1. Introduction
The prevalence of Parkinson’s disease (PD) is projected to rise by the year 2050. Recent estimates put the number of new cases of PD each year around 10 million people worldwide and the overall prevalence between 4.5 and 21.0 (1; 2; 3). The National Institute of Neurological Disorders and Stroke (NINDS) (ninds.nih.gov) estimates that every year in the United States, over half a million people are diagnosed with PD. This number is expected to reach one million by 2040. In Saudi Arabia as one of the AfrAbian countries, the prevalence of PD is notable, with an estimated rate of 27 per 100,000 (4). Based on the most recent statistics provided by the WHO in 2020, the number of fatalities attributed to PD in Saudi Arabia amounted to 776, accounting for about 0.58% of the total number of deaths. Saudi Arabia is ranked as the 25th country globally in terms of its age-adjusted death rate, which stands at 5.96 per 100,000 inhabitants (5). According to data collected in Egypt, which is another AfrAbian country, since 2005 (6), the prevalence of PD is steadily rising as the country’s population ages. As the populations of both nations age, the rising expense of treating those with PD and compensating for lost productivity is becoming more burdensome. By 2050, Egypt’s population will be much older and more likely to experience the onset of age-related diseases such as PD (7).
PD affects one percent of the global population. It disproportionately affects the elderly and severely affects their quality of life (2, 3). It is believed that neuron mortality in vulnerable brain regions and the development of Lewy bodies in the few surviving neurons result from a complex interaction between genetic and environmental factors (8). AfrAbian countries are undergoing a demographic transition that will result in an aging population by 2050 (an increase in the proportion of individuals aged 65 and over) and an increase in the prevalence of diseases afflicting the elderly disproportionately, such as PD (9).
AfrAbia is comprised of both Arab League and African Union members. Historical and geographical influences connect the two contiguous territories of Africa and the Arab world on a cross-cultural level (for more information, see 10,11). AfrAbia is marked by a higher frequency of illnesses, trauma, and violence, as well as greater exposure to toxic substances and malnutrition, against a backdrop of far greater genetic variety. All these variables present a potential challenge for the neuroscience community in AfrAbia to achieve breakthroughs in fundamental research and develop personalized therapies to boost brain health and well-being throughout the continent (12,13). A recent study highlighted why Africa has a low number of neurologists worldwide and how this low number negatively impacted neurological disorders’ management (14).
According to research released by the Global Burden of Disease Neurology Collaborators, neurological illnesses were identified as the primary contributor to disability-adjusted life-years lost, amounting to 276 million annually, and ranked as the second most significant cause of mortality, resulting in 9 million deaths globally in the year 2016. The three primary factors that significantly contribute to this worldwide burden are stroke, migraine, and neurodegenerative illnesses (15). Enhanced comprehension of modified pathways and biomarkers linked to environmental factors will aid in discovering efficacious strategies for preventing and managing neurological disorders, particularly neurodegenerative diseases. These conditions often exhibit a probable genetic susceptibility to abiotrophy, which can be compounded by acquired harm from prolonged heat exposure (16)(
Figure 1).
The primary objective of this study is to delineate the impact of global warming, air pollution, heavy metals, and pesticides on the prevalence of PD. Furthermore, this study aims to enhance comprehension of the pathophysiological mechanisms that underlie the association between these adverse events and PD in AfrAbian countries. Moreover, the current study aims to understand the mechanism for linking these factors and PD. We hypothesize that the combined effects of these environmental factors would lead to a decline in cognitive functions in PD and accelerate its progression.
2. Global warming and PD
Climate change is accelerating rapidly due to human activity (17; 18; 19 ). Global warming is one of the worst effects of climate change (20; 21). Previous studies predict a 0.3°C to 0.7°C increase in global ambient temperature over the next 30 years (22; 23). The Intergovernmental Panel on Climate Change (20) found that a 1°C increase in ambient temperature might worsen pre-existing conditions and cause early death. The WHO reported a 125 million increase in heat wave exposure between 2000 and 2016 (24). Unfortunately, the current trend predicts that heat waves may become more frequent and severe in the following decades (25). Global warming does not cause neurodegenerative disorders, but it worsens dementia, AD, and PD symptoms. The intricate relationship between global warming and the neurological system argues that climate change legislation must address both issues simultaneously.
Current trends suggest heat waves will become more frequent and severe in the following decades. Heat waves harmed over 125 million people between 2000 and 2016 (26). Heat exposure and acclimatization impact monoaminergic neurotransmitters in the brain, which regulate thermoregulation, food intake, and energy balance (27). Climate change interferes with sensory inputs and reactions, affecting cognitive processes and the brain. Further, high temperatures affect cognition, productivity, and work performance (28).
Buizza et al. (29) found a 25% correlation between global warming and three PD indices (deaths, prevalence, and disability-adjusted life years) from 1990 to 2016 in 185 countries, with more significant warming causing greater variations. High ambient temperatures may harm numerous physiological systems, including animal behavior (19). Lu et al. (30) explored heat stress’s effects on brain inflammation. Heat-exposed mice experienced cognitive impairment, neurotoxicity, and aberrant neurogenesis. The association between average monthly temperatures and nervous system illness fatalities was researched in ten Japanese prefectures from January 1, 2010, to December 31, 2019. The study found that cooler temperatures increased nervous system illness mortality. Parkinson’s patients 85 and older were the only heat-related deaths (Lin et al., 2023). Liu et al. (31) combined epidemiological literature to quantify the effects of high ambient temperatures and heat waves on mental health-related mortality and morbidity while studying sources of variance. Their data supports the hypothesis that heat waves and intense temperatures harm mental health. Warmer climates are expected to worsen climate change.
NDDs are strongly linked to climate change-induced heat waves (32). Classical heat stroke victims on a pilgrimage to Mecca showed cognitive impairment, with 29% entering a deep coma (33). In the 1990s, a deadly heat stroke in Chicago caused delirium, lethargy, disorientation, seizures, hypertonia, and hypotonia (34). In hospitalised people without neurological problems, heat stroke is associated with long-term neuronal damage (35). A French longitudinal research followed 83 heat stroke patients for two years. Six of the 27 survivors at one year had considerable functional impairment. Twenty-one had moderate to severe functional impairments (36). A separate Swedish research investigated 58 heat stroke victims. Of the 46 survivors, 35 had moderate to severe neurological impairment (35). Heat waves may accelerate neurological diseases, especially in dementia patients. Wei et al. (37) and Linares et al. (38) found that dementia hospital admissions increased with a 1.5°C increase in ambient temperature. Similarly, a 1°C temperature increase increased Alzheimer’s disease (AD)-related hospital admissions by 23.1% (39). Although there is no empirical evidence linking elevated temperatures to PD, a retrospective study in Spain from 2001 to 2009 found an 11.47% increase in PD hospital admissions and a 12.11% increase in mortality rate among PD patients after a temperature increase of 1°C above 34°C (38).
