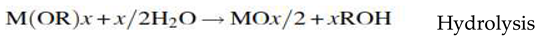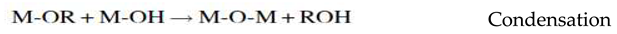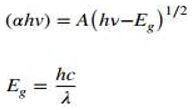1. INTRODUCTION
1.1. Background of the Review
Nanocomposites are a combination or matrix, in which different materials combine to develop new properties of the materials ensuring that one of the materials have size in range of 1-100nm [
1]. Nanocomposites (NC) materials have gained much attention and interest of scientists in recent years because of their improved properties than the single metal nanoparticles. There are hence two parts on NC i.e. continuous phase and discontinuous reinforcing phase. The nanocomposites hence can have a combination or have markedly different mechanical, electrochemical, electrical, catalytic, thermal and optical properties from the component materials. The nanocomposites have different phases as zero-dimensional (core shell), one-dimensional (nanowires and nanotubes), two- dimensional (lamellar) and three-dimensional (metal matrix composites). Nanocomposites materials are divided into three groups by their matrix materials. These are Ceramic matrix nanocomposites (CMNC), Polymer matrix nanocomposites (PMNC), and Metal matrix nanocomposites (MMNC) [
2]. These materials have promising properties, which makes them suitable for large number of structural and functional applications in other fields. The main advantages of nanocomposites over other composite materials are: high surface/volume ratio allows small filler size and distance between fillers; better mechanical properties- high ductility without strength loss, scratch resistance; Improved optical properties (light transmission depends on particle size).
Nanocomposites can be prepared from any combination of materials that can be categorized into three basic building blocks i.e. metals, ceramics and polymers. The synthetic methods of nanocomposites are frequently classified in to two classes i.e. top-down approach which includes physical methods and bottom up approach which encompasses wet methods. Bottom-up or constructive method is the build-up of material from atom to clusters to nanoparticles. Sol-gel, spinning, chemical vapour deposition (CVD), pyrolysis and biosynthesis are the most commonly used bottom-up methods for nanoparticle production. In the top-down approach, nanoparticles are synthesized by size reduction, disintegrating from the bulk material into fine particles [
3]. Mechanical milling, nanolithography, laser ablation, sputtering and thermal decomposition are some of the most widely used nanoparticle synthesis methods.
The synthesized nanocomposites are characterized using different techniques to get insight into the morphology, particle size, phase, composition, thermal stability, optical, magnetic, electrical and thermal properties. Some of the common techniques are ultra violet visible light spectroscopy (UV-Vis), Fourier transform infra-red spectroscopy (FTIR), X-ray diffraction analysis (X-RD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and thermal analysis (TA).
Recently, a lot of attention has been paid to nanocomposites than nanoparticles. This is why that nanocomposites show improved of properties and efficiencies than single nanoparticles. Nanocomposites avoid some limitations of nanoparticles such as high band gap energy, recombination of electron-hole pairs in photocatalytic activity to increase the reaction kinetics and eventually enhancement of system efficiency [
4,
5]. Even the applications of nanocomposites in different sectors such as environment, agriculture/ food and health/ medicine had been widely recognized [
1,
2]. Thus, this review focused on the synthesis methods, characterization methods and applications of nanocomposites.
2. NANOCOMPOSITES
Nanocomposites are composite materials in which one of the phases has a nanometer-scale dimension. Due to their superior qualities, nanocomposites are a viable alternative to microcomposites and monolithic materials. Scientists and engineers have been drawn to the field of nanocomposite materials in recent years. Nanocomposite materials now cover a wide range of systems, including one-dimensional, two-dimensional, three-dimensional (3D), and amorphous materials made of clearly diverse components and blended at the nanometre scale. At least one dimension of a constituent is on the nanometre scale. Nanocomposites’ constituents have a variety of geometries and compositions, therefore the materials made from them can be multifunctional. The idea of creating multiphase nanocomposites to improve material qualities and features is a novel one [
6].
Nanocomposites are made up of two or more distinct ingredients or phases separated by a discrete interface and with diverse physical and chemical properties. The matrix is the ingredient that is generally present in greater quantities. Reinforcement is an ingredient that is inserted into the matrix material in order to improve the mechanical properties of nanocomposites (or nanomaterials). Nanoscale filler materials are commonly used as reinforcement [
7].
Nanocomposites are a great alternative to traditional composite materials because of their unique features, and they have a wide range of applications in a variety of sectors. Since its discovery in 1991, carbon nanotubes-based nanocomposite systems have been the subject of contemporary research and development, with a steady and continual increase in the number of publications on the subject, including reviews and patents from time to time. In comparison to traditional composites, the area of the interface between the matrix and nano-reinforcement is often an order of magnitude larger. Clays are a kind of nanofiller materials that have been widely employed to make polymer matrix nanocomposites. Polymer/clay nanocomposites have recently received a lot of attention in academia and industry due to their increased properties [
1,
7].
2.1. Classification of Nanocomposites
Lateef, A., & Nazir, R [
2] classified nanocomposites according to the types of reinforcement materials and matrix materials used in their construction. In addition, Parameswaranpillai, J [
8] divides nanocomposites into three categories based on the type of matrix material used. Thus nanocomposites are generally classified into following three classes.
1. Polymer Matrix Nanocomposites (PMNC)
2. Ceramic Matrix Nanocomposites (CMNC)
3. Metal Matrix Nanocomposites (MMNC)
Table 1.
clssification of three types of Nanocomposite.
Table 1.
clssification of three types of Nanocomposite.
| PMNC |
CMNC |
MMNC |
| Thermoplastic/thermoset |
Al2O3/ SiO2
|
CdO/ZnO |
| Polymer/layered silicates |
SiO2/Ni |
NiO–ZnO–Ag |
| Polyester/TiO2
|
Al2O3/TiO2
|
ZnO/CuO/TiO2
|
| Polymer/CNT |
Al2O3/SiC |
NiO/CuO/ZnO |
| Polymer/layered double hydroxides |
Al2O3/CNT |
ZnO/Eu2O3/NiO |
2.1.1. Polymer Matrix Nanocomposites
Polymer nanocomposites are materials that have polymer as a matrix material and nanoadditives are as reinforcement material. The additives can be one dimensional (nanotubes and fibers), two-dimensional (layered materials like clay) or three-dimensional (spherical particles). Due to interaction between polymer matrix and nanofiler on molecular level effect of attraction between nanocomposites is observed [
7]. Consequently, addition of small amount of nanofiller with dimensions below 100 nm to matrix induces change on composite material properties. Of all nanomaterials, polymer nanocomposites (PNCs) are the most well-known and have been broadly investigated for a wide range of applications. Different from other materials such as metals and ceramics, polymers feature low manufacturing costs and a high specific strength, which means less energy needed for production and recycling. Nanocomposites exhibit a superior performance compared to their peer composites, and this creates opportunities for a number of industrial applications for PNCs [
6]. Polymers have outstanding properties such as lightweight, high durability, easy processing, corrosion resistance, ductility and low cost. Compared to ceramics and metals, polymers have relatively poor mechanical, thermal and electrical properties.
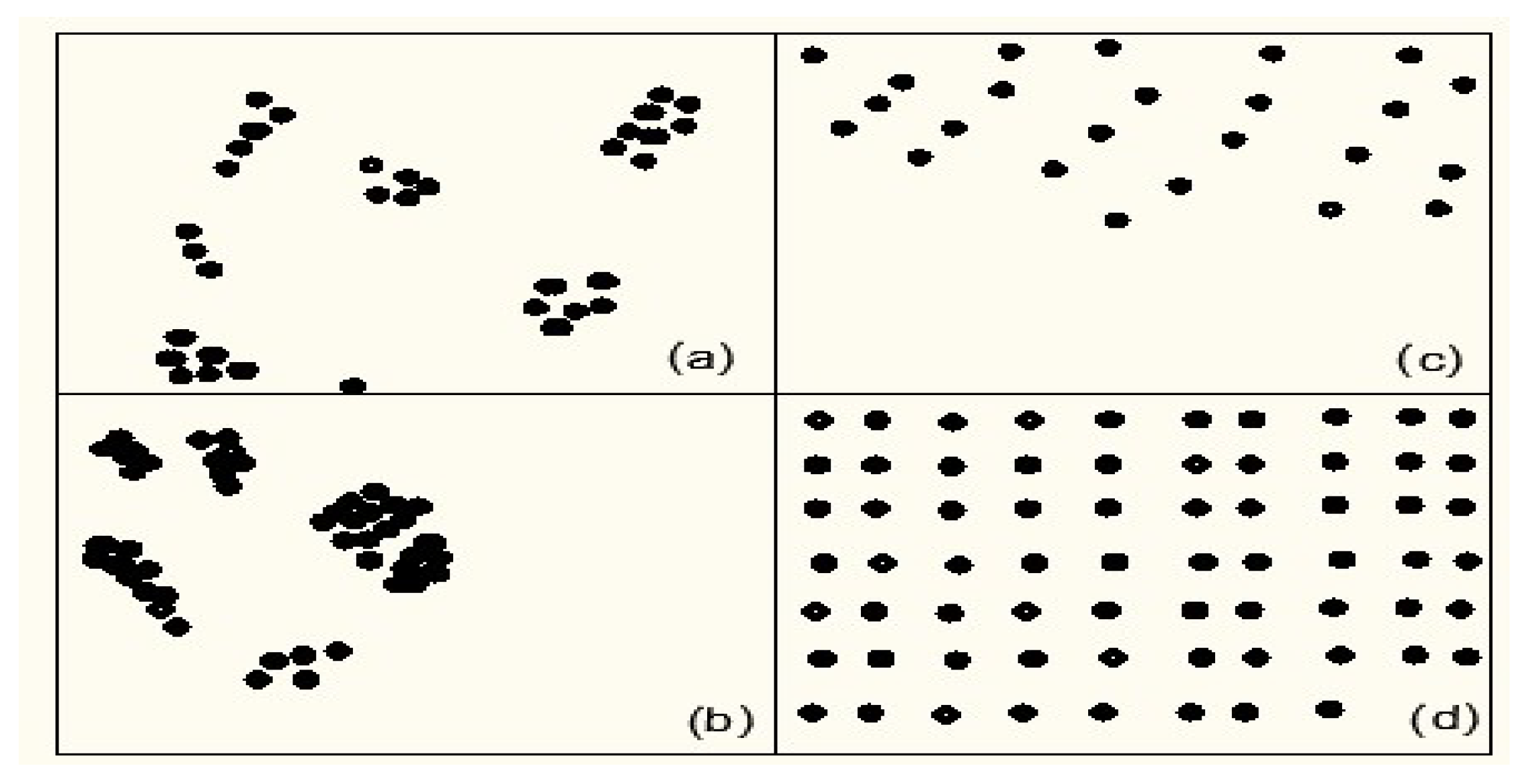
Nanosized particles must be appropriately dispersed and distributed in the matrix material to achieve enhanced attributes of nanocomposites; otherwise, particles would agglomerate and the properties of nanocomposites will deteriorate. These aggregates operate as defects, limiting the nanocomposite’s property enhancement. To obtain optimum property enhancement, the nanoparticles should be uniformly scattered throughout the matrix.
Figure 1 depicts various types of nanoparticles in the matrix material, with (a) good distribution but poor dispersion, (b) Poor distribution with poor dispersion, (c) a poor distribution with good dispersion, and (d) a good distribution with good dispersion. Polymer is the most important component in polymer matrix nanocomposites. Polymers of various types are employed in the production of polymer matrix nanocomposites. Polymers of various types are employed in the creation of polymer matrix nanocomposites. Thermoplastics, thermosets, elastomers, and biodegradable polymers are among these polymers [
8].
2.1.2. Ceramic Matrix Nanocomposites
The pioneering work of researchers Nihara and Gurnani [
9,
10] demonstrated the promise of ceramic matrix nanocomposites, particularly the aluminum oxide/silicon carbide (SiC) combination. The majority of investigations to far have confirmed that adding a low (i.e. 10%) volume fraction of silicon carbide particles of a suitable size to the aluminium oxide matrix and hot-pressing the resulting mixture strengthens the matrix significantly. The crack-bridging action of nanoscale reinforcements has been used in certain research to explain this toughening mechanism. As a result of incorporating high-strength nanofibres into ceramic matrices, improved nanocomposites with high toughness and superior failure characteristics have been developed in comparison to sudden ceramic material failures [
11].
AL
2O
3/SiO
2, SiO
2/Ni, Al
2O
3/TiO
2, and Al
2O
3/SiC are some of the most prevalent ceramic matrix nanocomposites. Carbon nanotubes (CNT) have been frequently used in nanocomposites manufacturing since their discovery. Al
2O
3/CNT, MgAl
2O
4/ CNT, and MgO/CNT are some examples of CNT-based ceramic matrix nanocomposite [
9,
10].
2.1.3. Metal Matrix Nanocomposites
Materials reinforced by nanoparticles are metal matrix nanocomposites, which are made up of a ductile metal or alloy matrix into which nanoparticle reinforcement is placed. These composites, which are made up of a metal/alloy matrix filled with nanoparticles, have completely different physical, chemical, and mechanical properties than the matrix material. Wear resistance, mechanical qualities, and damping characteristics are all improved using nanoparticles. Researchers have recently been investigating metal matrix nanocomposites, which, due to their improved characteristics due to nanoparticle embedment, are finding a wide range of applications in structural components [
12].
