Abstract Bioprospecting native Australian plants offers potential discovery of latent and novel bioactive compounds. Promising cytotoxic and antibacterial activity of methanolic extracts of Pittosporum angustifolium and Terminalia ferdinandiana led to further fractionation and isolation using our laboratory’s bioassay guided fractionation protocol. Hence, the aim of this study was to further evaluate the bioactivity of the fractions and subfractions and characterize bioactive compounds using liquid chromatography mass spectroscopy (LC-MS/MS) and gas chromatography MS (GC-MS). Compounds tentatively identified in P. angustifolium Fraction 1 using LC-ESI-QTOF-MS/MS were chlorogenic acid and/or neochlorogenic acid, bergapten, berberine, 8’-epitanegool and rosmarinic acid. GC-MS analysis data showed the presence of around 100 compounds, mainly comprising of carboxylic acids, sugars, sugar alcohols, amino acids, and monoalkylglycerols. Furthermore, the fractions obtained from T. ferdinandiana flesh extracts showed no cytotoxicity, except against HT29 cell lines, and only Fraction 2 exhibited some antibacterial activity. The reduced bioactivity observed in the T. ferdinandiana fractions could be attributed to the potential loss of synergy as compounds become separated within the fractions. As a result, further fractionation, and separation of compounds in these samples were not pursued. However, additional dose-dependent studies are warranted to validate the bioactivity of T. ferdinandiana flesh fractions, particularly since this is an understudied species. Moreover, LC-MS/GC-MS studies confirm the presence of bioactive compounds in P. angustifolium Fraction 1/subfractions which helps to explain the significant acute anti-cancer activity of this plant. The screening process designed in this study has the potential to pave the way for developing scientifically validated phytochemical/bioactivity information on ethnomedicinal plants, thereby facilitating further bioprospecting efforts, and supporting the discovery of novel drugs in modern medicine.
1. Introduction
Bioprospecting involves a multidisciplinary approach that entails the systematic discovery, isolation, and identification of new bioactive molecules from natural biological reserves, such as plants. Whilst medicinal plants have been used to treat various health conditions for centuries, conventional bioprospecting methods are often time consuming and expensive (Abdallah et al., 2021). Moreover, the complexity of plant matrices and the occurrence of numerous phytochemicals can make the separation and analysis process quite challenging.
The process of separating phytochemicals involves isolating the constituents in the plant extracts or effective parts and purifying them into monomer compounds using physical and chemical methods (Feng et al., 2019). Conventional isolation methods include solvent extraction, precipitation, crystallisation, fractional distillation, salting out, and dialysis. More modern techniques include column chromatography, high performance liquid chromatography, ultrafiltration, and high-performance liquid droplet counter current chromatography. There are several chromatographic techniques available for the identification and quantification of phenolic compounds in plants, including thin layer chromatography (TLC), gas chromatography (GC), high performance liquid chromatography alone or coupled with mass spectrometry (MS), capillary electrophoresis (CE) (2, 26–29) and micellar electro- kinetic chromatography (MEKC) (Nour et al., 2013).
Phenolic compounds are a highly complex class of naturally occurring molecules that possess a range of therapeutic properties. As a result, significant interest has been devoted to their analysis in medicinal plants and food samples. High performance liquid chromatography (HPLC) is the most commonly utilized separation technique for this purpose (Mekky et al., 2019; Naiker et al., 2020; Sochor et al., 2010; Zanatta et al., 2021).
High performance liquid chromatography equipped with a fraction collector was the analytical tool of choice for the separation and isolation of fractions/subfractions of extracts in this study due to its characteristic features of high efficiency, speed, and automation. The separation of compounds is based on the principles of the adsorptive capacity of column stationary phase to different compounds, molecular size of the compounds, difference of dissociation degrees of the chemical constituents, and different partition coefficients between stationary and mobile phase (Feng et al., 2019).
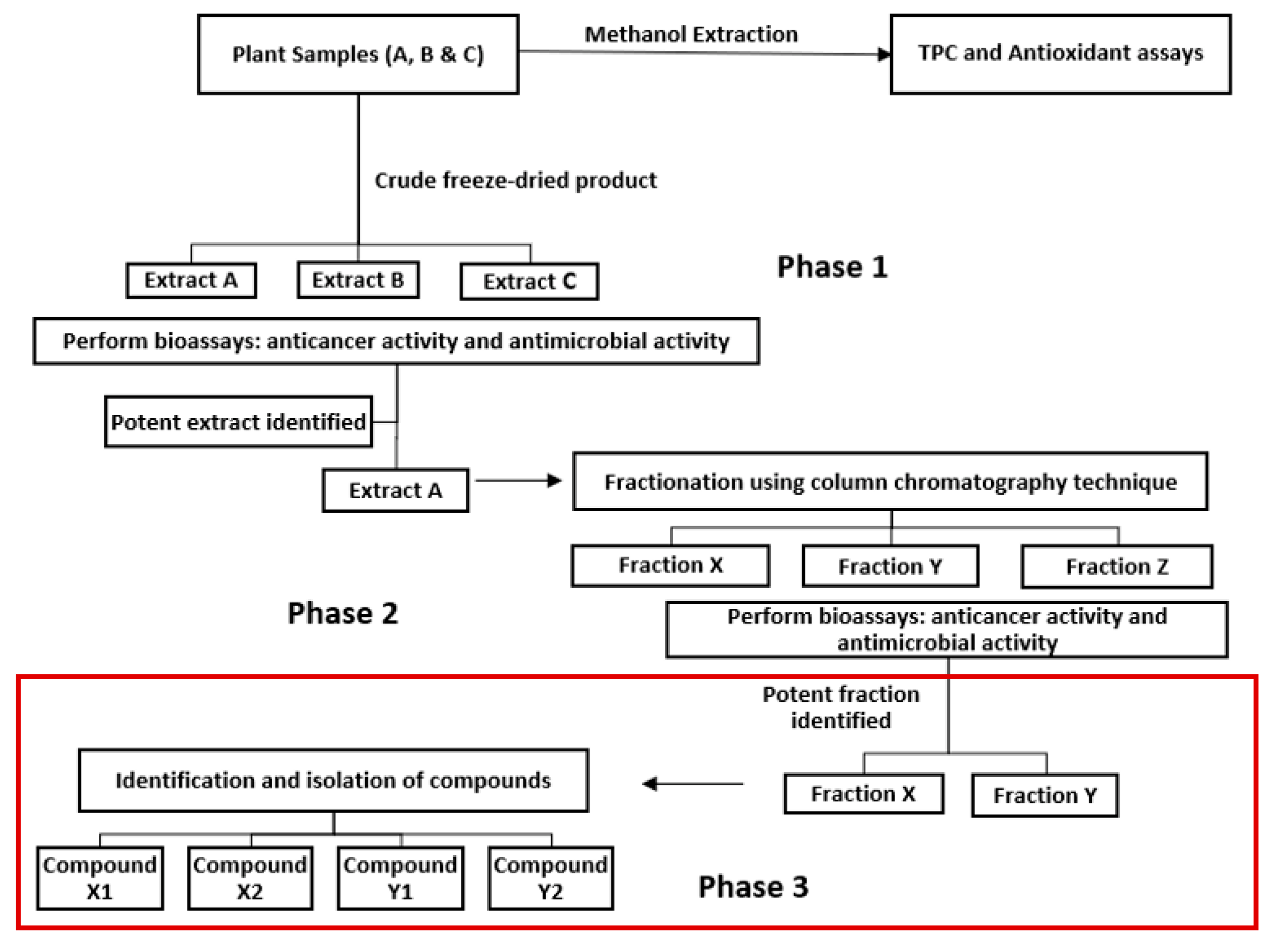
Based on the findings from our previous study (Mani, et al., 2022), the objective of this study was to further fractionate Fraction 1 of
P. angustifolium methanolic crude extracts into subfractions, utilizing phase 3 of the proposed bioassay guided fractionation protocol design (
Figure 1), and to perform LC-MS and GC-MS analysis for compound characterisation. Additionally, since
T. ferdinandiana flesh crude extracts had also shown some therapeutic potential, fractionation and bioassay testing on the fractions were also included.
2. Materials and Methods
2.1. Reagents
Hydrochloric acid and sodium carbonate were purchased from Chem-Supply (Australia). All other reagents including the HPLC grade methanol, were purchased from Sigma-Aldrich (Australia). Some reagents used in the cytotoxicity analysis which included the CellTiter 96® AQueous Assay (composed of solutions of tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)−5-(3-carboxymethoxyphenyl)−2-(4-sulfophenyl)−2H-tetrazolium, inner salt; MTS(a)] and an electron coupling reagent (phenazine methosulfate; PMS), commonly known as MTS reagent, and foetal bovine serum (FBS) were obtained from Promega (United States of America) and Scientifix (Australia), respectively. The Dulbecco's Modified Eagle's Medium - high glucose (DMEM), Dulbecco's Phosphate Buffered Saline (DPBS) solution was kept in the dark at 4°C, while the other reagents used in the bioassays were frozen until required for use. All dilutions and assay preparations used Milli-Q water. All reagents used were of analytical grade or higher purity.
2.1. Sample extraction
Approximately 2.5 g of powdered plant material was extracted in 75 mL of 90 % methanol as previously detailed (Mani, et al., 2022). The supernatant obtained was filtered using 0.45 µm Advantec filter paper and evaporated under reduced pressure at 27°C to a semi-solid consistency using a rotary evaporator. The semi-solid product was redissolved in approximately 25 mL Milli-Q water and freeze-dried under vacuum (Flexi-Dry Freeze-dryer, -47°C, 277 mTorr) for 72 h to obtain a fine lyophilized product, which was stored at 4°C in the dark until required.