Heat stroke causes delirium, convulsions, and coma due to core body temperature > 40°C and impaired CNS function (40). This condition may permanently damage neurons (41). Increased cerebral temperature from brain ischemia worsens prognosis. Blocking the middle cerebral artery (MCA) in monkeys elevated the ischemic penumbra temperature by 1.16°C, restricting tissue energy and oxygen delivery (42). Hyperthermic rats following localized brain ischemia had greater mortality and neurological impairments than normothermic controls (43). Keatinge et al. (44) found higher intracranial hypertension and cerebral ischemia in heat stroke animals. Hyperthermia causes matrix metalloproteinases to damage the basement membrane and microvasculature (45, 46). This increases infarction volume after middle cerebral artery closure (46). Heat increases blood-brain barrier permeability, reducing tPA benefits during ischemia. Edoema increases penumbra ischemia (43). Excitotoxicity, widespread necrosis, and apoptotic cell death are the main pathogenic changes in heat stroke-induced neurodegeneration (47; 48). At 41°C, rats were hyperthermic after lipopolysaccharide injection and electric heater. A significant increase in neuronal cell death was found (47). Ultrahigh temperatures impair cellular equilibrium, releasing calcium ions from the Golgi apparatus and endoplasmic reticulum. This triggers calpain proteases and caspases, releasing lysosomal cathepsins and killing the cell. Animals heated to 40°C had higher caspase-3 and lower Bcl-2 levels in their blood samples (48). Heat stress is a major environmental factor that may activate ROS (49).
Heat stress increases mitochondrial superoxide anions and decreases superoxide dismutase one mRNA and enzyme activity (50,51). This causes mitochondrial dysfunction, lipid oxidation, and brain antioxidant dysfunction (52). Heat stress-induced oxidative stress may promote neurodegeneration by hyperphosphorylating and aggregating tau proteins, which form neurofibrillary tangles (53). Studies have revealed that temperatures exceeding 40°C may promote amyloid fibril plaque development. Accelerated assembly and higher fibril binding affinity accelerate aggregation (54). Chen et al. (55) developed an explanatory PD brain thermal regulation paradigm. Mitochondrial malfunction causes neuroinflammatory stress and oxidative stress, according to this concept. This cascade results from cell death, slower metabolism, and lower intraventricular temperature. Elevated ambient temperatures may increase hospitalization, agitation, and death in Parkinson’s disease patients (56). High temperatures may cause heat stress, oxidative stress, and excitotoxicity, especially in older people with weaker thermoregulatory systems and neuroinflammatory processes (57; 19). Habibi et al. (32) found that heat stress degenerates neurons. According to many studies, heat stress affects neurons, their axons, glia, and brain blood vessel endothelial cells (58). Heat stress may cause heat stroke, dementia, stroke, migraine, and seizures (26). A study on rats found that heat stress damages the blood-brain barrier (BBB), causing moderate traumatic brain injury, hyperthermia, and long-term memory and learning deficits (59).
Due to prolonged heat stress, climatic changes in poor nations, notably Africa, worsen non-communicable illnesses such as NDDs. This may worsen NDDs, according to prior research (19). Climate change is associated with re-emerging diseases and viruses, including Dengue, Zika, Chikungunya, West Nile, and Yellow Fever, which have serious neurological effects. The tropical disease dengue affects 20% of people and causes neurological issues such as encephalitis and encephalopathy (60; 61). Yellow fever may cause encephalitis, acute neuroinflammation, and neuronal damage (62). The neurotropic West Nile virus may cause severe encephalitis in humans and horses. This drug endangers newborns, elderly individuals, and those with weaker immune systems (63). Microcephaly, cortical thinning, and vision impairment have been linked to Zika virus infection during pregnancy. Zika may cause adult meningoencephalitis and Guillain-Barre syndrome (64).
3. Air pollution and PD
The Global Burden of Disease Project estimated that air pollution killed 4.2 million people worldwide in 2015 (65). The 2019 Europe Air Quality Report found that air pollutants caused 22,000 to 537,000 premature deaths in Europe, depending on the pollutant. Industrialization has increased air pollution in recent decades (8; 66). Air pollution, especially particle matter, is strongly linked to NDDs by producing invisible contaminants and neurotoxins into the atmosphere (67; 68).
Recent study suggests that ambient air pollution may raise the risk of Parkinson’s disease (PD) by causing systemic inflammation, oxidative stress, and brain damage (69). Fine particulate matter may migrate to the brain via the olfactory system, which connects the nasal cavity to the brain (70) or via the autonomic nervous system or circulation (71). In toxicological and pathological investigations in extremely polluted Mexican cities, wild canines and youngsters had oxidative damage in their olfactory bulbs (72, 73). Additionally, postmortem human brain tissues showed aberrant amyloid-β, hyperphosphorylated tau, and α-synuclein protein accumulations (74, 73). Furthermore, particulate particles may enter the gastrointestinal system and alter the gut mucosa’s physiological state. These changes contribute to α-synuclein disease progression and microbiome disturbances (75). Similarly, Kwon et al. (76) found that modeled long-term exposure to traffic-related air pollution (CO) and multi-source fine particles (PM2.5) in central California residences and workplaces was linked with an elevated risk of PD. Brain damage from air pollution and occupational exposure may contribute to PD (77). Thus, urban tree restoration and preservation, green spaces, and green technology may improve environmental quality and atmospheric conditions while reducing direct and indirect pollution exposure. Brain hormone depletion must be reduced in all biogeographical cycles to improve well-being.
4. Pesticides and PD
Pollution from global warming has degraded air quality and been associated with PD disorders (78, 75). These chemicals, which are worsened by dirty air, may damage the brain (75) and increase PD risk (79). Pesticides stay in the environment, which is problematic considering their extensive use. Acute and chronic human toxicity (80) and significant environmental harm may result. Today’s most popular insecticides are organophosphorus (OP). Over 40 EPA-listed compounds make up these mixes (81). About 70% of US pesticides are OPs (82)—73 million pounds. Both high- and low-income countries worry about OP toxicity (83). OPs’ acute toxicity comes from blocking cholinesterase enzymes. Chronic Ops exposure may cause delayed neurotoxicity, developmental neurotoxicity, and organ damage (82). OPs like malathion (O, Odimethyl dithiophosphate of diethyl mercaptosuccinate) have several harmful effects (81). Malathion controls household insects and protects grain in worldwide storage (83). This treatment also treats animal ectoparasites, head lice, and body lice. Malaoxon, a bioactive malathion metabolite, promotes excitotoxicity, according to Acker et al. (84). Numerous studies show that malathion is neurotoxic in humans and animals (85; 86). Malathion and lead (Pb), another neurotoxicant, promote BBB permeability in comparable ways (87). New research reveals that Malathion during breastfeeding caused biochemical brain alterations and oxidative stress in rat pups. Malathion exposure in postnatal animals, including human neonates, has been linked to motor coordination, vestibular function, and muscular strength/coordination impairments in rat pups (84) and behavioral and morphological deformities in freshwater fish (88). Enzymes that deactivate organophosphates may be reduced in afflicted youngsters during crucial development. OP (containing Malathion) poisons by phosphorylating a serine residue in acetylcholinesterase (AChE), leaving it inactive. AChE converts acetylcholine (ACh) to choline and acetate during neurotransmission at cholinergic synapses (89).
