Submitted:
15 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Morphological observation of flower buds and leaf buds of A. bulbifer
2.2. Transcriptome analysis
2.2.1. Transcriptome sequencing data processing, transcript splicing and functional annotation
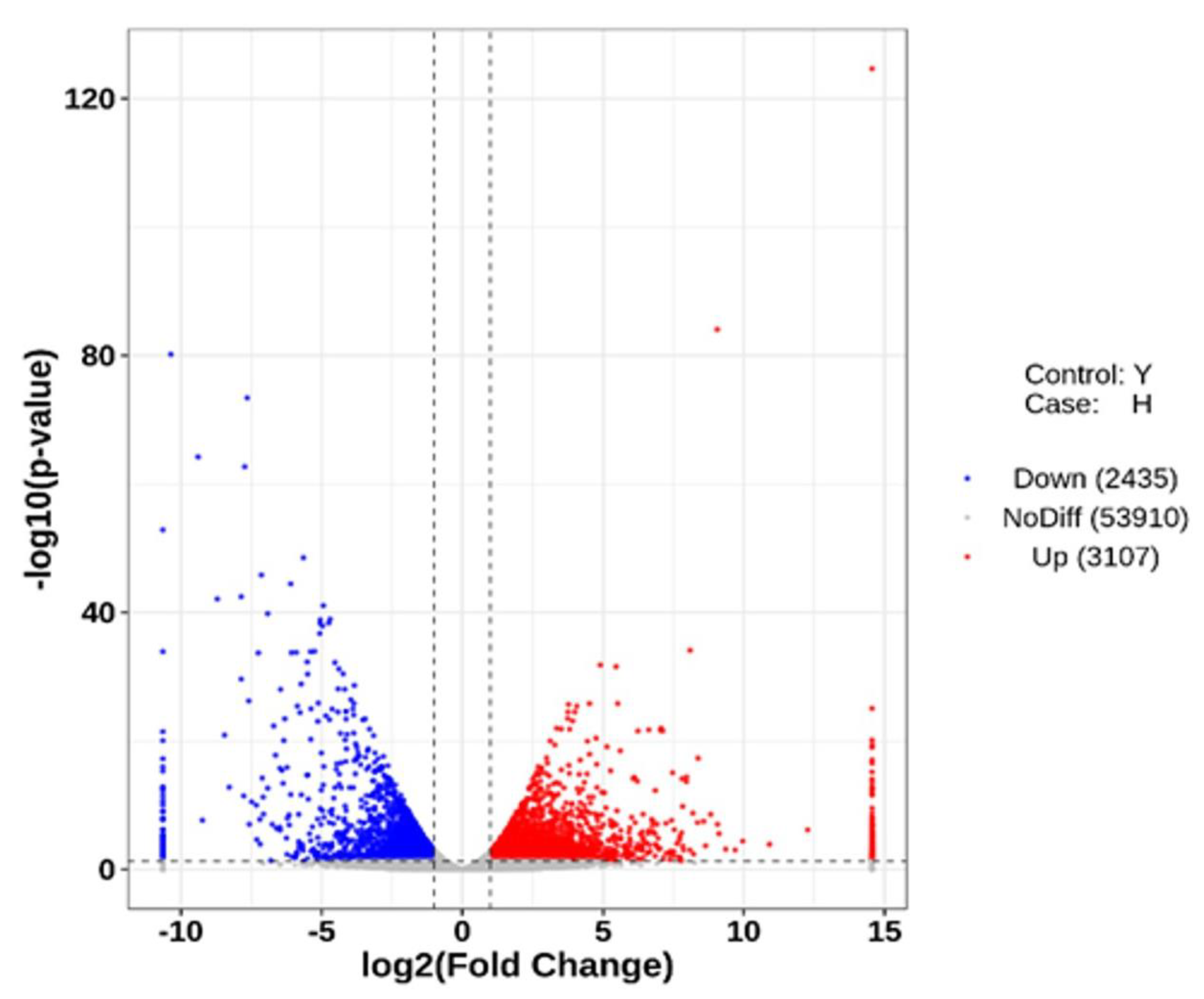
2.2.2. Screening of differentially expressed genes
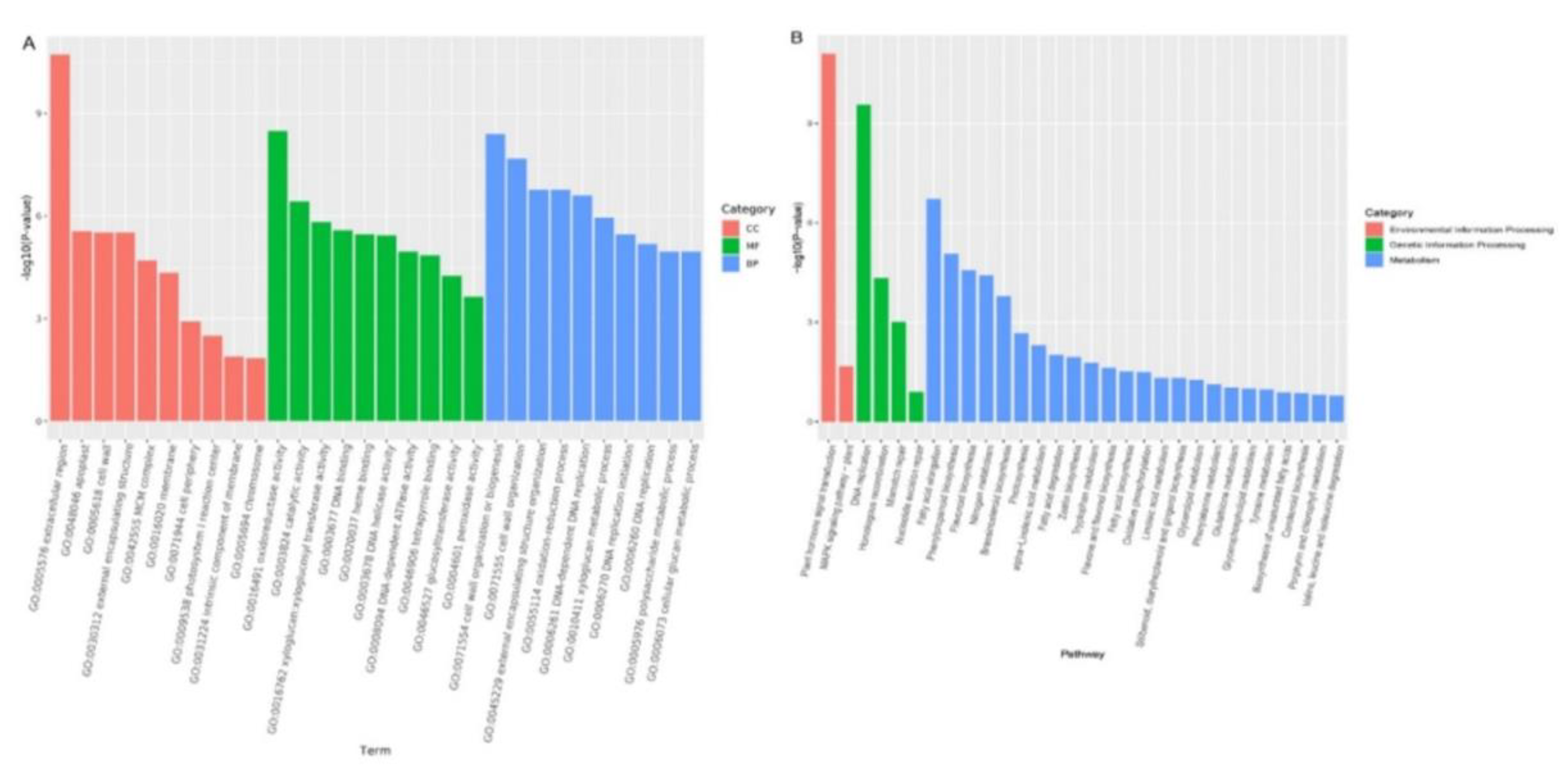
2.2.3. Functional annotation and classification of differentially expressed genes
