1. Introduction
Tuberculosis was one of the leading causes of death in human history and remains one of the leading infectious causes of death in the world [
1]. Globally, the number of new cases of tuberculosis exceeds 10 million, although the incidence of tuberculosis has fallen slowly over the past decade, and mortality has fallen by almost a third [
1].
Although there has been a downward trend in the incidence of tuberculosis, the achievements are below the indicators established by the World Health Organization [WHO] for the "END TB" strategy. The COVID-19 pandemic has interfered with the diagnosis and care program for tuberculosis, negatively impacting the progress achieved in combating the disease by 2019. An estimated 10.6 million people became ill with tuberculosis in 2021, compared to 10.1 million in 2020 and 1.6 million people have died of TB in 2021 compared to 1.5 million in 2020 [
1].
COVID-19 has epidemiological similarities with tuberculosis, both infections being airborne, favored by direct contact, crowded environments, and vulnerable biological status. Both tuberculosis and the COVID-19 infection predominantly affect the lung, although extrapulmonary manifestations are also known. The incubation period differs, with variations between 2-14 days for COVID-19 and 3-9 weeks for tuberculosis, although they may start with common manifestations, such as cough, chest pain and asthenia [
2]. Tuberculosis is a bacterial infection caused by Mycobacterium tuberculosis that can cause extensive destruction of lung tissue, with the formation of cavities, while COVID-19 is a viral infection caused by SARS-Cov-2 that alters lung cells, triggering inflammation and acute respiratory dysfunction [
3,
4]. The infection of COVID-19 can be diagnosed both before, during active tuberculosis, and post-tuberculosis, but the influence of COVID-19 or of anti-COVID-19 medication in the reactivation or worsening of active tuberculosis are still unclear [
5,
6]. Morbidity and mortality risks for COVID-19 and tuberculosis can be influenced by smoking and co-morbidities such as chronic lung disease, diabetes, or liver failure [
7,
8,
9]. The convergence of these infections constitutes major challenges for public health globally [
10].
In 2020, of the 33,148 cases of tuberculosis reported by 29 of 30 EU/EEA countries, 23.2% came from Romania [
11].
We hypothesized that COVID-19 increase the severity of tuberculosis and mortality. The purpose of our study is to evaluate the clinical, biological, radiological and evolutionary characteristics of patients hospitalized with tuberculosis-COVID-19 co-infection from a special hospital of pneumology in South-East Romania. To our knowledge, this is the first study on COVID-19 and tuberculosis co-infection from our region.
2. Materials and Methods
We have conducted a descriptive retrospective study on the cases of tuberculosis hospitalized during the COVID-19 pandemic period, from 1.07.2020 to 30.06.2022, from the Clinical Hospital of Pneumology Galati, located in the South-East of Romania. We specify that the hospital does not have an intensive care unit, and the complicated cases with organ dysfunctions are admitted/transferred to emergency hospitals. The diagnosis of tuberculosis (TB) was considered according to the case definitions established by the national tuberculosis management guideline, classified into new cases and relapses [
11]. The etiological diagnosis was based on the classical methods of sputum analysis through culture on Loewenstein-Jenssen medium and/or microscopic examination of Ziehl-Nielson colored smears. The laboratory diagnosis of TB was completed by molecular tests by quantitative polymerization reaction in real-time DNA chain - RT-PCR (GeneXpert MTB-Rif-Cepheid). All hospitalized patients with TB were tested for COVID-19 upon admission and during hospitalization, due to clinical or epidemiological suspicion for this infection. The diagnosis of COVID-19 was confirmed by RT-PCR SARS-CoV-2 test. The confirmation of COVID-19 was made simultaneously with the diagnosis of tuberculosis (≤7 days after hospitalization), or consecutively (7-60 days after hospitalization), during the same hospitalization.
The study was based on the analysis of data from the hospital’s archive of the clinical records, that were selected based on the diagnostic codes ICD-10-CM version 37.1 R1, specified A15 (respiratory tuberculosis), associated or not with U07.1 (COVID-19), for each case of co-infection, selecting a paired case of TB mono-infection (A15), with the proximate hospitalization date.
Demographic, clinical, biological, radiological, and therapeutic data were collected, used according to current hospital procedures for the diagnosis and treatment of TB and COVID-19 [
12,
13,
14].
The radiological changes were evaluated by three independent radiologists and were validated by the pneumologist physician. The cavitary, ulcerated, caseous, nodular, infiltrative, fibrous, pleural/extrapulmonary forms of TB have been notified. Chest CT examination was available in isolated cases. The BRIXIA radiological scores for severity were evaluated on the date of COVID-19 diagnosis (baseline) and at discharge (end of study). Extent of lung parenchyma damage evaluation by BRIXIA score, consists of division into six sections of the lung radiologic image, in which each section achieve a score of 0, 1, 2 or 3, while total score could sum from 0 to 18 [
15,
16,
17].
The inclusion criteria were age over 20 years, signing the informed consent for the use of personal data for the purpose of medical statistical studies, according to the standardized form from the clinical observation sheet.
They were excluded the patients vaccinated against COVID-19, patients with HIV infection, with neurocognitive dysfunctions who cannot express their consent, those re-hospitalized for the same episode of the disease of COVID-19 or tuberculosis, as well as cases with incomplete CXR examination of the lung (less than 3 radiological opinions).