Neurotransmitter system disruptions may cause neurodegeneration (90, 91). Toxic chemicals may enter neurons with a similar neurotransmitter via transporters. Dopamine transporters allow 1-Methyl-4-phenylpyridinium to enter neurons (92). Ball et al. (93) and Srivastav et al. (94) have linked NDDs to heavy metals, pesticides, and illicit drugs. Organochlorines and carbamates are being utilized globally. These chemicals may enter the body via the respiratory system, gastrointestinal tract, skin, and water (95). Ocular exposure is rare. However, pesticides may enter the bloodstream when chemicals accidentally touch the eyes or hands touch the eyes (96). Farmers and farmworkers are in danger of pesticide contamination. This group is directly exposed to these pollutants, whereas others risk food contamination (97). Ingesting and inhaling pollutants causes soft tissue gene expression patterns to deteriorate over time. Pesticides are one of the primary pollutants that may alter the regulatory system, causing epigenetic alterations and illness (98). Pesticide-induced neuron loss reduces cognitive and motor function (99). This relationship supports pesticide-related neurodegeneration. Labs and chemical and solvent handling may also allow neurotoxic substances to enter the body. The study conducted by Rosler et al. (100) confirmed that pesticide exposure is a risk factor for PD in a community in Egypt. The presence of the rs1126680 variant in the BCHE gene was shown to be associated with a reduced risk of PD, irrespective of exposure to pesticides. Additionally, it was discovered that the K-variant BCHE had the effect of decreasing the serum activity of butyrylcholinesterase, which is a recognized scavenger for pesticides. Therefore, individuals of Egyptian descent who possess the K-variant BCHE gene have a heightened susceptibility to Parkinson’s disease when subjected to pesticide exposure (100).
5. Heavey Metals and PD
Chronic placental insufficiency (CPI), hypoxia, heavy metals, and hormonal shifts throughout pregnancy might harm fetuses (101). It is generally known that fetal exposure during pregnancy may affect long-term health (102). Research suggests that umbilical cord CPI (cord prolapse or impairment) may obstruct the umbilicus in developing foetuses. Neurodegeneration and neonatal synaptic malfunction may result from this blockage (103). There is little human research on gestational or in-utero exposure and neurodegeneration. This lack of research restricts our knowledge of which trimester of pregnancy may cause such problems and how they impact the foetus. Mercury and lead exposure in umbilical cord blood during pregnancy has been demonstrated to impair visual processing (104). The BBB’s primary function is to keep heavy metals out of the brain (105, 106). Metals are sequestered by metal-protein complexes, protecting mature tissue from metal toxicity. Engwa et al. (107) and Jaishankar et al. (108) found that lead, aluminum, zinc, iron, copper, and mercury from soil, water, and other environmental sources cause NDDs. However, the findings are inconsistent, requiring more research to link neurometals to neurodegenerative diseases. Several researches have linked high levels of aluminum in drinking water to Alzheimer’s disease (AD), while others have not (109, 110). Furthermore, radioactive chemicals in contaminated areas transferred carbamate from the cornea to the retina via the aqueous humour. This supports the idea that radionuclide and pesticide exposure may disrupt the ocular pathway [
111].
Research has revealed that neurotoxicants may cause cognitive impairment, motor abnormalities, changed mental states, neuroendocrine changes, and sensory impairments (112, 113). Toxic chemicals that interrupt energy production harm the CNS and PNS, causing neurotoxicity. The central nervous system (CNS) is mainly impacted, with neurological effects appearing seconds after ingestion (114). Cyanide is known to impair mitochondrial complex IV cytochrome oxidase. The toxic substance’s effects on the neurological system may impair cellular respiration, neuron sensitivity, and immunological response (101). The loss of neprilysin (NEP) and insulin-degrading enzyme (IDE) efficiency causes lead build-up in brain tissue. NEP and IDE are essential to A Beta breakdown (115; 116).
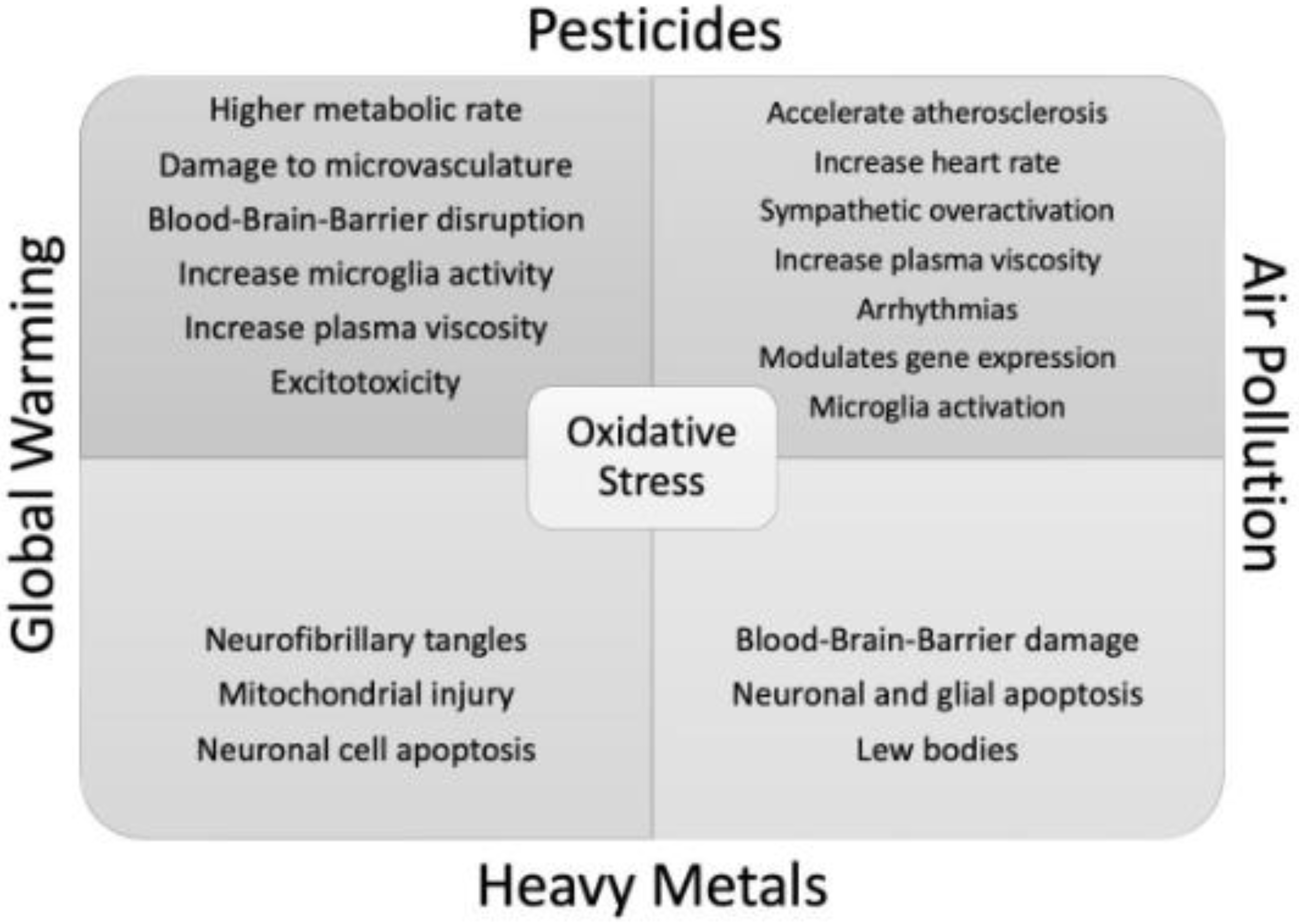
Figure 2.
provides a comprehensive overview of the intersection between the fundamental pathophysiological mechanisms resulting from global warming, air pollution, Heavy metals and Pesticides that have a direct or indirect impact on PD.
