1. Introduction
Heart rate variability (HRV) refers to the fluctuation in the time interval between adjacent heartbeats [
1]. Cardiac autonomic function can be noninvasively assessed by calculating HRV, which reflects the interaction of the sympathetic and parasympathetic parts of the autonomic nervous system (ANS) on the sinus node. HRV is an index of neurocardiac function and is generated by heart–brain interactions and dynamic nonlinear ANS processes; an emergent property of interdependent regulatory systems that operate at different timescales to facilitate adaptation to environmental and psychological challenges; and an indicator of the regulation of autonomic balance, blood pressure (BP), gas exchange, and gut, heart, and vascular tone, which reflects the diameter of the blood vessels that regulate BP [
2].
End-stage kidney disease (ESKD) is a global public health burden associated with high morbidity and mortality rates, and cardiovascular (CV) disease is a major cause of mortality in patients undergoing hemodialysis (HD) [
3]. In such patients, CV autonomic neuropathy and the related risk of arrhythmia may partially explain the observed high rate of CV mortality, in addition to traditional risk factors such as hypertension, diabetes, and dyslipidemia [
4,
5]. CV autonomic neuropathy can be evaluated using HRV, a measure of variations in heart rate [
5]. In practice, HRV is defined as variations of both instantaneous heart rate and R-R intervals on the electrocardiogram, and may provide a simple and noninvasive approach to assess the activities of the ANS [
1]. An abnormal HRV primarily reflects the dysregulation between the sympathetic and parasympathetic nervous systems, and higher HRV values indicate greater variation between two consecutive beats, thus reflecting higher parasympathetic activity [
6]. Frequency-domain analysis of HRV has gained popularity with a broad application as a functional indicator of the ANS because it is noninvasive and easily accessible. Low HRV, which indicates impaired autonomic function, has been reported in patients undergoing HD [
7].
Furthermore, reduced HRV has been shown to be associated with adverse CV outcomes and mortality in these patients [
3,
4], and HD itself has been suggested to improve HRV [
6,
7,
8]. However, data to predict HD efficiency and explain the association between electrolyte changes before/after HD and HRV in patients with ESKD are limited. Most studies analyzed 24-h electrocardiogram (ECG) recordings, which is a time-consuming and labor-intensive approach. Our study used a remote system for HRV recording and frequency/time-domain analysis of HRV to determine whether short HRV measurements during HD predict electrolyte changes (K
+ and P
+) after HD in patients with ESKD.
2. Methods
2.1. Participants
We recruited 75 patients (mean age, 62.1 ± 10.7 years) with ESKD undergoing routine follow-up at the nephrology outpatient clinic and hemodialysis center. Participants were recruited between October 2022 and December 2022. All patients were screened for medication use and medical conditions.
The study population comprised patients with ESKD aged >18 years who underwent HD. The main exclusion criteria were pregnancy, neurological disease, heart failure, chronic liver failure, uncontrolled diabetes mellitus (DM), thyroid disorder, and treatment that could influence HRV parameters. Finally, a total of 50 patients (20 men and 30 women; mean age: 66.3 ± 7.5 years) who completed the HRV measurements and electrolyte monitoring were included in the analysis.
The study protocol was approved by the ethical guidelines of the 1975 Declaration of Helsinki, and the research protocol (IRB No. 2022-06-016) was approved by the Ethics Committee of Kosin University Gospel Hospital. Written informed consent was obtained from all the patients.
2.2. Ethical Statement
The study protocol was approved and the requirement for informed consent of individual patients was approved by the Ethics Committee of Kosin University Gospel Hospital (IRB No. 2022-06-012). Written informed consent was obtained from all patients. This study was conducted according to the principles of the latest version (2013) of Declaration of Helsinki.
2.3. Data Collection
After ECG and chest radiography, the cardiovascular status of each patient was evaluated using echocardiography and blood laboratory data from the initial visit, as determined by the attending physicians. From the database, the following information were collected: (1) patient data, including sex, age, height, and weight; (2) cardiovascular risk factors, including hypertension (use of antihypertensive agents, systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg on admission) and DM (use of oral hypoglycemic agents or insulin, or glycosylated hemoglobin level ≥ 6.5%); (3) cardiovascular disease status, including structural heart disease, congestive heart failure, or a history of a disabling cerebral infarction or transient ischemic attack; and (4) use of medication.