The statistical analysis used the XLSTAT.2022.4.5 program. Continuous variables with normal distribution were compared based on t-tests, and categorical variables were compared with chi-squared tests or Fisher exact test. We calculated the frequency of the variables and the mean ±SD for the data with normal distribution or the median in the case of non-normally distributed data. We used the nonparametric Mann-Whitney U-test for data that did not correspond to the standard distribution.
The institutional approvement of this non-interventional study was approved by the Medical Council of the Pneumology Hospital from Galati no 1/291/9.01.2024.
3. Results
We analyzed 90 cases hospitalized with tuberculosis during the 2020-2022 pandemic period, of which 45 had the diagnosis of co-infection with COVID-19 and 45 had TB mono-infection.
3.1. Demographic data
The age of the patients varied between 20 and 86 years, with an average of 52.1±14.69 years, without significant statistical differences between the two groups (Mann Whitney test: p=0.272). The demographic characteristics indicated the predominance of male patients (78.8%), from rural areas (62.2%), with medium/higher education level (53.3%), professionally active (43.33%). The frequency of patients with a low level of education was significantly higher in the group with co-infection with COVID-19 compared to mono-infection with TB (p<0.001), without other demographic differences (
Table 1).
Smoking was reported in 71.11% of the patients, with an average lifetime smoking exposure of 29.48±11.74 packs of cigarettes/year. The alcohol use was reported by 74.33% of patients, with average of 4,164±1.82 IU/day. The health risk factors, such as smoking, alcohol consumption or altered nutritional status, did not influence the association with COVID-19.
3.2. Associated chronic co-morbidities
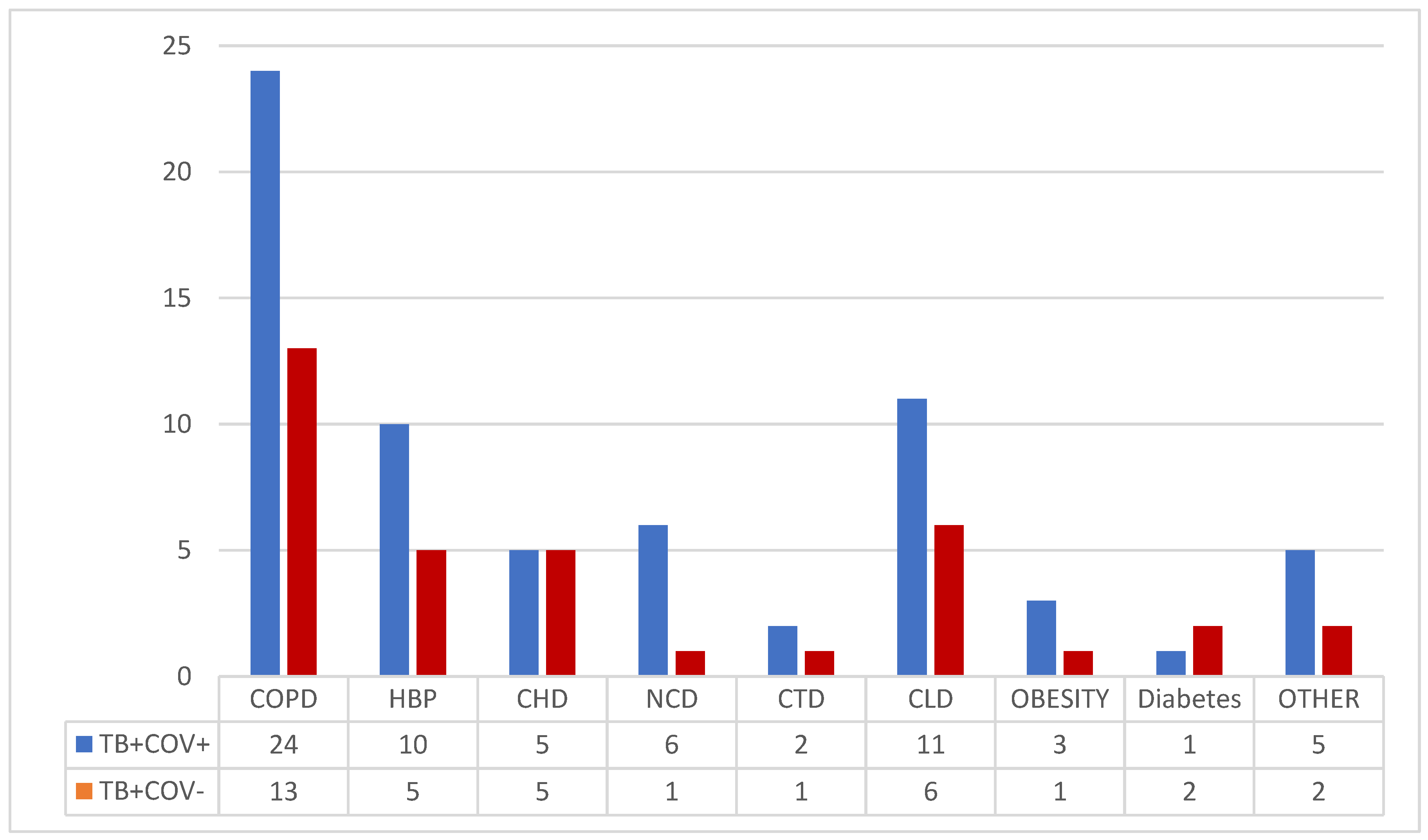
Chronic co-morbidities have been associated in 57.77% of patients (
Figure 1), mainly chronic lung diseases (41.11%), chronic liver disease (18.88%), hypertension (16.66%), chronic heart diseases (11.11%) (
Figure 1).