2.2. HPLC fractionation and sub-fractionation of P. angustifolium extract
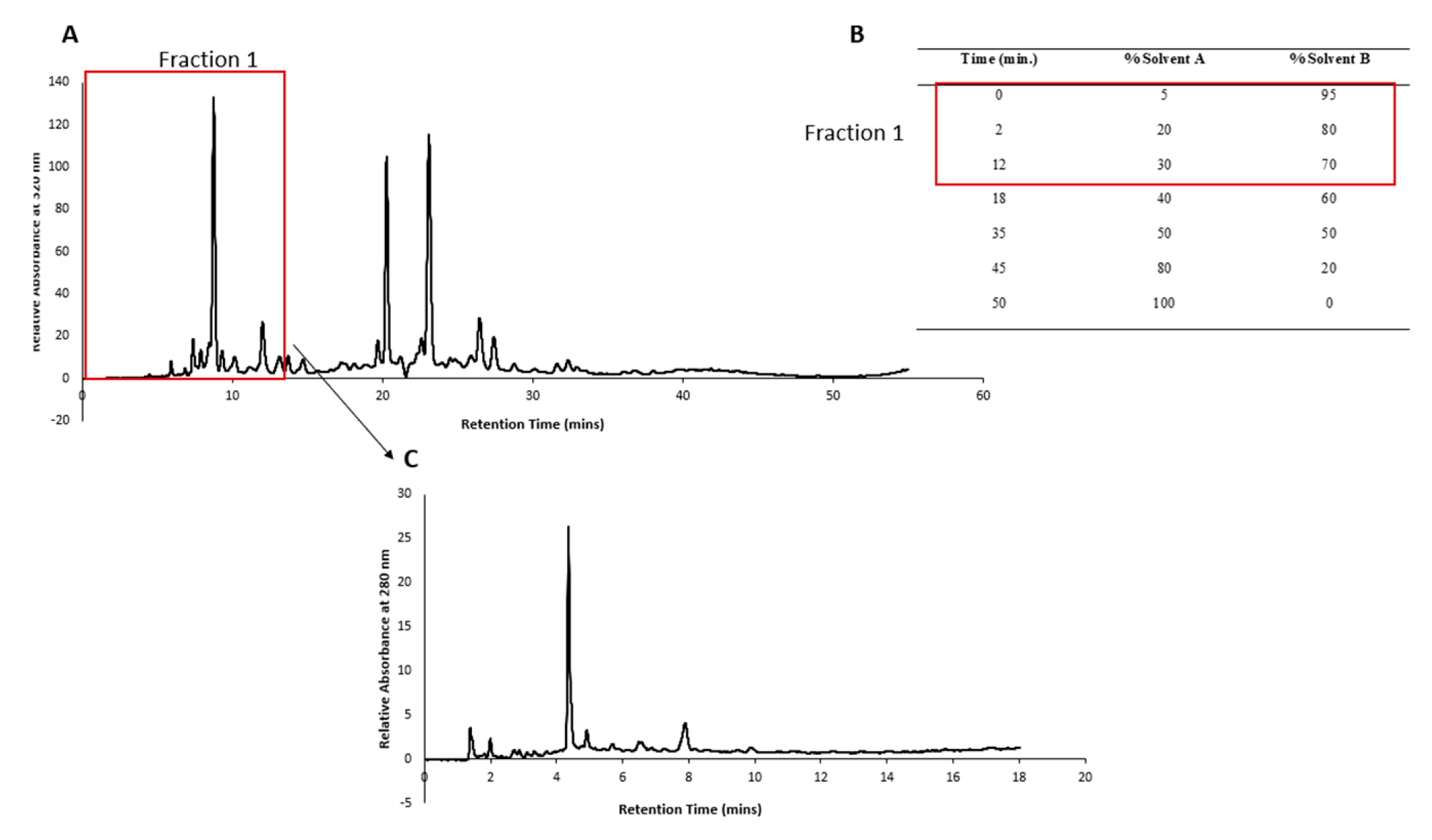
The HPLC conditions mentioned in our previous publication (Mani, et al., 2022) were followed with slight modifications. Briefly, a reversed-phase C18 column (Agilent Eclipse XDB-C18; 150 × 4.6 mm; 5 µm pore size) and guard cartridge (Gemini C18 4 × 2 mm) with an injection volume of 30 µL and a run time of mins with post run time of 5 mins was allowed for column flushing. The time slicing feature of the Agilent fraction collector was used to collect only Fraction 1 (0 to 12 min) from 20 mg mL
-1 of
P. angustifolium lyophilized product. The volume collected after multiple runs was then rotary evaporated to a semi-solid consistency and reconstituted in 30 mL Milli-Q water. This was then placed at -80 °C overnight and then freeze-dried for 72 hours. A crystalline product of a mass of 87.9 mg was obtained. The HPLC chromatogram of
P. angustifolium Fraction 1 is depicted in
Figure 2.
The crystalline product obtained from Fraction 1 was redissolved in Milli-Q water at a concentration of 43.95 mg mL
-1 and subjected to HPLC fractionation using gradient elution, as described in
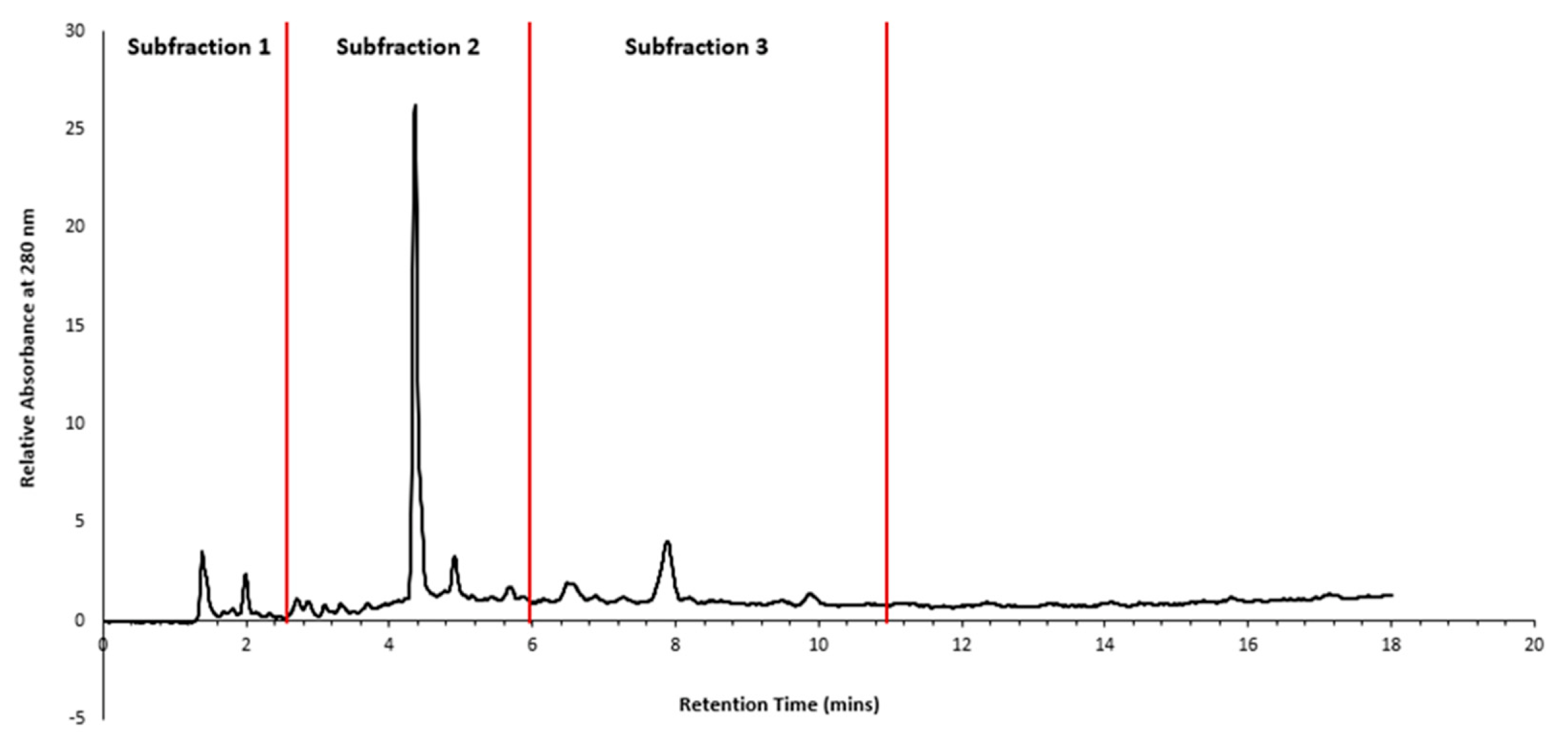
Figure 2 (B), and an injection volume of 30 µL. Retention time zones showing predominant peaks were selected for time slicing, and fractions were collected from 0-3 mins (Sub-fraction 1), 3-6 mins (Sub-fraction 2), and 6-11 mins (Sub-fraction 3), as depicted in
Figure 3.
2.3. HPLC fractionation of T. ferdinandiana flesh extract
The same HPLC conditions as described above (
Section 2.2) and our previous publication (Mani, et al., 2022), was followed with slight modifications to the gradient elution and injection volume. The gradient elution described in
Figure 2 (B) was used, and a sample injection volume of 30 µL was applied. A total run time of 50 mins was allowed to ensure that all eluents were captured in the chromatogram, and a post run time of 10 mins was allowed for column flushing.
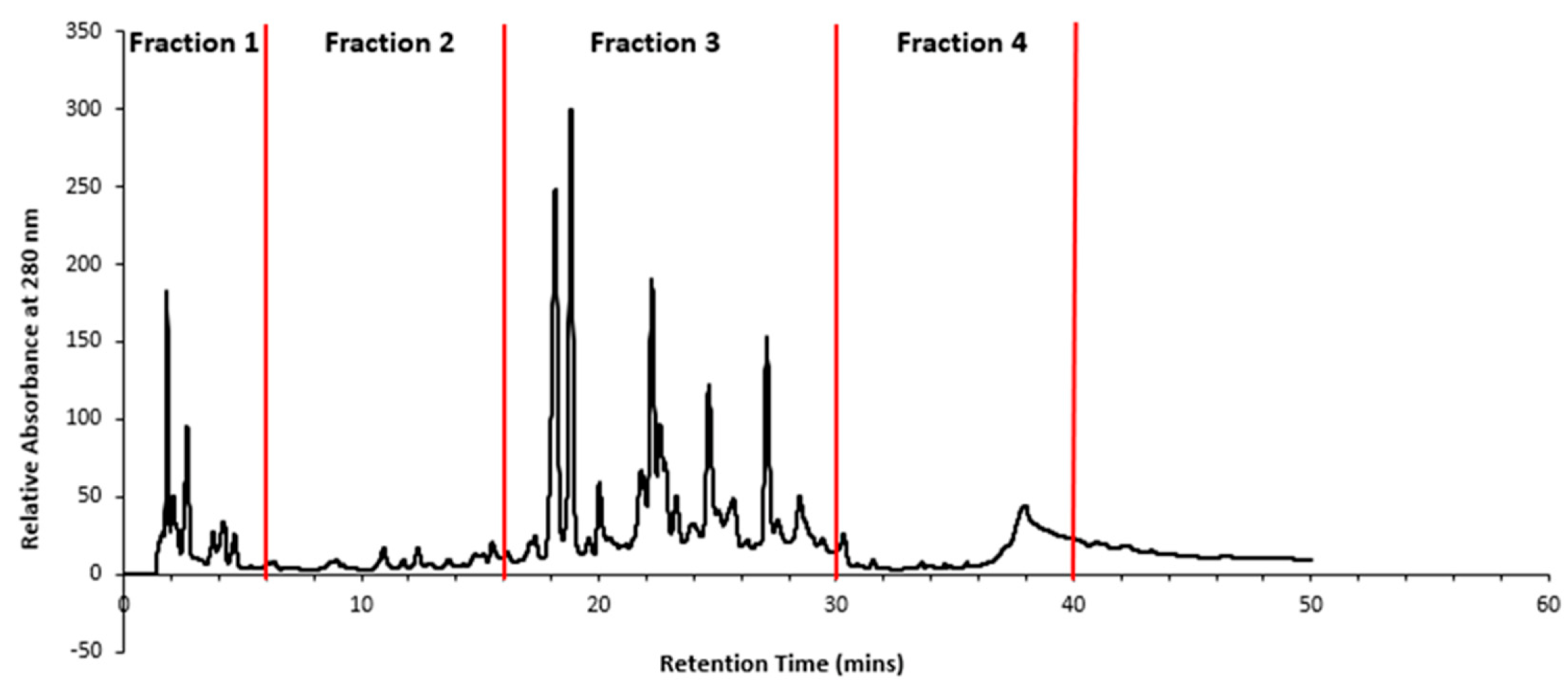
Retention times showing predominant peaks were selected for time slicing and collection of fractions from 0-6 mins (Fraction 1), 6-16 mins (Fraction 2), 16-30 mins (Fraction 3) and 30-40 mins (Fraction 4), as depicted in
Figure 4.
2.4. Cytotoxicity Assay
The cytotoxicity of the subfractions of P. angustifolium Fraction 1 and fractions of T. ferdinandiana were assessed against HeLa (human cervical carcinoma), HT29 (human colorectal carcinoma), HuH7 (human liver carcinoma) and PH5CH8 (human epithelial cell), obtained from the University of Adelaide, using MTS assay previously described (Mani, et al., 2022). However, it was observed that the proliferation and general health of the HuH7 cells were compromised, and they were nonviable for use in the cell culture assay of T. ferdinandiana fractions in the later trials.
2.5. Antimicrobial Activity
The antimicrobial activity of T. ferdinandiana Fractions (1–4) were tested against the four bacterial strains (Gram positive -Staphylococcus aureus and Gram negative -Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa) following the disk diffusion method (Mostafa et al., 2018) with slight modification as described previously (Mani, et al., 2022).