The nanoparticles act as a barrier in dislocation movement and thereby improve the mechanical properties. The common techniques used for the processing of metal matrix nanocomposites are spray pyrolysis, liquid metal infiltration, vapor techniques, rapid solidification, electrode position and chemical methods, which include colloidal and sol-gel methods [
13]. Some common metal matrix nanocomposites include Fe-Cr/Al
2O3, Ni/Al
2O
3, Fe/MgO, Al/CNT and Mg/CNT.
3. SYNTHESIS METHODS OF NANOCOMPOSITES
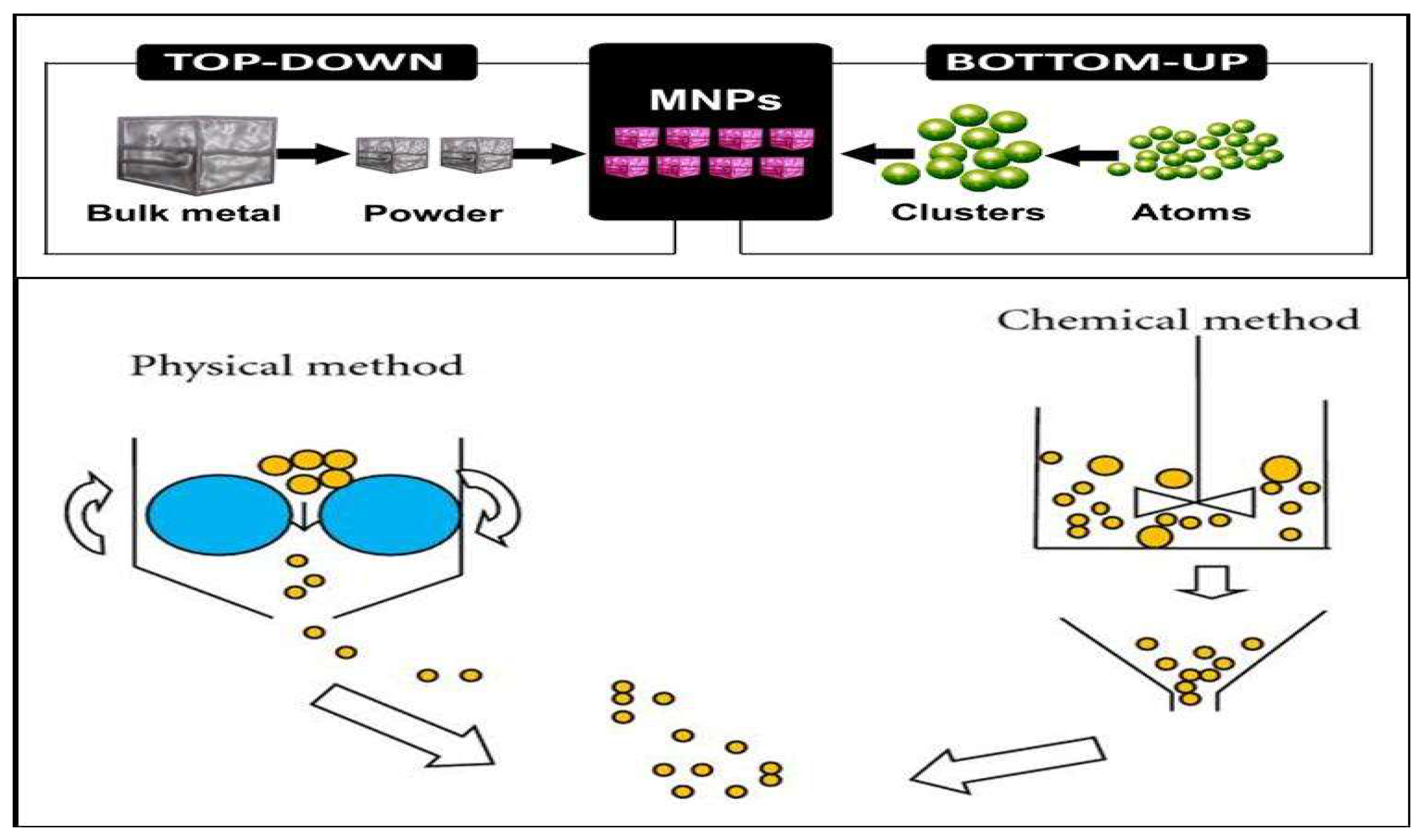
The synthesis of uniform sized nanocomposites is very important because their properties include optical, magnetic, electrical and biological properties depending on their size and dimensions. The synthetic methods are frequently classified in to three classes i.e. solution based synthesis, vapor phase synthesis and gas phase synthesis. Another approach is to divide these synthetic approaches into two broad categories (Figure 2) i.e. a) top-down approach which includes physical methods and b) bottom up approach which encompasses wet methods [
14].
The choice of synthetic approach depends on the desired characteristics required in the nanoparticles composites e.g. size, morphology, and crystal structure. But the benefit of physical methods is the production of large amount of nanocomposites but synthesis of equal sized nanocomposite particles is not easily attainable. In comparison, wet chemical methods give uniformity in size of nanocomposites as controlled particle size can be achieved easily. Although by varying conditions of reaction, different shapes (nano-rods, nanowires, nanotubes etc.) of nanocomposites can also be synthesized. There are several types of synthetic route for the preparation of different types of nanocomposites, the most important of which are discussed here [
2,
15].
Figure 2.
Schematic representations of top down and bottom up approaches.
Figure 2.
Schematic representations of top down and bottom up approaches.
3.1. SYNTHESIS METHODS OF POLYMER NANOCOMPOSITES
In literature, many processes have been described for the preparation of polymer nanocomposites including layered materials and those that contain carbon nanotubes [
14]. The common ones are 1) In situ polymerization; 2) Intercalation of polymer from solution; 3) Direct mixing of polymer and fillers; 4) Melt intercalation; 5) Template synthesis; and 6) Sol-gel process [
7].
3.1.1. Solution Blending Method
Solution blending is a simple and widely used method for nanocomposite fabrication. It involves mixing solutions of the polymer and fillers dispersed in suitable solvents. Solvents, such as water, chloroform, dimethylformamide, and toluene, are commonly used. The mixing of solutions and further removal of solvent yields the nanocomposite. The nanocomposite can be obtained by precipitation and simple filtration. Finding a suitable solvent and removing it in the last stage represent a drawback to this method. The solvents used may also be toxic [
16].
Graphene or graphene oxide nanoplatelet incorporated with polymers, such as polystyrene, polyimides, polymethyl methacrylate, and polyacrylamides, have been synthesized by blending their solutions. For instance, a recent report on their applications emphasized the novel polymer nanocomposite synthesis with polyacrylamide/graphene-based nanocomposite for use in water purification [
17].
3.1.2. Melt processing Method
Melt processing, also called melt compounding, or melt blending, is a comparatively cost effective and simple technique that removes the need for toxic solvents. It involves direct dispersion and mixing of the fillers in the melted polymer. The blending may be done by methods such as injection molding or extrusion. However, controlling the filler distribution in the matrix is difficult due to the high viscosity of the thermoplastic polymers. This process requires high temperatures to melt the polymer and mechanical or shear forces to uniformly disperse the fillers. For instance Zhang, Y. et al. [
17] was synthesized polyethylene terephthalate/montmorillonite clay nanocomposite by melt blending in a co rotating mini twin-screw extruder at 285°C. The authors studied the effect of various blending conditions on the resulting nanocomposite. Recently many reports on graphene/polymer, CNT/polymer, and clay polymer nanocomposites obtained via this method are also available [
16,
17].
3.1.3. In Situ Polymerization synthesis method
This method yields uniform distribution of the fillers in the polymer matrix and generally avoids the use of solvents. It involves preparation of a mixture of monomer and filler particles, which is then polymerized by standard polymerization techniques like mechanical methods (ultrasonication and stirring) or photoinitiation (UV curing). This technique was reported to produce nanocomposites with covalent or non-covalent bonding between the constituents. Polymers that cannot be processed by solution mixing and melt blending can be dealt with in this method. Insoluble and thermally unstable polymers can also be processed via this technique. The only drawback of this process is that high temperature synthesis causes decomposition of polymer [
7].
Bingzhen Li et al. [
18] prepared binary Fe
3O
4/PPy nanocomposite and ternary Fe
3O
4/PPy/PANI nanocomposite by this method. Pyrrole and aniline were polymerized in situ under appropriate conditions in the presence of Fe
3O
4 nanoparticles synthesized by the coprecipitation method. The nanocomposite was obtained by magnetic decantation. It was found that the PPy-PANI affinity by carbonyl groups lead to their strong interaction on the nanoparticle surface. The prepared ternary nanocomposites have shown an enhanced microwave absorbing property.
3.1.4. Sol- gel synthesis Method
Sol-gel method is a bottom up approach and it is based on an opposite principle than all the previous methods. The term sol-gel is associated to two relations steps, sol and gel. Sol is a colloidal suspension of solid nanoparticles in monomer solution and gel is the 3D interconnecting network formed between phases. In this method, solid nanoparticles are dispersed in the monomer solution, forming a colloidal suspension of solid nanoparticles (sol), they form interconnecting network between phases (gel) by polymerization reactions followed by the hydrolysis procedure. The polymer nanoparticle 3D network extends throughout the liquid. The polymer serves as a nucleating agent and promotes the growth of layered crystals. As the crystals grow, the polymer is seeped between layers and thus nanocomposite is formed. To summarize the different methods available for the synthesis of polymer nanocomposites and to compare the detailed methodologies formulated by various laboratories [
19].
3.1.5. Intercalation Method
Intercalation method generally involves the dispersion of nanoplatelets types of nanomaterials into the polymer matrix. It is well-known that incorporation of clays (nanomaterial) into polymer matrices improves the bulk properties such as stiffness, shrinkage and flammability. Intercalation is a top down approach and requires surface modification of nanoplatelets for homogeneous dispersion of plate-like nanofillers in the polymer matrix. Intercalated morphology occurs when polymer chains diffuse into the gallery spacing of layered structure. The nanoplatelets can be homogeneously dispersed by the following two techniques such as melt intercalation and melt blending [
7].
Melt intercalation is a promising method extensively used in the industry. This method involves mixing the nanofillers (clays) into the polymer matrix at molten temperature. In this method, mixture of polymer and nanofibers are annealed either statically or under shear. This method is compatible with current industrial processes, such as extrusion and injection molding and it allows the use of polymers, which are not suitable for in situ polymerization or solution intercalation. Melt blending is a similar process. It involves the melting of polymer powder or pellets to form a viscous solution and nanofillers are added into polymer solution by high shear rate combined with high temperature diffusion. The final shape of components can be fabricated by compression molding, injection molding or fiber production technique [
7].
Table 2.
An Elaborate Comparison of Different Polymer Nanocomposite Synthesis Methods.
Table 2.
An Elaborate Comparison of Different Polymer Nanocomposite Synthesis Methods.
| Method |
System |
Procedures |
Ref. |
| Microwave-assisted method |
Cellulose-silver nanocomposites |
Ethylene glycol is used as a reducing agent for the microwave absorbed silver particles and these particles are dispersed over the cellulose substrate homogeneously. |
[20] |
| In situ polymerization |
Clay nanocomposites
(N-methyl-polyamide 12)/organo-MMT nanocomposites. |
Intercalated MMT with lactam monomers and ADA were blended at 260°C by melting. |
[18] |
Co-precipitation
Method |
PEDOT/MWCNTs hybrid
Nanocomposite |
Poly(3,4-ethylenedioxythiophene) hollow spheres (b-PEDOT) were grafted on MWCNTs and to wrap MnO2 nanograins on the b-PEDOT. As a result, MnO2/b-PEDOT/MWCNTs hybrid nanocomposite was synthesized. |
[17] |
| Melt intercalation process |
Poly(propylene-g-maleic
anhydride) (PPMA)/expanded
graphite oxide (EGO)
nanocomposites |
poly(propylene maleic anhydride) (PPMA)/graphite oxide (EGO) was heated and mixed for 30min at 200°C. |
[17] |
Table 3.
Advantages and limitations of polymer-based nanocomposite processing methods.
Table 3.
Advantages and limitations of polymer-based nanocomposite processing methods.
| Process |
Advantages |
Limitations |
Ref |
In-situ Intercalative
Polymerization |
Easy procedure, based on the dispersion of the filler in the polymer precursors. |
Difficult control of intragallery polymerization. Limited applications. |
[18] |
| Melt Intercalation |
Environmentally benign; use of polymers not suited for other processes; compatible with industrial polymer processes. |
Limited applications to polyolefins, who represent the majority of used polymers. |
[7] |
| Sol-Gel Process |
Simple, low processing temperature; versatile; high chemical homogeneity; rigorous stoichiometry control; high purity products; formation of three dimensional polymers containing metal-oxygen bonds. Single or multiple matrices. Applicable specifically for the production of composite materials with liquids or with viscous fluids that are derived from alkoxides. |
Greater shrinkage and lower amount of voids, compared to the mixing method. |
[19] |
3.2. SYNTHESIS METHODS OF CERAMIC NANOCOMPOSITES
A key point to achieve excellent mechanical properties in nanocomposite ceramics is a proper design and fabrication process to yield tailored sintered nanostructures. When designing a composite structure, besides considering the chemical composition of matrix and second-phase, the amount, distribution, morphology of the reinforcement phase should be properly evaluated. A key point is achieving a homogenous distribution of the second-phase in the matrix material, but this is still challenging when nanocrystalline particles are used, due to their extremely high specific surface and, hence, intrinsically high tendency to agglomeration. This means that all manufacturing steps towards the elaboration of nanocomposite ceramics should be carried out with particular care, from the synthesis of the composite powders, to the forming of green bodies and their sintering [
21].