3.3. Clinical presentation and diagnostic
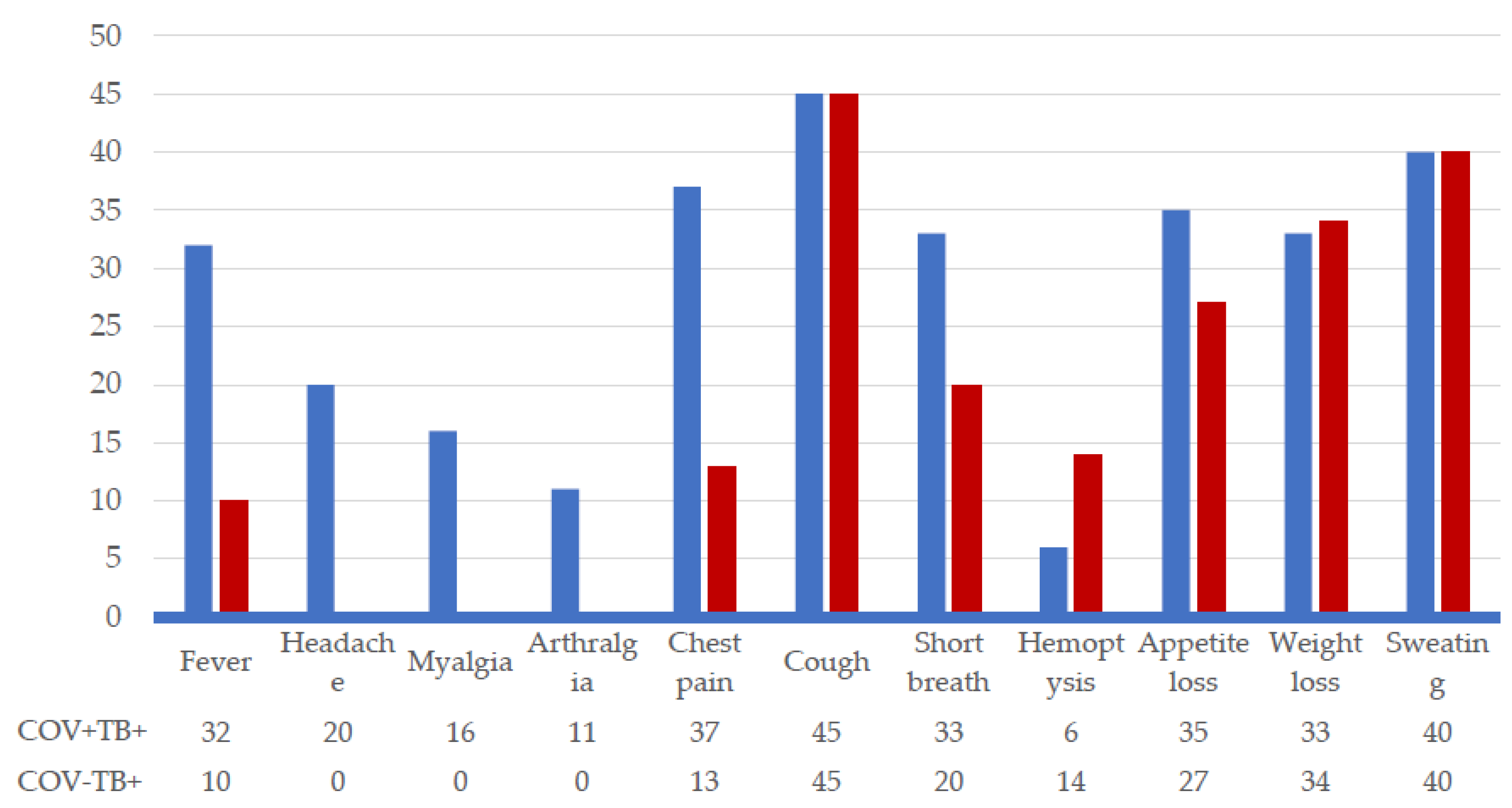
The inventory of clinical symptoms identified manifestations exclusively associated with COVID-19, such as headache (44.4%), myalgias (35.5%), arthralgias (24.4%), as well as manifestations common to the two groups of patients, but with a higher frequency in the case of co-infection COVID-19/TB: fever (71.1% vs 22.2%), chest pain (82.2% vs 28.8%), shortness of breath (73.3% vs 44.4%), decreased appetite (77.7% vs 60%). Other manifestations had equal frequencies, for example cough (100%), sweating (88.8%), weight loss (73-74%). Hemoptysis was reported in 22.2%, but the frequency was higher in patients with TB monoinfection (
Figure 2).
Diagnosis of COVID-19 was confirmed at hospital admissition, concomitant with tuberculosis in 62.2% of cases, or during hospitalization, as a healthcare-associated infection, in 37.7% of patients.
Recurrences constituted 30% of TB cases, more frequent in patients with COVID-19 co-infections compared to TB mono-infections (38% vs 22%).
The microscopic examination of acid-alcohol-resistant bacilli (AARB) was positive in 91.1% of the cases.