2.4. ECG-Monitoring Device
Hicardi® (MEZOO Co., Ltd., Wonju-si, Gangwon-do, Republic of Korea) is an 8-g, 42 × 30 × 7 mm (without disposable electrodes) wearable ECG-monitoring patch device certified as a medical device by the Ministry of Food and Drug Safety of Korea. This wearable device monitors and records single-lead ECGs, respiration, skin-surface temperature, and activity. The ECG signal was recorded at a 250-Hz sampling frequency and 14-bit resolution. Data from the wearable patch were transferred through Bluetooth Low Energy to a mobile gateway, which was implemented as a smartphone application. The mobile gateway transmitted the data to a cloud-based monitoring server. After obtaining informed consent from the patient, a wearable patch was attached to the left sternal border. The ECG signals and the above-mentioned data were continuously recorded, and all ECG signals were reviewed by a cardiologist using a cloud-based monitoring server.
2.5. HRV Parameters
HRV analysis was performed in the time and frequency domains of wearable ECG recordings according to international guidelines [
8]. On average, 225.7 ± 107.3 h of ECG data were recorded per patient, and the HRV analysis was performed by excerpting the five-minute segment before the time of glucose measurement. To calculate HRV parameters, R-R intervals must be computed from wearable ECG recordings. The following steps were performed to obtain the R-R interval time series. First, the R peaks were detected using the geometric angle between two consecutive samples of the ECG signal [
9]. The detected R peaks were then used to generate an R-R interval time series. To remove abnormal intervals caused by ectopic beats, arrhythmic events, missing data, and noise, the intervals <80% or >120% of the average of the last six intervals were excluded. Time-domain parameters were calculated from the R-R interval time series. Second, the R-R interval time series was resampled at 4 Hz using linear interpolation. The resulting series was detrended by eliminating linear trends [
10]. After detrending, the power spectral density for the R-R interval time series was estimated using the Burg autoregressive model, where the order of the model was 33. In the time domain, we analyzed the R-R intervals, standard deviations of the R-R intervals, square root of the mean squared difference of successive R-R intervals, and the percentage of adjacent N-N intervals that differed by more than 50 ms (NN50). In the frequency-domain analyses, we analyzed low frequency (LF, 0.04–0.15 Hz), which was an index of both sympathetic and parasympathetic activity, and high frequency (HF, 0.15–0.4 Hz), which represented the most efferent vagal (parasympathetic) activity to the sinus node. Very low frequency (VLF; 0.003–0.04 Hz) partially reflects thermoregulatory mechanisms, fluctuations in the activity of the renin–angiotensin system, and the function of peripheral chemoreceptors. The LF/HF ratio, which reflects sympathovagal balance, was also calculated.
2.6. Assessment of Electrolytes
Electrolyte measurements were performed before and after HD in all patients. In patients who required assessments of electrolyte levels and other blood sample parameters, including complete blood count and liver function tests, we performed the measurements several times individually.
2.7. Statistical Analysis
All continuous variables are expressed as mean ± standard deviation or median (25th and 75th interquartile range), depending on the distribution. For continuous data, the statistical significance of the differences was evaluated using Student’s t-test or the Mann–Whitney U test, depending on the data distribution. Categorical variables were presented as frequencies (percentages) and were analyzed using the chi-squared test. To determine whether any of the variables were independently related to HRV based on the glucose levels, a multivariate analysis of variables with p-values < 0.05 in the univariate analysis was performed using linear logistic regression analysis. All correlations were calculated using Spearman’s rank correlation test. All statistical analyses were conducted using SPSS statistical software (version 19.0; SPSS Inc., Chicago, IL, USA), and statistical significance was set at p < 0.05 (two-sided).