The most investigated structural ceramic nanocomposites are Al
2O
3/SiC and Si
3N
4/SiC. However, many other different phases such as TiN, TiC, TiO
2, ZrO
2, Cr
3C
2, YAG, can be used as nano-reinforcements in Al
2O
3, Si
3N
4, MgO, mullite (SiAlON) ceramic matrix. In the following, the mainly used synthesis routes for the above composite systems are reviewed. For sake of clarity,
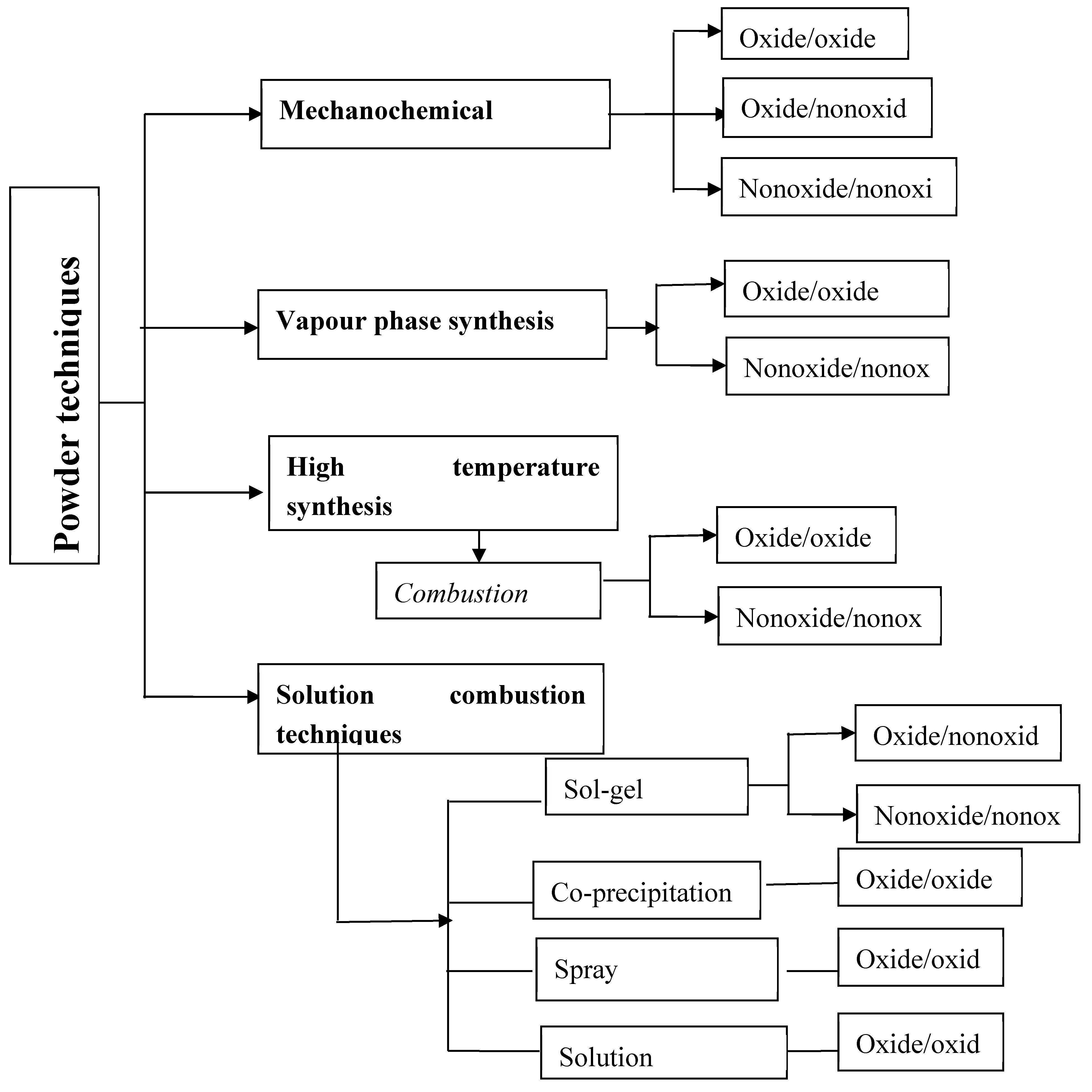
Table 4 summarizes direct composite powder synthesis methods and provides some examples of compositions already developed. Some of the techniques suitable for the preparation of different types of ceramic nanocomposites by powder process are shown in the following
Figure 3 [
18].
3.2.1. Vapor Phase Reaction Technique
This technique involves evaporation of a solid to form a supersaturated vapor and a subsequent condensation, which results in a nanomaterial with a variety of particle sizes, shapes, and compositions [
14]. The supersaturated vapor can be achieved by Joule’s heating, electron beam evaporation, arc-discharge and laser ablation. The processes involved are chemical reaction, mass transfer, nucleation, coagulation, and condensation. No calcination step is needed in this method. Vapor phase methods are widely used for commercial production of nanocomposite powders. The nature of the gas used determines the type of powder produced. Generally, oxygen-containing gases are used to synthesize metal oxide powders, whereas inert gases are used to produce nonoxide powders [
17].
Figure 3.
Various powder process techniques for the synthesis of ceramic nanocomposites.
Figure 3.
Various powder process techniques for the synthesis of ceramic nanocomposites.
Si
3N
4/SiC nanocomposite powders have been prepared by vapor phase pyrolytic reaction [
22]. According to these authors, [Si (CH
3)
3]
2NH or [Si(CH
3)
2NH]
3, are mixed with NH
3, under N
2 used as carrier gas, and then passed into a reaction chamber at 1000 °C. The amorphous powder is collected and crystallized to Si
3N
4/SiC at 1500 °C for 6 h. Also, ZrO
2/SiO
2 powder has been produced by vapor decomposition route, in which the precursors (namely, [Si(OC
2H
5)
4] and zirconium tert-butoxide) are introduced in the reactor as vapors, obtained by bubbling a carrier gas through the precursor solution.
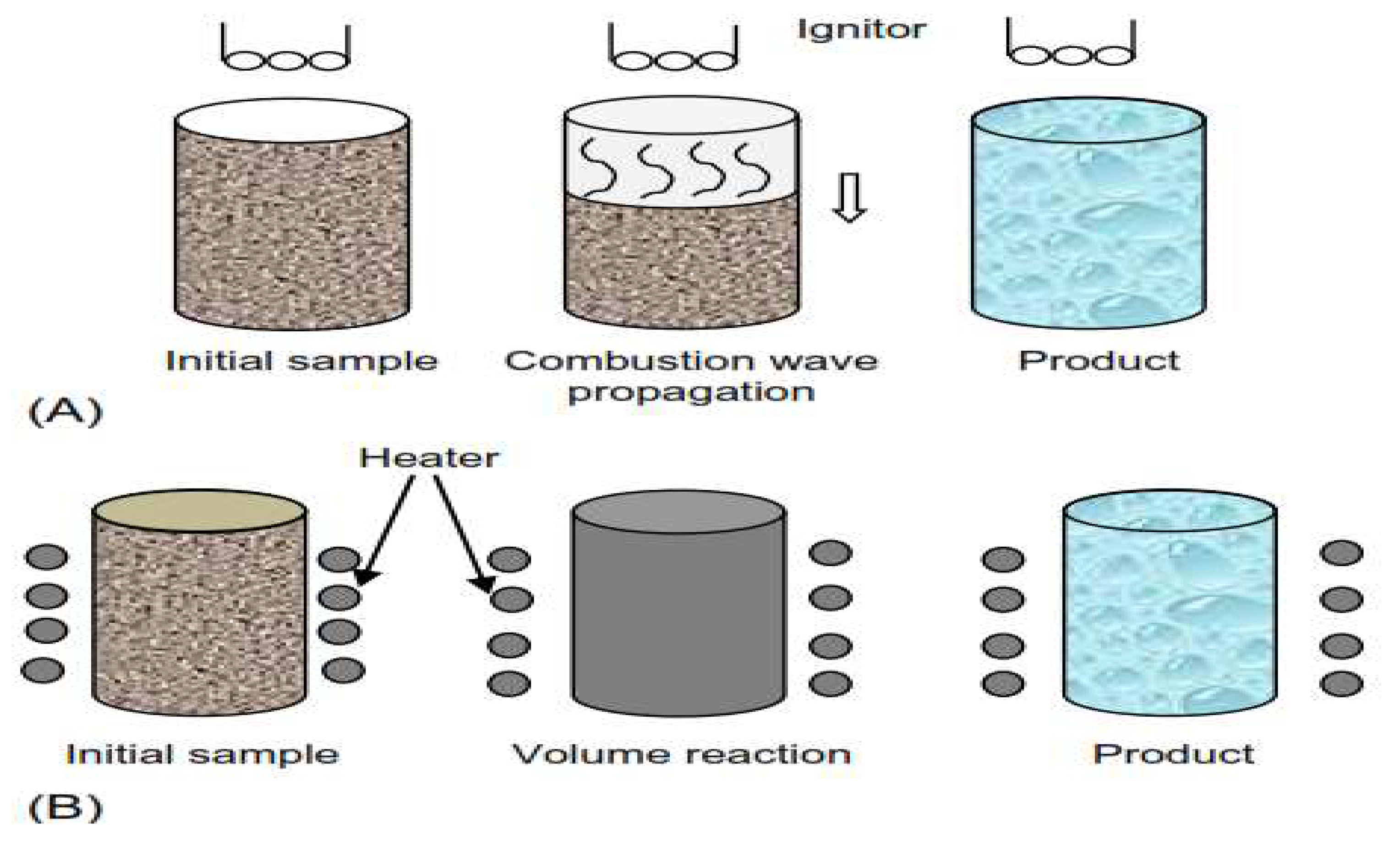
3.2.2. Self-Propagating High-Temperature Synthesis (SHS)
SHS involves the ignition of powder mixtures that exhibit an exothermic reaction and produce temperatures in the range 1000–3000 °C under adiabatic conditions. Besides powders production, this technique is also used for direct production of ceramic bodies. In addition, by this route, both single and multiphasic ceramic compositions can be produced. In fact, the method has been applied for in situ synthesizing of a number of refractory materials and composites, as reported. An example is provided by the following reaction, used to prepare cermets compositions [
14].
Figure 4.
Self-propagating high-temperature synthesis (SHS) method. (B) Volume combustion synthesis method (VCS).
Figure 4.
Self-propagating high-temperature synthesis (SHS) method. (B) Volume combustion synthesis method (VCS).
Recent work of Ryu, H. Y. et al. [
23], bi-, tri- and tetra-phase powders, such as ZrC/SiC, ZrB
2/SiC/ZrC, ZrB
2/SiC/ZrC/ZrSi, were synthesized as fine powder via combustion synthesis using a mixture made by ZrSiO4, Mg, C, B and NaCl as raw materials. In particular, Mg was used to reduce ZrSiO
4, whereas NaCl was used as diluents to control the particle size and phase composition of the composite powders. The authors showed that the combustion temperature and particle size decreased by increasing the NaCl amount in the starting mixture.
3.2.3. Sol-Gel Synthesis Method
Sol-gel is one of the simple wet chemical techniques widely used for the preparation of nanocomposites (mostly oxides) and ceramics. The sol-gel process is a chemical transformation of a sol into gel (three-dimensional polymer) state and a subsequent transition into solid oxide material caused by suitable post treatment. This method is based on inorganic polymerization reaction including hydrolysis, polycondensation, gelation, aging, drying, and calcination or sintering (densification). The sol-gel synthesis steps for the preparation of oxide nanocomposites are:- Hydrolysis, Poly condensation, Gelation, Aging, Drying, Dehydration and Densification process [
24].
A recent study compares the microstructural development and mechanical properties of (Y, Ce) ZrO
2/Al
2O
3 composites prepared by sol-gel and mechanical mixing methods [
22].
3.2.4. Co-precipitation method
In co-precipitation process, the required metal cations from a common medium are coprecipitated generally as hydroxides, carbonates, oxalates, or citrates. These precipitates are subsequently calcined at appropriate temperatures to obtain the required final nanopowder [
17]. As the calcination temperature required is low, co-precipitation results in smaller particle size. However, each synthesis requires its own special conditions and precursor reactions. The structure, morphology, and the composition of nanomaterials can be controlled in a better way if each reaction step is appropriately controlled. The chemical reaction starts just by mixing the reactants in a beaker. The crucial parameters that determine the characteristics of the final nano product are concentration of reactants, time and order of addition of reactants to the solution, process temperature, pH of the solution, and the surfactants used. When reaction products get supersaturated, spontaneous nucleation occurs and subsequently, they go through the growth mechanism.