The majority of cases (86.66%) had complex radiological images, including 46.66% cavitary forms, 40% caseous, 26.66% ulcerated, 21.11% infiltrative, 36.66% fibrous, 43.33% nodular and 7.7% pleural or associated extrapulmonary locations (
Table 2).
Recurrences constituted 30% of TB cases, more frequent in patients with COVID-19 co-infections compared to TB mono-infections (38% vs 22%). The microscopic examination of acid-alcohol-resistant bacilli (AARB) was positive in 91.1% of the cases. The majority of cases (86.66%) had complex radiological images, highlighting 46.66% cavitary forms, 40% caseous, 26.66% ulcerated, 21.11% infiltrative, 36.66% fibrous, 43.33% nodular and 7.7% pleurisy or associated extrapulmonary locations (
Table 2).
The biological picture evidenced higher values of inflammation markers and of the Neutrophils/Lymphocytes ratio in patients with co-infection with COVID-19. Overmore, significantly higher differences of values in creatinine, ALT and Brixia scores, both at baseline and during the follow-up examination, were found in COVID-19 group (
Table A1).
Non-TB bacterial examination of sputum was performed in 17.77% of patients, with 43.75% showing negative results. Isolates from sputum included Streptococcus spp (3), Moraxella catharalis (1), Klebsiella spp (3), Pseudomonas spp (1), and E. coli (1). A higher number of Gram-negative bacteria (BGN) isolates were observed in cases with COVID-19 (3:1), while TB monoinfection had a higher proportion of Gram-positive bacteria (CGP) (3:2).
3.4. Drug therapy and outcomes
Treatment with the combination of Isoniazid, Rifampicin, Pyrazinamide, and Ethambutol, was administered to 96.6% of TB patients. Individualized antituberculous regimens were provided for 2 patients with TB-MDR and for one case of TB rifampicin resistance detected in the GeneXPERT test.
Antibiotics for non-tuberculous infections were used in 33.3% of patients, including ceftriaxone (14.4%), amoxicillin (2.2%), tienamicin (3.3%), moxifloxacin (3.3%), levofloxacin (2.2%), linezolid (1.1%), gentamicin (1.1%), sulfamethoxazole (1.1%). Vancomycin (14.4%) and metronidazole (6.6%) were used for the treatment of patients with Clostridioides difficile-associated diarrhea.
Oxygen support via nasal cannula or face mask was provided to 31.1% of patients with COVID-19, compared to 6.6% in patients with TB monoinfection. Patients with COVID-19 received antivirals (77.7%) with favipiravir or remdesivir, dexamethasone (51.1%), and anticoagulants (62.2%).
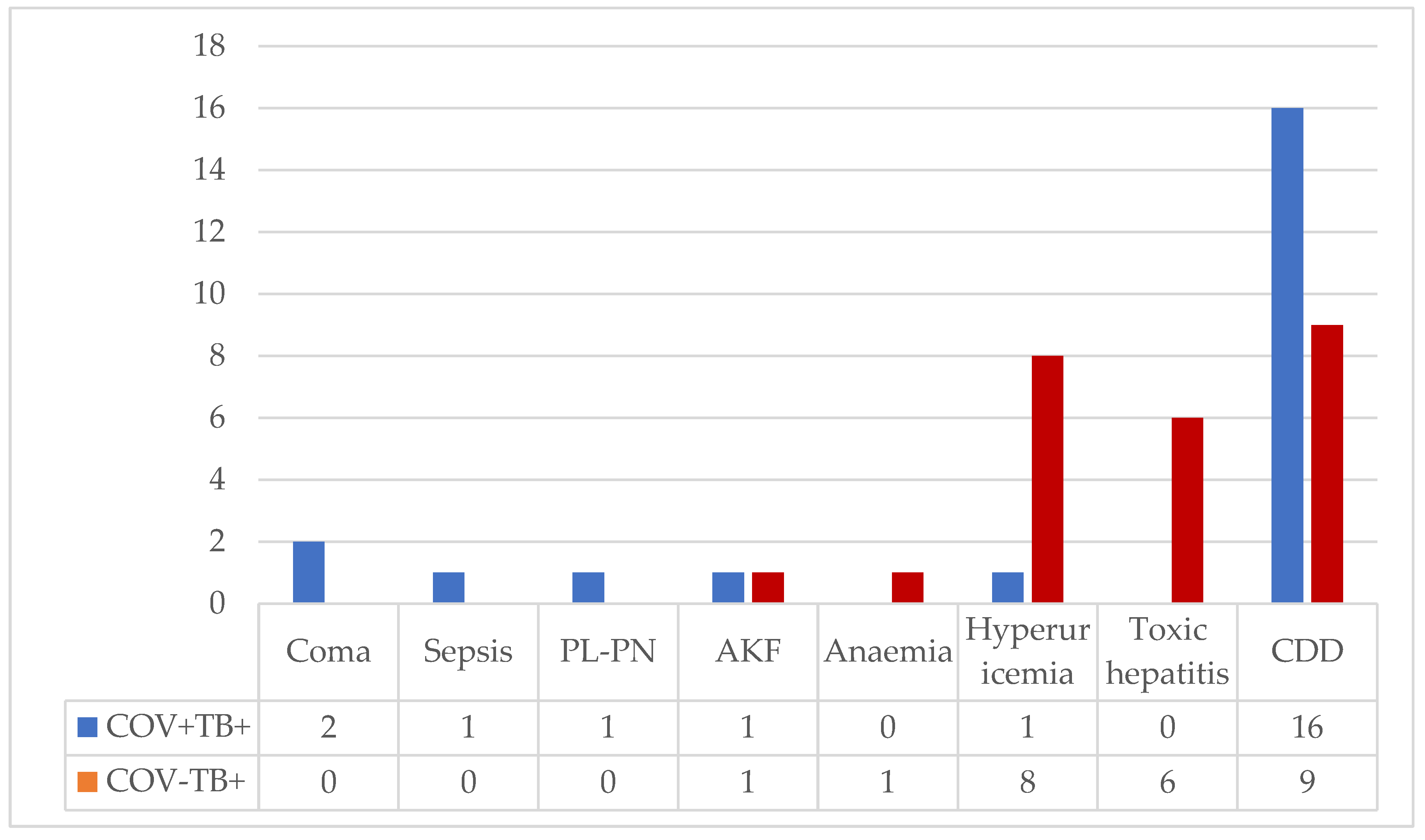
Complications associated with the severe evolution of COVID-19 were observed in 8.8% of cases (coma, sepsis, and pleuropneumothorax), and medication toxicity was reported in 20% of patients (hyperuricemia, renal failure, toxic hepatitis, anemia).