2.6. LC-MS/MS Analysis of P. angustifolium fraction
The methanolic extract of P. angustifolium was analyzed for targeted phenolic compounds using liquid chromatography tandem mass spectroscopy (LC-MS/MS). The analysis was performed using a Nexera X2 chromatography system, which was coupled with a Shimadzu LCMS-8040 system comprising of a CBM-20A communications bus module, DGU-20A5R degassing unit, LC-30AD pumps, SIL-30AC autosampler, and CTO-20AC column oven. The analytical method used a Raptor biphenyl column (100 mm ꓫ 2.1 mm, 2.7 µm), 5 µL injection volume, 40˚ C column temperature and flow rate of 0.6 mL min-1. The mobile phase comprised water (phase A) and methanol (phase B), each containing 5 mM ammonium formate and 0.1% formic acid. The eluent was directed to the electrospray ionization (ESI) module.
Shimadzu LCMS-8040 model triple quadrupole mass spectrometer, equipped with an electrospray ionization (ESI) source was used to perform targeted tandem mass spectrometry on the eluting compounds. Both positive and negative ionization modes were used depending on the ionization characteristics of each analyte. The ESI conditions used were interface temperature of 350 °C, DL (Dissolution line) temperature 250 °C, and 400 °C in ESI source. Nitrogen was used as the nebulizing gas and drying gas, at flow rates of 3 L min-1 and 15 L min-1, respectively and the interface voltage used was 4.50 kV. The LC-MS/MS data were collected and analysed in the LabSolutions software (Shimadzu, Kyoto, Japan). The PubChem database and offline version (accessed 21 March 2023) of the National Institute of Standards and Technology (NIST) Library was used to match the MS/MS spectra of phenolic compounds in the P. angustifolium extract.
2.7. GC-MS/MS Analysis of P. angustifolium fraction 1 and subfractions
All four samples were dissolved in 1 mL chilled MS grade water and mixed via vortex and kept on ice throughout processing where possible. Twelve aliquots were created for each sample. Three aliquots each of 1 μL, 10 μL, 100 μL and 200 μL were generated by transferring the sample into glass vial inserts. The aliquots were then frozen prior to lyophilisation at 1 mbar (room temperature) for 3.5 hrs until dry. The sample aliquots were then sealed in auto sampler vials and stored at -20 °C until analysis.
Samples were analysed on a Shimadzu TQ8050 NX system following automated trimethylsilyl (TMS) derivatisation using an AOC 6000 plus auto-sampler. The derivatisation was accomplished by adding 25 μL of 30 mg mL-1 methoxyamine hydrochloride in pyridine to 10 μL of dried metabolite extract. The samples were then incubated at 37 °C for 2 hrs with continuous agitation. Following this, 25 μL of N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) was added, with further incubation at 37 °C for 1 hr with continuous agitation. Derivatised sample was then incubated at room temperature for 1 hr prior to injection. One microliter of the derivatised samples were injected onto the GC-MS/MS by the auto-sampler at a split ratio of 5:1 and a constant flow of 1.10 mL min-1 and the oven temperature was maintained at 100˚C. The samples were analysed in Multiple Reaction Monitoring (MRM) mode using the Shimadzu Smart Metabolite Database containing 521 MRM metabolite targets. Prior to each sample being analysed, four hexane blanks were analysed to ensure there was no carry over between samples.
Data produced from the analysis of samples was extracted using LabSolutions Insight software. Metabolites that were within ± 0.1 min of the predicted retention time and within ± 25% of the relative ion ratio had their area under the curve reported for further statistical analysis. Where metabolites did not need these criteria, the area under the curve was not reported.
2.8. Statistical Analysis
All data were presented as means ± SEM for triplicate samples. Statistical analyses were performed using RStudio version 2022.12.0-353 software, with a one-way analysis of variance to determine the significance between the control and treated groups. A value of p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Pittosporum angustifolium sub fractions
3.1.1. Cytotoxic Activity and HPLC Profiling
The sub-fractionated products obtained from
P. angustifolium Fraction 1 (GGLX F1 S1-3) were subjected to an anticancer bioassay, and the results are presented in
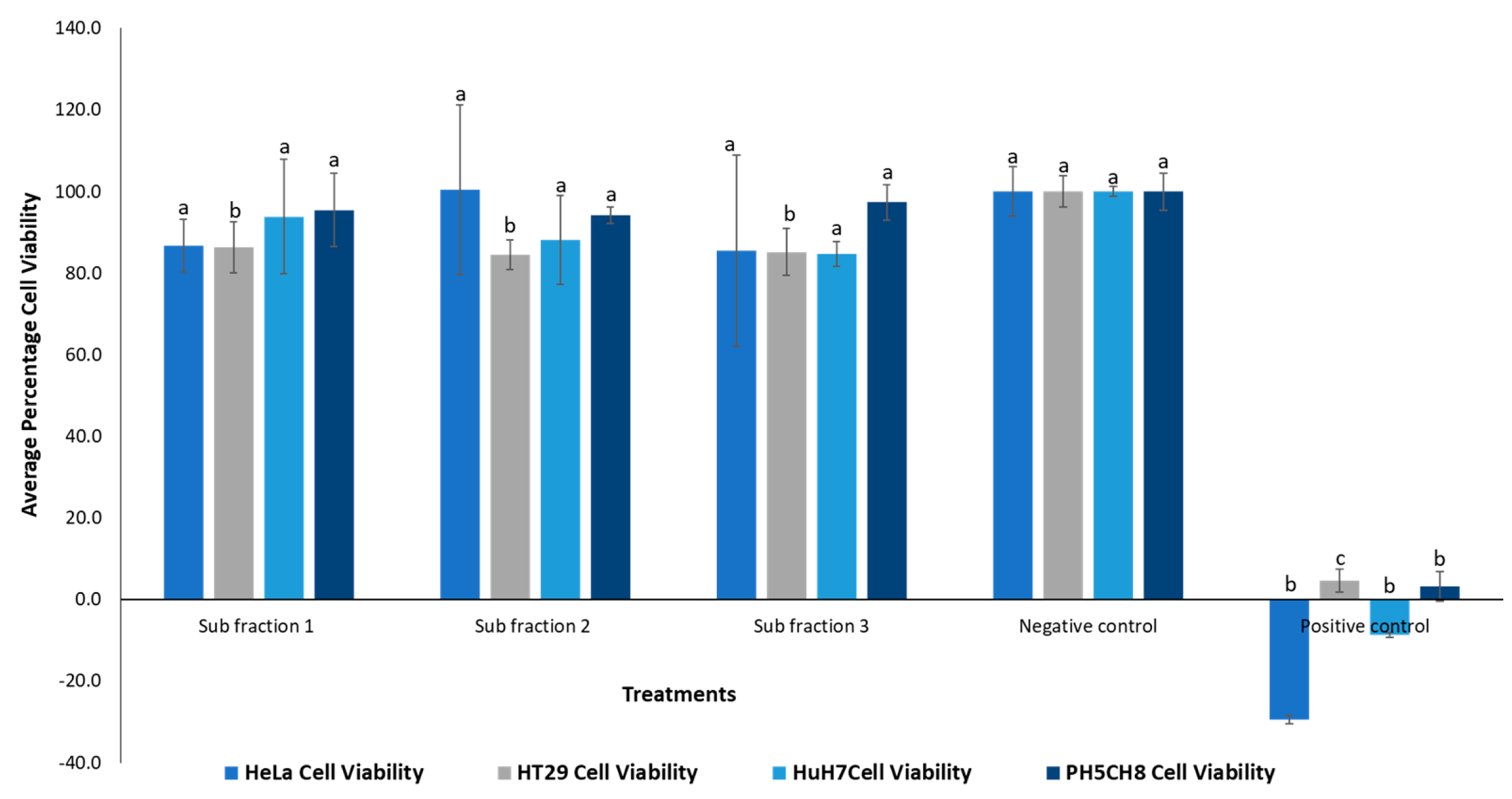
Figure 6. There was no significant difference (p > 0.05) in the percentage cell viability between of the treated cells and negative control for all the tested cell lines, except for the HT29 cells (p < 0.05), where all three subfractions demonstrated some cytotoxic activity.
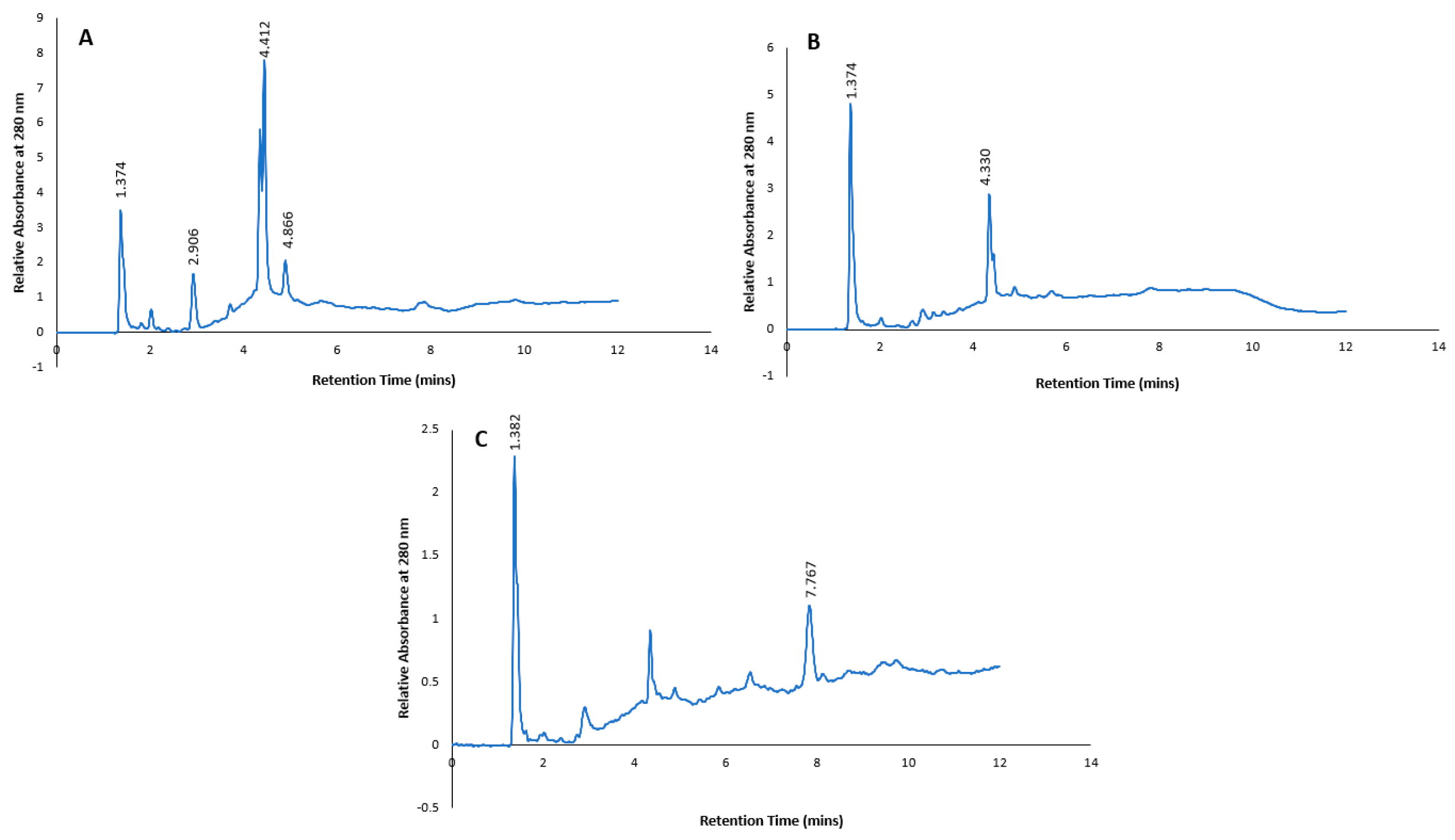
The low bioactivity observed in the subfractions may be attributed to the low doses of compounds present in these subfractions, as indicated by low peak signals in the HPLC chromatograms shown in
Figure 7. Additionally, the cytotoxic effect observed in the methanolic extracts of
P. angustifolium and its fractions may be due to the synergistic action of different compounds. On the other hand, the subfractions contain perhaps fewer isolated compounds, which may explain the low or no cytotoxicity observed.
