Submitted:
13 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
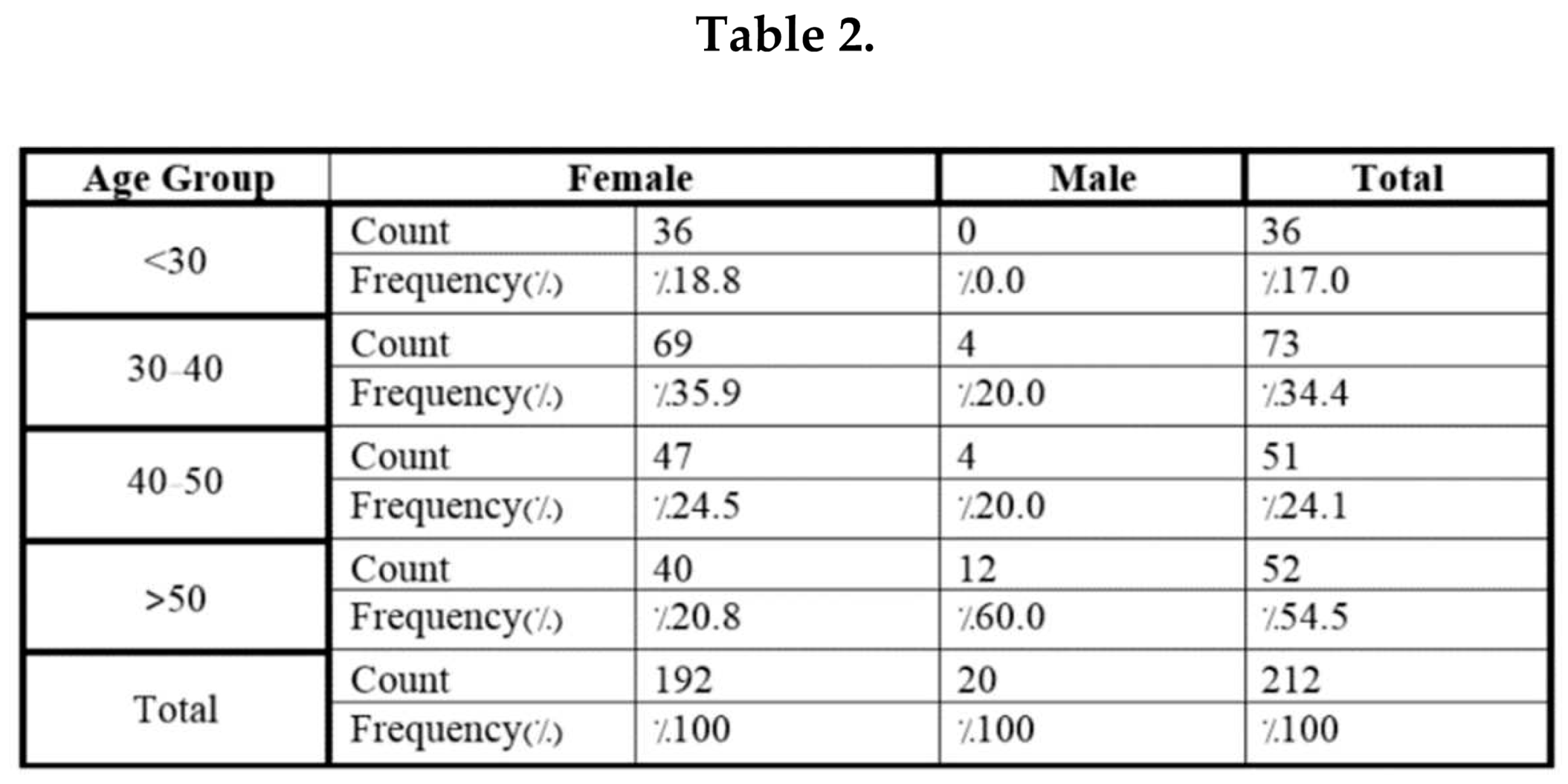
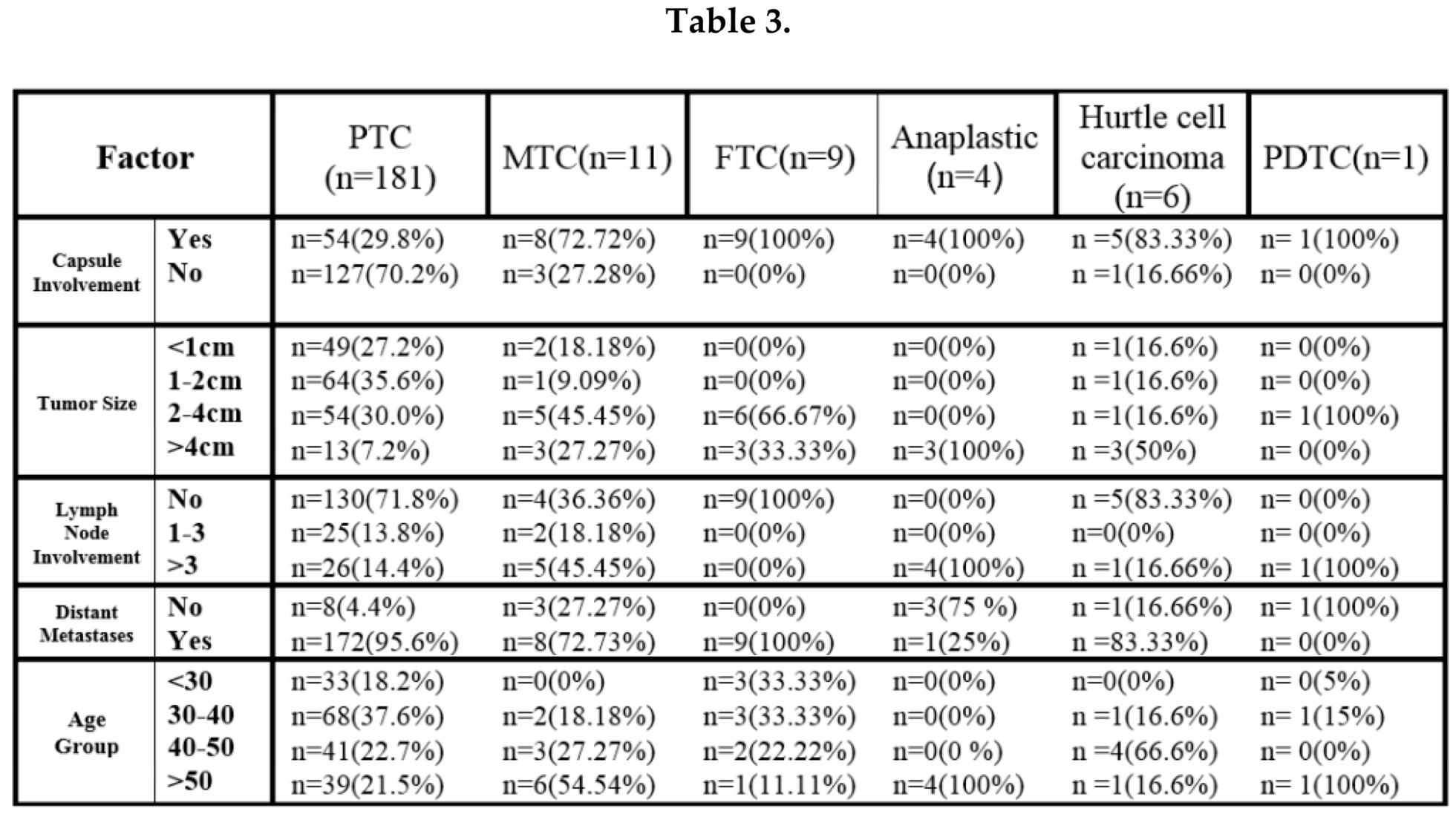