Clostridioides difficile associated diarrhea occurred in 27.7% of cases, more frequently in the group with COVID-19 (16/9 vs.), but the difference was not statistically significant (
Figure A1).
The outcomes were favorable in 92.2% of patients who were discharged with improved conditions. However, 5.5% of patients with COVID-19 deteriorated, requiring transfer to intensive care units in emergency hospitals, and 2.2% of patients died (1 case of TB-COVID co-infection and 1 case of monoinfection).
4. Discussion
Impact of COVID-19 on Tuberculosis Outcomes
Mycobacterium tuberculosis Adaptation and Latent Infections:
Mycobacterium tuberculosis, a well-adapted pathogen to humans, has coexisted throughout human history. Approximately 2 billion people worldwide carry latent tuberculosis infections, with an estimated 10% risk of developing active disease during their lifetime, varying geographically and with comorbidities [
18].
COVID-19 induces pulmonary lesions but also affects other compartments of the immune system. In this context, tuberculosis reactivation can be attributed to various mechanisms, such as the immunosuppressive effects of corticosteroid therapy, cytokine storms, and T lymphocyte depletion [
19]. An observational case-control study in China highlighted that both active and latent tuberculosis are risk factors for the progression of COVID-19 [
20].
Conversely, studies from the early pandemic period provided evidence that COVID-19 increases the frequency and severity of tuberculosis [
18,
21,
22]. The mortality rate in our study for patients with co-infection was 2.2%, comparable to the monoinfection TB group. This mortality rate was lower than reported in some multicenter studies, where rates ranged from 9.2% to 14.2% [
21,
23]. The majority of radiological lesions observed in our study were cavitary, evenly distributed between the co-infection and monoinfection TB groups, constituting 46.6%. Over 60% of these were new cases of tuberculosis, suggesting that the lesions had been present for at least one month before the onset of the COVID infection, whose incubation period is short [
23].
In view of these observations, our study results in line with the patterns observed in countries with a high prevalence of tuberculosis. In such settings, the diagnosis of COVID-19 might be more of a coincidental revelation of tuberculosis rather than a causative factor for reactivating latent tuberculosis [
18,
22,
24,
25].
Considerations of COVID-19 impact on Tuberculosis appearance in Romanian patients
In comparison with a report from Serbia, patients with tuberculosis-COVID-19 co-infection in our study exhibited more intense symptoms, with a higher frequency of cough (100% vs. 69.8%), fever (71.1% vs. 30.2%), and shortness of breath (73.3% vs. 30.2%). However, the frequency of cases diagnosed with COVID-19 following tuberculosis was lower in our study (37.7% vs. 45%) [
26]. A noteworthy proportion of patients in both the monoinfection TB and co-infection COVID-19 groups received non-tuberculosis antibiotics. Despite the global alert on excessive antibiotic use for COVID-19 without clear evidence of bacterial infections, our study reported lower antibiotic utilization rates compared to other studies [
27].
Demographic characteristics and risk factors such as alcohol and smoking did not influence the occurrence of COVID-19 co-infection. Nevertheless, lower adherence to COVID-19 prevention rules could be related to lower education level, that was found more prevalent in co-infected group. Chronic comorbidities, especially chronic obstructive pulmonary disease (COPD), hypertension, chronic liver diseases, or chronic neurological diseases, were more frequent in patients with COVID-19 co-infection, consistent with previous findings [
28,
29].
Clinical symptoms highlighted an overlap between manifestations typical of both COVID-19 and tuberculosis, such as cough, sweating, and weight loss. Fever, chest pain, shortness of breath, and loss of appetite were more common in patients with COVID-19 co-infection, whereas hemoptysis was more frequent in monoinfection TB. Algic syndrome, characterized by headache, myalgia, and arthralgia, was mentioned exclusively in patients with COVID-19 co-infection. However, the specificity of clinical manifestations for COVID-19 is limited, with none of the symptoms having the accuracy to confirm or rule out this infection [
30]. Moreover, the clinical spectrum of COVID-19 varies from asymptomatic to severe forms, correlated with epidemiological factors and host characteristics [
31]. Hemoptysis is an uncommon manifestation of COVID-19, reported with frequencies between 0.9% and 3.3% [
31,
32]. Hemoptysis in tuberculosis can be caused by bronchiectasis, bronchial artery erosions, and, more rarely, pulmonary artery erosions [
33]. The proportion of hemoptysis revealing the onset of tuberculosis and/or the unfavorable evolution of fibrocavitary secondary TB was 20.71%, in line with recent studies in Romania [
34].