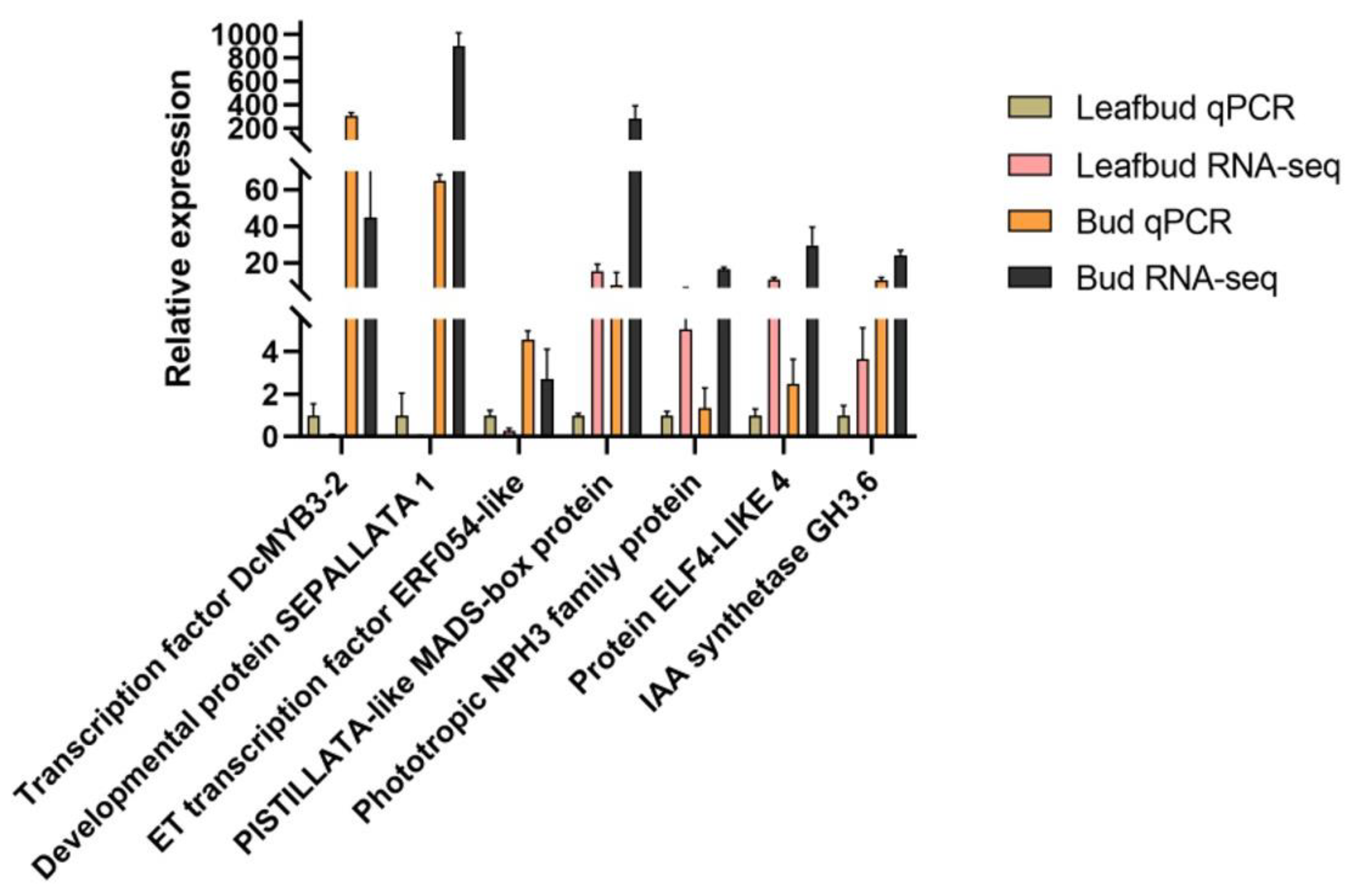
2.2.4. Validation of the transcriptome data
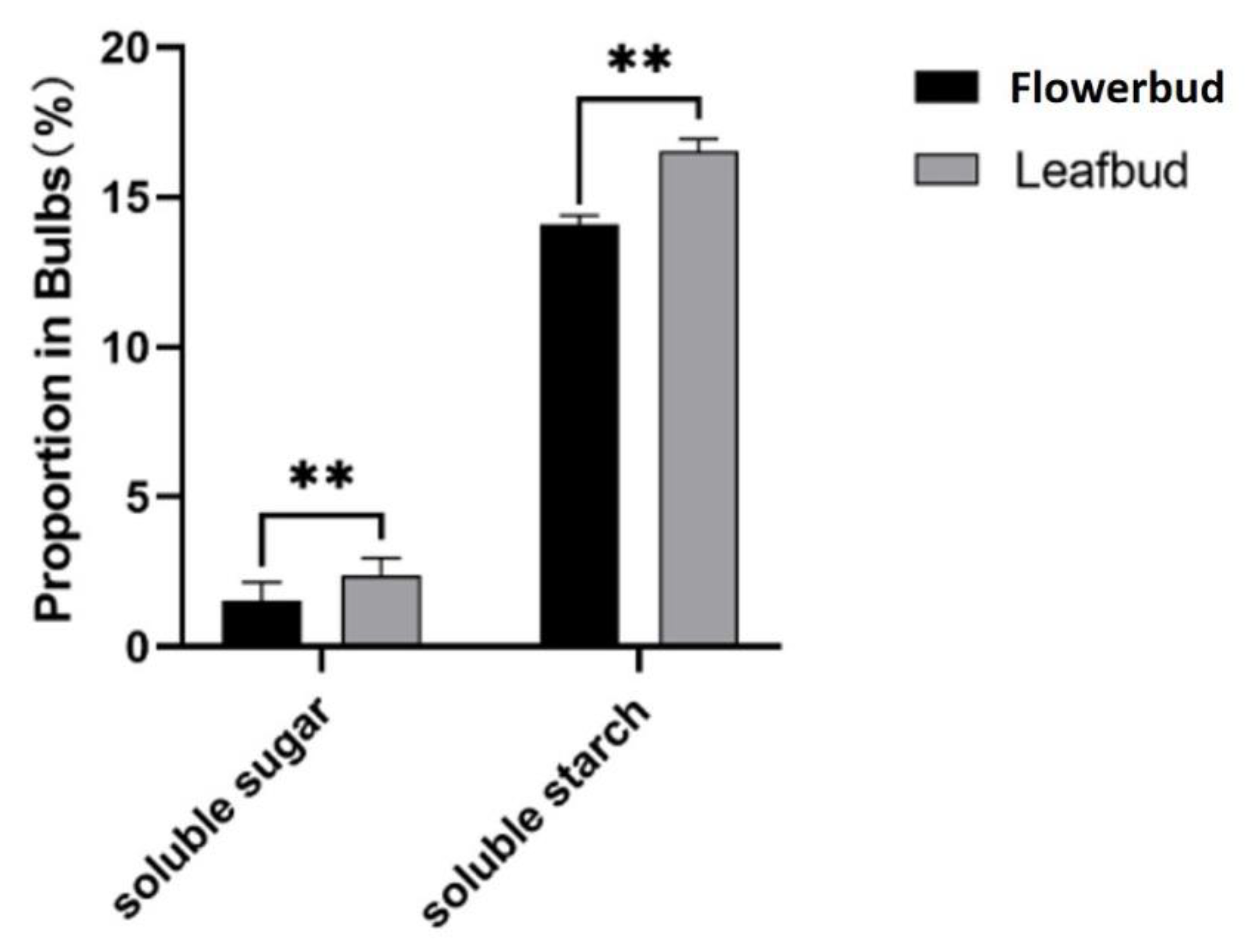
2.3. Determination of total soluble sugar and starch content in A. bulbifer corms
2.4. Metabolomic analysis
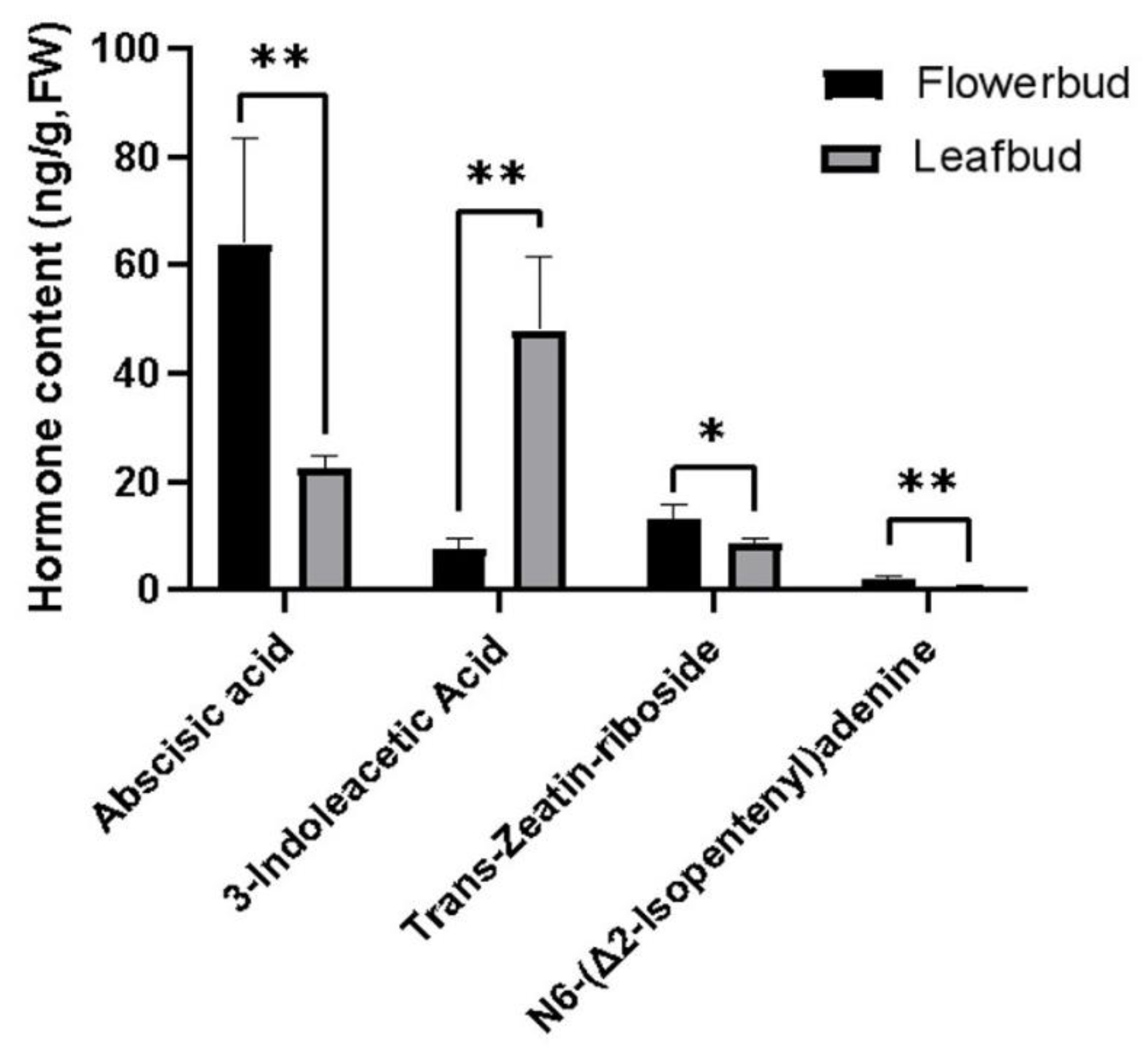
2.4.1. Targeted metabolic analysis of endogenous hormone contents in flower buds and leaf buds of A. bulbifer with corms
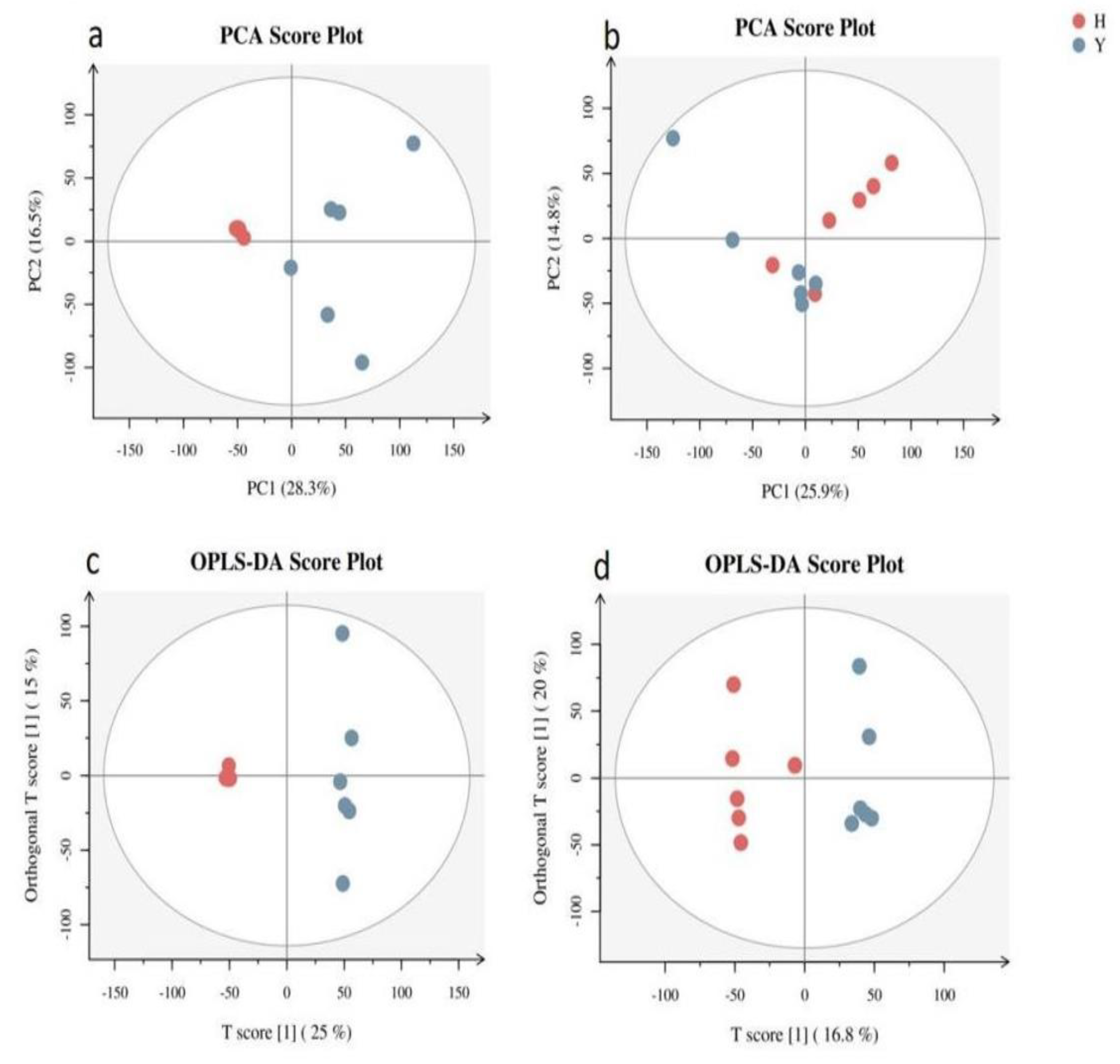