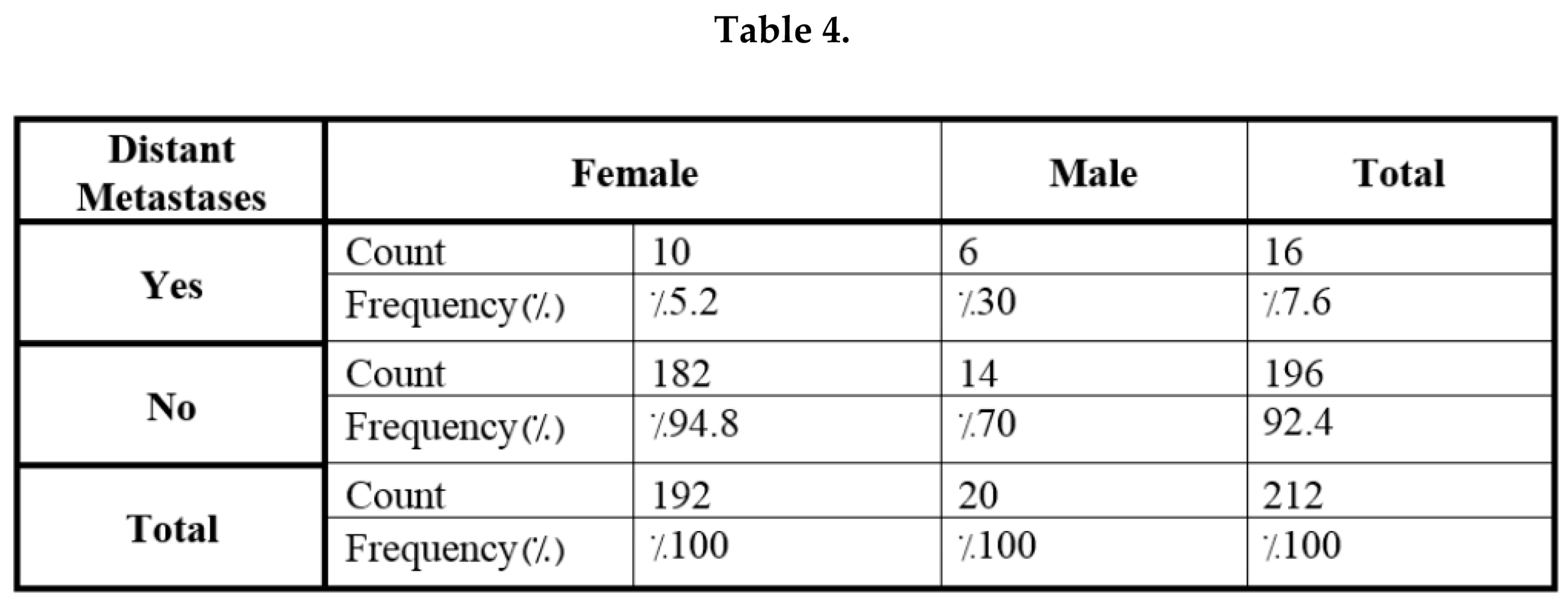
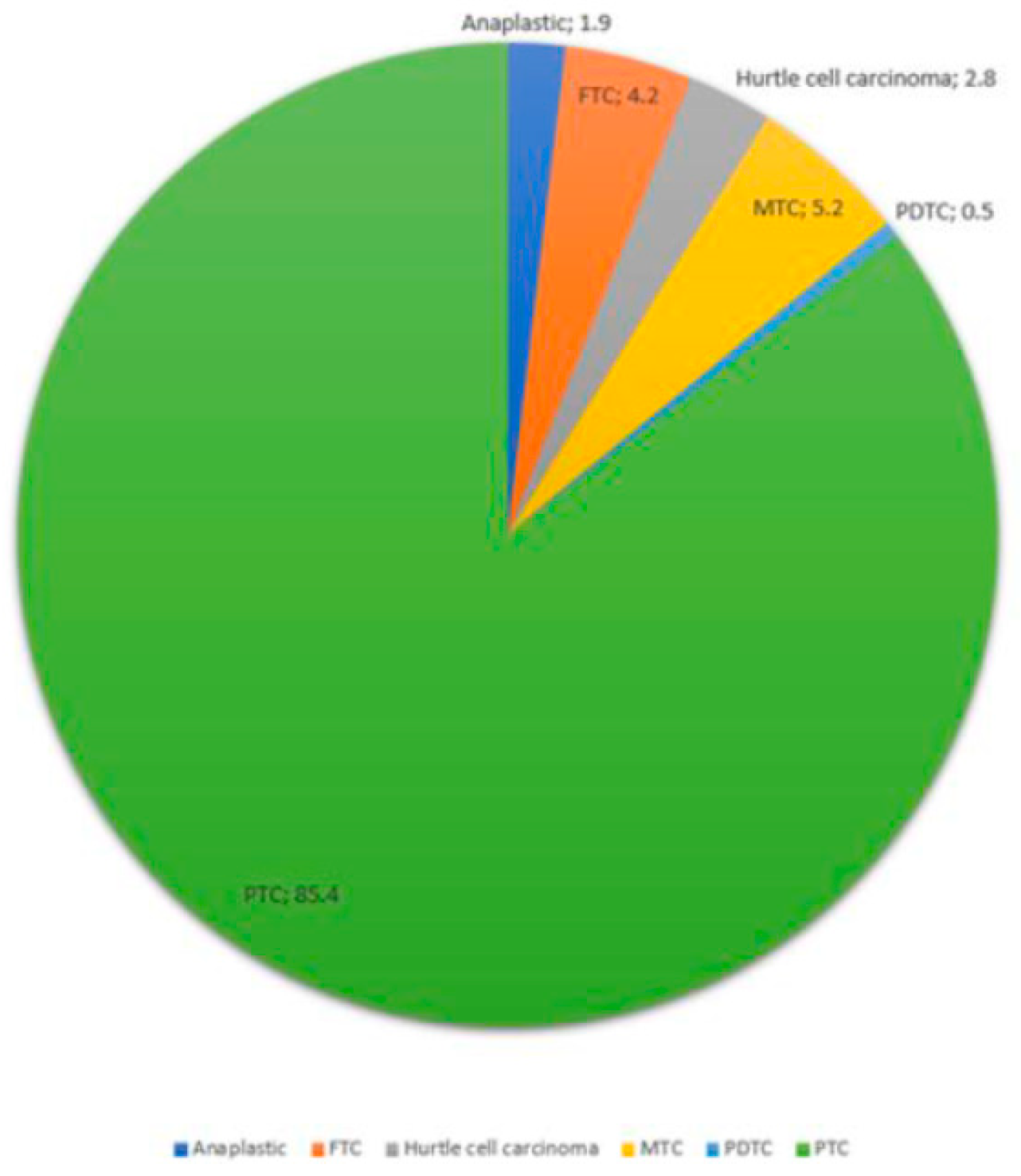
4. Discussion
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Bikas, A.; Burman, K.D. Epidemiology of thyroid cancer. The Thyroid and Its Diseases: A Comprehensive Guide for the Clinician 2019, 541–547.