Bilateral lung lesions were significantly more frequent in the COVID-19 group compared to monoinfection TB (p=0.003), consistent with a higher BRIXIA radiologic score. Although the BRIXIA score decreased in both groups upon discharge, the difference in values accentuated, likely due to the slow regression of COVID-19 pulmonary lesions. Prospective evaluation of these cases may be useful to clarify whether there is an additional risk for post-COVID fibrosis and respiratory dysfunction in the case of tuberculosis co-infection.
The average NLR was elevated in most patients in our study, but the differences between the COVID-19 co-infection and monoinfection TB groups were not significant, possibly because critical COVID-19 pattern were not evaluated in the study [
35,
36].
Clostridioides difficile infection was reported in 27.7% of tuberculosis patients, possibly favored by rifampicin use, listed as one of the antibiotics that can induce diarrhea with this etiology, especially in elderly individuals with chronic comorbidities (underlying conditions) [
37]. The frequency of
Clostridioides difficile-associated diarrhea was higher in the COVID-19 co-infection group (35.5% vs. 20%), likely explained by the twice as frequent use of non-tuberculosis antibiotics and the occurrence of this type of diarrhea. It could be favored by rifampicin or broad-spectrum antibiotics use, as well as by changes in the intestinal microbiome during COVID-19 infection (fecal microbiota disruption by SARS-CoV-2 infection) [
38].
Limitations of the Study
The retrospective design of the study could have limited the accuracy of collecting the data. The number of cases is small, limiting the statistical significance of the results.
There were only hospitalized TB cases, with symptomatic COVID-19 during the first 4 to 8 weeks from the admission. Symptomatic or asymptomatic COVID-19 cases preceding tuberculosis were not analyzed. The potential impact of prior asymptomatic COVID-19 infections on tuberculosis, with potential effects on the biological and radiological changes found in the monoinfection TB group, could not be assessed.
5. Conclusions
The hypothesis of our study is not confirmed. Symptomatic non-severe infection COVID-19 had a mild impact on the clinical and biological expression of tuberculosis diagnosed in the pandemic context. The mortality of hospitalized tuberculosis patients was not influenced by COVID-19 co-infection in a country with a high tuberculosis prevalence. Persistent structural lung damage is suggested by radiological lesions according to the BRIXIA score, could be an indicator for COVID-19 additional risk on tuberculosis sequelae. Tuberculosis control strategies should consider intensifying efforts to evaluate tuberculosis in COVID-19 patients, as common clinical manifestations and some pathogenic mechanisms continue to pose challenges for medical research and public health.
Author Contributions
Conceptualization, G.C.P. and M.A.; methodology, G.C.P. and M.A.; software, O.M.M. and M.C.V.; validation, C.V.G., C.I.V. and M.A; formal analysis, M.C.V. and O.M.M.; investigation, G.C.P.; data curation, C.I.V. and C.V.G.; writing—original draft preparation, G.C.P., C.V.G., C.I.V., O.M.M., M.C.V.; writing—review and editing, M.A.; visualization, O.M.M., M.C.V.; supervision, M.A.; funding acquisition, C.I.V. and C.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The institutional approvement of this non-interventional study was approved by the Medical Council of the Pneumology Hospital from Galati no 1/291/9.01.2024.
Informed Consent Statement
Written informed consent was obtained from all participants as a routine procedure of the hospital for permission to use the personal data for statistical analyzes and research.
Data Availability Statement
Data supporting reported results are available on request from the first author GCP.
Acknowledgments
We kindly acknowledge the bord of “Dunarea de Jos” University from Galati for the financial support for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1.
Comparative features of biological data and radiologic scores in TB monoinfection and TB-COVID-19 co-infection.
Table A1.
Comparative features of biological data and radiologic scores in TB monoinfection and TB-COVID-19 co-infection.
| |
NRV |
Average±SD |
Median |
2t-test |
CI 0.95 |
| COV+ |
COV- |
COV+ |
COV- |
Leucocytes
*103/mm3
|
4.5-11 |
13270±8947 |
12300±922 |
11400 |
11400 |
0.504 |
-1.920;3.860 |
| NLR |
<3 |
7.648±8.346 |
5.468±4.502 |
4.764 |
4.229 |
0.127 |
-0.641;5.002 |
| CRP [mg/L] |
<3 |
14.448±21.208 |
8.611±5.63 |
7 |
6.7 |
0.080 |
-0.732;12.408 |
| ESR [mm/h] |
0-22 |
62.71±31.430 |
73.2±32.460 |
55 |
65 |
0.123 |
-23.877;2.898 |
| Creatinine [mg/dL] |
0.7-1.3 |
1.295±1.924 |
0.678±0.172 |
0.77 |
0.66 |
0.037 |
0.036;1.197 |
| ALT [U/L] |
7-55 |
72.71±86.69 |
33.77±27.93 |
38 |
22 |
0.005 |
11.700;66.166 |
| AST [U/L] |
8-48 |
66.711±73.621 |
47.667 |
43 |
30 |
0.126 |
-5.520;43.609 |
| BRIXIA Score-B |
0 |
9.288±3.307 |
7.688±2.556 |
9 |
8 |
0.012 |
0.360;2.839 |
| BRIXIA Score C |
0 |
7.266±3.326 |
3.911±2.172 |
7 |
3 |
<0.001 |
2.175;4.535 |
Figure A1.