Although numerous studies have identified triterpenoid saponins, terpenoids, phenols, and coumarin compounds isolated from P. angustifolium as having potential anticancer properties (Bäcker, et al., 2014a; Beh & Teoh, 2022; Blonk & Cock, 2019; Phan et al., 2020; Winnett et al., 2017), only a few have been shown to be effective in vitro. Backer et al (2016) screened ten acylated saponins for their ability to inhibit human DNA-topoisomerase I, an enzyme responsible in resolving torsional stress associated with DNA replication, transcription, and chromatin condensation. Inhibitors of DNA-topoisomerase I can inhibit the proliferation of cancer cells, and such agents are commonly used in chemotherapy for their antiproliferative effects. However, their effects on the metastasis of cancer cells remain unclear (Liu et al., 2019).
In previous work, Backer et al (2015) isolated two new taraxastane-type triterpene saponins, which were evaluated against four cell lines. However, no cytotoxic activity was observed up to a concentration of 130 µM. In a similar sub fractionation study of Syzygium polyanthum (Wight.), the crude methanol extract showed higher bioactivity in terms of hypoglycemic effect compared to the fraction, subfraction, and squalene (the major chemical compound and a triterpene isolated from S. polyanthum leaf extract) (Widyawati et al., 2022). Therefore, the cytotoxic properties of P. angustifolium could possibly be from a synergistic effect, similar to that seen in other studies.
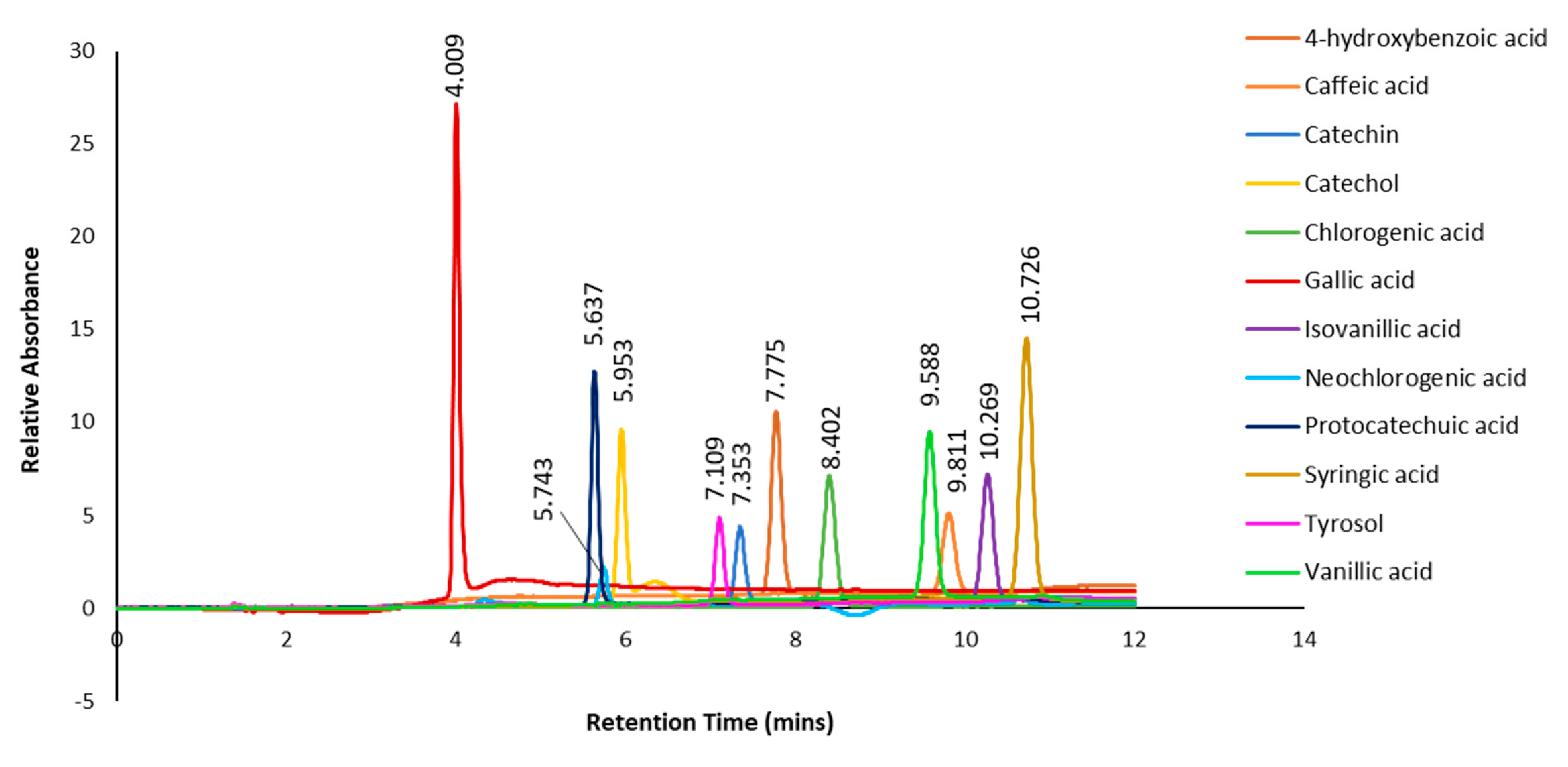
In addition, authentic standards of selected polyphenols (4-hydroxybenzoic acid, caffeic acid, catechin, catechol, chlorogenic acid, gallic acid, isovanillic acid, neochlorogenic acid, protocatechuic acid, syringic acid, tyrosol and vanillic acid) were subjected to the same HPLC gradient elution as the subfractions, and the combined chromatograms with retention times are shown in Figure 8. When compared to the retention times of the predominant peaks in the chromatograms of the subfractions (Figure 7), only one peak at retention time 4.412 min in subfraction 1 could tentatively be identified as gallic acid based on similar UV spectrum as the standard. However, there were some discrepancies between the retention time of the standard gallic acid (4.009 mins) and the subfraction (4.412 mins). In subfraction 2, none of the retention time peaks or UV spectra matched any of the authentic standards. On the other hand, the retention time of a predominant peak at 7.768 mins in subfraction 3 was tentatively identified as 4-hydroxybenzoic acid, as similar retention time (7.775 mins) and UV spectrum was evident (Figure 8).
As the subfractions of P. angustifolium did not show any significant cytotoxic activity, further separation and isolation was deemed impractical and hence were not pursued.
3.1.2. LC- MS Analysis of P. angustifolium Fraction 1
While the subfractions of P. angustifolium fraction 1 did not demonstrate significant cytotoxic properties or contain compounds of interest, fraction 1 was found to be effective in reducing cell viability in the tested cancer cell lines, and it was also safer in terms of its selectivity index compared to the other fractions (Mani et al., 2022). Moreover, given the possibility of a synergistic effect at play in dictating the cytotoxic behaviour of P. angustifolium fraction 1, characterizing the potential phenolic metabolites in the fraction was considered a valuable pursuit.
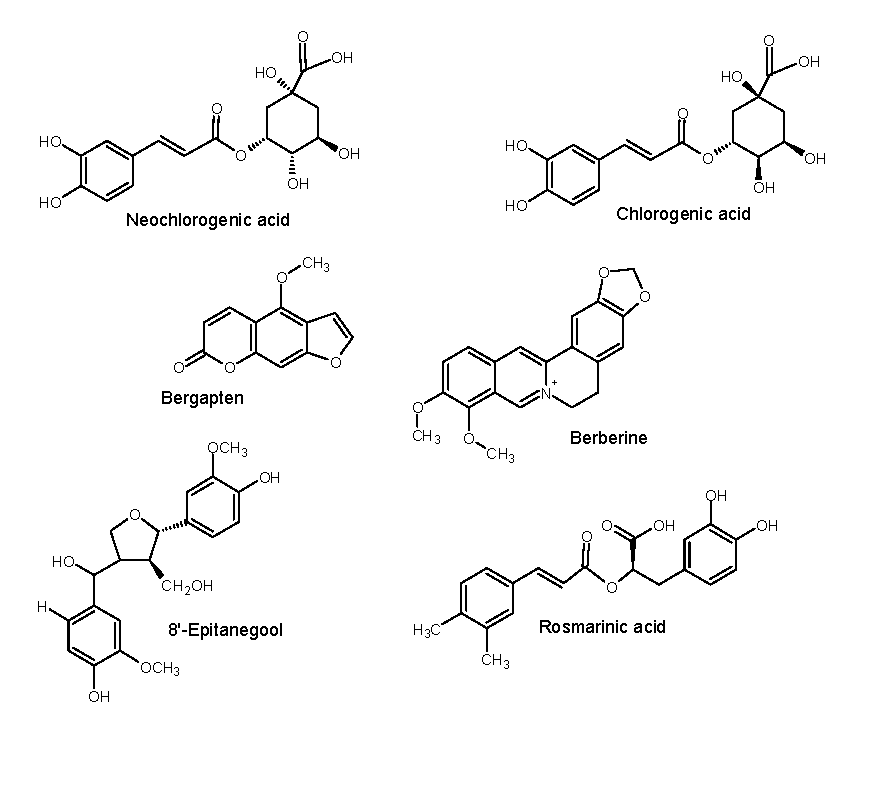
Using LC-ESI-QTOF-MS/MS, untargeted screening, and characterization of phenolic compounds in Fraction 1 was performed. The obtained MS/MS spectra were compared with NIST and PubMed database libraries, as well as published literature, to putatively confirm the presence of phenolic metabolites (Table 3). Among ten prominent peaks, only peaks 1, 4, 7-9 were tentatively identified (Figure 9) in Fraction 1.
Figure 9.
Proposed phenolic metabolites in Pittosporum angustifolium Fraction 1.
Figure 9.
Proposed phenolic metabolites in Pittosporum angustifolium Fraction 1.