The major difficulty in the chemical precipitation method is the presence of contamination, particularly due to the by-products generated in the chemical reaction. The working conditions, such as stirring speed, vibration, exposure to light, and cleanliness of glassware, can significantly affect the quality of nanomaterial formed. This is a simple, direct process suitable for the synthesis of fine metal nanocomposites. However, this process is not suitable for the preparation of a high-purity phase with accurate stoichiometry. Moreover, this method does not work well if the reactants have incompatible solubility values [
25]. Investigated the role of precipitant and drying method on Al
2O
3/ZrO
2 powders synthesizes by co-precipitation. In particular, NH
4HCO
3, NH
4OH, and (NH
3)
2CO
3 were used as precipitant agents.
Table 4.
Advantages and limitations of ceramic nanocomposite processing methods.
Table 4.
Advantages and limitations of ceramic nanocomposite processing methods.
| Method |
Advantages |
Limitations |
Ref. |
| Powder Process |
Simple |
Low formation rate, high temperature, agglomeration, poor phase dispersion, formation of secondary phases in the product. |
[22] |
Polymer Precursor
Process |
Possibility of preparing finer particles; better reinforcement dispersion |
Inhomogeneous and phase-segregated materials due to agglomeration and dispersion of ultra-fine particles |
[26] |
| Sol-Gel Process |
Simple, low processing temperature; versatile; high chemical homogeneity; rigorous stoichiometry control; high purity products; formation of three dimensional polymers containing metal-oxygen bonds. Single or multiple matrices. Applicable specifically for the production of composite materials with liquids or with viscous fluids that are derived from alkoxides. |
Greater shrinkage and lower amount of voids, compared to the mixing method.
|
[24] |
3.3. Synthesis of Metal Matrix Nanocomposites
Metal matrix nanocomposites (MMNC) refer to materials consisting of a ductile metal or alloy matrix in which some nanosized reinforcement material is implanted. These materials combine metal and ceramic features, that is, ductility and toughness, with high strength and modulus. Thus, MMNC are suitable for production of materials with high strength in shear/compression processes and with high service temperature capabilities [
27].
Metal matrices used for MMNC production are Al, Mg, Pb, Sn, W and Fe. Reinforcements for MMNC are same as for CMNC and PMNC. The most used techniques in preparation of MMNC are; spray pyrolysis, liquid metal infiltration, rapid solidification, vapor techniques (PVD, CVD), electrodeposition and chemical methods, which include colloidal and sol-gel processes.
Table 5 gives systems prepared by these methods.
3.3.1. Spray Pyrolysis
Spray pyrolysis is a process in which a thin film is deposited by spraying a solution on a heated surface, upon which the constituents react to form a chemical compound. The chemical reactants are selected such that the products other than the desired compound are volatile at the temperature of deposition [
29]. The fabrications steps include: (i) dissolution of the inorganic precursors (starting materials) in a suitable solvent to get the liquid source; (ii) generation of a mist from this liquid source using an ultrasonic atomizer; (iii) use of a carrier gas to carry the mist into a preheated chamber; (iv) vaporization of the droplets in the chamber and trapping with a filter, promoting their decomposition to give the respective oxide materials; and (v) selective reduction of the metal oxides to produce the respective metallic materials [
28,
29].
3.3.2. Liquid Infiltration
Infiltration is a liquid state method of composite materials fabrication, in which a preformed dispersed phase (ceramic particles, fibers) are soaked in a molten matrix metal, which fills the space between the dispersed phase inclusions. The motive force of an infiltration process may be either the capillary force of the dispersed phase (spontaneous infiltration) or an external pressure (gaseous, mechanical, electromagnetic, centrifugal, or ultrasonic) applied to the liquid matrix phase (forced infiltration) [
13].
3.3.3. Rapid Solidification
Rapid solidification is loosely defined in the scientific literature as the rapid extraction of thermal energy to include both super heat and latent heat during the transition from a liquid state at high temperature to a solid material at room or ambient temperature. Ultrasonics is used for mixing and for improving wet ability between the matrix and the reinforcements [
13].
3.3.4. High-Energy Ball Milling
High-energy ball milling is a ball milling process in which a powder mixture placed in a ball mill is subjected to high-energy collisions from the balls. High-energy ball milling, also called mechanical alloying, can successfully produce fine, uniform dispersions of oxide particles in nickel-base super alloys that cannot be made by conventional powder metallurgy methods. High-energy ball milling is a way of modifying the conditions in which chemical reactions usually take place, either by changing the reactivity of as-milled solids or by inducing chemical reactions during milling [
30].
3.3.5. Chemical Vapor Deposition and physical vapor deposition Methods
Chemical vapor deposition is a chemical process used to produce high-quality, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical chemical vapor deposition, the water (substrate) is exposed to one or more volatile precursors that react and/ or decompose on the substrate surface to produce the desired deposit. Frequently, volatile byproducts are also produced, which are removed by gas flow through the reaction chamber [
31].
Physical vapor deposition describes a variety of vacuum deposition methods that can be used to produce thin films and coatings. Physical vapor deposition is characterized by a process in which the material goes from a condensed phase to a vapor phase and then back to a thin film condensed phase. The most common physical vapor deposition processes are sputtering and evaporation [
28]. The procedure for physical vapor deposition is as follows: (i) sputtering/evaporation of different components to produce a vapor phase; (ii) super-saturation of the vapor phase in an inert atmosphere to promote the condensation of metal nanoparticles; and (iii) consolidation of the nanocomposite by thermal treatment under inert atmosphere. Examples: Fe/MgO, Pb/Cu, Al/Pb, Al/SiC, Cu/Al
2O
3, Al/Mo, and Ag/Au [
28].
Table 6.
Advantages and limitations of processing methods for metal-based nanocomposites.
Table 6.
Advantages and limitations of processing methods for metal-based nanocomposites.
| Process |
Advantages |
Limitations |
Ref. |
| Spray Pyrolysis |
Effective preparation of ultra fine, spherical and homogeneous powders in multicomponent systems, reproductive size and quality. |
High cost associated with producing large quantities of uniform, nanosized particles. |
[28,29] |
| Liquid Infiltration |
Short contact times between matrix and reinforcements; moulding into different and near net shapes of different stiffness and enhanced wear resistance; rapid solidification; both lab scale and industrial scale production. |
Use of high temperature; segregation of reinforcements; formation of undesired products during processing.
|
[13]
|
| High Energy Ball Milling |
Homogeneous mixing and uniform distribution. |
|
[30] |
| CVD/PVD |
Capability to produce highly dense and pure materials; uniform thick films; adhesion at high deposition rates; good reproducibility. |
Optimization of many parameters; cost; relative complexity. |
[31] |
Chemical Processes
(Sol-Gel, Colloidal) |
Simple; low processing temperature; versatile; high chemical homogeneity; rigorous stoichiometry control; high purity products. |
Weak bonding, low wear-resistance, high permeability and difficult control of porosity. |
[24] |
4. CHARACTERIZATION METHODS OF NANOCOMPOSITES
Nanocomposites are characterized using different techniques to get insight into the morphology, particle size, phase, composition, thermal stability, optical, magnetic, electrical and thermal properties. Some of the common techniques are X-ray diffraction analysis, microscopic techniques, thermal analysis (TA), spectroscopic techniques and strength measurements. These are discussed briefly.
4.1. X-Ray Diffraction Analysis
X-ray diffraction has played a central role in identifying and characterizing solids. The X-ray diffraction pattern of an amorphous polymer will not show any sharp peak, whereas the nanocomposites show sharp peaks due to crystalline characteristics. X-ray diffraction has been most commonly used for routine characterization as well as for detailed structural elucidation. In order to obtain detailed structural information, knowledge of X-ray diffraction intensities is also essential. Powder X-ray Diffraction (XRD) is used for phase determination and unit cell information of the nanocomposites under investigation. The technique is also frequently employed to determine particle size using Scherrer’s formula. The size of nanoparticles and nanocomposite is calculated by the well-known Debye–Scherrer equation as follows [
32].
Here, D is the crystallite size, K is the shape factor, calculated for a spherical particle is 0.9, and β is full width at half maxima of the highest peak in radian.
For instance, in the work of Gerawork, M. [
32] the crystallite size and structure of ZnO, ZnS, MnO
2, and ZnO/ZnS/MnO
2 nanomaterials were measured by powder XRD, as shown in
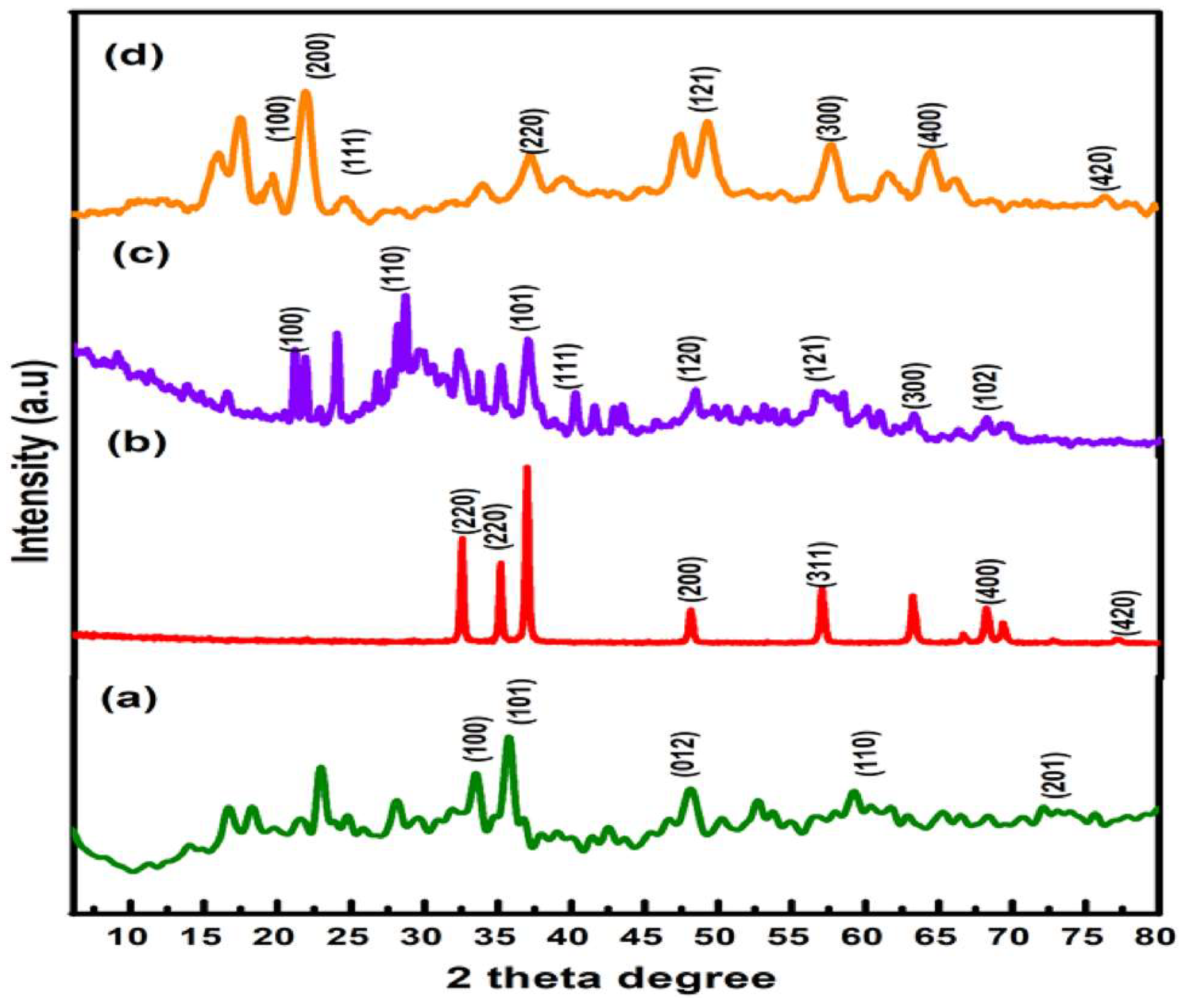
Figure 1. As this Author the average crystallite size of ZnO, ZnS, MnO
2, and ZnO/ZnS/MnO
2 was 6.35 nm, 26.231 nm, 5.65 nm, and 2.8914 nm respectively.
Figure 5.
XRD patterns of a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2 nanocomposite.
Figure 5.
XRD patterns of a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2 nanocomposite.
4.2. Thermal Analysis
Thermal analysis including thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), dynamic mechanical thermal analysis (DMTA), thermal mechanical analysis (TMA) etc. is a useful tool to determine the stability of the nanocomposite materials and various phenomena occurring because of doping, curing and annealing. The steps leading to thermal decomposition of the nanocomposites, endothermal or exothermic nature of decomposition process, solvent/ moisture loss, weight loss at each step, final decomposed matter etc. are the usual information obtained by the thermal analysis technique [
33].