- Xue, L.; Gong, Z.; Vlantis, A.C.; Chan, J.Y.; Meehan, K.; van Hasselt, C.A.; et al. Autophagy regulates anti-angiogenic property of lenvatinib in thyroid cancer. American journal of cancer research 2023, 13, 1457–1470. [Google Scholar] [PubMed]
- Boukheris, H.; Bettayeb, A.; Anderson, L.A.; Achour, Z.; Benbachir, F.Z.; Attar, S.; et al. Changes in the demographic and clinicopathological characteristics of thyroid cancer: a population-based investigation in Algeria, 1993-2013. Journal of cancer epidemiology 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Rendl, G.; Rodrigues, M.; Schweighofer-Zwink, G.; Hutter, J.; Hittmair, A.; Zellinger, B.; et al. Clinicopathological characteristics of thyroid cancer in the federal state of Salzburg. Wiener klinische Wochenschrift 2017, 129, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Chmielik, E.; Rusinek, D.; Oczko-Wojciechowska, M.; Jarzab, M.; Krajewska, J.; Czarniecka, A.; et al. Heterogeneity of Thyroid Cancer. Pathobiology: journal of immunopathology, molecular and cellular biology 2018, 85, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. The Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Fauci, A.S.; Kasper, D.L.; Hauser, S.; Longo, D.; Jameson, J.L. Harrison's Principles of Internal Medicine, (Vol. 1 & Vol. 2): McGraw Hill Professional; 2022.
- Abdul-Sater, Z.; Mukherji, D.; Adib, S.M.; Shamseddine, A.; Abu-Sitta, G.; Fadhil, I.; et al. Cancer registration in the Middle East, North Africa, and Turkey (MENAT) region: A tale of conflict, challenges, and opportunities. Frontiers in Oncology 2022, 12, 1050168. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.Y.; Choi, H.S.; Park, Y.J.; Lim, J.A.; Ahn, H.Y.; Lee, E.K.; et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid: official journal of the American Thyroid Association 2013, 23, 797–804. [Google Scholar] [CrossRef]
- Bukhari, U.; Sadiq, S.; Memon, J.; Baig, F. Thyroid carcinoma in Pakistan: a retrospective review of 998 cases from an academic referral center. Hematology/Oncology and Stem Cell Therapy 2009, 2, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Current thyroid cancer trends in the United States. JAMA otolaryngology–head & neck surgery 2014, 140, 317–322. [Google Scholar]
- Du, L.; Wang, Y.; Sun, X.; Li, H.; Geng, X.; Ge, M.; et al. Thyroid cancer: trends in incidence, mortality and clinical-pathological patterns in Zhejiang Province, Southeast China. BMC cancer 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Shobab, L.; Burman, K.D.; Wartofsky, L. Sex differences in differentiated thyroid cancer. Thyroid: official journal of the American Thyroid Association 2022, 32, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; de Vathaire, F.; Boutron-Ruault, M.-C.; Journy, N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocrine-Related Cancer 2020, 27, 245–259. [Google Scholar]
- Moeller, L.C.; Führer, D. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr Relat Cancer 2013, 20, R19–R29. [Google Scholar] [CrossRef] [PubMed]
- Laurberg, P.; Knudsen, N.; Andersen, S.; Carlé, A.; Pedersen, I.B.; Karmisholt, J. Thyroid function and obesity. European thyroid journal 2012, 1, 159–167. [Google Scholar] [CrossRef]
- Brandt, F.; Thvilum, M.; Almind, D.; Christensen, K.; Green, A.; Hegedüs, L.; et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PloS one 2013, 8, e66711. [Google Scholar] [CrossRef]
- Jonklaas, J.; Nsouli-Maktabi, H.; Soldin, S.J. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid: official journal of the American Thyroid Association 2008, 18, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Moazezi, Z.; Mahmoudi, M.; Yahyahpour, Y.; Alaleh, A. Risk factors of thyroid cancer in Babol, Northern Iran. Caspian Journal of Internal Medicine 2011, 2, 171. [Google Scholar] [PubMed]