3. Results
A total of 50 patients (age, 66.3 ± 7.5 yr) with ESKD underwent continuous real-time ECG monitoring (225.7 ± 107.3 h) for HRV using a remote-monitoring system during HD. We compared HRV in relation to the electrolyte profile and changes after HD. HRV, ambulatory heart rate, and respiratory rate were measured every 15 min in all patients during real-time ECG monitoring.
During monitoring, we simultaneously analyzed 2374 ECG data points for HRV, ambulatory heart rate, and respiration rate for all patients. The baseline characteristics and echocardiographic parameters of all patients with ESKD are shown in
Table 1.
Both time- and frequency-domain HRVs, except for nHF, were higher in patients with ESKD with lower K
+ level changes than in those with higher K
+ level changes after HD (
Table 2). As shown in
Table 3, both time- and frequency-domain HRVs were higher in patients with lower P
+ level changes than in those with higher P
+ level changes after HD.
In addition, the mean heart rates and incidence of arrhythmic events during HD were higher in patients with lower K
+ and P
+ level changes than in those with higher K
+ and P
+ level changes after HD (
Table 2 and
Table 3). As shown in
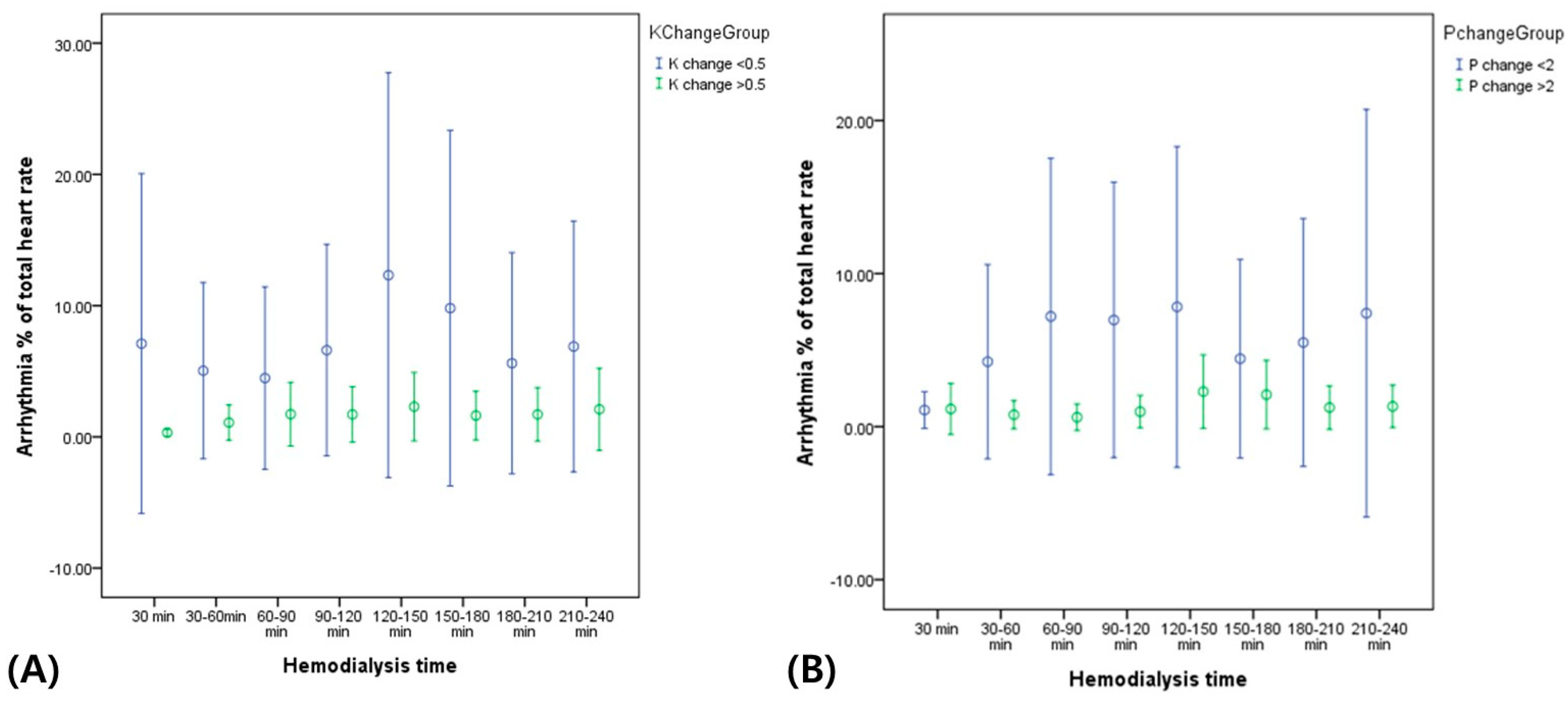
Figure 2, the total arrhythmic burden (percentage, %) of the total heart rate was constantly higher during HD in patients with lower K
+ and P
+ level changes than in those with higher K
+ and P
+ level changes after HD.