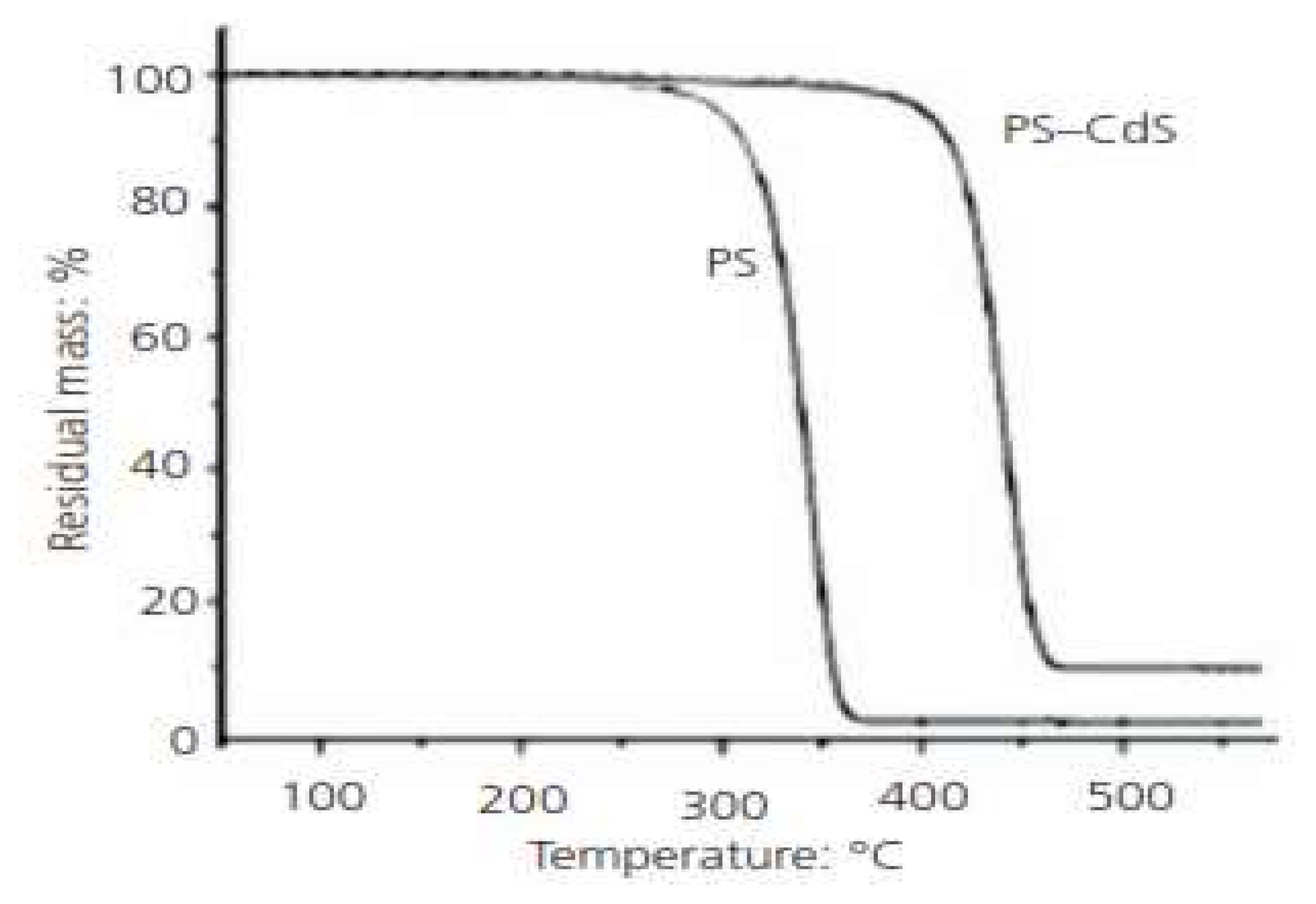
For example, [
32] the influence of the content of cadmium sulfide (CdS) on the thermal degradation of polystyrene (PS)–cadmium sulfide composites was examined.76 TGA curves (
Figure 8) show that the thermal stability of PS/cadmium sulfide composites is higher than that of pure PS.76.
Figure 6.
TGA curves of pure PS and PS–cadmium sulfide nanocomposites.
Figure 6.
TGA curves of pure PS and PS–cadmium sulfide nanocomposites.
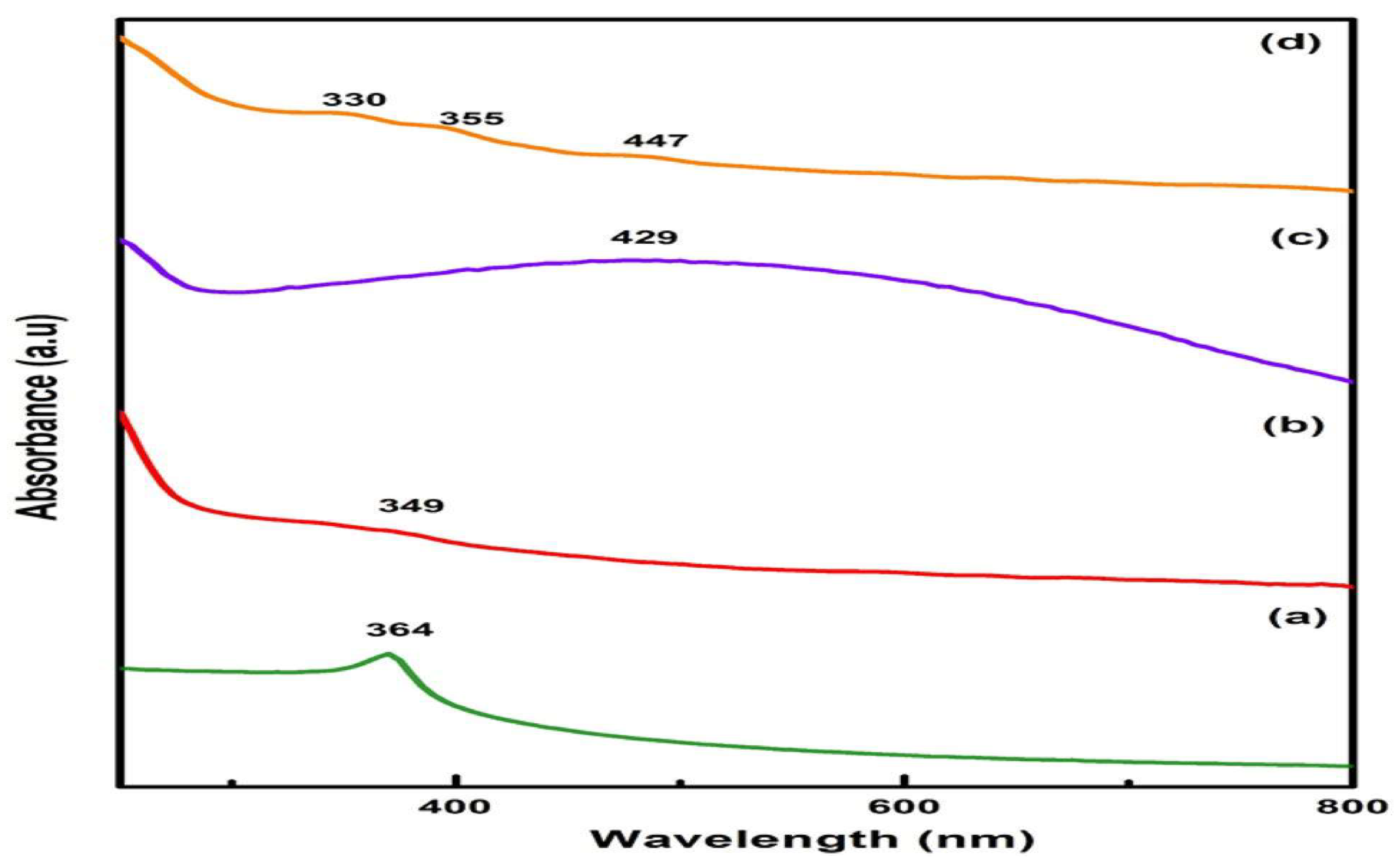
4.3. Ultraviolet-visible spectra (Optical studies)
The optical properties of the as-prepared photocatalysts were characterized to know their band gap energy. The optical analysis and bandgap of ZnO, ZnS, MnO
2, and ZnO/ZnS/MnO
2 were investigated from UV–visible spectrophotometer and spectrum shown in
Figure 4. The absorption values of ZnO, ZnS, MnO
2, ZnO/ZnS/MnO
2 are 364 nm, 349 nm, and 429 nm [
34]. These peaks are due to the transition of an electron from the valence band to the conduction band. The absorption peak of ZnO, ZnS, and MnO
2 appear in the composite at 330 nm (blue shift), 355 nm (blue shift), and 447 nm (Red shift). The change in the absorption pattern shows the formation of the ZnO/ZnS/MnO
2 nanocomposite. The bandgap energy of nanostructures was measured by Tauc plot creating a tangent using the following equations:
Where, α, h, ν, c, Eg, and A are absorption coefficient, Planks constant, light frequency, optical band gap, speed of light, and absorption.
Figure 7.
UV–Visible spectra of a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2.
Figure 7.
UV–Visible spectra of a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2.
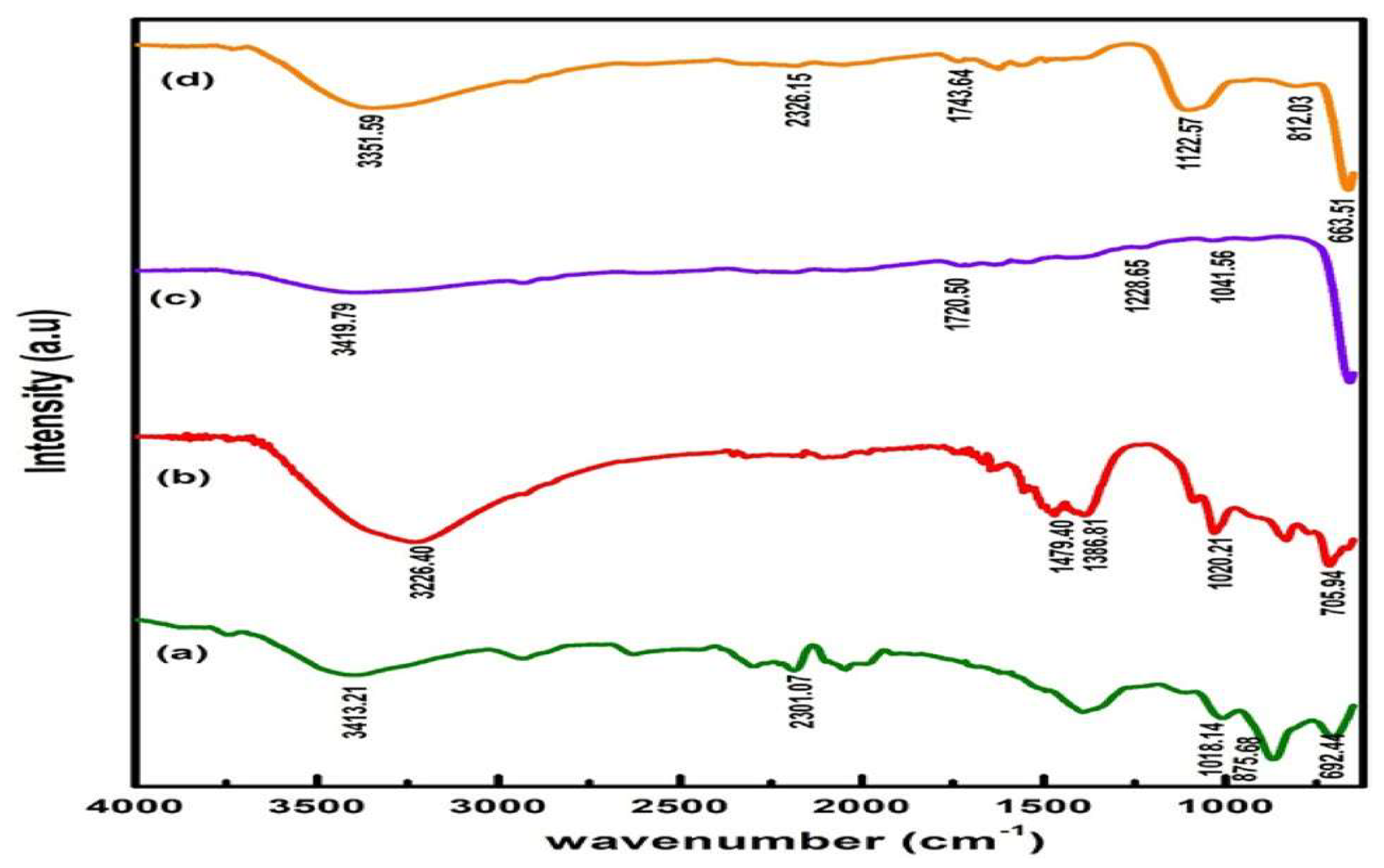
4.4. Fourier Transforms Infrared Spectroscopy (FTIR)
The possible physicochemical interaction between the filler and the matrix can be found out with the help of Fourier transform infrared (FTIR) spectroscopy. By introducing nanofillers into the polymer matrix, the vibrational frequencies are changed and shift to a lower frequency [
34]. Moreover, FTIR analysis was used to measure the existence of functional group vibration bands in fabricated materials.
Figure 2.
FTIR spectra of synthesized a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2 nanocomposite.
Figure 2.
FTIR spectra of synthesized a ZnO, b ZnS, c MnO2 and d ZnO/ZnS/MnO2 nanocomposite.
4.5. Transmission Electron Microscopy (TEM)
TEM is widely employed, in its simplest bright-field mode, as a tool for direct visualization of the nanocomposite structure of PNCs. This is possible because a sufficient contrast exists, for the transmitted electrons, between the polymer matrix and most filler (inorganic materials such as metal oxides). In the extreme case, high-resolution TEM can even provide a qualitative picture of the inorganic filler crystal structure. As is well known, to obtain a transmission electron micrograph, only a small piece of the material is required and the result may not be representative of the whole [
32].