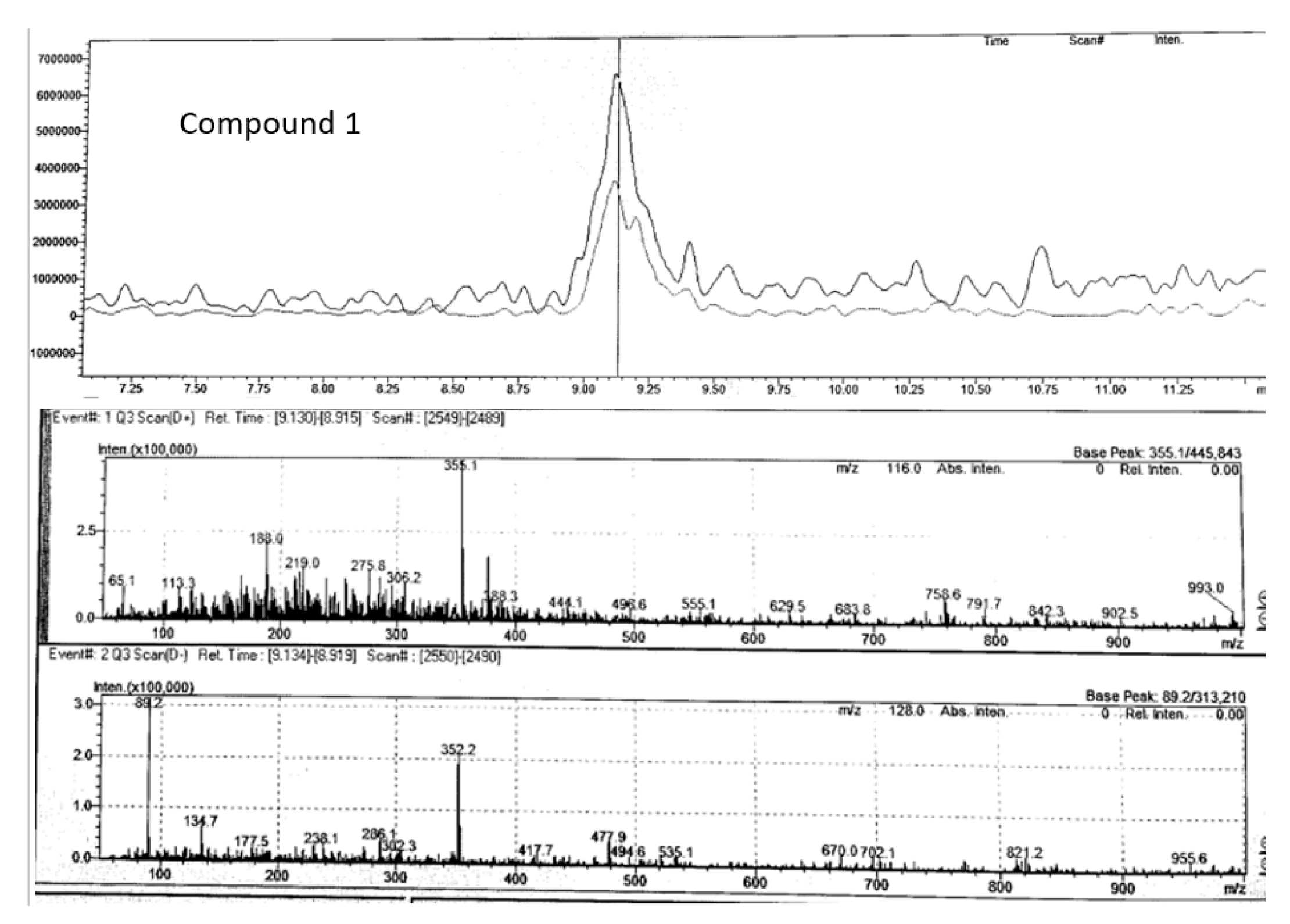
Compound 1 (
m/z 355.10) chromatogram and mass spectrum as shown in
Figure 10 was identified as either chlorogenic acid or its isomer, neochlorogenic acid, both of which belong to the caffeoylquinic acid class of molecules. These compounds are known for their strong antioxidant, anticancer, anti-inflammatory, and antifungal properties (Jiao et al., 2018). Previous studied have also identified chlorogenic acid in
P. angustifolium leaves (Bäcker, Jenett-Siems, Bodtke, et al., 2014; Beh & Teoh, 2022; Phan et al., 2020). Additionally, our previous work have suggested the presence of chlorogenic acid in crude MeOH extracts of
P. angustifolium leaves (Mani, et al., 2022; Mani, et al., 2022a).
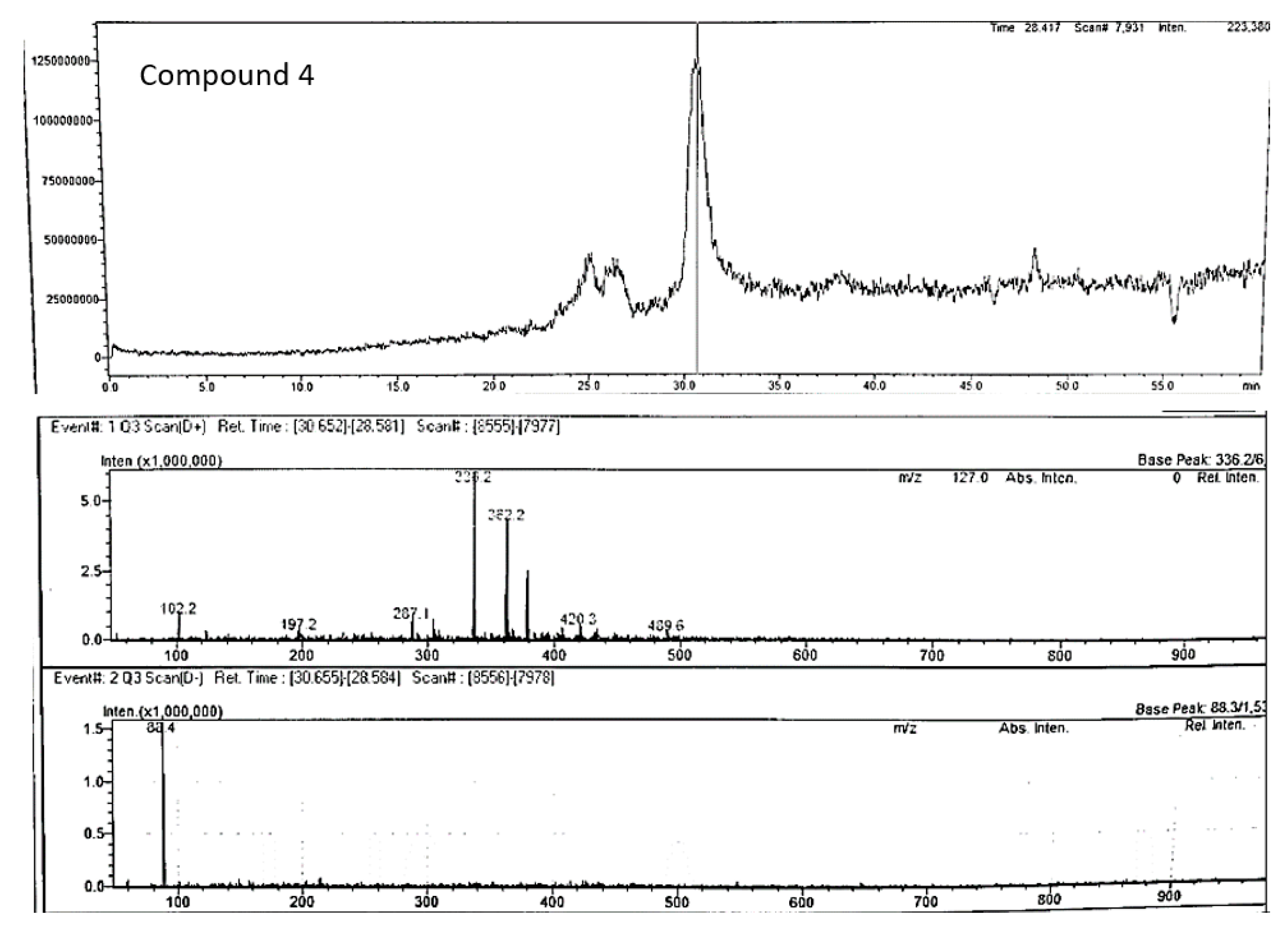
Compound 4 (m/z 336.20) chromatogram and mass spectrum as shown in
Figure 11 was tentatively identified as berberine (2,3-methylenedioxy-9,10-dimethoxyprotoberberine chloride), a benzyl tetra isoquinoline alkaloid. Berberine been previously extracted from roots of various plants such as Berberis vulgaris, B. aristotle, B. aquifolium, Hydrastus canadensis, Pellodendron chenins, and Coptidis rhizomes (Rauf et al., 2021). Numerous authors have reported the broad spectrum therapeutic potential of berberine due to its action against diabetes, hypertension, depression, obesity, inflammation, and cancer (Jang et al., 2008; Kulkarni & Dhir, 2010; Rauf et al., 2021; Samadi et al., 2020). However, this is the first study to speculate the occurrence of berberine in P. angustifolium. Therefore, further investigation to confirm this finding is warranted.
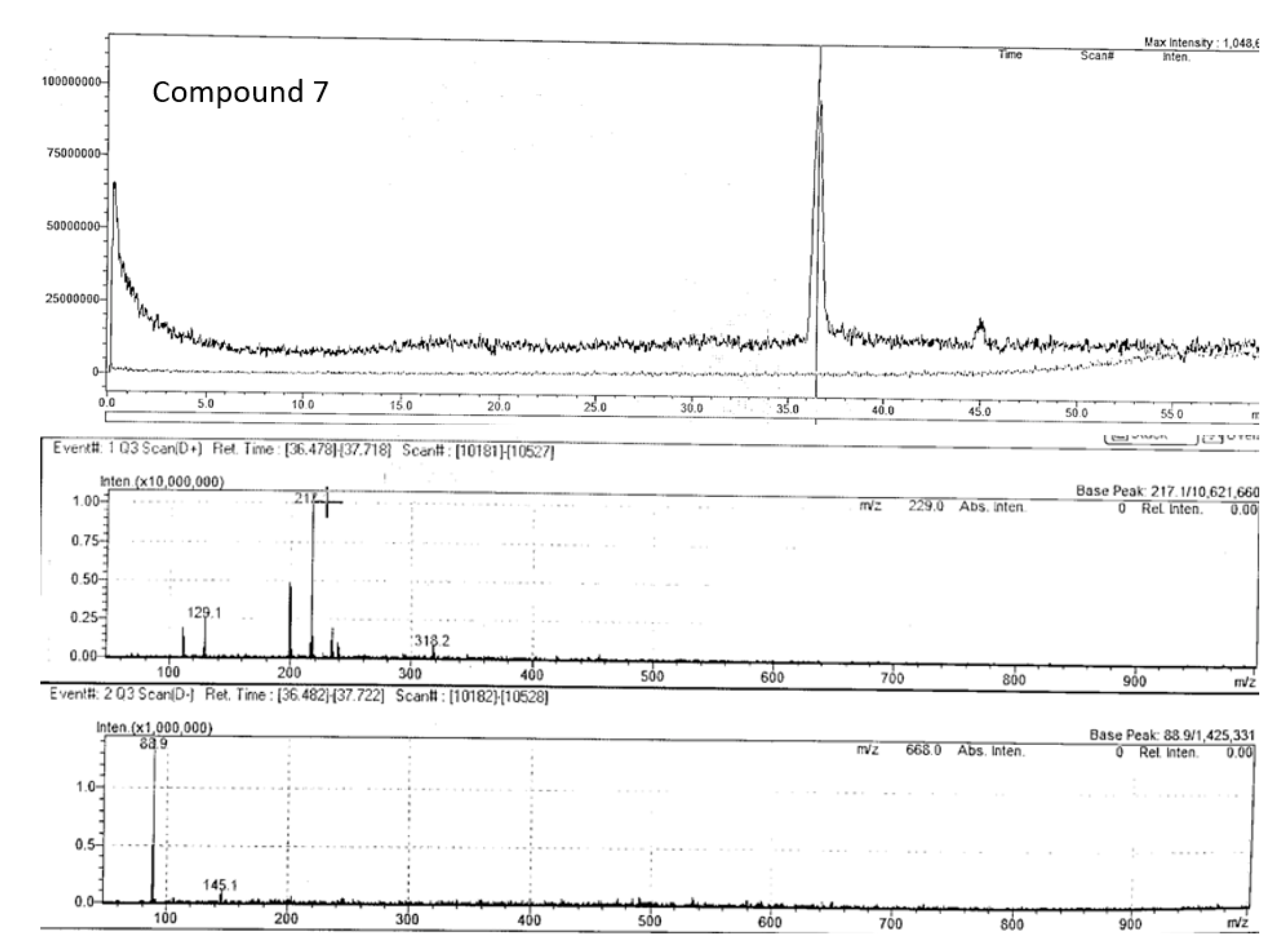
Compound 7 (m/z 217.10) chromatogram and mass spectrum as shown in
Figure 12 was identified as bergapten
(5-methoxypsoralen), which belongs to the class furocoumarin. This furanocoumarin derivate is commonly found in bergamot essential oil, other citrus essential oils, and grapefruit juice, as well as in a wide variety of medicinal plants from the
Rutaceae and
Umbelliferae families such as figs, parsley, celery, and anise (Quetglas-Llabrés et al., 2022)
. Pharmacological studies have shown that bergapten has various properties, including neuroprotection, organ protection, anticancer, anti-inflammatory, antimicrobial, and antidiabetic effects (Liang et al., 2021; Quetglas-Llabrés et al., 2022). However, this is the first to report its potential occurrence in P. angustifolium, and further investigations are required to confirm this claim.