Figure 1.
HiCardi device for HRV measurement.
Figure 1.
HiCardi device for HRV measurement.
Univariate analysis revealed that SDNN, min NN, nHF, and QTc changes were associated with K
+ changes after HD. In multivariate analysis, min NN and normalized HF were independent predictors of K
+ changes after HD in patients with ESKD (
Table 4(A)). Similarly, in the univariate analysis, SDNN, min NN, nLF, nHF, and the LF/HF ratio were associated with P
+ changes after HD. In the multivariate analysis, SDNN and min NN were independent predictors of P
+ changes after HD in patients with ESKD (
Table 4(B)).
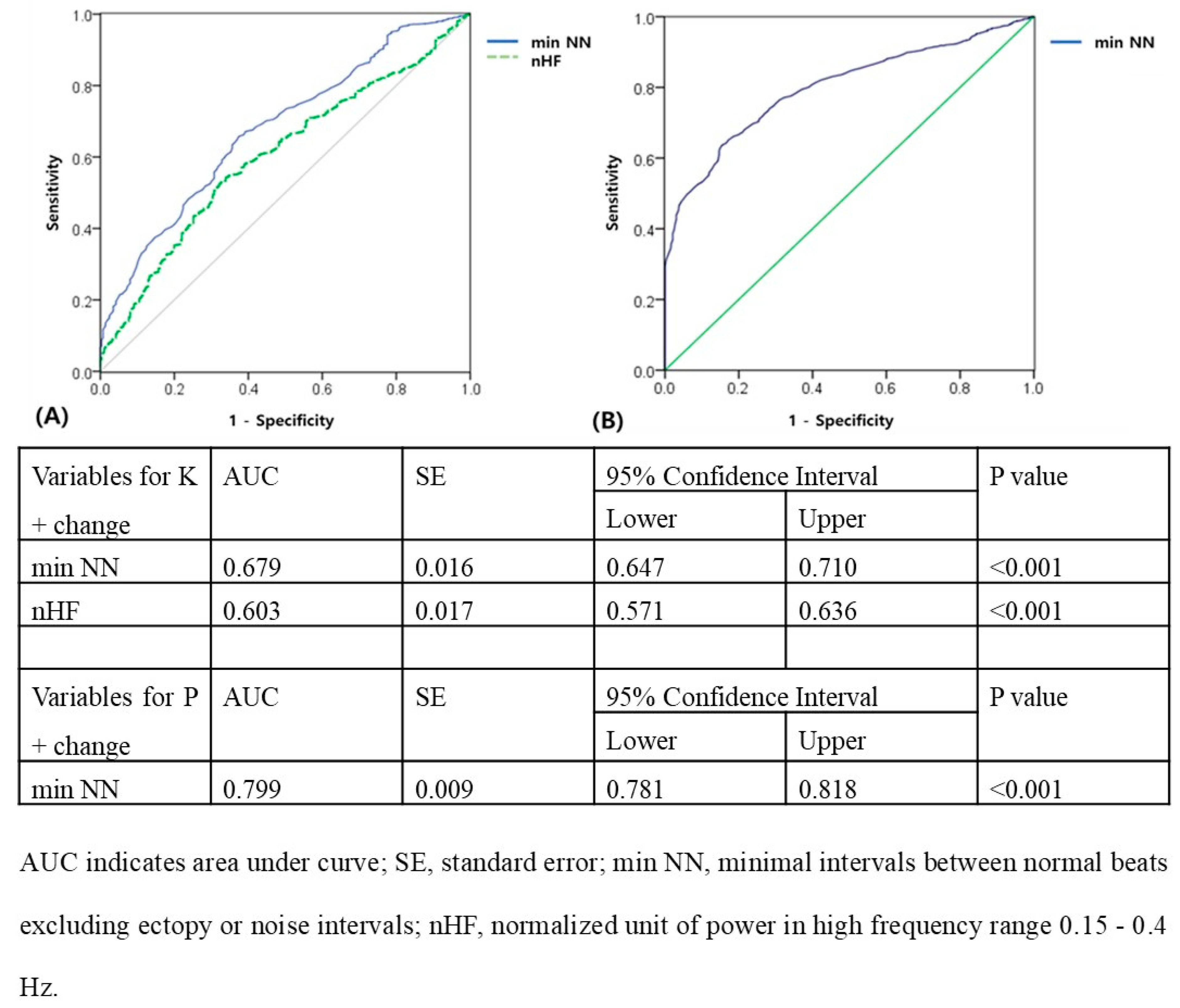
The ROC curve in
Figure 3A showed that a min NN of ≥474 ms and normalized HF of ≥0.0732 N.U. predicted effective HD (K
+ change ≥ 0.5 after HD; for min NN: AUC = 0.679; 95% confidence interval = 0.647–0.710,
p < 0.001; for normalized HF: AUC = 0.603; 95% confidence interval = 0.571–0.636,
p < 0.001). The ROC curve in
Figure 3B showed that a min NN of ≥ 598 ms predicted effective HD (P
+ change ≥ 2 after HD; for min NN: AUC = 0.799, 95% confidence interval = 0.781–0.818,
p < 0.001).
4. Discussion
In this study, we simultaneously evaluated heart rate and HRV during HD in relation to K+ and P+ changes after HD in patients with ESKD. The results demonstrate that poorly controlled K+ and P+ levels during HD are independently associated with higher HRV in patients with ESKD. This was further substantiated by independent continuous associations between real-time measurements of K+ and P+ levels and higher HRV. These associations may be independent of HD efficiency. Therefore, our results support the concept that cardiac autonomic dysfunction in real time before assessment of electrolytes routinely occurs when poorly controlled K+ and P+ levels after HD are measured, and may play a role in predicting lower HD efficiency earlier in the course of ESKD.
HRV is a noninvasive measure of the ANS that reflects beat-to-beat variabilities in heart rate and has been successfully applied in chronic dialysis patients [
5]. A previous study used a standard 10-s ECG recording to analyze the time domain of HRV, and suggested that the root mean square of successive differences between R-R intervals independently predicts mortality in patients with ESKD [