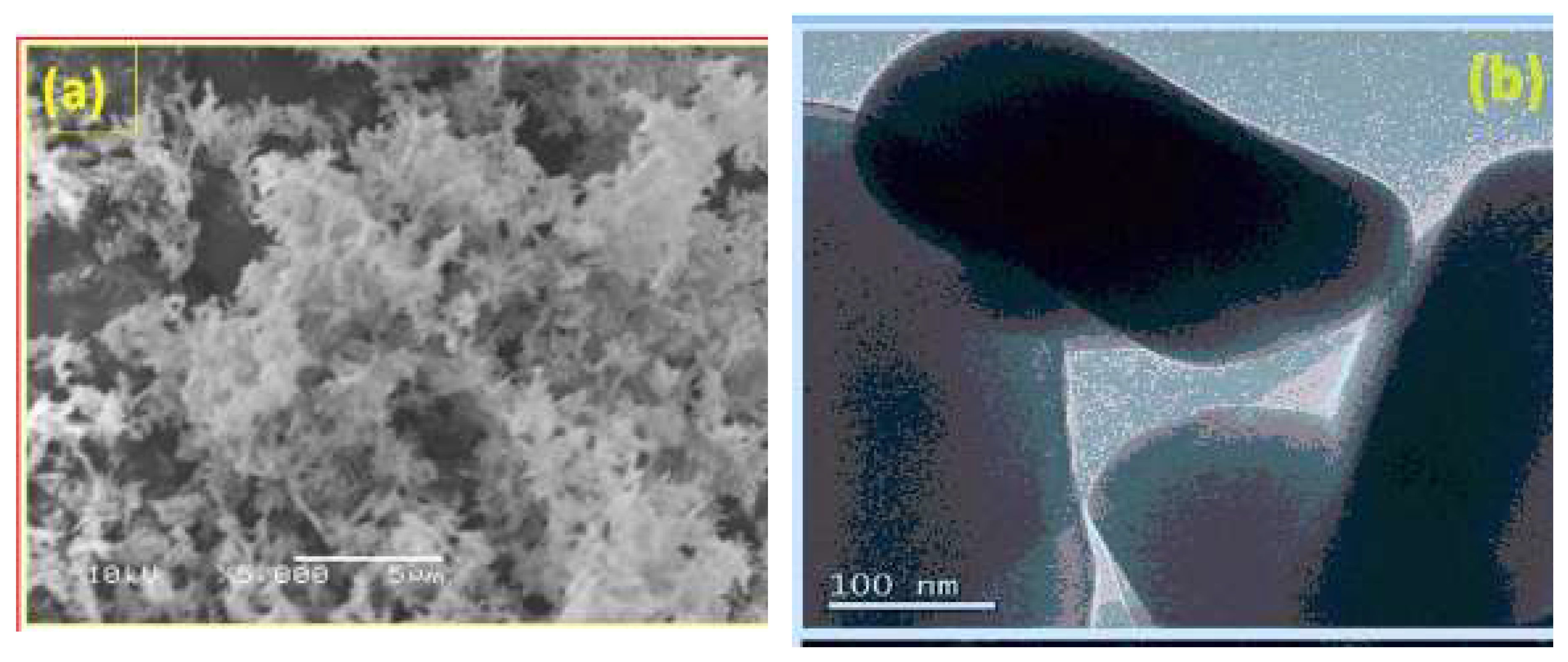
By two authors T.N. Ravishankar & S.R. Teixeira [
35] effective and low-cost photocatalysts CuO/TiO
2 nanocomposites have been prepared at room temperature by surfactant assisted sol-gel method using titanium (IV) isopropoxide and copper (II) nitrate as precursors. In the current work, surfactant like Hexadecyltrimethylammonium bromide had used in the sol-gel method to reduce agglomeration of CuO/TiO
2 nanocomposites. The morphology was examined using table top Hitachi 3000 scanning electron microscopy (SEM). The nanostructure of the product was observed by transmission electron microscopy (TEM) performed. Samples for TEM were prepared by dropping the dispersion of 2-propanol metal oxide nanoparticle on a holey carbon grid and drying the grids under vacuum for 24 h.
Figure 12.
(a) SEM image and (b) high magnification TEM image of CuO/TiO2 nanocomposite prepared by surfactant assisted sol-gel method.
Figure 12.
(a) SEM image and (b) high magnification TEM image of CuO/TiO2 nanocomposite prepared by surfactant assisted sol-gel method.
4.6. Scanning Electron Microscopy (SEM)
SEM is an important technique for providing a visual image of materials at the micron and in some cases the submicron level of resolution. One primary reason for SEM’s usefulness is the high resolution that can be obtained. Another feature of SEM images is the 3D appearance of the specimen. Therefore, SEM is particularly powerful in characterizing the crystallographic, magnetic and electrical characteristics of the specimen and in determining if any change in the particle morphology has occurred when the specimen surface is modified by other molecules [
32]. SEM determines the morphology and shape of the surface of the material in a polymer matrix nanocomposite, especially when a low amount of nanoparticles is added to the polymer; polymeric chains cover the nanoparticle. Thus, in a SEM surface image, nanoparticles cannot be determined in the matrix.
5. APPLICATIONS OF NANOCOMPOSITES
Nanotechnology is revolutionizing the world of materials. It has a very high impact in developing a new generation of composites with enhanced functionality and a wide range of applications such as biosensors, antibacterial activities, photocatalytic, anticancer etc.
5.1. Biosensor
Nanosensors are sensing devices that are dependent on the unique properties of nanomaterials to recognize and detect the behavior of matter within a given environment at the nanoscale to macromolecular level. These sensing devices can be large or small devices that utilize the nanomaterials incorporated as recognition elements to detect changes at a nanoscale. The sensing mechanism follows either the optical, electrochemical or mechanical route of detection, as a result of the combination of the recognition element and the detector used. Evidently, as key technological and economic drivers, various nanosensors have been developed for the detection of chemical and toxic gases, food packaging, medical diagnostics, and water quality monitoring, amongst other applications [
22].
Interestingly, the recognition element has been identified amongst these applications as the key element giving the identity of the nanosensor. Owing to their unique physico-chemical properties enhancing the signal detection, nanomaterials have been incorporated as recognition elements in sensors. Nanomaterials allow for the amplified binding of target molecules on their available active sites, which lead to detectable signals. It is worth noting that if the recognition element is a nanostructured material, then a nanosensor is obtained. To this end, various classes of nanostructured materials ranging from carbon-based nanomaterials to organic-based nanomaterials, inorganic-based nanomaterials, and nanocomposite materials (incorporated in
Figure 11) have been synthesized and deployed in the afore-mentioned applications [
36].
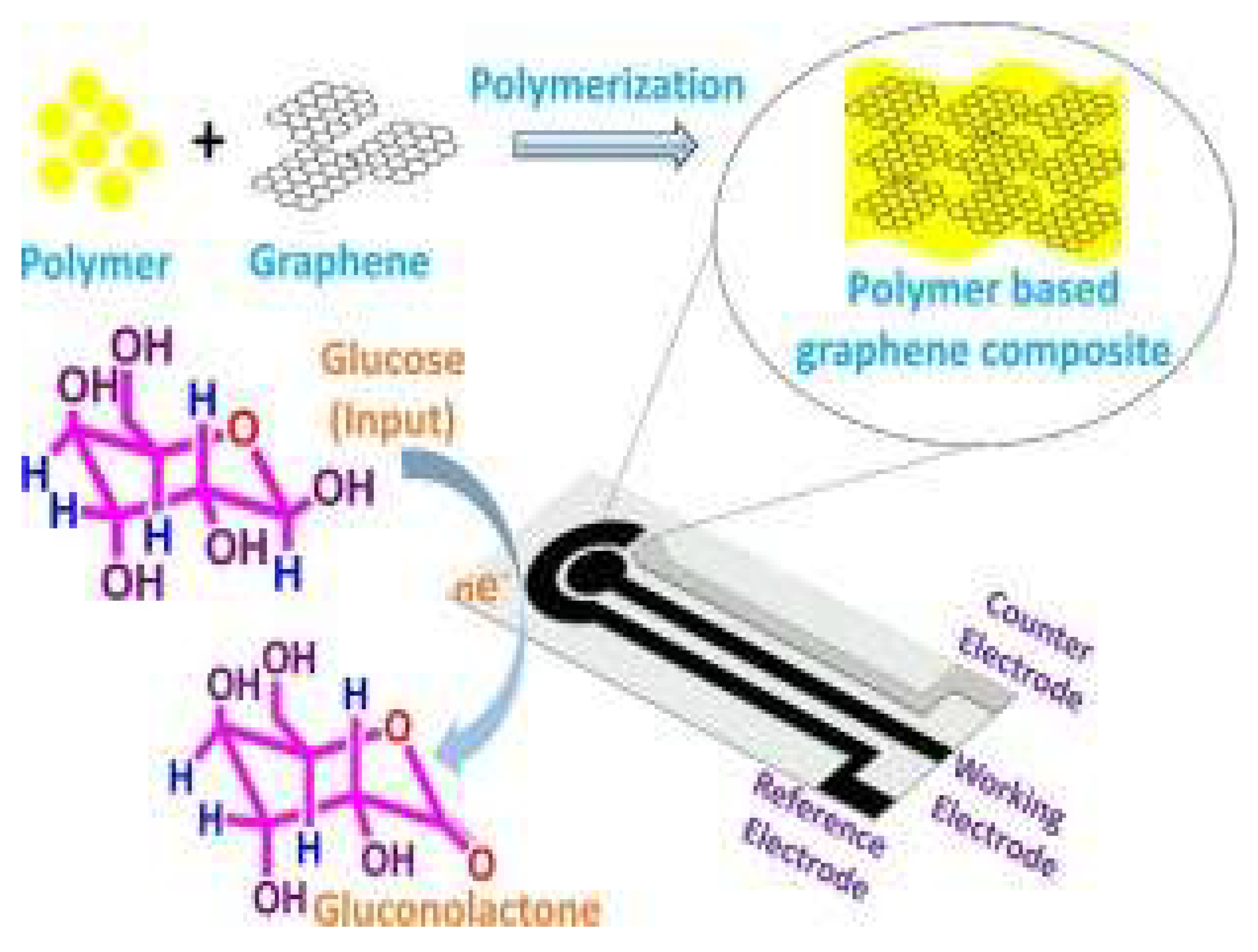
Figure 8.
Graphene and its derivative based polymer nanocomposites for glucose sensing.
Figure 8.
Graphene and its derivative based polymer nanocomposites for glucose sensing.
5.2. Antibacterial Activity
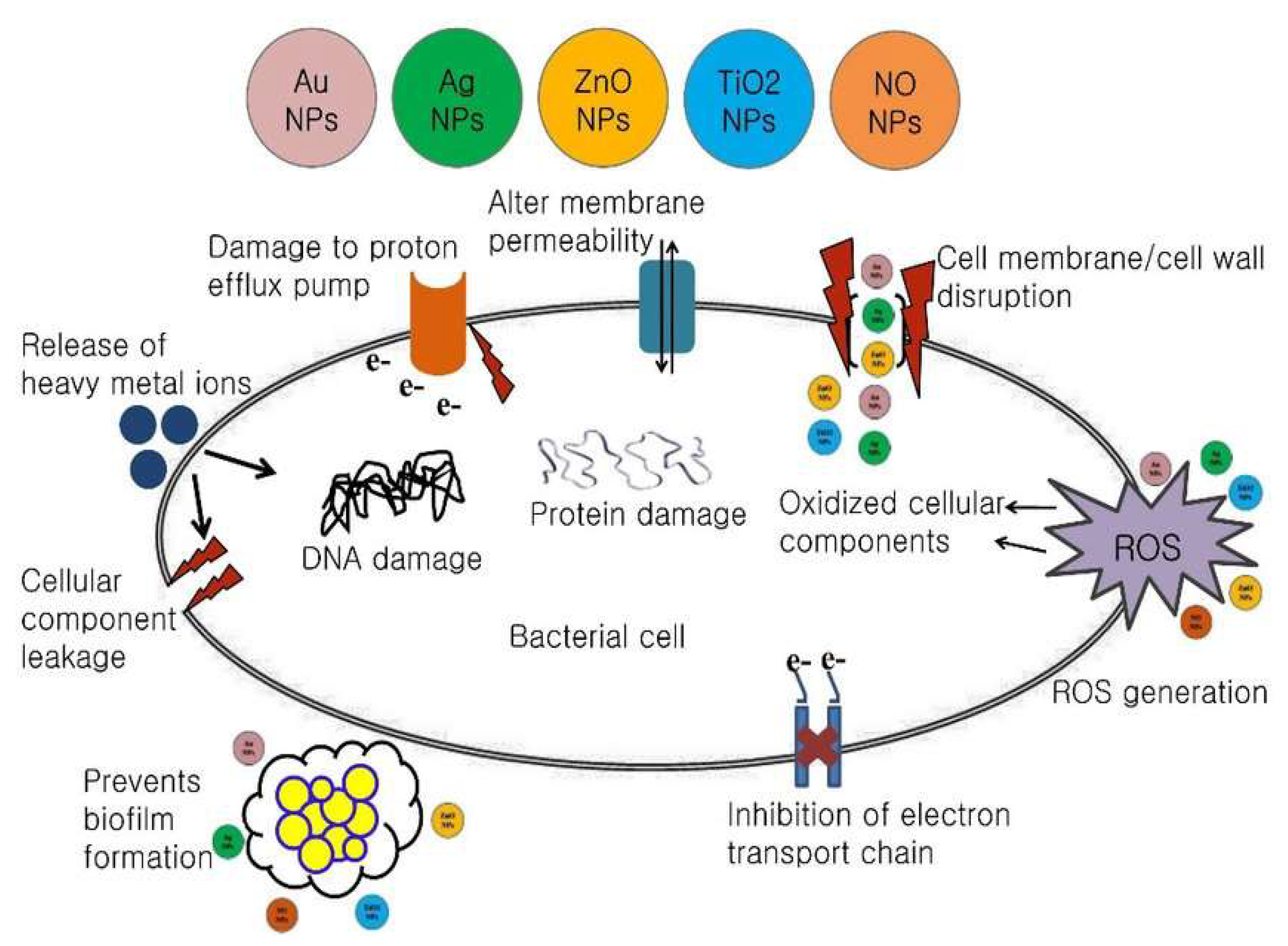
The increasing use of nanocomposites in medicine has led to a growing number of studies exploring potential antibacterial mechanisms of nanocomposites. For example, metal nanocomposites can change the metabolic activity of bacteria. This capacity represents a huge advantage in terms of eliminating bacteria to cure diseases. Nanocomposites need to be in contact with bacterial cells to achieve their antibacterial function. The accepted forms of contact include electrostatic attraction van der Waals forces, and receptor–ligand and hydrophobic interactions [
22]. Nanocomposites then cross the bacterial membrane and gather along the metabolic pathway, influencing the shape and function of the cell membrane. Thereafter, nanocomposites interact with the bacterial cell’s basic components, such as DNA, lysosomes, ribosomes, and enzymes, leading to oxidative stress, changes in cell membrane permeability, enzyme inhibition, protein deactivation, and changes in gene expression. The oxidative stress mechanisms are the most frequently proposed in current research [
37].