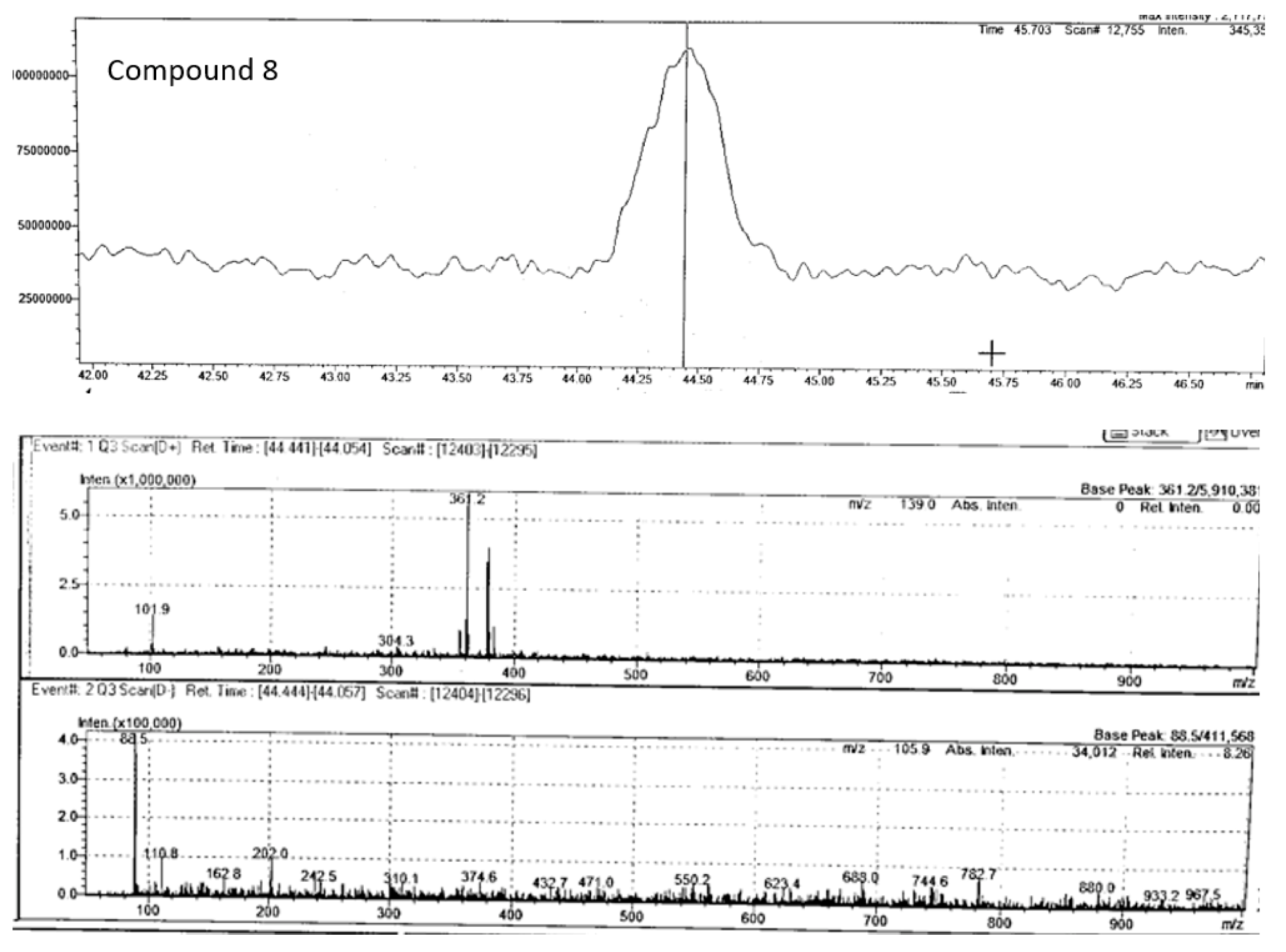
Compound 8 (m/z 361.20) chromatogram and mass spectrum as shown in
Figure 13 was tentatively identified as rosmarinic acid, which belongs to the hydroxycinnamic acid class of phenolic acids and is commonly found in fruits, herbs and medicinal plants (Ali et al., 2022). It is produced by
Boraginaeceae and subfamily
Nepetoideae of the
Lamiaceae plant species. Initially, it was extracted as a pure compound from rosemary (
Rosmarinus officinalis) (Nunes et al., 2017). This compound has shown potent biological activities in combating human diseases such as cancer, diabetes, neurodegenerative disorders, cardiovascular disease, and inflammatory disorders (Noor et al., 2022). While this is the first study to tentatively report its occurrence in
P. angustifolium, further investigation is needed to confirm this finding.
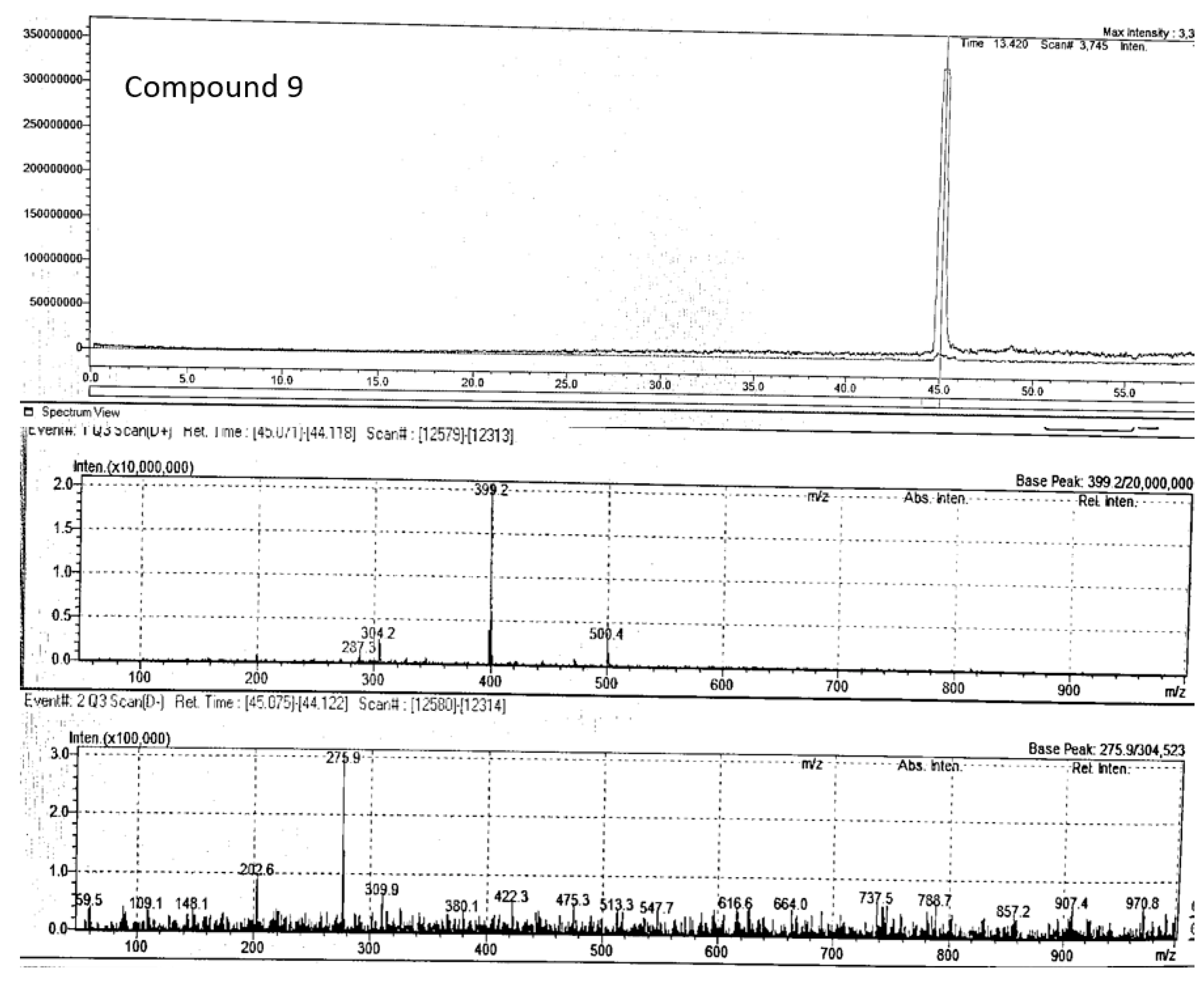
Compound 9 (m/z 403.2) chromatogram and mass spectrum as shown in
Figure 14 was tentatively identified as 8'-epitanegool, classified as phenylpropanoids. Previous study has demonstrated promising
in silico antiviral results similar to the main alkaloids (Omar et al., 2023). This compound has been previously identified in
Tinospora sinensis, a type of Chinese folk medicine (Jiao et al., 2018), and literature on its therapeutic potential is limited or non-existent. Additionally, this is the first study to tentatively identify this compound in
P. angustifolium.
If the identities of these compounds are confirmed in Fraction 1 of P. angustifolium, then it will not only strongly support its antioxidant and anticancer properties, as determined in this study, but also the anecdotal claims of Indigenous Australians (Phan et al., 2020). Furthermore, detailed studies to confirm the identity of these compounds and/or to discover other bioactive compounds in P. angustifolium crude, fractions and subfractions were conducted using GC-MS.