11]. In comparison with HRV measurements obtained by 24-h Holter ECG, a short measurement of the resting HRV consistently predicts the long-term outcome (>10 years) in the ESKD population [
12]. Giordano et al. studied HRV changes during dialysis sessions in patients with ESKD. In their study, healthy controls had lower resting LF/HF ratios than did patients with ESKD. During dialysis therapy, patients with ESKD showed increasing LF/HF, which indicated an increase in sympathetic activity during ultrafiltration [
13], and previous studies reported that HRV measurement, which reflects various aspects of ANS activities, is a simple and useful tool to predict long-term mortality among patients undergoing HD [
14,
15]. However, data to predict HD efficiency and electrolyte changes after HD are limited. In our study, we compared HRV in relation to electrolyte changes (K
+ and P
+) after HD. ANS dysfunction has been reported to occur in more than 50% of patients with chronic HD [
16]. Damage to the autonomic innervations of the heart and blood vessels can lead to lethal arrhythmias and sudden cardiac death [
17], and ischemia can induce atrophy of the autonomic nerve fibers innervating the cardiac and vascular tissues [
18]. Both divisions of the ANS are typically affected, with parasympathetic impairment preceding sympathetic dysfunction [
6]. Loss of HRV is one of the earliest manifestations of this process. In the Framingham Heart Study, HRV was found to be inversely associated with the risk of mortality [
19]. Similarly, the Atherosclerosis Risk in Communities study found that decreased HRV was independently associated with the risk of developing coronary heart disease [
20], and that lower HRV was also associated with the total burden of cerebral small vessel disease (CSVD) and each of the magnetic resonance imaging markers of CSVD in patients with DM [
21]. Adaptation to stress is characterized by an increase in sympathetic activity and a decrease in parasympathetic activity, inducing a state of alertness [
22]. Interestingly, common diseases such as depression, metabolic syndrome, and cancer; smoking habits; and obesity are associated with decreased parasympathetic activity and activation of sympathetic activity [
7,
23].