Reactive Oxidative stress (ROS) is an important antibacterial mechanism of nanocomposites. ROS is a generic term for molecules and reactive intermediates that have strong positive redox potential, and different types of NPs produce different types of ROS by reducing oxygen molecules [
38]. The four ROS types are the superoxide radical (O
2−), the hydroxyl radical (⋅OH), hydrogen peroxide (H
2O
2), and singlet oxygen (O
2), which exhibit different levels of dynamics and activity. For example, calcium oxide and magnesium oxide NPs can generate O
2−, whereas zinc oxide nanocomposites can generate H
2O
2 and OH but not O
2−. Meanwhile, copper oxide based nanocomposites can produce all four types of reactive oxygen [
37].
The main causes of ROS production are restructuring, defect sites, and oxygen vacancies in the crystal. Under normal circumstances, the production and clearance of ROS in bacterial cells are balanced. In contrast, with excessive production of ROS, the redox balance of the cell favors oxidation. This unbalanced state produces oxidative stress, which damages the individual components of bacterial cells [
36]
Figure 9.
Schematic representations of the antimicrobial mechanisms of various nanoparticles.
Figure 9.
Schematic representations of the antimicrobial mechanisms of various nanoparticles.
5.3. Photocatalytic Activity
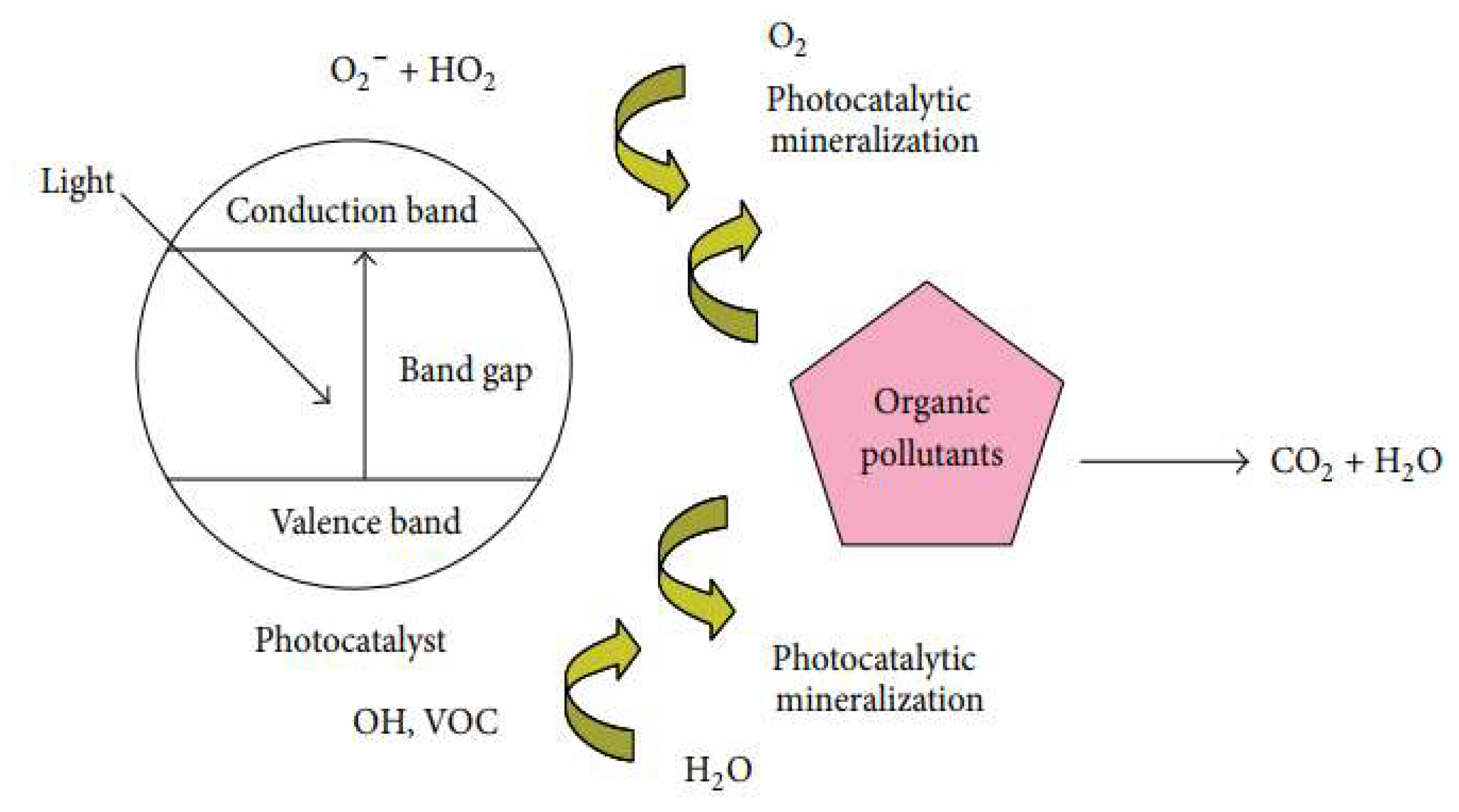
The problem of release of toxic from industrial, agricultural and domestic waste leads to surface and ground water contamination. This problem has drawn much attention in recent years and is considered as one of the growing concerns facing scientists today. One of the best ways to reduce contamination of water is by photocatalytic treatment [
39].
Figure 10.
Schematic diagram of photocatalytic degradation of organic effluent.
Figure 10.
Schematic diagram of photocatalytic degradation of organic effluent.
Recently, numerous studies have been devoted to the use of photocatalysis in the removal of dyes from wastewaters, particularly, because it can initiate reactions to decompose organic contaminants under ultraviolet (UV) or even sunlight irradiation without consuming chemicals or producing chemical wastes. Thus, photocatalytic reactions are considered as a sustainable approach for the removal of a variety of environmental pollutants.
In the work of Ardalan Azimi-Fouladi et al. [
40] research, TiO
2-CdO-Ag nanoparticles were prepared via sol-gel method and employed as a superior nano-photocatalyst for the photodegradation of methylene blue (MB), as a model of organic dye in water. In addition [
41], investigate one of the major disadvantages of the application of ZnO nanoparticles as photocatalyst in photocatalytic systems is photoinstability due to the photocorrosion under UV light irradiation resulting in the significant reduction in their photocatalytic activity. Therefore, in the present study, SiO
2 nanopowder was incorporated into the ZnO nanoparticles to enhance their photocatalytic activity for the decolorization of methylene blue (MB) dye in comparison with pure ZnO/UV process. The efficiency of UV/ZnO/SiO
2 process was compared with UV/ZnO process for the decolorization of MB dye and the removal of chemical oxygen demand (COD).
Kumar, A. et al.[
42] many metal oxide nanoparticles such as TiO
2, ZnO, SnO and CuO have been used to degrade various organic dyes such as methylene blue (MB), and methylene orange (MO) in wastewater. The possible photocatalytic mechanism for the degradation reaction with the assistance of SnO
2-CuO nanocomposite is proposed as illustrated in fig 14. Under UV light illumination, SnO
2 nanoparticles can be excited to produce photo-generated electrons (e-) and holes (h
+). The e- was then injected into the conduction band of CuO due to the match of energy. The defined interfaces between SnO
2 nanoparticles and CuO nanoparticles in the obtained catalyst can supply an optional environment and the force to drive the transfer of the electrons/holes to the surfaces of the catalysts. Then the photo-generated electrons reacted with the adsorbed oxygen (or dissolved in the solution) to produce active ˙O
2- radicals. Therefore, efficient separation of electron–hole pairs in the as-achieved SnO
2-CuO nanocomposite facilitates enhanced stability and photocatalytic activity towards photodegradation of malachite green under UV light. The concurrent active species of h
2+, and other radicals produced in the solution, such as ˙OH, can directly oxidize dye molecules in the system [
42].
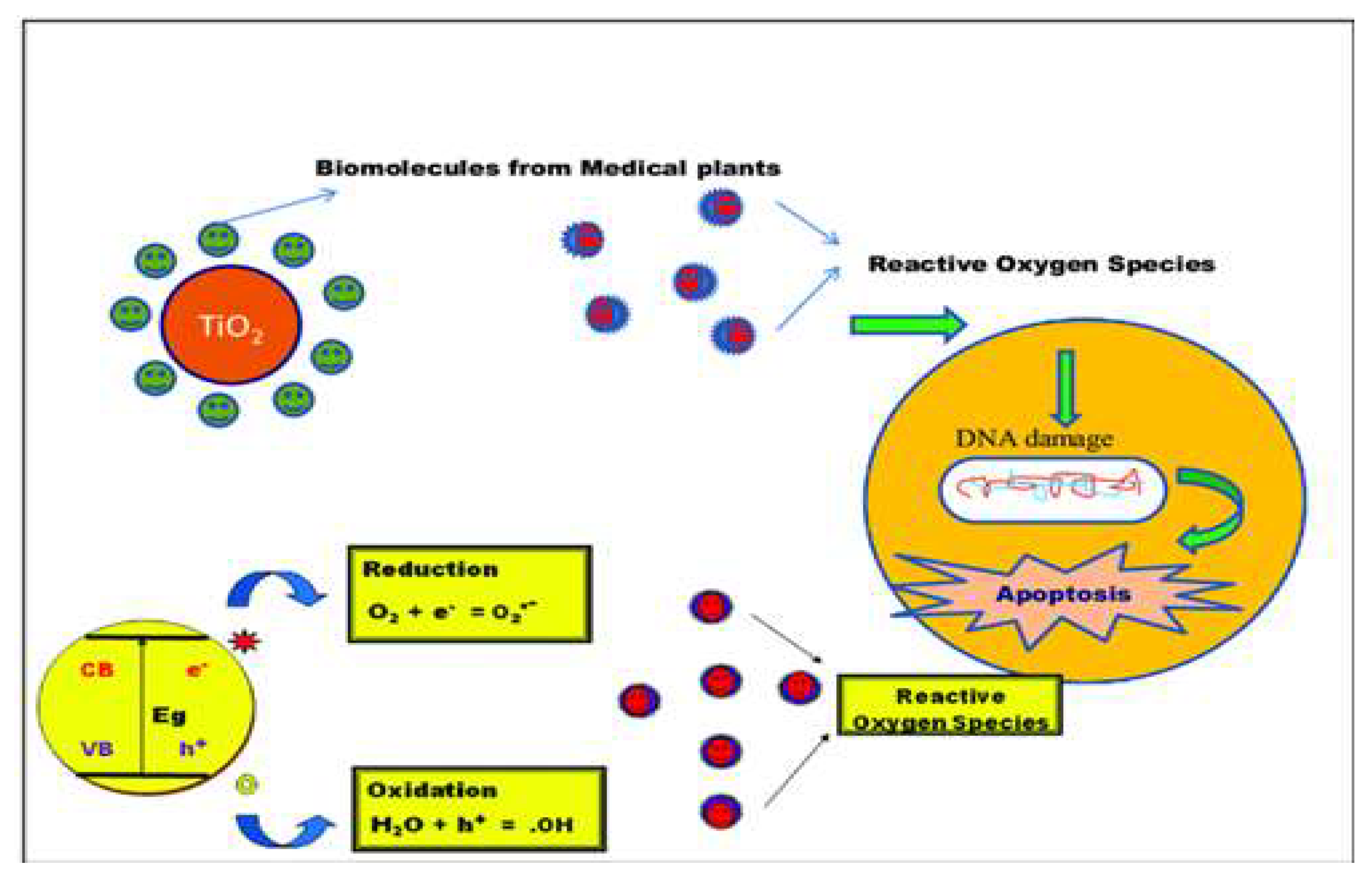
5.4. Anticancer Activity
Figure 11.