Comparative Complications in Patients Hospitalized with Co-infection TB-COVID-19 and Monoinfection TB. Legend: PL-PN: pleuropneumonia; AKF: acute kidney failure; CDD; Clostridioides difficile diarrhea.
Figure A1.
Comparative Complications in Patients Hospitalized with Co-infection TB-COVID-19 and Monoinfection TB. Legend: PL-PN: pleuropneumonia; AKF: acute kidney failure; CDD; Clostridioides difficile diarrhea.
References
- Global tuberculosis report 2022. World Health Organization: 2022. Available online: https://iris.who.int/bitstream/handle/10665/363752/9789240061729-eng.pdf?sequence=1 (accessed on 11 December 2023).
- Cioboata, R.; Biciusca, V.; Olteanu, M.; Vasile, C. M. , COVID-19 and Tuberculosis: Unveiling the Dual Threat and Shared Solutions Perspective. J. Clin. Med. 2023, 12, 4784. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P. G.; Qin, L.; Puah, S. H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A. I.; Mocanu, H.; Moldovan, C.; Soare, I.; Niculet, E.; Tatu, A. L.; Vasile, C. I.; Diculencu, D.; Postolache, P. A.; Nechifor, A. Some Manifestations of Tuberculosis in Otorhinolaryngology-Case Series and a Short Review of Related Data from South-Eastern Europe. Infect. Drug Resist. 2022, 15, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, S. B.; Jonkman, A. H.; Kugler, M. C.; Munger, J. S.; Kaufman, D. A. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Visca, D.; Ong, C. W. M.; Tiberi, S.; Centis, R.; D'Ambrosio, L.; Chen, B.; Mueller, J.; Mueller, P.; Duarte, R.; Dalcolmo, M.; et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 2021, 27, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Al Maqbali, M.; Al Badi, K.; Al Sinani, M.; Madkhali, N.; Dickens, G. L. Clinical Features of COVID-19 Patients in the First Year of Pandemic: A Systematic Review and Meta-Analysis. Biol. Res. Nurs. 2022, 24, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S. S.; Awal, S. S.; Awal, S. K. COVID-19 and pulmonary tuberculosis-A diagnostic dilemma. Radiol. Case Rep. 2021, 16, 3255–3259. [Google Scholar] [CrossRef] [PubMed]
- Vasile, M. C.; Arbune, A. A.; Lupasteanu, G.; Vlase, C. M.; Popovici, G. C.; Arbune, M. Epidemiologic and Clinic Characteristics of the First Wave of the COVID-19 Pandemic in Hospitalized Patients from Galați County. J. Clin. Med. 2021, 10, 4210. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M. T.; Guzmán-Beltrán, S.; Bobadilla, K.; Santos-Mendoza, T.; Flores-Valdez, M. A.; Gutiérrez-González, L. H.; González, Y. Human Pulmonary Tuberculosis: Understanding the Immune Response in the Bronchoalveolar System. Biomolecules 2022, 12, 1148. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2022–2020 data. Copenhagen: WHO Regional Office for Europe and Stockholm: European Centre for Disease Prevention and Control; 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Tuberculosis-surveillance-monitoring-europe-2022_0.pdf (accessed on 26 December 2023).
- MINISTERUL SĂNĂTĂȚII ORDIN, nr.; 956 din 31 martie 2022 privind modificarea Ordinului ministrului sănătății nr. 1.171/2015 pentru aprobarea unor reglementări privind controlul tuberculozei: GHID METODOLOGIC DE IMPLEMENTARE A PROGRAMULUI NAŢIONAL DE PREVENIRE, SUPRAVEGHERE ȘI CONTROL AL TUBERCULOZEI ȘI ALTOR MICOBACTERIOZE. MONITORUL OFICIAL AL ROMÂNIEI, PARTEA I, Nr. 326 bis/4.IV.2022. Available online: https://legislatie.just.ro/Public/DetaliiDocument/253597?msclkid=09174c29c24711ec95524134f8dfd6f2 (accessed on 21 October 2023).
- Ministerul Sănătății. ORDIN nr. 487 din 23 martie 2020 pentru aprobarea protocolului de tratament al infecției cu virusul SARS-Cov-2. MONITORUL OFICIAL nr. 242 din 24 martie 2020. Available online: https://legislatie.just.ro/Public/DetaliiDocument/224341 (accessed on 21 October 2023).
- Ministerul Sănătății. ORDIN nr. 533 din 22 aprilie 2021 privind modificarea anexei la Ordinul ministrului sănătății nr. 487/2020 pentru aprobarea protocolului de tratament al infecției cu virusul SARS-CoV-2. MONITORUL OFICIAL nr. 434 din 23 aprilie 2021. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/241318 (accessed on 21 October 2023).