- Dolidze, D.D.; Shabunin, A.V.; Mumladze, R.B.; Vardanyan, A.V.; Covantsev, S.D.; Shulutko, A.M.; et al. A narrative review of preventive central lymph node dissection in patients with papillary thyroid cancer-a necessity or an excess. Frontiers in Oncology 2022, 12, 906695. [Google Scholar] [CrossRef]
- Larijani, B.; Aghakhani, S.; Khajeh-Dini, H.; Baradar-Jalili, R. Clinico-pathological features of thyroid cancer as observed in five referral hospitals in Iran. Acta Oncologica 2003, 42, 334–337. [Google Scholar] [CrossRef]
- Capezzone, M.; Tosti Balducci, M.; Morabito, E.M.; Durante, C.; Piacentini, P.; Torregrossa, L. ; et al. High incidence of thyroid Cancer in southern Tuscany (Grosseto Province, Italy): potential role of environmental heavy metal pollution. Biomedicines 2023, 11, 298. [Google Scholar] [CrossRef]
- Jayarajah, U.; Fernando, A.; Prabashani, S.; Fernando, E.A.; Seneviratne, S.A. Incidence and histological patterns of thyroid cancer in Sri Lanka 2001-2010: an analysis of national cancer registry data. BMC cancer 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.S.; Hamid, G.A. Diagnosis and Treatment of Patients with Thyroid Cancer in Yemen during the National war 2017-2021.
- Flemban, A.F.; Kabrah, S.; Alahmadi, H.; Alqurashi, R.K.; Turaes, A.S.; Almaghrabi, R.; et al. Patterns of Thyroid Cancer Mortality and Incidence in Saudi Arabia: A 30-Year Study. Diagnostics 2022, 12, 2716. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA: a cancer journal for clinicians 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nature Reviews Endocrinology 2016, 12, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Risk patterns of distant metastases in follicular, papillary and medullary thyroid cancer. Hormone and Metabolic Research 2022, 54, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Fujimoto, Y.; Yamamoto, N. Papillary thyroid carcinoma with distant metastases: survival predictors and the importance of local control. Surgery 2008, 143, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Levy, S.; Tsvetov, G.; Gorshtein, A.; Slutzky-Shraga, I.; Akirov, A.; et al. Long-term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocrine Practice 2017, 23, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Lee, J.S.; Yun, H.J.; Chang, H.; Kim, S.M.; Lee, Y.S.; et al. Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis. Cancers 2022, 14, 5784. [Google Scholar] [CrossRef]
- Sugino, K.; Ito, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; et al. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid: official journal of the American Thyroid Association 2011, 21, 751–757. [Google Scholar] [CrossRef]
- Qari, F.A. Pattern of thyroid malignancy at a University Hospital in Western Saudi Arabia. Saudi med J 2004, 25, 866–870. [Google Scholar]
- Aziz, A.; Khan, S.A.; Suchal, Z.A.; Islam, N. Clinicopathological Characteristics and Treatment Outcome of Patients with Metastatic Differentiated Thyroid Cancer. Indian journal of endocrinology and metabolism 2022, 26, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Williams, V.L.; Hallanger Johnson, J.; Valderrabano, P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid: official journal of the American Thyroid Association 2020, 30, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Saeidi, M.; Zarei, A.; Hasani, M. Investigating the demographic characteristics and pathological manifestations of thyroid Cancer during the last two decades (1997–2017) in patients referred to Baqiyatallah hospital, Tehran, Iran. Journal of Diabetes & Metabolic Disorders 2020, 19, 1165–1172. [Google Scholar]
- Kent, W.D.; Hall, S.F.; Isotalo, P.A.; Houlden, R.L.; George, R.L.; Groome, P.A. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. Cmaj 2007, 177, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA oncology 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M., Jacob, J., Eds. Invasion in thyroid cancer: controversies and best practices. Seminars in Diagnostic Pathology; 2020: Elsevier.