2.4.2. Nontargeted analysis of the metabolite content in flower buds and leaf buds of A. bulbifer
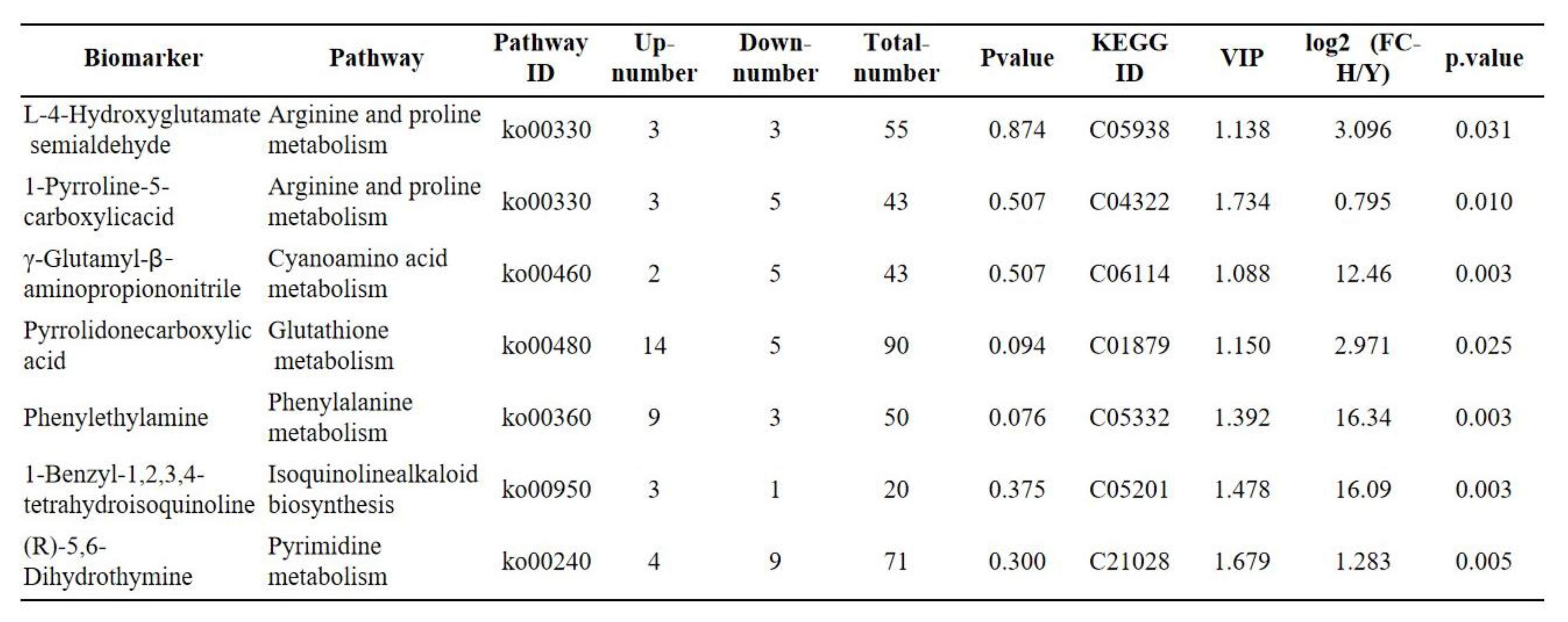
2.4.3. Identification of differentially accumulated metabolites
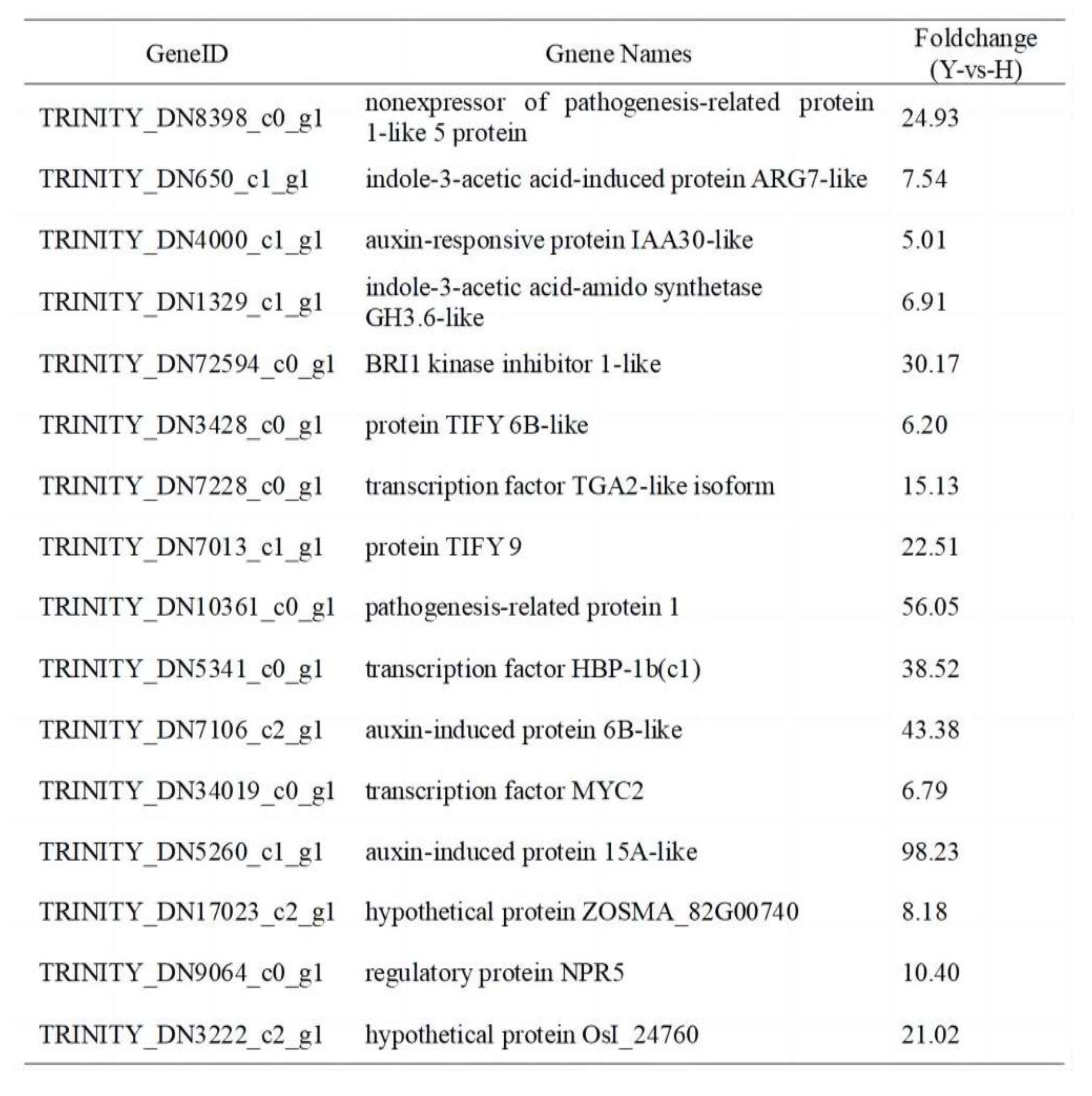
2.5. Correlation analysis between differentially expressed genes and differentially accumulated metabolites
3. Discussion
4. Conclusion
5. Materials and methods
5.1. Plant material cultivation
5.2. Transcriptome analysis
5.2.1. RNA extraction from flower buds and leaf buds of A. bulbifer
5.2.2. Transcriptome sequencing analysis
5.2.3. Quantitative RT‒PCR (qRT‒PCR) analysis
5.3. Determination of sugar content in A. bulbifer corms
5.4. Targeted metabolic analysis of A. bulbifer flower buds and leaf bud hormone content
5.4.1. Sample extraction
5.4.2. Liquid-phase parameters
5.4.3. Mass spectrometry parameters
5.5. Nontargeted metabolic analysis of A. bulbifer flower bud and leaf bud metabolite contents
5.5.1. Metabolite extraction
5.5.2. UPLC‒MS analysis
5.5.3. Data processing and analysis
5.6. Combined transcriptomic and metabolomic analysis
Supplementary Materials
Funding
CRediT authorship contribution statement
Data availability statement
Conflicts of Interest
Declaration of competing interests
References
- Jie, P. A STUDY ON CHINESE AMORPHOPHALLUS RESOURCES. Resources Science 2001, 23(05), 87–89. [Google Scholar]
- Liu, P. Y. Konjac. China Agricultural Press: 2004; p348.