Arrhythmias during HD are common, and dialysis-related ECG artifacts mimicking arrhythmias have been reported previously [
24]. Studies using advanced ECG analyses have examined the impact of HD on selected repolarization descriptors and HRV indices. Despite the challenges related to the impact of fluctuations in fluid and electrolyte status on conventional and advanced ECG parameters, further research on ECG monitoring during dialysis has the potential to provide clinically meaningful and practically useful information for diagnostic and risk-stratification purposes. In our study, patients with poorly controlled K
+ and P
+ levels during HD showed a higher incidence of arrhythmic events, including atrial/ventricular premature complexes, although their mean heart rate did not differ from those with well-controlled electrolyte levels after HD (
Figure 2).
Although no study has previously assessed this relationship in patients with ESKD based on electrolyte changes after HD, conflicting results have been reported in the general population, with high BP associated with an increase in all spectral parameters or a decrease in HRV [
25,
26]. A decrease in autonomic nervous function has also been suggested to precede the development of clinical hypertension [
27]. However, our study showed no significant difference in HRV in relation to hypertension and BP.
To the best of our knowledge, this is the first study to simultaneously investigate the HRV and electrolyte changes (K
+ and P
+) in patients with ESKD after HD by using a remote monitoring system. Importantly, in contrast to previous population-based studies [
28,
29], our study showed that virtually all time- and frequency-domain measures of HRV, either as composite scores or individual measurements, were associated with worsening electrolyte levels after HD. This may be explained by the fact that we used a more accurate real-time remote-monitoring ECG-derived HRV as opposed to the HRV derived from short-term ECG recordings. In addition, we adjusted for a large number of potential confounders, including real-time respiration and physical activity, which were objectively measured in a live studio at our institute using a remote-monitoring system.
Limitations
Our study had some limitations that require consideration. First, the relatively small sample size was a limiting factor in generalizing the findings to patients with ESKD undergoing HD. However, it was sufficient to identify significant correlations between HRV and electrolyte changes in individuals with ESKD using a remote HRV system. Despite the small number of patients, our analysis demonstrated significant and interesting relationships, particularly between the HRV parameters and electrolyte changes (K+ and P+) associated with HD efficiency. Thus, the results of our study should be considered hypothesis-generating, and future prospective studies are warranted to confirm these results. Second, the electrolyte indices were only measured twice, i.e., before and after each HD session. Third, sympathetic tone was not estimated via direct methods, such as muscle sympathetic nerve activity or plasma catecholamine levels, which may have helped verify the activities of the sympathetic nervous system. However, these direct methods are invasive and less clinically useful, and their predictive values have not yet been established.
5. Conclusions
Higher HRV was independently associated with poor controlled K+ and P+ level during hemodialysis in patients with ESKD. This is further substantiated by independent continuous associations between real-time measures of higher HRV and K+ and P+ level. These data strongly suggest that cardiac autonomic dysfunction can be caused by lower change of electrolytes before/after hemodialysis alone.