- Samargandy, S.; Qari, R.; Aljadani, A.; Assaqaf Sr, D.; Etaiwi, A.; Alghamdi, D.; et al. Clinicopathological characteristics of thyroid cancer in a Saudi academic hospital. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Izadi, B.; Jalilian, S.; Ramezani, M.; Sadeghi, M.; Khazaei, S. A study of clinicopathologic features of thyroid cancer in Western Iran: A 9-year experience. Clinical cancer Investigation journal 2019, 8. [Google Scholar]
| Variables | total | Frequency (%) | |
|---|---|---|---|
| n =212 | |||
| Gender | male | 20 | %9.4 |
| female | 192 | %90.6 | |
| Pathology | PTC | 181 | %85.4 |
| FTC | 9 | %4.2 | |
| MTC | 11 | %5.2 | |
| Anaplastic | 4 | %1.9 | |
| Hurtle cell carcinoma | 6 | %2.8 | |
| PDTC | 1 | %0.5 | |
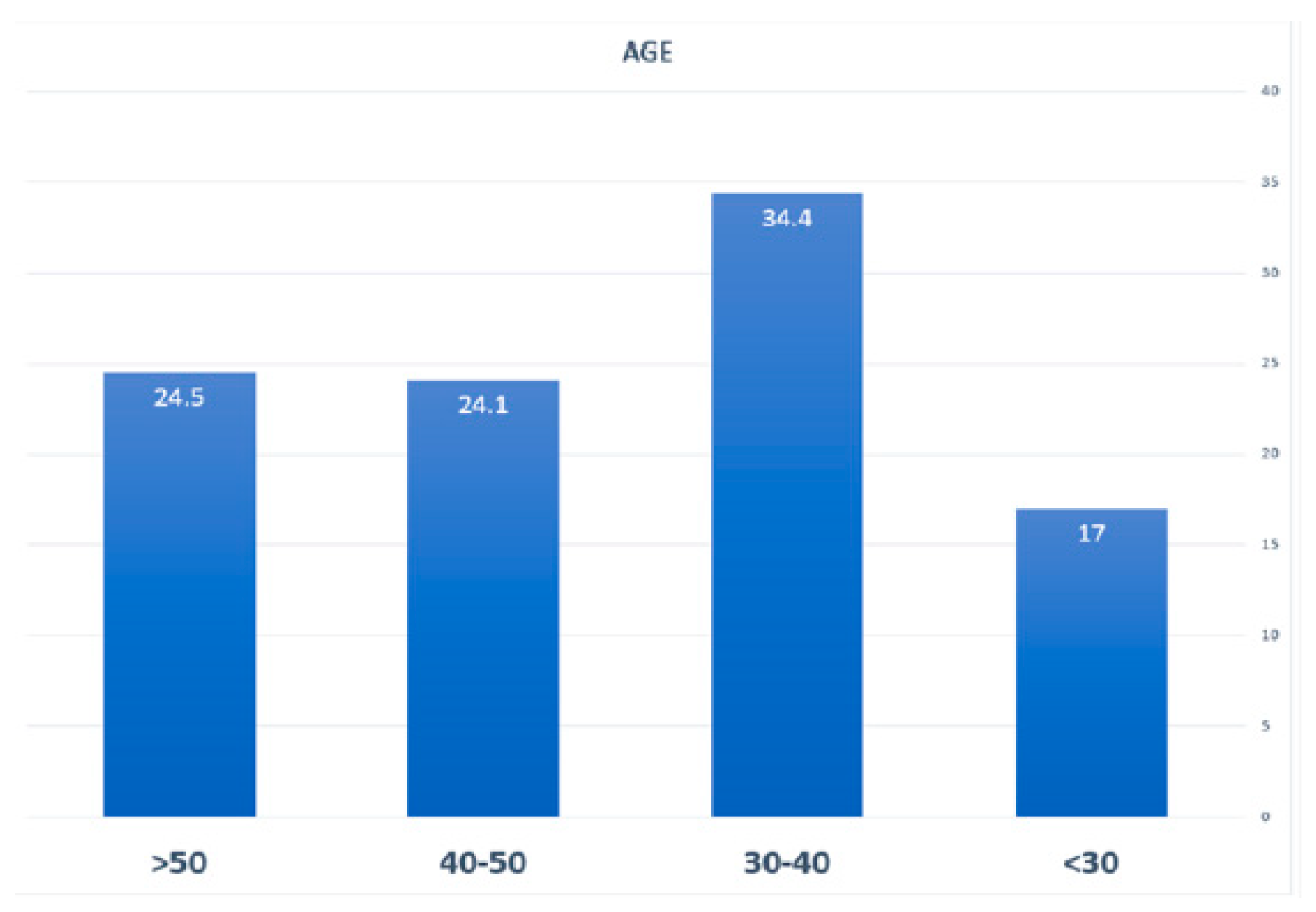
| Age Group | < 30 years | 36 | %17 |
| 30-40 | 73 | %34.4 | |
| 50-40 | 51 | %24.1 | |
| >50 | 52 | %24.5 | |
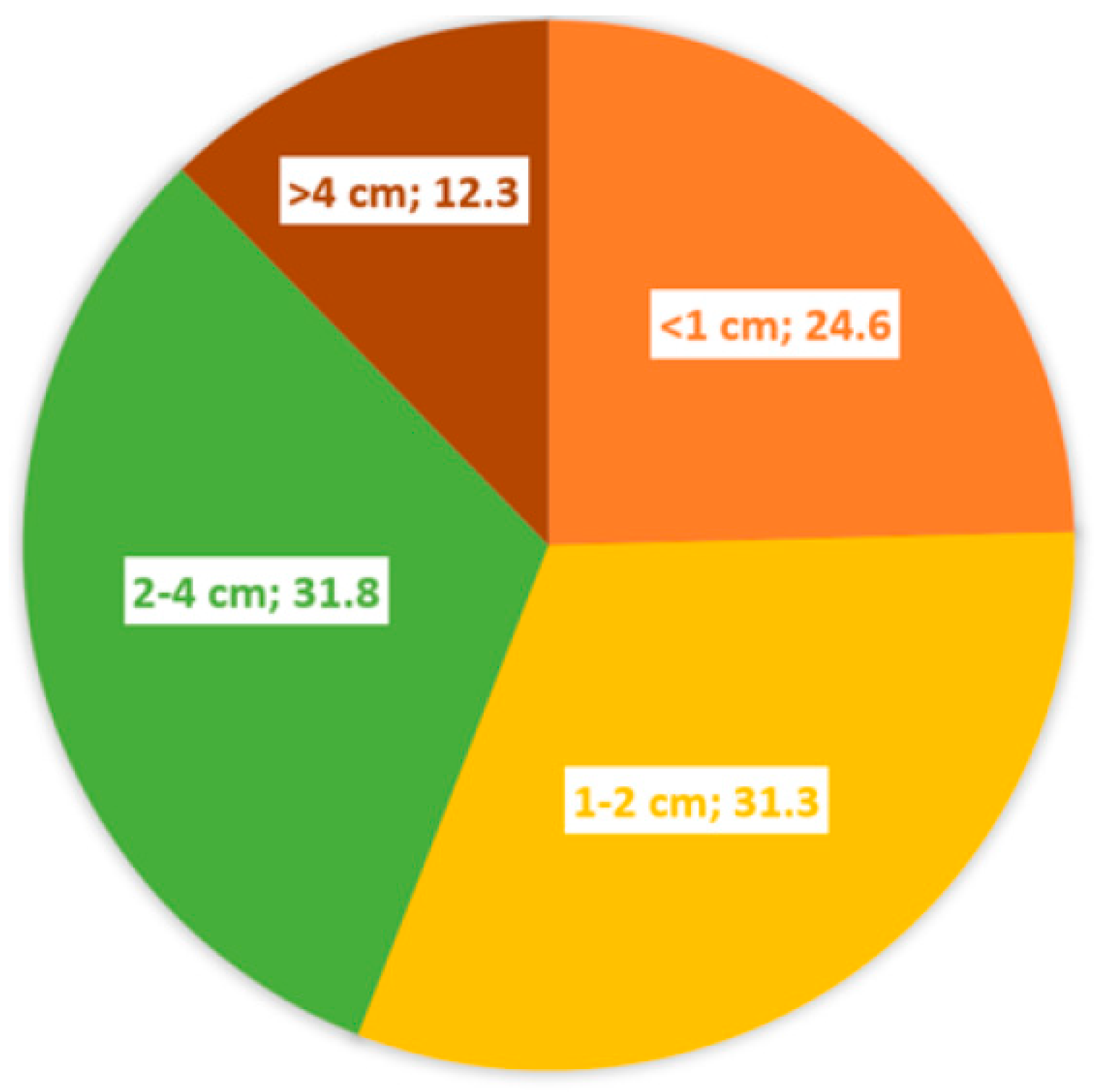
| Tumor size | <1cm | 52 | %24.6 |
| 1-2 cm | 66 | %31.3 | |
| 2-4 cm | 67 | %31.8 | |
| >4 cm | 27 | %12.3 | |
| Lymph node involvement | No | 147 | %69.3 |
| 1-3 Lymph node | 28 | %13.2 | |
| >3 Lymph node | 37 | %17.5 | |