- Du, Q.; Liu, J.; Ding, Y. Recent progress in biological activities and health benefits of konjac glucomannan and its derivatives. Bioact. Carbohydrates Diet. Fibre 2021, 26, 100270. [Google Scholar] [CrossRef]
- Zhao, C.; Harijati, N.; Liu, E.; Jin, S.; Diao, Y.; Hu, Z. First report on DNA content of three species of Amorphophallus. J. Genet. 2020, 99, 1–6. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Zhao, J. P.; Srzednicki, G.; Borompichaichartkul, C.; Kanlayanarat, S. Morphological and growth characteristics of Amorphophallus muelleri blume A commercially important konjac species. Acta Horticulturae 2010, 875, 501–508. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, Y.; Huang, L.; Huang, Y.; Chen, J.; Zhou, H.; Zhang, X. Comparative analysis of buds transcriptome and identification of two florigen gene AkFTs in Amorphophallus konjac. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Susila, H.; Nasim, Z.; Ahn, J.H. Ambient Temperature-Responsive Mechanisms Coordinate Regulation of Flowering Time. Int. J. Mol. Sci. 2018, 19, 3196. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Li, C.; Lin, H.; Joe, A.; Padilla, M.; Woods, D.P.; Dubcovsky, J. EARLY FLOWERING 3 interactions with PHYTOCHROME B and PHOTOPERIOD1 are critical for the photoperiodic regulation of wheat heading time. PLOS Genet. 2023, 19, e1010655. [Google Scholar] [CrossRef]
- Gai, Z.; Zhang, M.; Zhang, P.; Zhang, J.; Liu, J.; Cai, L.; Yang, X.; Zhang, N.; Yan, Z.; Liu, L.; et al. 2-Oxoglutarate contributes to the effect of foliar nitrogen on enhancing drought tolerance during flowering and grain yield of soybean. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shao-Hua, Y. J. G. a. S. Review on Mechanism of Strawberry Flower Bud Differentiation and Application of Regulation Techniques. 2011.
- Lu, X.; Li, J.; Chen, H.; Hu, J.; Liu, P.; Zhou, B. RNA-seq analysis of apical meristem reveals integrative regulatory network of ROS and chilling potentially related to flowering in Litchi chinensis. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Wang, X.; Liu, Q.; Gu, L.; Oka, Y.; Lin, C. Beyond the photocycle-how cryptochromes regulate photoresponses in plants? Curr Opin Plant Biol 2018, 45 (Pt A), 120–126. [Google Scholar] [CrossRef]
- Gao, D.; Ji, X.; Yuan, Q.; Pei, W.; Zhang, X.; Li, F.; Han, Q.; Zhang, S. Effects of total daily light integral from blue and broad-band red LEDs on flowering of saffron (Crocus sativus L.). Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Deng, X.W. Phytochrome Signaling Mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Woods, D.P.; Li, W.; Sibout, R.; Shao, M.; Laudencia-Chingcuanco, D.; Vogel, J.P.; Dubcovsky, J.; Amasino, R.M. PHYTOCHROME C regulation of photoperiodic flowering via PHOTOPERIOD1 is mediated by EARLY FLOWERING 3 in Brachypodium distachyon. PLOS Genet. 2023, 19, e1010706. [Google Scholar] [CrossRef] [PubMed]
- Lin, C. Photoreceptors and Regulation of Flowering Time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. The flower to fruit transition in Citrus is partially sustained by autonomous carbohydrate synthesis in the ovary. Plant Sci. 2019, 285, 224–229. [Google Scholar] [CrossRef]
- Gu, J.; Zeng, Z.; Wang, Y.; Lyu, Y. Transcriptome Analysis of Carbohydrate Metabolism Genes and Molecular Regulation of Sucrose Transport Gene LoSUT on the Flowering Process of Developing Oriental Hybrid Lily ‘Sorbonne’ Bulb. Int. J. Mol. Sci. 2020, 21, 3092. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, Y.; Zhang, N.; Zhang, B.; Niu, Y. Expression of the Amorphophallus albus heat stress transcription factor AaHsfA1 enhances tolerance to environmental stresses in Arabidopsis. Ind. Crop. Prod. 2021, 174, 114231. [Google Scholar] [CrossRef]
- Liu, T.; Hu, Y.; Li, X. Comparison of dynamic changes in endogenous hormones and sugars between abnormal and normal Castanea mollissima. Prog. Nat. Sci. 2008, 18, 685–690. [Google Scholar] [CrossRef]
- Park, S.Y.; Jeong, D.-H. Comprehensive Analysis of Rice Seedling Transcriptome during Dehydration and Rehydration. Int. J. Mol. Sci. 2023, 24, 8439. [Google Scholar] [CrossRef]
- Yan, B.; Hou, J.; Cui, J.; He, C.; Li, W.; Chen, X.; Li, M.; Wang, W. The Effects of Endogenous Hormones on the Flowering and Fruiting of Glycyrrhiza uralensis. Plants 2019, 8, 519. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Chen, Y.; Yang, W.; Li, M.; Sun, B.; Song, H.; Tang, W.; Zhang, Y.; Gong, R. Joint metabolome and transcriptome analysis of the effects of exogenous GA3 on endogenous hormones in sweet cherry and mining of potential regulatory genes. Front. Plant Sci. 2022, 13, 1041068. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Ma, D.; Lv, S.; Xu, S.; Zhong, B.; Peng, T.; Liu, D.; Liu, Y. Comparative Transcriptome and sRNAome Analyses Reveal the Regulatory Mechanisms of Fruit Ripening in a Spontaneous Early-Ripening Navel Orange Mutant and Its Wild Type. Genes 2022, 13, 1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, T.; Zhang, H.; Shao, J.; Peng, J.; Sun, J. Variation of Endogenous Hormones during Flower and Leaf Buds Development in ‘Tianhong 2’ Apple. HortScience 2020, 55, 1794–1798. [Google Scholar] [CrossRef]
- Liang, Y.; Bai, J.; Xie, Z.; Lian, Z.; Guo, J.; Zhao, F.; Liang, Y.; Huo, H.; Gong, H. Tomato sucrose transporter SlSUT4 participates in flowering regulation by modulating gibberellin biosynthesis. Plant Physiol. 2023, 192, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, N.; Jia, Y.; Zhang, Y.; Feng, M.; Jiang, C.-Z.; Ma, C.; Gao, J. An Ethylene-Induced Regulatory Module Delays Flower Senescence by Regulating Cytokinin Content. Plant Physiol. 2016, 173, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, Z.; Li, Z.; Jiao, P.; Zhai, J.; Liu, S.; Han, X.; Zhang, S.; Sun, J.; Gai, Z.; et al. Multi-omics analysis reveals spatiotemporal regulation and function of heteromorphic leaves in Populus. Plant Physiol. 2023, 192, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Li, S.; Qian, C.; Wang, H. Regulatory effects of polysaccharides and hormones on multi-leaf formation of Amorphophallus bulbifer. Journal of Northwest A & F University ( Natural Science Edition ) 2022, 50 (09), 69-79.