- Nukovic, J. A.; Opancina, V.; Zdravkovic, N.; Prodanovic, N.; Pejcic, A.; Opancina, M.; Nukovic, J. J.; Vojinovic, R.; Dulovic, D.; Jukovic, F.; et al. Brixia Chest X-ray Score, Laboratory Parameters and Vaccination Status for Prediction of Mortality in COVID-19 Hospitalized Patients. Diagnostics 2023, 13, 2122. [Google Scholar] [CrossRef]
- Au-Yong, I.; Higashi, Y.; Giannotti, E.; Fogarty, A.; Morling, J. R.; Grainge, M.; Race, A.; Juurlink, I.; Simmonds, M.; Briggs, S.; et al. Chest Radiograph Scoring Alone or Combined with Other Risk Scores for Predicting Outcomes in COVID-19. Radiology 2022, 302, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Little, B. P. Disease Severity Scoring for COVID-19: A Welcome Semiquantitative Role for Chest Radiography. Radiology 2022, 302, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Mousquer, G. T.; Peres, A.; Fiegenbaum, M. Pathology of TB/COVID-19 Co-Infection: The phantom menace. Tuberculosis (Edinb) 2021, 126, 102020. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Sheikh, J. A.; Quadir, N.; Sharma, N.; Hasnain, S. E.; Ehtesham, N. Z. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022, 31. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Tadolini, M.; Codecasa, L. R.; García-García, J. M.; Blanc, F. X.; Borisov, S.; Alffenaar, J. W.; Andréjak, C.; Bachez, P.; Bart, P. A.; Belilovski, E.; et al. Active tuberculosis, sequelae and COVID-19 co-infection: First cohort of 49 cases. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Motta, I.; Centis, R.; D'Ambrosio, L.; García-García, J. M.; Goletti, D.; Gualano, G.; Lipani, F.; Palmieri, F.; Sánchez-Montalvá, A.; Pontali, E.; et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology 2020, 26, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Group, T. C.-G. S. Tuberculosis and COVID-19 co-infection: Description of the global cohort. Eur. Respir. J. 2022, 59. [Google Scholar] [CrossRef]
- Khurana, A. K.; Aggarwal, D. The (in)significance of TB and COVID-19 co-infection. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Stochino, C.; Villa, S.; Zucchi, P.; Parravicini, P.; Gori, A.; Raviglione, M. C. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Adzic-Vukicevic, T.; Stosic, M.; Antonijevic, G.; Jevtic, M.; Radovanovic-Spurnic, A.; Velickovic, J. Tuberculosis and COVID-19 co-infection in Serbia: Pandemic challenge in a low-burden country. Front Med (Lausanne) 2022, 9, 971008. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V. M.; Gandhi, T. N.; Petty, L. A.; Patel, P. K.; Prescott, H. C.; Malani, A. N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S. A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin Infect Dis 2021, 72, e533–e541. [Google Scholar] [CrossRef] [PubMed]
- Parolina, L.; Pshenichnaya, N.; Vasilyeva, I.; Lizinfed, I.; Urushadze, N.; Guseva, V.; Otpushchennikova, O.; Dyachenko, O.; Kharitonov, P. Clinical characteristics of COVID-19 in patients with tuberculosis and factors associated with the disease severity. Int. J. Infect. Dis. 2022, 124 (Suppl. S1), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Sheng, X.; Justice, J. L.; Lum, K. K.; Metzger, P. J.; Cook, K. C.; Kostas, J. C.; Cristea, I. M. Intercellular communication within the virus microenvironment affects the susceptibility of cells to secondary viral infections. Sci. Adv. 2023, 9, eadg3433. [Google Scholar] [CrossRef] [PubMed]
- Martínez Orozco, J. A.; Sánchez Tinajero, Á.; Becerril Vargas, E.; Delgado Cueva, A. I.; Reséndiz Escobar, H.; Vázquez Alcocer, E.; Narváez Díaz, L. A.; Ruiz Santillán, D. P. COVID-19 and Tuberculosis Coinfection in a 51-Year-Old Taxi Driver in Mexico City. Am. J. Case Rep. 2020, 21, e927628. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Guan, W. J.; Ni, Z. Y.; Hu, Y.; Liang, W. H.; Ou, C. Q.; He, J. X.; Liu, L.; Shan, H.; Lei, C. L.; Hui, D. S. C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Quigley, N.; Gagnon, S.; Fortin, M. Aetiology, diagnosis and treatment of moderate-to-severe haemoptysis in a North American academic centre. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Chirana, C.; Vlase, I. Hemopthisis in Patients with Lung Tuberculosis. ARS Medica Tomitana 2021, 27. [Google Scholar] [CrossRef]
- Kim, M. A.; Park, Y. E.; Chong, Y. P.; Shim, T. S.; Jo, K. W. Neutrophil-Lymphocyte Ratio and Monocyte-Lymphocyte Ratio According to the Radiologic Severity of. J. Korean Med. Sci. 2022, 37, e292. [Google Scholar] [CrossRef]
- Sarkar, S.; Khanna, P.; Singh, A. K. The Impact of Neutrophil-Lymphocyte Count Ratio in COVID-19: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2022, 37, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A. K.; Aggarwal, D. The (in)significance of TB and COVID-19 co-infection. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Rosołowski, M.; Kaniewska, M.; Kucha, P.; Meler, A.; Wierzba, W.; Rydzewska, G. Clostridioides difficile infection in coronavirus disease 2019 (COVID-19): An underestimated problem? Pol Arch Intern Med 2021, 131, 121–127. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).