| Lymph node involvement | Yes | 70 | %33 |
| No | 142 | %67 | |
| Distance metastases | No | 196 | %92.4 |
| Yes | 16 | %7.6 | |
| Capsule involvement | No | 131 | %61.8 |
| Yes | 81 | %38.2 | |
| Family history of thyroid cancer | No | 191 | %90.1 |
| Yes | 81 | %9.9 | |
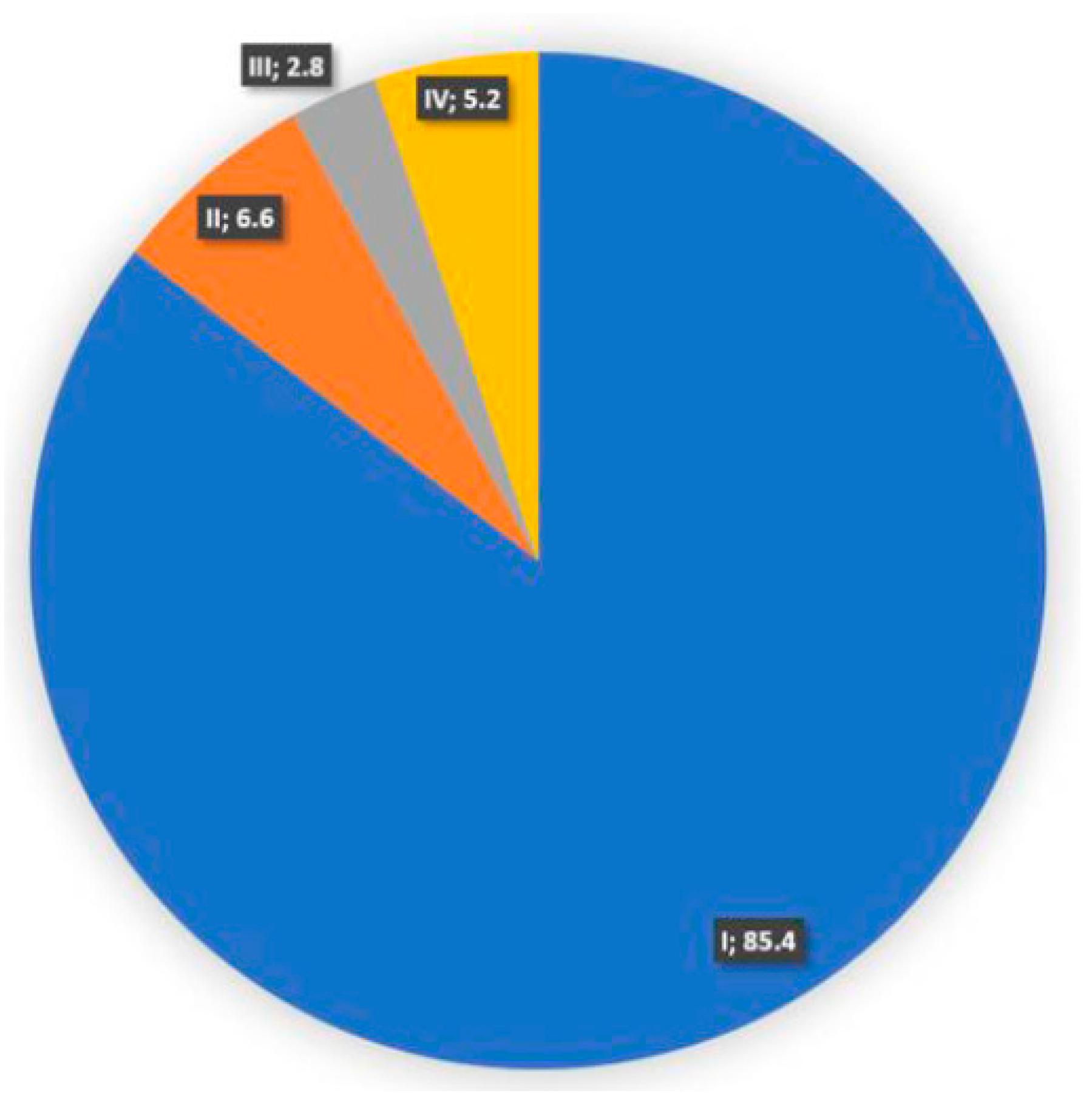
| Stage | I | 181 | %85.4 |
| II | 14 | %6.6 | |
| III | 6 | %2.8 | |
| IV | 11 | %5.2 | |
| T | T1a | 58 | %27.4 |
| T1b | 58 | %27.4 | |
| T2 | 64 | %30.2 | |
| T3a | 16 | %7.5 | |
| T3b | 11 | %5.2 | |
| T4a | 2 | %0.9 | |
| T4b | 3 | %1.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