Figure 2.
provides a comprehensive overview of the intersection between the fundamental pathophysiological mechanisms resulting from global warming, air pollution, Heavy metals and Pesticides that have a direct or indirect impact on PD.
6. Discussion and Insights
The etiology of late-onset sporadic Parkinson’s disease (PD) is not yet fully understood, and it is believed that PD is the outcome of many genetic and environmental factors, as well as their interplay, within the context of age-related changes in the brain. Studying the environmental factors that contribute to and influence the development of PD has significant academic significance due to many compelling rationales. Initially, it is essential to note that late-onset sporadic PD is a condition that requires a considerable amount of time to manifest. Consequently, when individuals get a diagnosis, the neurodegenerative alterations have already progressed to a point where it is no longer feasible to impede, halt, or reverse them. Hence, our efforts to combat PD are contingent upon the timely detection and intervention of the illness, which, in turn, need a comprehensive comprehension of its genesis and its impact on modifiable risk factors. Furthermore, while significant advancements have been in identifying the genetic underpinnings of late-onset sporadic PD in recent years, it is essential to note that these genetic discoveries may only account for a limited proportion of cases, and their implications for disease prevention are not straightforward. However, it is essential to note that during the prodromal stage of PD, many environmental variables may potentially contribute to the onset and development of the disease (117). These factors might vary in their impact and timing, thereby influencing the pathogenesis of PD and altering its course. It is a justifiable proposition to posit that in a significant proportion, if not the whole, of instances involving the beginning of PD, occurring later in life, environmental factors have a role in determining or altering the risk and age at which PD symptoms manifest clinically. Regrettably, the identification of these components, the delineation of their respective functions, and the quantification of their contributions remain elusive now.
Additionally, in contrast to hereditary determinants, environmental influences provide the possibility for modification, yielding significant implications for the prevention and treatment of PD. In light of the accelerated expansion of elderly populations worldwide, PD has emerged as the neurological disorder, seeing the most rapid rise in terms of both its prevalence and mortality rates (15; 118). In addition to those above possible public health issue, emerging but inconclusive data indicates a rising trajectory in the prevalence of PD over the last three decades (119). This trend potentially signifies the involvement of environmental variables in the development of PD. Collectively, funding agencies and researchers in the field of PD must take immediate action to investigate the role of environmental factors in the onset and progression of PD.
Over the last twenty years, researchers have successfully identified several environmental variables that are linked to the likelihood of acquiring PD. Furthermore, the results related to the bulk of these factors have shown a reasonable level of consistency across many studies (120). Plausible biological theories have been offered for the majority of these correlations. Nevertheless, establishing causal inference for these epidemiological data has proven challenging. In addition to limited and sometimes conflicting experimental evidence, many of these epidemiological facts might be potentially explained by reverse causation. This suggests that the development of PD before clinical diagnosis may alter lifestyle and behavior, rather than the opposite causal relationship. There may be exceptions about the use of certain insecticides. Other significant inquiries have yet to be addressed, even in the realm of pesticides. Hence, while its significance and a substantial body of research, our comprehension of the environmental factors that contribute to Parkinson’s disease remains nascent.
Lack of knowledge of the prodromal period of late-onset sporadic PD, which takes decades to develop, makes it challenging to determine environmental factors contributing to the disease. The disease development paradigm for PD may need decades of exposure to commence the pathogenic process, which is typically unattainable in epidemiological investigations. Moreover, environmental and genetic variables may influence PD progression during the prodromal stage for decades after neurodegeneration begins. Thus, even epidemiological solid data, such as those from longitudinal cohorts, may have alternate causes.
Climate change, air pollution, heavy metals, pesticide use and ingestion, detergents, chemicals and solvents, and other industrial bio-products contribute to the global burden of diseases (GBD) in many non-communicable illnesses, including PD (121, 122, 123). Breaching the blood-brain barrier (BBB) disrupts CNS and PNS function (124). Climate change, which disrupts the body’s thermoregulation, may cause mental illness, according to Cheshire (125). A climate change study predicts 250,000 more deaths yearly from 2030 to 2050. Aging, genetic susceptibility, behavioral habits, environmental factors, and other neurological diseases have been linked to PD (126, 94). Environmental jobs like blacksmithing and drinking metal-polluted well water may contribute to PD, according to Oluwole et al. (126).
This article analyzes scientific facts to prove that environmental factors harm human health. Global warming and pesticide contamination are known to cause PD and increase its symptoms. Increased ambient temperatures promote acute cognitive impairment and worsen neurodegenerative diseases over time (32, 35, 37). Global warming and pesticide contamination affect the cardiovascular and cerebrovascular systems, creating a pro-thrombotic environment. These events cause brain inflammation and programmed neuron and glial cell death. This, together with neurofibrillary tangles and amyloid plaque deposition, increases the risk of neurodegenerative diseases (53, 54; 127).
Heat activates molecular pathways, causing functional decline in PD patients and older people with thermoregulation issues. Additionally, several tracks may contribute to similar situations. Temperature changes and epidemiological and molecular-level data might be linked to estimate neurodegenerative disease incidence in places with different temperatures. Note that no conclusive research links recent mean temperature changes to neurodegenerative disease prevalence. ,Data gathering issues may affect the quality of life in poor nations like most AfrAbian ones. Neurodegenerative disease data from 1990 to 2016 covers 195 countries (13). However, several governments have gathered annual average climatic data from 1970 to the present. Still, climatic data is very new and variable. Heat treatment and acclimatization boost heat shock protein (HSP) expression, which may be used in climate change adaptation tactics (128). These therapies have neuroprotective benefits in healthy and neurologically damaged people. Regular sauna bathers are less likely to acquire dementia and AD (129). This discovery suggests that passive heating may prevent neurodegenerative diseases. Due to the inability to conduct rigorous clinical research without retrospective observations, the contrasting effects of chronic and acute thermal stress may occur within a temperature and duration range that has yet to be determined. This intriguing idea needs further evidence.
AfrAbian PD case-control and cohort studies are lacking. Thus, the disease’s environmental and genetic risk factors are unknown. As shown (130), AfrAbians with PD and other mobility impairments require the same healthcare as people in wealthy nations. Thus, AfrAbian data on drug responsiveness, quality of life, survival, and other social and economic repercussions of PD are limited. International cooperation may assist AfrAbian researchers in overcoming resource constraints (money, people, and supplies). Genetic, archeological, and anthropological data suggest modern humans originated in Africa 200,000 years ago (131). Linguistic, genomic, and archeological data indicate that AfrAbia has more private alleles than anywhere else and a significant genetic diversity. Thus, AfrAbia and Europe differ significantly in race and ethnicity. Due to considerable environmental variability, AfrAbia communities have many cultural and phenotypic differences, making them attractive for gene-environment studies. This might revolutionize biological research by revealing gene-gene and gene-environment relationships. The quantity and scope of high-quality PD prevalence studies in AfrAbia are lacking. researchNations must collaborate and share resources and samples to recruit enough AfrAbian patients for genetic study. A centralized mutation screening center for AfrAbian PD patients would boost genetics research in AfrAbia. Finding AfrAbia PD families for whole exome sequencing may help find more PD genes. Time and money are required to educate more AfrAbian neurologists and movement disorder experts.