Author Contributions
S.I.I. and H.S.S. are the lead investigators who conceived the research idea and methodology. They are the guarantors for the overall content. Funding acquisition was done by H.S.S., H.S.K., S.J.K., S.H.B., B.J.K., J.H.H., Y.N.K., Y.J., H.R., S.P.C. and J.H.P. conducted data acquisition. S.I.I., S.P.C. and H.S.S. performed the analyses and wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported technically by MEZOO.
Institutional Review Board Statement
The study protocol was approved and the requirement for informed consent of in-dividual patients was approved by the Ethics Committee of Kosin University Gospel Hospital (IRB No. 2022-06-012).
Informed Consent Statement
Written informed consent was obtained from all patients. This study was conducted according to the principles of the latest version (2013) of Declaration of Helsinki.
Data Availability Statement
Data availability statements are available after contact with the corresponding author; 67920@naver.com
Acknowledgments
We thank all members of division of Cardiology, nephrology and MEZOO for providing data and technical support, and providing follow-up information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Shah, A.S.; El Ghormli, L.; Vajravelu, M.E.; Bacha, F.; Farrell, R.M.; Gidding, S.S.; Katz, L.E.L.; Tryggestad, J.B.; White, N.H.; Urbina, E.M. Heart Rate Variability and Cardiac Autonomic Dysfunction: Prevalence, Risk Factors, and Relationship to Arterial Stiffness in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care 2019, 42, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, T.; Nakai, S.; Akiba, T.; Yamagami, S.; Yamazaki, C.; Kitaoka, T.; Kubo, K.; Maeda, K.; Morii, H. Report on the annual statistical survey of the Japanese Society for Dialysis Therapy in 1996. Kidney Int. 1999, 55, 700–712. [Google Scholar] [CrossRef]
- Oikawa, K.; Ishihara, R.; Maeda, T.; Yamaguchi, K.; Koike, A.; Kawaguchi, H.; Tabata, Y.; Murotani, N.; Itoh, H. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int. J. Cardiol. 2009, 131, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Huang, J.-C.; Tsai, Y.-C.; Mai, R.N.H.-C.; Chen, R.N.J.-H.; Kuo, P.-L.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Heart Rate Variability Change Before and After Hemodialysis is Associated with Overall and Cardiovascular Mortality in Hemodialysis. Sci. Rep. 2016, 6, 20597. [Google Scholar] [CrossRef] [PubMed]
- Sudo, S.Z.; Montagnoli, T.L.; Rocha, B.d.S.; Santos, A.D.; de Sá, M.P.L.; Zapata-Sudo, G. Diabetes-Induced Cardiac Autonomic Neuropathy: Impact on Heart Function and Prognosis. Biomedicines 2022, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta–analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Song, M.-H.; Cho, S.-P.; Kim, W.; Lee, K.-J. New real-time heartbeat detection method using the angle of a single-lead electrocardiogram. Comput. Biol. Med. 2015, 59, 73–79. [Google Scholar] [CrossRef]
- Tarvainen, M.; Ranta-Aho, P.; Karjalainen, P. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef]
- Drawz, P.E.; Babineau, D.C.; Brecklin, C.; He, J.; Kallem, R.R.; Soliman, E.Z.; Xie, D.; Appleby, D.; Anderson, A.H.; Rahman, M.; et al. Heart Rate Variability Is a Predictor of Mortality in Chronic Kidney Disease: A Report from the CRIC Study. Am. J. Nephrol. 2013, 38, 517–528. [Google Scholar] [CrossRef]
- Kuo, G.; Chen, S.-W.; Huang, J.-Y.; Wu, C.-Y.; Fu, C.-M.; Chang, C.-H.; Liu, S.-H.; Chan, Y.-H.; Wu, I.-W.; Yang, H.-Y. Short-term heart rate variability as a predictor of long-term survival in patients with chronic hemodialysis: A prospective cohort study. J. Formos. Med. Assoc. 2018, 117, 1058–1064. [Google Scholar] [CrossRef]