Proposed mechanisms for anticancer activity shown by TiO2 based nanocomposites.
Figure 11.
Proposed mechanisms for anticancer activity shown by TiO2 based nanocomposites.
Recent work Maheswari, P. et al. [
43] Titanium dioxide based nanocomposites were found to be good anticancer and antibacterial agents. In this study, the antibacterial and anticancer activities of pure TiO
2, turmeric, ginger and garlic modified TiO
2 nanoparticles were investigated. Antibacterial activities were performed against five bacterial strains namely Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruoginosa, Staphylococcus aureus and Streptococcus mutans. The results of the modified TiO
2 NPs indicate a greater efficacy on anticancer and antibacterial properties compared to the pure TiO
2 nanocomposites.
6. CONCLUSION
This review paper provides an overview of nanocomposites, including their types, synthesis, characterization, and applications. The definition of nanocomposite materials has broadened significantly to encompass a large variety of systems such as one-dimensional, two dimensional, three-dimensional (3D) and amorphous materials made of distinctly dissimilar components and mixed at the nanometer scale. Polymer matrix, metal matrix, and ceramic matrix nanocomposites are the three basic kinds of nanocomposites based on their matrix materials. Various nanocomposites have been designed with the aim to achieve high thermal, electrical and mechanical properties. Despite their nano dimensions, most of the processing techniques of the three types of nanocomposites remain almost the same as in microcomposites. Spray pyrolysis, liquid metal infiltration, rapid solidification, vapor techniques (PVD, CVD), electrodeposition, and chemical procedures, which include colloidal and sol-gel processes, are the most commonly utilized techniques in the fabrication of MMNC. For the synthesis of CMNC, a variety of approaches have been devised. Some methodologies include: conventional powder method; polymer precursor route; spray pyrolysis; vapor techniques (CVD and PVD). Chemical methods include: sol-gel process; colloidal and precipitation technique, template synthesis. Several methods have been adopted for the preparation of polymer nanocomposites. The most used are: Intercalation of the polymer; In-situ intercalative polymerization; Melt intercalation and Template synthesis (Sol-gel technology). The synthesized nanocomposites can be characterized using different techniques to get insight into the morphology (SEM& TEM), particle size (XRD), phase composition (XPS), thermal stability (TGA), optical and magnetic, properties. Nanocomposites have become essential materials for a variety of applications, such as antibacterial, anticancer, photocatalytic, sensors, super capacitors, and so on.
Author Contributions
Lema Yadeta performed collecting review papers and writing the draft of the review paper. Raji Fayisa was a major contributor in editing the review paper. All authors read and approved the final manuscript.”
Funding
This research did not receive any specific grant from funding agencies.
Acknowledgments
The authors, would like to thank the almighty God who help us through all to complete this review.
Conflicts of Interest
The authors declare that they have no competing interests.
Availability of data
All data mentioned in this paper.
References
- Twardowski, T. E. Introduction to nanocomposite materials: properties, processing, characterization. DEStech Publications, Inc. 2007.
- Lateef, A., & Nazir, R. Metal nanocomposites: Synthesis, characterization and their applications. Science Application Tailored Nanostructures. 2017, 239-256.
- Ahmed, S., Ahmad, M., Swami, B. L., & Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. Journal of advanced research. 2016, 7(1), 17-28. [CrossRef]
- Feng. C, F. Qu, J. Liu, L. Zhu, & Y. Lin. Electrospun nanofibers of p-type NiO/n-type ZnO heterojunction with different NiO content and its influence on trimethylamine sensing properties. Sensors and Actuators Biochemical journal. 2015, 207(6), 90-96. [CrossRef]
- Kanjwal. M. A and Barakat N. A. Electrospun NiO, ZnO and composite NiO–ZnO nanofibers/photocatalytic degradation of dairy effluent. Ceramics International. 2015, 41, 12229-12236. [CrossRef]
- Singh, N. B., & Agarwal, S. Nanocomposites: an overview. Emerging Materials Research. 2016, 5(1), 5-43. [CrossRef]
- Khan, W. S., Hamadneh, N. N., & Khan, W. A. Polymer nanocomposites–synthesis techniques, classification and properties. Science and applications of Tailored Nanostructures. 2016, 50-67.
- Parameswaranpillai, J., Hameed, N., Kurian, T. Nanocomposite materials: synthesis, properties and applications. Chemical Rubber Company Press. 2016.
- Niihara, K. Development of high performance ceramic base nanocomposites. Metal Powder Report. 1999, 3(54), 37.
- Gurnani, L., & Mukhopadhyay, A. Development of Carbon Nanotube-Reinforced Ceramic Matrix Nanocomposites for Advanced Structural Applications. Handbook of Advanced Ceramics and Composites: Defense, Security, Aerospace and Energy Applications. 2020, 82(7), 929-974. [CrossRef]
- Parida, S. K. Polymer Nanocomposites and Applications: A Brief Review. Polymer. 2018, 6(3), 75-78. [CrossRef]
- Yu, W. H., Sing, S. L., Chua, C. K., Kuo, C. N., & Tian, X. L. Particle-reinforced metal matrix nanocomposites fabricated by selective laser melting: A state of the art review. Progress in Materials Science. 2019, 104, 330-379. [CrossRef]
- Mohanty, P., Mahapatra, R., Padhi, P., Ramana, C. V., & Mishra, D. K. Ultrasonic cavitation: An approach to synthesize uniformly dispersed metal matrix nanocomposites -A review. Nano-Structures & Nano-Objects. 2020, 23, 100475. [CrossRef]
- Ravichandran, K., Praseetha, P. K., Arun, T., & Gobalakrishnan, S. Synthesis of nanocomposites. In Synthesis of Inorganic Nanomaterials. 2018,141-168).
- Pirayesh, A., Salami-Kalajahi, M., Roghani-Mamaqani, H., & Najafi, F. Polysulfide polymers: synthesis, blending, nanocomposites, and applications. Polymer Reviews. 2019, 59(1), 124-148. [CrossRef]
- Akpan, E. I., Shen, X., Wetzel, B., & Friedrich, K. Design and synthesis of polymer nanocomposites. Polymer Composites with Functionalized Nanoparticles. 2019, 34(5), 47-83. [CrossRef]
- Zhang, Y., Rhee, K. Y., Hui, D., & Park, S. J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Composites Part B: Engineering. 2018, 143, 19-27. [CrossRef]
- Bingzhen Li., Weng, X., Wu, G., Zhang, Y., Lv, X., & Gu, G. Synthesis of Fe3O4/polypyrrole/polyaniline nanocomposites by in-situ method and their electromagnetic absorbing properties. Journal of Saudi Chemical Society. 2017, 21(4), 466-472. [CrossRef]
- El Nahrawy, A. M., Abou Hammad, A. B., Shaheen, T. I., & Mansour, A. M. Sol–gel synthesis and physical characterization of high impact polystyrene nanocomposites based on Fe2O3 doped with ZnO. Applied Physics A. 2020, 126(8), 1-11. [CrossRef]
- Siva, V., Murugan, A., Shameem, A., Thangarasu, S., & Bahadur, S. A. A facile microwave-assisted combustion synthesis of NiCoFe2O4 anchored polymer nanocomposites as an efficient electrode material for asymmetric supercapacitor application. Journal of Energy Storage. 2022, 48, 103965. [CrossRef]
- Palmero, P. Structural ceramic nanocomposites: a review of properties and powders’ synthesis methods. Nanomaterials. 2015, 5(2), 656-696. [CrossRef]
- Huang, J. L., & Nayak, P. K. Strengthening alumina ceramic matrix nanocomposites using spark plasma sintering. Advances in ceramic matrix composites. 2018, 23(12), 231-247. [CrossRef]
- Ryu, H. Y., Nersisyan, H. H., & Lee, J. H. Preparation of zirconium-based ceramic and composite fine-grained powders. International Journal of Refractory Metals and Hard Materials. 2012, 30(1), 133-138. [CrossRef]
- Dong, Y., Ren, K., Lu, Y., Wang, Q., Liu, J., & Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. Journal of the European Ceramic Society. 2019, 39(7), 2574-2579. [CrossRef]
- Han, X., Liang, Z., Feng, L., Wang, W., Chen, J., Xue, C., & Zhao, H. Co-precipitated synthesis of Al2O3–ZrO2 composite ceramic nanopowders by precipitant and drying method regulation: A systematic study. Ceramics International. 2015, 41(1), 505-513. [CrossRef]
- Arai, Y., Inoue, R., Goto, K., & Kogo, Y. Carbon fiber reinforced ultra-high temperature ceramic matrix composites: A review. Ceramics International. 2019, 45(12), 14481-14489. [CrossRef]
- Tabandeh-Khorshid, M., Kumar, A., Omrani, E., Kim, C., & Rohatgi, P. Synthesis, characterization, and properties of graphene reinforced metal-matrix nanocomposites. Composites Part B: Engineering. 2020, 183, 107664. [CrossRef]
- Rane, A. V., Kanny, K., Abitha, V. K., & Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of inorganic nanomaterials. 2018, 121-139. [CrossRef]
- Vojoudi, H., Ghasemi, J. B., Hajihosseinloo, A., Bastan, B., & Badiei, A. One-pot synthesis of hematite-alumina hollow sphere composite by ultrasonic spray pyrolysis technique with high adsorption capacity toward PAHs. Advanced Powder Technology. 2021, 32(4), 1060-1069. [CrossRef]
- Fathy, A., Wagih, A., & Abu-Oqail, A. Effect of ZrO2 content on properties of Cu-ZrO2 nanocomposites synthesized by optimized high energy ball milling. Ceramics International. 2019, 45(2), 2319-2329. [CrossRef]
- Saxena, A., Singh, N., Kumar, D., & Gupta, P. Effect of ceramic reinforcement on the properties of metal matrix nanocomposites. Materials Today: Proceedings. 2017, 4(4), 5561-5570. [CrossRef]
- Gerawork, M. Photodegradation of methyl orange dye by using Zinc Oxide–Copper Oxide nanocomposite. Optik. 2020, 216, 164864. [CrossRef]
- Ishaq, S., Moussa, M., Kanwal, F., Ehsan, M., Saleem, M., Van, T. N., & Losic, D. Facile synthesis of ternary graphene nanocomposites with doped metal oxide and conductive polymers as electrode materials for high performance supercapacitors. Scientific reports. 2019, 9(1), 1-11. [CrossRef]
- Abdullah, M., John, P., Ahmad, Z., Ashiq, M. N., Manzoor, S., Ghori, M. I., & Ahmed, S. Visible-light-driven ZnO/ZnS/MnO2 ternary nanocomposite catalyst: synthesis, characterization and photocatalytic degradation of methylene blue. Applied Nanoscience. 2021, 11(8), 2361-2370. [CrossRef]
- T.N. Ravishankar & S.R. Teixeira. The effect of surfactant on sol-gel synthesis of CuO/TiO2 nanocomposites for the photocatalytic activities under UV-Visible and Visible Light Illuminations. New Journal of Chemistry. 2020, 39, 333. [CrossRef]
- Munonde, T. S., & Nomngongo, P. N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors. 2021, 21(1), 131. [CrossRef]
- Wang, L., Hu, C., & Shao, L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International journal of nanomedicine. 2017, 12, 1227. [CrossRef]
- He, Y., Ingudam, S., Reed, S., Gehring, A., Strobaugh, T. P., & Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. Journal of nanobiotechnology. 2016, 14(1), 1-9. [CrossRef]
- Gnanaprakasam, A., Sivakumar, V. M., & Thirumarimurugan, M. Influencing parameters in the photocatalytic degradation of organic effluent via nanometal oxide catalyst: a review. Indian Journal of Materials Science. 2015, 6(4), 215. [CrossRef]
- Ardalan Azimi-Fouladi, S.A. Hassanzadeh-Tabrizi & A.Saffar-Teluri. Sol-gel synthesis and characterization of TiO2-CdO-Ag nanocomposite with superior photocatalytic efficiency. Ceramics International. 2017, 42, 14121–14125. [CrossRef]
- R. Darvishi Cheshmeh Soltani, Gh. Shams Khoramabadi, H. Godini & Z. Noorimotlagh. The application of ZnO/SiO2 nanocomposite for the photocatalytic degradation of a textile dye in aqueous solutions in comparison with pure ZnO nanoparticles. Desalination and Water Treatment. 2015, 1944-3994. [CrossRef]
- Kumar, A., Rout, L., Achary, L. S. K., Mohanty, A., Marpally, J., Chand, P. K., & Dash, P. Design of binary SnO2-CuO nanocomposite for efficient photocatalytic degradation of malachite green dye. In AIP Conference Proceedings. 2016, 1724(1), 020027. [CrossRef]
- Maheswari, P., Ponnusamy, S., Harish, S., Ganesh, M. R., & Hayakawa, Y. Hydrothermal synthesis of pure and bio modified TiO2: Characterization, evaluation of antibacterial activity against gram positive and gram negative bacteria and anticancer activity against KB Oral cancer cell line. Arabian Journal of Chemistry. 2020, 13(1), 3484-3497. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).