- Xiao, M.; Zhang, Y.; Chen, X.; Lee, E.-J.; Barber, C.J.; Chakrabarty, R.; Desgagné-Penix, I.; Haslam, T.M.; Kim, Y.-B.; Liu, E.; et al. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. J. Biotechnol. 2013, 166, 122–134. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Han, Y.; Yan, G.-L.; Wu, F.-F.; Wang, X.-J. Metabolomics biotechnology, applications, and future trends: a systematic review. RSC Adv. 2019, 9, 37245–37257. [Google Scholar] [CrossRef]
- Coneva, V.; Zhu, T.; Colasanti, J. Expression differences between normal and indeterminate1 maize suggest downstream targets of ID1, a floral transition regulator in maize. J. Exp. Bot. 2007, 58, 3679–3693. [Google Scholar] [CrossRef]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiology and Biochemistry 2007, 45(9), 705–710. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Minami, A.; Arakawa, K.; Fujikawa, S.; Takezawa, D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J. Plant Physiol. 2005, 162, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiology and Biochemistry 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, L.-N.; Hao, J.-B.; Zhang, Y.; Huang, J.-C. Comparative transcription profiles reveal that carbohydrates and hormone signalling pathways mediate flower induction in Juglans sigillata after girdling. Ind. Crop. Prod. 2020, 153, 112556. [Google Scholar] [CrossRef]
- Wei, L.; Yu, B.; Song, E. Responses of Endogenous Hormones and Amino Acids to Flower Bud Differentiation of Cassava. Southwest China Journal of Agricultural Sciences 2021, 34(07), 1400–1406. [Google Scholar]
- Su, H.; Xu, K.; Liu, W. Changes of endogenous homones during the process of flower bud differentiation of Welsh onion. Acta Horticulturae Sinica 2007, 34, 671–676. [Google Scholar]
- Liu, Z.; Zeng, L.; Du, X.; Peng, Y.; Tao, Y.; Li, Y.; Qin, J. Flower Bud Differentiation and Endogenous Hormone Changes of RosaAngela’. Bulletin of Botanical Research 2021, 41(1), 37–43. [Google Scholar]
- Skogerbo, G. Effects of root pruning and trunk girdling on xylem cytokinin content of apple (Malus x domestica Borkh.). Norweigain J Agric Sci 1992, 499-527.
- Kojima, K.; Yamada, Y.; Yamamoto, M. Effects of Abscisic Acid Injection on Sugar and Organic Acid Contents of Citrus Fruit. J. Jpn. Soc. Hortic. Sci. 1995, 64, 17–21. [Google Scholar] [CrossRef]
- Ito, A.; Yaegaki, H.; Hayama, H.; Kusaba, S.; Yamaguchi, I.; Yoshioka, H. Bending Shoots Stimulates Flowering and Influences Hormone Levels in Lateral Buds of Japanese Pear. HortScience 1999, 34, 1224–1228. [Google Scholar] [CrossRef]
- Goldschmidt, E.E. Endogenous abscisic acid and 2-trans-abscisic acid in alternate bearing ?Wilking? mandarin trees. Plant Growth Regul. 1984, 2, 9–13. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Jameson, P. E.; Dhandapani, P.; Novak, O.; Song, J. Cytokinins and Expression of SWEET, SUT, CWINV and AAP Genes Increase as Pea Seeds Germinate. International journal of molecular sciences 2016, 17 (12).
- Corbesier, L.; Prinsen, E.; Jacqmard, A.; Lejeune, P.; Van Onckelen, H.; Périlleux, C.; Bernier, G. Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. J. Exp. Bot. 2003, 54, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Loh, C. Induction of early bolting in Arabidopsis thaliana by triacontanol, cerium and lanthanum is correlated with increased endogenous concentration of isopentenyl adenosine (iPAdos). J. Exp. Bot. 2002, 53, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, L.; Zhang, H. Changes of endogenous hormones during flower bud formation of different blueberry varieties. Fruit trees in southern China 2014, 43 (05), 106-108+114.
- Du, J.; Li, P.; Gu, Y. Study on the changes of endogenous hormones and carbohydrate content during flower bud differentiation of olive. Western Forestry Science 2018, 47(01), 122–126. [Google Scholar]
- Ai, J.; Wang, Y. P.; Li, C. Y.; Guo, X. W.; Li, A. M. The changes of three endogenous hormones during flower bud differentiation of Schisandga chinensis. China journal of Chinese materia medica 2006, 31(1), 24–6. [Google Scholar] [PubMed]
- Kinet, J. M. Environmental, Chemical, and Genetic Control of Flowering. Horticultural Reviews, Volume 15: 2010.
- Grochowska, M.J.; Hodun, M. The dwarfing effect of a single application of growth inhibitors to the root-stem connection—“the collar tissue”—of five species of fruit trees. J. Hortic. Sci. 1997, 72, 83–91. [Google Scholar] [CrossRef]
- Li, Y.; Xing, L.; Zhang, D.; Shen, Y.; Zhang, S.; Han, M. Mechanism of Spraying IAA Inhibiting Flower Bud Inoculation of Fuji Young Trees. Acta Agriculturae Boreali-Occi-dentals Sinica 2015, 24 (04), 84-89.
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nature Reviews Molecular Cell Biology 2022, 23, 516–516. [Google Scholar] [CrossRef]
- Wen, Z.; Li, M.; Meng, J.; Miao, R.; Liu, X.; Fan, D.; Lv, W.; Cheng, T.; Zhang, Q.; Sun, L. Genome-Wide Identification of the MAPK and MAPKK Gene Families in Response to Cold Stress in Prunus mume. Int. J. Mol. Sci. 2023, 24, 8829. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, Y. F.; Zeng, T.; Sun, R.; Yang, J. Y.; Li, Y.; Ren, D. T.; Ma, H.; Xu, Z. H.; Bai, S. N. Phosphorylation of SPOROCYTELESS/NOZZLE by the MPK3/6 Kinase Is Required for Anther Development. Plant Physiol. 2017, 173(4), 2265–2277. [Google Scholar] [CrossRef]
- Han, X.; Li, S.; Zhang, M.; Yang, L.; Liu, Y.; Xu, J.; Zhang, S. Regulation of GDSL Lipase Gene Expression by the MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factors in Arabidopsis Immunity. Molecular plant-microbe interactions : MPMI 2019, 32 (6), 673-684.