- Giordano, M.; Manzella, D.; Paolisso, G.; Caliendo, A.; Varricchio, M.; Giordano, C. Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol. Dial. Transplant. 2001, 16, 566–573. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Huang, Y.-T.; Chen, I.-L.; Yang, C.-L.; Leu, S.-C.; Su, H.-L.; Kao, J.-L.; Tsai, S.-C.; Jhen, R.-N.; Shiao, C.-C. Heart rate variability as an independent predictor for 8-year mortality among chronic hemodialysis patients. Sci. Rep. 2020, 10, 881. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Chang, Y.-M.; Chen, I.-L.; Yang, C.-L.; Leu, S.-C.; Su, H.-L.; Kao, J.-L.; Tsai, S.-C.; Jhen, R.-N.; Tang, W.-R.; et al. Correction: Heart rate variability during hemodialysis is an indicator for long-term vascular access survival in uremic patients. PLoS ONE 2017, 12, e0181283. [Google Scholar] [CrossRef]
- Ewing, D.; Winney, R. Autonomic Function in Patients with Chronic Renal Failure on Intermittent Haemodialysis. Nephron 1975, 15, 424–429. [Google Scholar] [CrossRef]
- Ewing, D.J.; Boland, O.; Neilson, J.M.M.; Cho, C.G.; Clarke, B.F. Autonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetic patients. Diabetologia 1991, 34, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Loiacono, G.; Mohn, A.; Chiarelli, F. New insights in diabetic autonomic neuropathy in children and adolescents. Eur. J. Endocrinol. 2009, 161, 811–818. [Google Scholar] [CrossRef]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events: The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef]
- Liao, D.; Carnethon, M.; Evans, G.W.; Cascio, W.E.; Heiss, G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: The atherosclerosis risk in communities (ARIC) study. Diabetes 2002, 51, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Song, W.; Zhou, X.; Yu, Z.; Wang, M.; Hao, H.; Pan, D.; Luo, X. Heart rate variability is associated with cerebral small vessel disease in patients with diabetes. Front. Neurol. 2022, 13, 989064. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, C.; Chambres, P.; Bertrand, P.R.; Dutheil, F. The Need for Objective Measures of Stress in Autism. Front. Psychol. 2017, 8, 64. [Google Scholar] [CrossRef]
- Lin, K.-D.; Chang, L.-H.; Wu, Y.-R.; Hsu, W.-H.; Kuo, C.-H.; Tsai, J.-R.; Yu, M.-L.; Su, W.-S.; Lin, I.-M. Association of depression and parasympathetic activation with glycemic control in type 2 diabetes mellitus. J. Diabetes Complicat. 2022, 36, 108264. [Google Scholar] [CrossRef]
- Poulikakos, D.; Malik, M. Challenges of ECG monitoring and ECG interpretation in dialysis units. J. Electrocardiol. 2016, 49, 855–859. [Google Scholar] [CrossRef]
- Askin, L.; Cetin, M.; Turkmen, S. Ambulatory blood pressure results and heart rate variability in patients with premature ventricular contractions. Clin. Exp. Hypertens. 2017, 40, 251–256. [Google Scholar] [CrossRef]
- de Andrade, P.E.; Amaral, J.A.T.D.; da Silva Paiva, L.; Adami, F.; Raimudo, J.Z.; Valenti, V.E.; de Abreu, L.C. Reduction of heart rate variability in hypertensive elderly. Blood Press. 2017, 26, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.B.; Liao, D.; Chambless, L.E.; Prineas, R.J.; Evans, G.W.; Heiss, G. Hypertension, blood pressure, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Hypertension 2003, 42, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Voss, A.; Rathmann, W.; Strom, A.; Perz, S.; Roden, M.; Peters, A.; Meisinger, C. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: The KORA S4 survey. Diabetologia 2015, 58, 1118–1128. [Google Scholar] [CrossRef]
- Meyer, M.L.; Gotman, N.M.; Soliman, E.Z.; Whitsel, E.A.; Arens, R.; Cai, J.; Daviglus, M.L.; Denes, P.; González, H.M.; Moreiras, J.; et al. Association of glucose homeostasis measures with heart rate variability among Hispanic/Latino adults without diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Cardiovasc. Diabetol. 2016, 15, 45. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).