We agree with Popejoy and Fullerton (132) that genetic diversity requires bottom-up and top-down approaches. Genetic variation and socioeconomic and environmental variables should be considered when setting research aims and designing robust studies. Community participation and healthcare solutions attract underrepresented populations to scientific research. Due to the variability of PD, genetic research on persons of different races and cultures is necessary. Genetic variety should be linked with phenotypic description and inclusion criteria standardization to optimize study etiology. Trans-ethnic fine-mapping and other analytic methods improve understanding. Funding organisations should also give specialized resources, increase research participation for marginalised communities, and communicate research findings to healthcare professionals and policymakers (133). Ethical monitoring by culturally competent agents in disadvantaged populations, a well-thought-out informed consent process, local rules, data protection, and value creation are essential (134, 135). New regulations to protect indigenous people from genetic research abuse include the Human Heredity and Health Guidelines for Community Engagement (136), the San Code of Ethics (137), and the Navajo Nation’s genetic research and data sharing policy (138). Research objectives, financing, governance, and publishing criteria should also be widely available. A clear career development strategy should be established to help younger academics write first-author or senior-author publications instead of co-authoring multi-authored research (135). This might be done by collaborating with higher-income nations or creating regional development plans with hubs. International organizations help link economically impoverished countries with economically prosperous ones via regional firms and collaborations. Journals and editors should better reflect society on editorial boards by diversifying them. URP-centric versions with strict inclusion requirements may be proposed. Finally, peer review must be done carefully to be productive, effective, and inclusive.
7. Conclusions and Future Perspectives
The terms “exposome” and “neuroexposome” are especially intriguing for addressing repeated exposures over time. Chris Wild used the term “exposome” over a decade ago to describe the sum of environmental exposures a person experiences throughout their lifetime (139). Heffernan and Hare (140) have expanded this paradigm to neurological illnesses. They suggested combining “omics” technologies (e.g., genomes and metabolomics) with epidemiological surveys and clinical examinations at all ages, considering brain characteristics like BBB permeability and neuron longevity. Despite its challenges, the exposome concept offers a theoretical framework for studying environmental causes of PD and developing early interventions to prevent motor dysfunction.
AfrAbians believe environmental labor, such as mining gold and cobalt and drinking metal-contaminated well water, may induce PD. Many hazardous human actions, such as cross-contamination of dirty agro-allied sites and radioactive materials in contaminated areas, may increase PD prevalence. Nevertheless, it is essential to acknowledge that exposure assessment, both in terms of methodology and technology, must be complemented by developing novel approaches for data analysis and interpretation. Additionally, it is crucial to establish rules that ensure the ethical sharing of sensitive data in the AfrAbian context. Inhibiting disease-associated protein deposition, which accelerates PD, may be a future treatment option. A molecular chaperone called heat shock protein 104 can do this. This strategy showed neuroprotective benefits by eliminating amyloid conformations, pre-amyloid oligomer deposits, and precise activities (141).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary AfrAbia+plus Parkinson Disease Genomic Consortium (AA-PD-GC) members based on the country.
Funding
This project was supported by the Global Parkinson’s Genetics Program (GP2). GP2 is funded by the Aligning Science Across Parkinson’s (ASAP) initiative and implemented by The Michael J. Fox Foundation for Parkinson’s Research (
https://gp2.org). For a complete list of GP2 members, see https://gp2.org.
Acknowledgments
We express our sincere gratitude to GP2 and MJF for their support of the AA+plus-PD-GC consortium. All consortium members served as authors, contributing equally and actively to the writing and revision of this manuscript. There is no relevant financial disclosure.
Competing Interest
We declare no competing interests.
Appendix
It includes name and affiliation of members of AA+plus -PD-GC.
References
- Ray Dorsey, E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016, a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, K.H.; Tandberg, E.; Årsland, D.; Larsen, J.P. Health related quality of life in Parkinson’s disease: a prospective longitudinal study. J. Neurol. Neurosurg. Psychiatry 2000, 69, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Global Parkinson’s Disease Survey (GPDS) Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov. Disord. 2002, 17, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Al Rajeh, S.; Bademosi, O.; Ismail, H.; Awada, A.; Dawodu, A.; Al-Freihi, H.; Assuhaimi, S.; Borollosi, M.; Al-Shammasi, S. A Community Survey of Neurological Disorders in Saudi Arabia: The Thugbah Study. Neuroepidemiology 1993, 12, 164–178. [Google Scholar] [CrossRef] [PubMed]
- World Life Expectancy. 2020. Available online: https://www.worldlifeexpectancy.com/saudi-arabia-parkinson-disease.
- El-Tallawy, H.N.; Farghaly, W.M.; Shehata, G.A.; Rageh, T.A.; Hakeem NM, A.; al Hamed, M.A.; Badry, R. Prevalence of Parkinson’s disease and other types of Parkinsonism in Al Kharga district, Egypt. Neuropsychiatr. Dis. Treat. 2013, 9, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Sharrow, D.J.; Clark, S.J.; Collinson, M.A.; Kahn, K.; Tollman, S.M. M. The Age Pattern of Increases in Mortality Affected by HIV: Bayesian Fit of the Heligman-Pollard Model to Data from the Agincourt HDSS Field Site in Rural Northeast South Africa. Demogr. Res. 2013, 29, 1039. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, X.; Wang, C.; Xu, Q.; Wang, W.; Luo, Y.; Tao, L.; Gao, Q.; Guo, J.; Chen, S.; et al. PM2.5 Spatiotemporal Variations and the Relationship with Meteorological Factors during 2013–2014 in Beijing, China. PLoS ONE 2015, 10, e0141642. [Google Scholar] [CrossRef] [PubMed]
- Heligman, L.; Chen, N.; Babakol, O. Shifts in the structure of population and deaths in less developed regions. In The Epidemiological Transition. Policy and Planning Implications for Developing Countries; Gribble, J.N., Preston, S.H., Eds.; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Mohamed, W. Leveraging genetic diversity to understand monogenic Parkinson’s disease’s landscape in AfrAbia. Am. J. Neurodegener. Dis. 2023, 12, 108–122. [Google Scholar] [PubMed]
- Mohamed, W. Parkinson’s genetics research on underrepresented AfrAbia populations: current state and future prospects. Am. J. Neurodegener. Dis. 2023, 12, 23–41. [Google Scholar]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; et al. Global, regional, and national burden of neurological disorders during 1990–2015, A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016, A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Kissani, N.; Liqali, L.; Hakimi, K.; Mugumbate, J.; Daniel, G.M.; Ibrahim, E.A.A.; Yewnetu, E.; Belo, M.; Wilmshurst, J.; Mbelesso, P.; et al. Why does Africa have the lowest number of Neurologists and how to cover the Gap? J. Neurol. Sci. 2022, 434, 120119. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016, a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Peinkhofer, C.; Amiri, M.; Othman, M.H.; De Vecchi, T.; Nersesjan, V.; Kondziella, D. Global warming and neurological practice: systematic review. MedRxiv 2020, 2020, 1–23. [Google Scholar]
- Cook, J.; Oreskes, N.; Doran, P.T.; Anderegg, W.R.; Verheggen, B.; Maibach, E.W.; Carlton, J.S.; Lewandowsky, S.; Skuce, A.G.; Green, S.A.; et al. Consensus on consensus: a synthesis of consensus estimates on human-caused global warming. Environ. Res. Lett. 2016, 11, 048002. [Google Scholar] [CrossRef]
- Mann, M.E.; Rahmstorf, S.; Kornhuber, K.; Steinman, B.A.; Miller, S.K.; Coumou, D. Influence of anthropogenic climate change on planetary wave resonance and extreme weather events. Sci. Rep. 2017, 7, 45242. [Google Scholar] [CrossRef] [PubMed]
- Bongioanni, P.; Del Carratore, R.; Corbianco, S.; Diana, A.; Cavallini, G.; Masciandaro, S.M.; Dini, M.; Buizza, R. Climate change and neurodegenerative diseases. Environ. Res. 2021, 201, 111511. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change (IPCC). Climate Change 2014, Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; p. 1132. [Google Scholar]
- Patz, J.A.; Frumkin, H.; Holloway, T.; Vimont, D.J.; Haines, A. Climate change: challenges and opportunities for global health. JAMA 2014, 312, 1565–1580. [Google Scholar] [CrossRef]
- Haustein, K.; Allen, M.R.; Forster, P.M.; Otto, F.E.L.; Mitchell, D.M.; Matthews, H.D.; Frame, D.J. A real time Global Warming Index. Sci. Rep. 2017, 7, 15417. [Google Scholar] [CrossRef] [PubMed]
- Folland, C.K.; Boucher, O.; Colman, A.; Parker, D.E. Causes of irregularities in trends of global mean surface temperature since the late 19th century. Sci. Adv. 2018, 4, eaao5297. [Google Scholar] [CrossRef] [PubMed]
- WHO. Information and public health advice: heat and health. Available online: https://www.who.int/globalchange/publications/heat-andhealth/en/ (accessed on 1 August 2020).