- Duan, P. G.; Rao, Y. C.; Zeng, D. L.; Yang, Y. L.; Xu, R.; Zhang, B. L.; Dong, G. J.; Qian, Q.; Li, Y. H. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant Journal 2014, 77(4), 547–557. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Duan, P.; Yu, H.; Zhou, Z.; Zhang, B.; Wang, R.; Li, J.; Zhang, G.; Zhuang, S.; Lyu, J.; et al. Control of Grain Size and Weight by the OsMKKK10-OsMKK4-OsMAPK6 Signaling Pathway in Rice. Mol. Plant 2018, 11, 860–873. [Google Scholar] [CrossRef]
- Pei, L.; Gao, Y.; Feng, L.; Zhang, Z.; Liu, N.; Yang, B.; Zhao, N. Phenolic Acids and Flavonoids Play Important Roles in Flower Bud Differentiation in Mikania micrantha: Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2023, 24, 16550. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3(1), 2–20. [Google Scholar] [CrossRef]
- Wei, L.; Yu, B.; Song, E. ; Xhtan; Zheng, H. Response of endogenous hormones and amino acids to cassava flower bud differentiation. Southwest China Journal of Agrtcultural Sciences 2021, 34(07), 1400–1406. [Google Scholar]
- Liu, J.; Zhang, S. Changes of nucleic acid content in fruit and unfruitful trees during citrus flower bud differentiation. Journal of Southwest University ( Natural Science Edition ) 2008, (06), 56-59.
- Yu, Z.; Jia, D.; Liu, T. Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef]
- Tyagi, S.; Shumayla; Verma, P. C.; Singh, K.; Upadhyay, S.K. Molecular characterization of ascorbate peroxidase (APX) and APX-related (APX-R) genes in Triticum aestivum L. Genomics 2020, 112, 4208–4223. [Google Scholar] [CrossRef]
- Vaish, S.; Gupta, D.; Mehrotra, R.; Mehrotra, S.; Basantani, M.K. Glutathione S-transferase: a versatile protein family. 3 Biotech 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gho, Y.-S.; Kim, S.-J.; Jung, K.-H. Phenylalanine ammonia-lyase family is closely associated with response to phosphate deficiency in rice. Genes Genom. 2019, 42, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, I.; Ishikawa, M.; Nozawa, M.; Yoshida, M.-A.; Ikeo, K. Transcriptomic changes with increasing algal symbiont reveal the detailed process underlying establishment of coral-algal symbiosis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, S.; Sagong, H.-Y.; Son, H.F.; Jin, K.S.; Kim, I.-K.; Kim, K.-J. Structural basis for cytokinin production by LOG from Corynebacterium glutamicum. Sci. Rep. 2016, 6, 31390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- McGrance, S.J.; Cornell, H.J.; Rix, C.J. A Simple and Rapid Colorimetric Method for the Determination of Amylose in Starch Products. 50. [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Grund, B.; Gassner, A.-L.; Menin, L.; Henry, H.; Bromirski, M.; Schütz, F.; Mcmullen, J.; Rochat, B. Validation of the Mass-Extraction-Window for Quantitative Methods Using Liquid Chromatography High Resolution Mass Spectrometry. Anal Chem 2016, 88 (6), 3264-3271. [CrossRef]
- Glauser, G.; Grund, B.; Gassner, A.-L.; Menin, L.; Henry, H.; Bromirski, M.; Schütz, F.; McMullen, J.; Rochat, B. Validation of the Mass-Extraction-Window for Quantitative Methods Using Liquid Chromatography High Resolution Mass Spectrometry. Anal. Chem. 2016, 88, 3264–3271. [Google Scholar] [CrossRef]
- Vasilev, N.; Boccard, J.; Lang, G.; Grömping, U.; Fischer, R.; Goepfert, S.; Rudaz, S.; Schillberg, S. Structured plant metabolomics for the simultaneous exploration of multiple factors. Sci. Rep. 2016, 6, 37390. [Google Scholar] [CrossRef]

| DAM | Mean_H | Mean_Y | FoldChange_H/Y | log2(FC_H/Y) | p value |
|---|---|---|---|---|---|
| L-4-Hydroxyglutamate semialdehyde | 14854137.1 | 1737381.2 | 8.55 | 3.10 | 0.03 |
| N,N-Dimethylaniline | 5953932280.7 | 118333143.3 | 50.32 | 5.65 | 0.01 |
| 2-Heptanone | 478865629.4 | 988.8 | 484260.0 | 18.89 | 0.01 |
| 1-Pyrroline-5-carboxylic acid | 80591608.6 | 46463691.7 | 1.73 | 0.79 | 0.01 |
| 1-Benzyl-1,2,3,4-tetrahydroisoquinoline | 68788352.0 | 988.8 | 69564.0 | 16.09 | 0.00 |
| 2-Methylbenzoic acid | 949974505.8 | 57384295.4 | 16.56 | 4.05 | 0.01 |
| gamma-Glutamyl-beta-aminopropiononitrile | 5588130.7 | 988.8 | 5651.1 | 12.46 | 0.00 |
| Pyrrolidonecarboxylic acid | 30079215.7 | 3836016.5 | 7.84 | 2.97 | 0.02 |
| Ketoleucine | 26500401.0 | 988.8 | 26799.0 | 14.71 | 0.00 |
| (R)-5,6-Dihydrothymine | 279791870.5 | 114946419.6 | 2.43 | 1.28 | 0.01 |
| Fenfluramine | 10258146.5 | 5721365.4 | 1.79 | 0.84 | 0.01 |
| Sulfamethazine | 22024321.9 | 12887857.9 | 1.71 | 0.77 | 0.01 |
| Porphobilinogen | 156535147.6 | 115434618.3 | 1.36 | 0.44 | 0.01 |
| Gyromitrin | 284576546.4 | 988.8 | 287790.0 | 18.14 | 0.00 |
| 3-Methyl-2-oxovaleric acid | 180493405.8 | 988.8 | 182530.0 | 17.48 | 0.00 |
| Phenylethylamine | 81993597.7 | 988.8 | 82918.0 | 16.34 | 0.00 |
| Capsidiol | 120373230.9 | 105812770.2 | 1.14 | 0.19 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