3.1.3. GC- MS Analysis of Pittosporum angustifolium Fraction 1 and sub-fractions
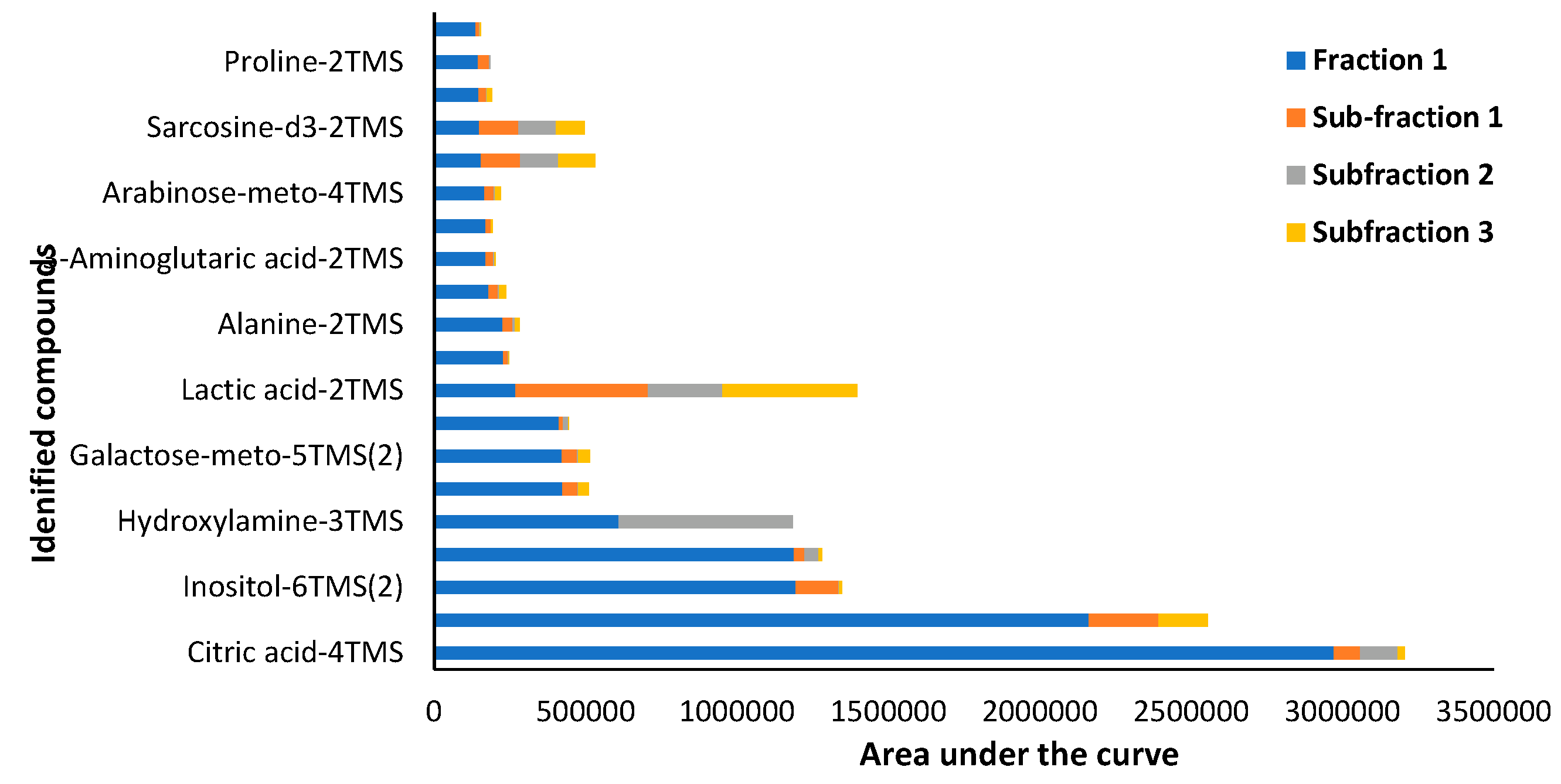
Targeted GC-MS/MS analysis in MRM acquisition mode identified a total of 103 compounds, belonging to various classes of primary and secondary plant metabolites. The main primary metabolites identified are classified as carbohydrates, amino acids, proteins, lipids, purines and pyrimidines of nucleic acids. On the contrary, the secondary metabolites identified were classified into three main groups; (a) nitrogen-containing compounds such as alkaloids, glucosinolates, and cyanogenic glycosides, (b) phenolic compounds such as phenylpropanoids and flavonoids, and (c) terpenes (Rabizadeh et al., 2022). However, only twenty predominantly found compounds in Fraction 1 and Fraction 1 subfractions are reported in
Figure 15 and
Table 4 which mainly belong to the class of carbohydrates and amino acids.
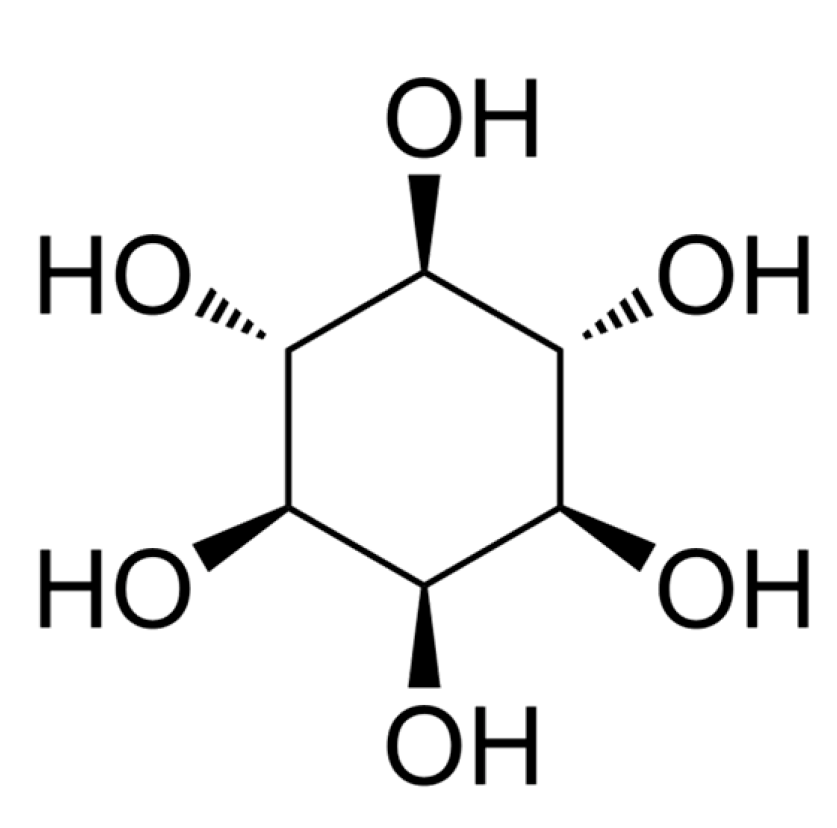
Inositol (
Figure 16), the third most abundant compound identified in Fraction 1 was of interest due to its previously reported anti-atherogenic, anti-oxidative, anti-inflammatory and anti-cancer properties (Siracusa & Napoli, 2022). Clinical trials using inositol in pharmacological doses have shown promising results in the management of gynaecological diseases, respiratory stress syndrome, Alzheimer’s disease, metabolic syndrome, and cancer (Bizzarri et al., 2016). Inositol occurs naturally in all eukaryotes and is involved in several biological processes (Siracusa & Napoli, 2022). In mammals inositol is produced in the liver and kidney and myo-inositol (inositol isomer) and its MDPIderivatives in particular are involved in biological functions which include modulation of glucose metabolism, calcium release in cell signalling, chromatin and CSK remodelling, gene transcription, proliferation, apoptosis, and proper structural development (Bizzarri et al., 2016). In plants, inositol is well known for acting a stress ameliorator and controls multiple aspects of plant signalling and physiology (Amaral & Brown, 2022). On this premise the cytotoxic effects of Fraction 1 may likely be due to the predominant occurrence of inositol. However, in our knowledge this study is the first to report the occurrence of inositol in
P. angustifolium and thus further investigation are required to confirm this finding.
3.2. Terminalia ferdinandiana Fractions
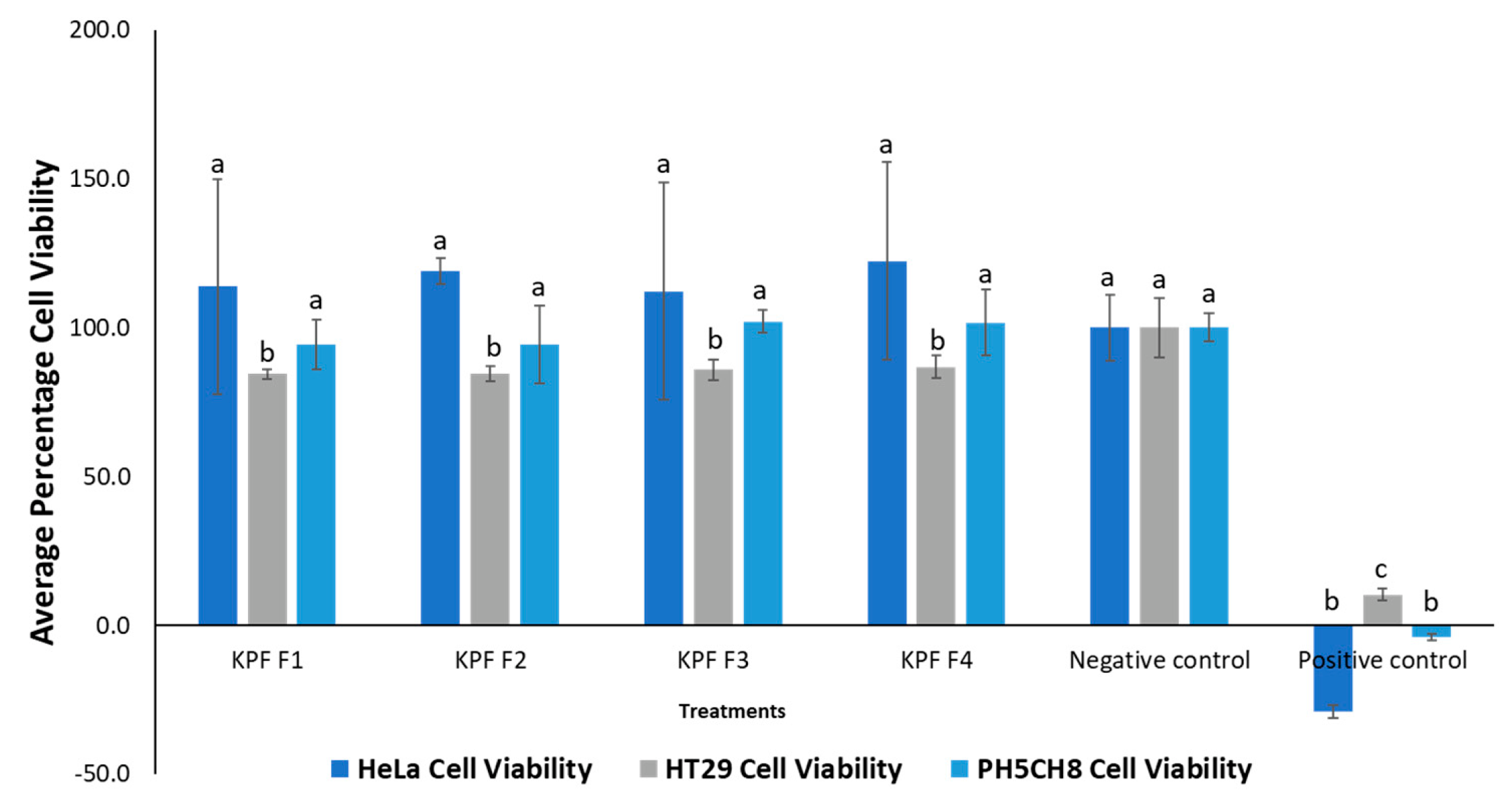
3.2.1. Cytotoxic Activity
The lyophilised methanolic flesh extract of
T. ferdinandiana was fractionated into four fractions, as listed in
Table 5. The cytotoxicity of these fractions was evaluated against HeLa, HT29 and PH5CH8 cell lines using the concentrations given in
Table 5. The results of this experiment are presented in
Figure 17.
None of the fractions showed significant (p > 0.05) cytotoxic activity against HeLa and PH5CH8 cell lines, except for slight cytotoxicity observed against the HT29 cell line. This cytotoxicity was significantly different from the negative control (p < 0.05). To minimize matrix effect, lower doses of the fractions were used in the bioassay. However, in future studies, higher doses could be utilized to investigate potential higher toxicity.
While previous literature (Akter et al., 2018; Deo et al., 2016; Tan, Konczak, Ramzan, & Sze, 2011) has shown cytotoxic properties of T. ferdinandiana , there are limited studies on their fractions (Tan, et al., 2011). As such this study is crucial in paving the way for future fractionation studies of this species.
3.2.2. Antibacterial Activity
Considering the promising antibacterial activity demonstrated by the crude flesh extract of T. ferdinandiana (Mani et al., 2022), the fractions were also evaluated against four bacterial strains. The results are presented in
Table 6.
The antibacterial activity exhibited by the fractions was relatively mild, and in some cases, (Fraction 3), no activity was observed. Fraction 2 demonstrated the highest activity and was the only fraction that inhibited the growth of all tested bacterial strains compared to the other fractions, possibly because it was the most concentrated fraction. Overall, even though the flesh extracts of T. ferdinandiana fruit have previously demonstrated antibacterial activity in several studies (Akter et al., 2021; Gorman et al., 2019; Noé et al., 2019) including our previous studies (Mani et al., 2022), the low activity of the fractions suggests that the bioactive compounds in the lyophilised extract may work synergistically to produce its antibacterial property.