- AR5 Synthesis Report: Climate Change 2014—IPCC. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 1 August 2020).
- Zammit, C.; Torzhenskaya, N.; Ozarkar, P.D.; Calleja Agius, J. Neurological disorders vis-à-vis climate change. Early Hum. Dev. 2021, 155, 105217. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ishiwata, T. Effect of short- and long-term heat exposure on brain monoamines and emotional behavior in mice and rats. J. Therm. Biol. 2021, 99, 102923. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, B.; Webb, J.K. Incubation under climate warming affects learning ability and survival in hatchling lizards. Biol. Lett. 2017, 13, 20170002. [Google Scholar] [CrossRef] [PubMed]
- Buizza, R.; del Carratore, R.; Bongioanni, P. Evidence of climate change impact on Parkinson’s disease. J. Clim. Change Health 2022, 6, 100130. [Google Scholar] [CrossRef]
- Lu, J.; Sun, F.; Ma, H.; Qing, H.; Deng, Y. Quantitative detection of dopamine, serotonin and their metabolites in rat model of Parkinson’s disease using HPLC-MS/MS. In Proceedings of the 2015 IEEE International Conference on Mechatronics and Automation (ICMA), Beijing, China, 2–5 August 2015. [Google Scholar]
- Liu, J.; Varghese, B.M.; Hansen, A.; Xiang, J.; Zhang, Y.; Dear, K.; Gourley, M.; Driscoll, T.; Morgan, G.; Capon, A.; et al. Is there an association between hot weather and poor mental health outcomes? A systematic review and meta-analysis. Environ. Int. 2021, 153, 106533. [Google Scholar] [CrossRef] [PubMed]
- Habibi, L.; Perry, G.; Mahmoudi, M. Global warming and neurodegenerative disorders: speculations on their linkage. Bioimpacts 2014, 4, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, B.A. Neurologic manifestations of heatstroke at the Mecca pilgrimage. Neurology 1987, 37, 1004–1006. [Google Scholar] [CrossRef]
- Dematte, J.E.; K O’Mara; Buescher, J. ; Whitney, C.G.; Forsythe, S.; McNamee, T.; Adiga, R.B.; Ndukwu, I.M. Nearf atal heat stroke during the 1995 heat wave in Chicago. Ann. Intern. Med. 1998, 129, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.G. Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environ. Health Perspect. 2016, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Argaud, L.; Ferry, T.; Le, Q.H.; Marfisi, A.; Ciorba, D.; Achache, P.; Ducluzeau, R.; Robert, D. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch. Intern. Med. 2007, 167, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Lin, C.-K.; Yin, K.; Yang, J.; Shi, L.; Li, L.; Zanobetti, A.; Schwartz, J.D. Associations between seasonal temperature and dementia-associated hospitalizations in New England. Environ. Int. 2019, 126, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Martinez-Martin, P.; Rodríguez-Blázquez, C.; Forjaz, M.J.; Carmona, R.; Díaz, J. Effect of heat waves on morbidity and mortality due to Parkinson’s disease in Madrid: A time-series analysis. Environ. Int. 2016, 89–90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Culqui, D.R.; Linares, C.; Ortiz, C.; Carmona, R.; Díaz, J. Association between environmental factors and emergency hospital admissions due to Alzheimer’s disease in Madrid. Sci. Total Environ. 2017, 592, 451–457. [Google Scholar] [CrossRef]
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Dhopesh, V.P.; Burns, R.A. Letter: Loss of nerve conduction in heat stroke. N. Engl. J. Med. 1976, 294, 557–558. [Google Scholar] [PubMed]
- Sun, Z.; Zhang, J.; Chen, Y.; Zhang, Y.; Zhang, X.; Guo, H.; Yu, C. Differential temporal evolution patterns in brain temperature in different ischemic tissues in a monkey model of middle cerebral artery occlusion. J. Biomed. Biotechnol. 2012, 2012, 980961. [Google Scholar] [CrossRef] [PubMed]
- Noor, R.; Wang, C.X.; Shuaib, A. Hyperthermia masks the neuroprotective effects of tissue plaminogen activator. Stroke 2005, 36, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Keatinge, W.R.; Coleshaw, S.R.; Easton, J.C.; Cotter, F.; Mattock, M.B.; Chelliah, R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am. J. Med. 1986, 81, 795–800. [Google Scholar] [CrossRef]
- Alam, M.; Mohammad, A.; Rahman, S.; Todd, K.; Shuaib, A. Hyperthermia up-regulates matrix metalloproteinases and accelerates basement membrane degradation in experimental stroke. Neurosci. Lett. 2011, 495, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; He, C.; Shuaib, A.; Wang, C.X. Hyperthermia worsens ischaemic brain injury through destruction of microvessels in an embolic model in rats. Int. J. Hyperthermia. 2012, 28, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Kislal, F.M.; Demirol, M.; Girgin, F.I.; Kabakus, N.; Kocak, M. Neuronal rat brain damage caused by endogenous and exogenous hyperthermia. Arch. Turk. Dermatol. Venerol. 2012, 18, 11–16. [Google Scholar]
- Mohamed, W.A.M.; Ismail, T.; Farouk, S. The ameliorative potential of ethanolic extract of propolis on hematotoxicity and structural neuronal damage in hyperthermia-exposed rats. Iran. J. Basic. Med. Sci. 2016, 19, 875–882. [Google Scholar] [PubMed]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperthermia. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Sato, K.; Akiba, Y.; Toyomizu, M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006, 85, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Pumford, N.R.; Bottje, W.; Nakagawa, K.; Miyazawa, T.; Akiba, Y.; Toyomizu, M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 2007, 44, 439–445. [Google Scholar] [CrossRef]
