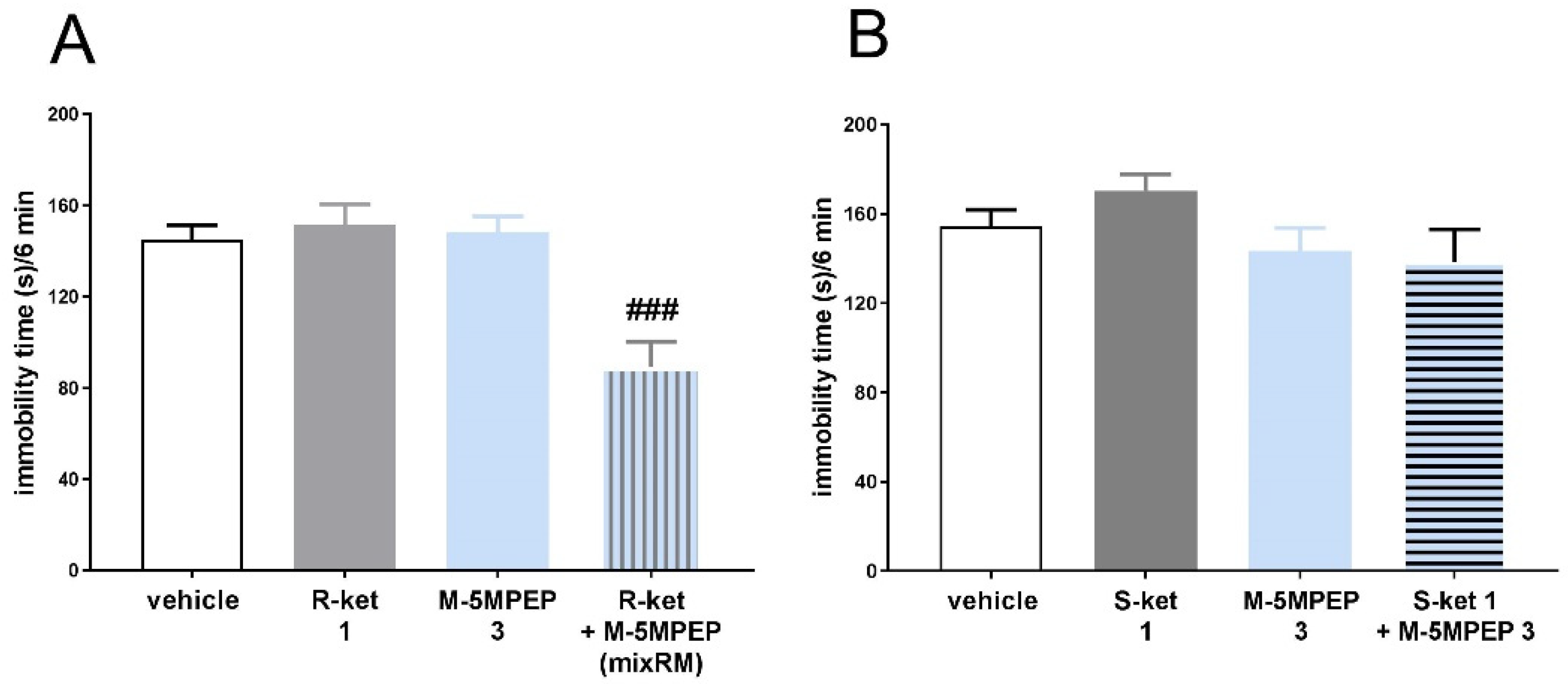
39]. Thus, in the next stage, we checked whether M-5MPEP potentiates ketamine enantiomers. We found that a subeffective dose of
(R)-ketamine (1 mg/kg) was potentiated by a subeffective dose of M-5MPEP (3 mg/kg) in the TST. This rapid antidepressant-like effect was fully antagonized by prior administration of an AMPA antagonist, NBQX, and a TrkB receptor antagonist, ANA-12, indicating that it was related to BDNF activity and AMPA receptor activation. On the other hand,
(S)-ketamine was not potentiated by M-5MPEP in the same experimental scheme, which indicates that only one ketamine enantiomer, namely
(R)-ketamine, can be potentiated by the partial mGlu5 NAM, through a mechanism dependent on the activation of the TrkB receptor. Since
(R)-ketamine is much safer than
(S)-ketamine due to the approximately four times lower affinity for the NMDA receptor [
37,
40], the blockade of which is related to ketamine-induced undesirable effects, the combination of drugs we used seems to be promising due to its high safety of use. This finding requires further research and confirmation in models of depression designed to study the mechanisms of rapid antidepressant actions. Altogether, our findings open further prospects for the practical use of M-5MPEP in the effective and safe therapy of depression.