- Chang, C.-K.; Chang, C.-P.; Liu, S.-Y.; Lin, M.-T. Oxidative stress and ischemic injuries in heat stroke. Neurobiology of Hyperthermia. Prog. Brain Res. 2007, 162, 525–546. [Google Scholar] [PubMed]
- Chauderlier, A.; Delattre, L.; Buée, L.; Galas, M.-C. In vivo hyperthermic stress model: an easy tool to study the effects of oxidative stress on neuronal tau functionality in mouse brain. Methods Mol. Biol. 2017, 1523, 369–373. [Google Scholar] [PubMed]
- Ghavami, M.; Rezaei, M.; Ejtehadi, R.; Lotfi, M.; Shokrgozar, M.A.; Abd Emamy, B.; Raush, J.; Mahmoudi, M. Physiological temperature has a crucial role in amyloid β in the absence and presence of hydrophobic and hydrophilic nanoparticles. ACS Chem. Neurosci. 2013, 4, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Yamada, K.; Sakai, K.; Lu, C.H.; Chen, M.H.; Lin, W.C. Alteration of brain temperature and systemic inflammation in Parkinson’s disease. Neurol. Sci. 2020, 41, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Franklin, G.M.; Longstreth, W.T.; Swanson, P.D.; Checkoway, H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann. Neurol. 2013, 74, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H. Diverse Misfolded Conformational Strains and Cross-seeding of Misfolded Proteins Implicated in Neurodegenerative Diseases. Front. Mol. Neurosci. 2019, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Hoopes, P.J. Hyperthermia induced pathophysiology of the central nervous system. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group. 2003, 19, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Titus, D.J.; Furones, C.; Atkins, C.M.; Dietrich, W.D. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp. Neurol. 2015, 263, 254–262. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. Int. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Coates, S.J.; Norton, S.A. The effects of climate change on infectious diseases with cutaneous manifestations. Int. J. Women Dermatol. 2021, 7, 8–16. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Guedes, B.F.; Sulleiman, J.; de Oliveira, F.T.M.; de Souza, I.O.M.; Nogueira, J.S.; Marcusso, R.M.N.; Fernandes, E.G.; do Olival, G.S.; de Figueiredo, P.; et al. Neurologic Disease after Yellow Fever Vaccination, São Paulo, Brazil, 2017–2018. Emerg. Infect. Dis. 2021, 27, 1577–1587. [Google Scholar]
- Suen, W.W.; Prow, N.A.; Hall, R.A.; Bielefeldt-Ohmann, H. Mechanism of West Nile virus neuroinvasion: A critical appraisal. Viruses 2014, 6, 2796–2825. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.E. Severe thunderstorms and climate change. Atmos. Res. 2013, 123, 129–138. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks,1990–2015, a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Freedman, G.; Frostad, J.; van Donkelaar, A.; Martin, R.V.; Dentener, F.; van Dingenen, R.; Estep, K.; Amini, H.; Apte, J.S.; et al. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ. Sci. Technol. 2016, 50, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kinney, P.L. Interactions of Climate Change, Air Pollution, and Human Health. Curr. Environ. Health Rep. 2018, 5, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public. Health 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Kieltyka, M.; Roman, A.; Nalepa, I. The air we breathe: air pollution as a prevalent proinflammatory stimulus contributing to neurodegeneration. Front. Cell. Neurosci. 2021, 15, 647643. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Ozturk, S. Evaluation of the effect of air pollution on cognitive functions, cognitive decline, and dementia. Ann. Indian Acad. Neurol. 2022, 25, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. 2010, 62, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Kavanaugh, M.; Block, M.; D’Angiulli, A.; Delgado-Chávez, R.; Torres-Jardón, R.; González-Maciel, A.; Reynoso-Robles, R; Osnaya, N. ; Villarreal-Calderon, R.; et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J. Alzheimers. Dis. 2012, 28, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cognit. 2008, 68, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Barnhill, L.M.; Bronstein, J.M. Air Pollution and the Risk of Parkinson’s Disease: A Review. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 894–904. [Google Scholar] [CrossRef]
- Kwon, D.; Paul, K.; Yu, Y.; Zhang, K.; Folle, A.; Wu, J.; Bronstein, J.; Ritz, B. Traffic-related air pollution and Parkinson’s disease in central California. Environ. Res. 2023, 240, 117434. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Spencer, P.S.; Román, G.C.; Buguet, A. Environmental neurology in the tropics. J. Neurol. Sci. 2021, 421, 117287. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, Y.J.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Kim, B.J.; Chung, S.J. J. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol. 2021, 78, 800–808. [Google Scholar] [CrossRef]
- Islam, M.S.; Azim, F.; Saju, H.; Zargaran, A.; Shirzad, M.; Kamal, M.; Fatema, K.; Rehman, S.; Azad MA, M.; Ebrahimi-Barough, S. Pesticides and Parkinson’s disease: Current and future perspective. J. Chem. Neuroanat. 2021, 115, 101966. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Calugi, S.; Brambilla, F.; Abbate-Daga, G.; Fassino, S.; Marchesini, G. The effect of inpatient cognitive-behavioral therapy for eating disorders on temperament and character. Behav. Res. Ther. 2007, 45, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- EPA. Environmental Protection Agency of USA guidelines. 2002. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/epa-2002-guidelines-ensuring-and-maximizing-quality.
- Jokanović, M.; Oleksak, P. ; Kuca, K. Multiple neurological effects associated with exposure to organophosphorus pesticides in man. Toxicology 2023, 484, 153407. [Google Scholar] [CrossRef] [PubMed]
- Maroni, M.; Colosio, C.; Ferioli, A.; Fait, A. Toxicology: Introduction. Toxicology 2000, 143, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Rohlman DS chertler Ismail, A.A.; Abdel-Rasoul, G.; Lasarev, M.; Hendy, O.; Olson, J.R. Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metab. Brain Dis. 2014, 29, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Acker, C.I.; Souza AC, G.; Pinton, S.; da Rocha, J.T.; Friggi, C.A.; Zanella, R.; Nogueira, C.W. W. Repeated malathion exposure induces behavioral impairment and AChE activity inhibition in the brains of rat pups. Ecotoxicol. Environ. Saf. 2011, 74, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Vidair, C.A. Age dependence of organophosphate and carbamate neurotoxicity in the postnatal rat: Extrapolation to the human. Toxicol. Appl. Pharmacol. 2004, 196, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Selmi, S.; El-Fazaa, S.; Gharbi, N. Oxidative stress and cholinesterase inhibition in plasma, erythrocyte and brain of rats’ pups following lactational exposure to malathion. Environ. Toxicol. Pharmacol. 2012, 34, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Li, W.; Ehrich, M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood-brain barrier: cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology 2011, 32, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.K.; David, M. Behavioral and morphological endpoints: as an early response to sublethal malathion intoxication in the freshwater fish, Labeo rohita. Drug Chem. Toxicol. 2010, 33, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Ye, W.H.; Zhou, S.S.; Lin, K.D.; Zhao, M.R.; Liu, W.P. Acute and chronic toxicity of organophosphate monocrotophos to Daphnia magna. J. Environ. Sci Health B 2009, 44, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA 1985, 82, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Anand, B.G.; Fatima, M.; Prajapati, K.P.; Yadav, S.S.; Kar, K.; Mondal, A.C. Piperine-Coated Gold Nanoparticles Alleviate Paraquat-Induced Neurotoxicity in Drosophila melanogaster. ACS Chem. Neurosci. 2020, 11, 3772–3785. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Parrón, T.; Tsatsakis, A.M.; Requena, M.; Alarcón, R.; López-Guarnido, O. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 2013, 307, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Martinez-Moral, M.P.; Kannan, K. Temporal variability in urinary pesticide concentrations in repeated-spot and firstmorning-void samples and its association with oxidative stress in healthy individuals. Environ. Int. 2019, 130, 104904. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Rösler, T.W.; Salama, M.; Shalash, A.S.; Khedr, E.M.; El-Tantawy, A.; Fawi, G.; El-Motayam, A.; El-Seidy, E.; El-Sherif, M.; El-Gamal, M.; et al. K-variant BCHE and pesticide exposure: Gene-environment interactions in a case-control study of Parkinson’s disease in Egypt. Sci Rep. 2018, 8, 16525. [Google Scholar] [CrossRef] [PubMed]
- Modgil, S.; Lahiri, D.K.; Sharma, V.L.; Anand, A. Role of early life exposure and environment on neurodegeneration: Implications on brain disorders. Transl. Neurodegener. 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Saidj, S.; Henderson, M.; Ruchat, S.-M.; Paradis, G.; Hulst, A.V.; Zappitelli, M.; Mathieu, M.-E. Are Suboptimal in utero Conditions Associated with Obesity and Cardiometabolic Risk Factors in Caucasian children? medRxiv 2020, 2837. [Google Scholar]
- Rubio, E.I. MR Imaging of the Fetal Chest and Abdomen: How to Provide Value-Added Imaging. Curr. Radiol. Rep. 2017, 5, 54. [Google Scholar] [CrossRef]
- Sakamoto, M.; Murata, K.; Domingo, J.L.; Yamamoto, M.; Oliveira, R.B.; Kawakami, S.; Nakamura, M. Implications of mercury concentrations in umbilical cord tissue in relation to maternal hair segments as biomarkers for prenatal exposure to methylmercury. Environ. Res. 2016, 149, 282–287. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Mak, R.; Lee, J.; Buckland, M.E.; Harding, A.J.; Kum Jew, S.; Paterson, D.J.; Jones, M.W.M.; Lay, P.A. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PLoS ONE 2020, 15, e0233300. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.; Ferdinand, P.; Nwalo, N.; Unachukwu, M. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. Poisoning Mod. World New Tricks Old Dog 2019, 10, 70–90. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Meleleo, D.; Notarachille, G.; Mangini, V.; Arnesano, F. Concentration-dependent effects of mercury and lead on A 42, Possible implications for Alzheimer’s disease. Eur. Biophys. J. 2019, 48, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 124, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Mihalopoulos, C.; Erskine, H.E.; Roberts, J.; Rahman, A. Childhood Mental and Developmental Disorders. In Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, 3rd ed.; Patel, V., Chisholm, D., Dua, T., Laxminarayan, R., Medina-Mora, M.E., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 4. [Google Scholar]
- Jiang, J.; Chan, A.; Ali, S.; Saha, A.; Haushalter, K.J.; Lam, W.L.; Glasheen, M.; Parker, J.; Brenner, M.; Mahon, S.B.; et al. Hydrogen Sulfide–Mechanisms of Toxicity and Development of an Antidote. Sci. Rep. 2016, 6, 20831. [Google Scholar] [CrossRef] [PubMed]
- Dosunmu, R.; Alashwal, H.; Zawia, N.H. Genome-wide expression and methylation profiling in the aged rodent brain due to early-life Pb exposure and its relevance to aging. Mech. Ageing Dev. 2012, 133, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Brew, B.J.; Guillemin, G.J. Lead dysregulates serine/threonine protein phosphatases in human neurons. Neurochem. Res. 2011, 36, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. The changing landscape of Parkinson epidemiologic research. J. Park. Dis. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.L.; Raphael, K.G.; Brundin, P.; Matthews, H.; Wyse, R.K.; Chen, H.; Bloem, B.R. Parkinson Matters. J. Park. Dis. 2018, 8, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Time trends in the incidence of Parkinson’s disease: A 30-year study. JAMA Neurol. 2017, 32, 227–234. [Google Scholar]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Tanner Caroline, M.; Kamel, F.; Ross, G.W.; Hoppin Jane, A.; Goldman Samuel, M.; Korell, M.; Marras, C.; Bhudhikanok Grace, S.; Kasten, M.; Chade Anabel, R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015, A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M. Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr. Environ. Health Rep. 2017, 4, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinson Dis. 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. Basic Clin. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.G.; Kuivaniemi, H.; Carr, J.A.; Ross, O.A.; Olaogun, M.O.B.; Bardien, S.; Komolafe, M.A. Parkinson’s disease in Nigeria: A review of published studies and recommendations for future research. Park. Relat. Disord. 2019, 62, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Lucking, A.J.; Lundbäck, M.; Barath, S.L.; Mills, N.L.; Sidhu, M.K.; Langrish, J.P.; Boon, N.A.; Pourazar, J.; Badimon, J.J.; Gerlofs-Nijland, M.E.; et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 2011, 123, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.P.; Minett, G.M.; Gibson, O.R.; Kerr, G.K.; Stewart, I.B. Could heat therapy Be an effective treatment for alzheimer’s and Parkinson’s diseases? A narrative review. Front. Physiol. 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.; Kauhanen, J.; Laukkanen, J.A. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing 2017, 46, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Teshome, M.; Melaku, Z.; Zenebe, G. Frequency of movement disorders in an Ethiopian university practice. Mov. Disord. 2005, 20, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.C.; Tishkoff, S.A. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu. Rev. Genom. Hum. Genet. 2008, 9, 403–33. [Google Scholar] [CrossRef] [PubMed]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, N. Tiered informed consent: respecting autonomy, agency and individuality in Africa. BMJ Glob Health 2018, 3, e001249. [Google Scholar] [CrossRef] [PubMed]
- Claw, K.G.; Anderson, M.Z.; Begay, R.L.; Tsosie, K.S.; Fox, K.; Garrison, N.A. A framework for enhancing ethical genomic research with indigenous communities. Nat. Commun. 2018, 9, 2957. [Google Scholar] [CrossRef] [PubMed]
- H3Africa. Available online: https://h3africa.org/index.php/ consortium/consortium-documents/ (accessed on 8 April 2022).
- The San Code. Global Code of Conduct. 2019. Available online: https://www.globalcodeofconduct.org/ affiliated-codes/ (accessed on 8 April 2022).
- Reardon, S. Navajo. nation reconsiders ban on genetic research. Nature 2017, 550, 165–166. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.L.; Hare, D.J. Tracing Environmental exposure from neurodevelopment to neurodegeneration. Trends Neurosci. 2018, 41, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Jackrel, M.E.; Shorter, J. Engineering enhanced protein disaggregases for neurodegenerative disease. Prion 2015, 9, 90–109. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).