Despite the small amount of existing literature on the therapeutic potential of T. ferdinandiana, this study provides the first report on the bioactivity of its fractions. However, further rigorous testing in necessary to validate the data obtained, especially in terms of dose-dependent anticancer and antibacterial effects. Therefore, more elaborate investigation on this understudied native fruit is warranted.
4. Conclusion
Whilst P. angustifolium crude leaf extracts and fractions demonstrated strong cytotoxic activities, no significant activity was evident in the subfractions. Thereby suggesting that the bioactivity may be attributed to the synergistic effect of the phenolic compounds present. Moreover, LC-MS/GC-MS studies confirm the presence of bioactive compounds in P. angustifolium fraction 1/subfractions which helps to explain the significant acute anti-cancer activity of this plant. Compounds tentatively identified in P. angustifolium Fraction 1 using LC-ESI-QTOF-MS/MS were chlorogenic acid and/or neochlorogenic acid, bergapten, berberine, 8’-epitanegool and rosmarinic acid. GC-MS analysis indicated predominant occurrence of compound inositol in P. angustifolium fraction 1 which may be responsible for its anticancer properties. This is the first study to report the occurrence of the above-mentioned compounds in P. angustifolium, and further investigations are required to confirm these findings. Furthermore, fractions of T. ferdinandiana flesh extracts showed no cytotoxicity, except against HT29 cell lines and only Fraction 2 showed some antibacterial activity against the bacterial strains tested. The reduced bioactivity in the T. ferdinandiana fractions may again also be due to loss of synergy as compounds become separated in the fractions. Further dose dependent studies to validate the bioactivity T. ferdinandiana flesh fractions is warranted for this understudied species.
References
- Abdallah, M. S., Mustafa, M., Nallappan, M. A., Choi, S., Paik, J. H., & Rusea, G. (2021). Determination of Phenolics and Flavonoids of Some Useful Medicinal Plants and Bioassay-Guided Fractionation Substances of Sclerocarya birrea (A. Rich) Hochst Stem (Bark) Extract and Their Efficacy Against Salmonella typhi. Frontiers in Chemistry, 9(July), 1–13. [CrossRef]
- Akter, S., Netzel, M. E., Fletcher, M. T., Tinggi, U., & Sultanbawa, Y. (2018). Chemical and nutritional composition of terminalia ferdinandiana (kakadu plum) kernels: A novel nutrition source. Foods, 7(4). [CrossRef]
- Akter, S., Sultanbawa, Y., & Cozzolino, D. (2021). High throughput screening to determine the antibacterial activity of Terminalia ferdinandiana (Kakadu plum): A proof of concept. Journal of Microbiological Methods, 182(February), 106169. [CrossRef]
- Ali, A., Cottrell, J. J., & Dunshea, F. R. (2022). LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites, 12(11). [CrossRef]
- Amaral, D. C., & Brown, P. H. (2022). Foliar Application of an Inositol-Based Plant Biostimulant Boosts Zinc Accumulation in Wheat Grains: A μ-X-Ray Fluorescence Case Study. Frontiers in Plant Science, 13(April), 1–9. [CrossRef]
- Bäcker, C., Jenett-Siems, K., Bodtke, A., & Lindequist, U. (2014). Polyphenolic compounds from the leaves of pittosporum angustifolium. Biochemical Systematics and Ecology, 55, 101–103. [CrossRef]
- Bäcker, C., Jenett-Siems, K., Siems, K., Wurster, M., Bodtke, A., & Lindequist, U. (2014). Cytotoxic saponins from the seeds of Pittosporum angustifolium. Zeitschrift Fur Naturforschung - Section C Journal of Biosciences, 69 C(5–6), 191–198. [CrossRef]
- Beh, C. C., & Teoh, W. H. (2022). Recent Advances in the Extraction of Pittosporum angustifolium Lodd. Used in Traditional Aboriginal Medicine: A Mini Review. Nutraceuticals, 2(2), 49–59. [CrossRef]
- Bizzarri, M., Fuso, A., Dinicola, S., Cucina, A., & Bevilacqua, A. (2016). Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opinion on Drug Metabolism and Toxicology, 12(10), 1181–1196. [CrossRef]
- Blonk, B., & Cock, I. E. (2019). Interactive antimicrobial and toxicity profiles of Pittosporum angustifolium Lodd. extracts with conventional antimicrobials. Journal of Integrative Medicine, 17(4), 261–272. [CrossRef]
- Deo, P., Hewawasam, E., Karakoulakis, A., Claudie, D. J., Nelson, R., Simpson, B. S., Smith, N. M., & Semple, S. J. (2016). In vitro inhibitory activities of selected Australian medicinal plant extracts against protein glycation, angiotensin converting enzyme (ACE) and digestive enzymes linked to type II diabetes. BMC Complementary and Alternative Medicine, 16(435). [CrossRef]
- Feng, W., Li, M., Hao, Z., & Zhang, J. (2019). Analytical Methods of Isolation and Identification. In Phytochemicals in Human Health (p. 13). IntechOpen. [CrossRef]
- Gorman, J. T., Wurm, P. A. S., Vemuri, S., Brady, C., & Sultanbawa, Y. (2019). Kakadu Plum (Terminalia ferdinandiana) as a Sustainable Indigenous Agribusiness. Economic Botany, X, 1–18. [CrossRef]
- Jang, M. H., Piao, X. L., Kim, J. M., Kwon, S. W., Park, J. H., Raju, M., Kulkarni, Y. A., & Wairkar, S. (2008). Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Journal of Functional Foods, 61(August), 103517.
- Jiao, Q. S., Xu, L. L., Zhang, J. Y., Wang, Z. J., Jiang, Y. Y., & Liu, B. (2018). Rapid Characterization and Identification of Non-Diterpenoid Constituents in Tinospora sinensis by HPLC-LTQ-Orbitrap MSn. Molecules, 23(2). [CrossRef]
- Kulkarni, S. K., & Dhir, A. (2010). Berberine: A Plant Alkaloid with Therapeutic Potential for Central Nervous System Disorders. Phytotherapy Research, 24, 317–324. [CrossRef]
- Liang, Y., Xie, L., Liu, K., Cao, Y., Dai, X., Wang, X., Lu, J., Zhang, X., & Li, X. (2021). Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytotherapy Research, 35(11), 6131–6147. [CrossRef]
- Liu, J., Qu, L., Meng, L., & Shou, C. (2019). Topoisomerase inhibitors promote cancer cell motility via ROS-mediated activation of JAK2-STAT1-CXCL1 pathway. Journal of Experimental and Clinical Cancer Research, 38(1), 1–12. [CrossRef]
- Mani, J., Johnson, J., Hosking, H., Hoyos, B. E., Walsh, K. B., Neilsen, P., & Naiker, M. (2022). Bioassay Guided Fractionation Protocol for Determining Novel Active Compounds in Selected Australian Flora. Plants, 11(21), 2886. [CrossRef]
- Mani, J., Johnson, J., Hosking, H., Walsh, K., Neilsen, P., & Naiker, M. (2022a). In vitro Cytotoxic Properties of Crude Polar Extracts of Plants Sourced from Australia. Clinical Complementary Medicine and Pharmacology, 2(1), 100022. [CrossRef]
- Mani, J., Johnson, J., Hosking, H., Walsh, K., Neilsen, P., & Naiker, M. (2022b). In vitro Cytotoxic Properties of Crude Polar Extracts of Plants Sourced from Australia. Clinical Complementary Medicine and Pharmacology, 2(1), 100022. [CrossRef]
- Mekky, R. H., Abdel-Sattar, E., Segura-Carretero, A., & Del Mar Contreras, M. (2019). Phenolic compounds from sesame cake and antioxidant activity: A new insight for agri-food residues’ significance for sustainable development. Foods, 8(10). [CrossRef]
- Naiker, M., Anderson, S., Johnson, J. B., Mani, J. S., Wakeling, L., & Bowry, V. (2020). Loss of trans-resveratrol during storage and ageing of red wines. Australian Journal of Grape and Wine Research, 26(4), 385–387. [CrossRef]
- Noé, W., Murhekar, S., White, A., Davis, C., & Cock, I. E. (2019). Inhibition of the growth of human dermatophytic pathogens by selected australian and asian plants traditionally used to treat fungal infections. Journal de Mycologie Medicale, 29(4), 331–344. [CrossRef]
- Noor, S., Mohammad, T., Rub, M. A., Raza, A., Azum, N., Yadav, D. K., Hassan, M. I., & Asiri, A. M. (2022). Biomedical features and therapeutic potential of rosmarinic acid. Archives of Pharmacal Research, 45(4), 205–228. [CrossRef]
- Nour, V., Trandafir, I., & Cosmulescu, S. (2013). HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. Journal of Chromatographic Science, 51(9), 883–890. [CrossRef]
- Nunes, S., Madureira, A. R., Campos, D., Sarmento, B., Gomes, A. M., Pintado, M., & Reis, F. (2017). Therapeutic and nutraceutical potential of rosmarinic acid—Cytoprotective properties and pharmacokinetic profile. Critical Reviews in Food Science and Nutrition, 57(9), 1799–1806. [CrossRef]
- Omar, R., El-salam, M. A., Elsbaey, M., & Hassan, M. (2023). Fourteen immunomodulatory alkaloids and two prenylated phenylpropanoids with dual therapeutic approach for COVID-19 : molecular docking and dynamics studies. Journal of Biomolecular Structure and Dynamics, 0(0), 1–18. [CrossRef]
- Phan, A. D. T., Chaliha, M., Hong, H. T., Tinggi, U., Netzel, M. E., & Sultanbawa, Y. (2020). Nutritional value and antimicrobial activity of Pittosporum angustifolium (gumby gumby), an australian indigenous plant. Foods, 9(7). [CrossRef]
- Quetglas-Llabrés, M. M., Quispe, C., Herrera-Bravo, J., Catarino, M. D., Pereira, O. R., Cardoso, S. M., Dua, K., Chellappan, D. K., Pabreja, K., Satija, S., Mehta, M., Sureda, A., Martorell, M., Satmbekova, D., Yeskaliyeva, B., Sharifi-Rad, J., Rasool, N., Butnariu, M., Bagiu, I. C., … Cho, W. C. (2022). Pharmacological Properties of Bergapten: Mechanistic and Therapeutic Aspects. Oxidative Medicine and Cellular Longevity, 2022(December 2021). [CrossRef]
- Rabizadeh, F., Mirian, M. S., Doosti, R., Kiani-Anbouhi, R., & Eftekhari, E. (2022). Phytochemical Classification of Medicinal Plants Used in the Treatment of Kidney Disease Based on Traditional Persian Medicine. Evidence-Based Complementary and Alternative Medicine, 2022. [CrossRef]
- Rauf, A., Abu-Izneid, T., Khalil, A. A., Imran, M., Shah, Z. A., Bin Emran, T., Mitra, S., Khan, Z., Alhumaydhi, F. A., Aljohani, A. S. M., Khan, I., Rahman, M. M., Jeandet, P., & Gondal, T. A. (2021). Berberine as a potential anticancer agent: A comprehensive review. Molecules, 26(23), 1–19. [CrossRef]
- Samadi, P., Sarvarian, P., Gholipour, E., Asenjan, K. S., Aghebati-Maleki, L., Motavalli, R., Hojjat-Farsangi, M., & Yousefi, M. (2020). Berberine: A novel therapeutic strategy for cancer. IUBMB Life, 72(10), 2065–2079. [CrossRef]
- Siracusa, L., & Napoli, E. (2022). Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules, 27(5), 1525. [CrossRef]
- Sochor, J., Zitka, O., Skutkova, H., Pavlik, D., Babula, P., Krska, B., Horna, A., Adam, V., Provaznik, I., & Kizek, R. (2010). Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules, 15(9), 6285–6305. [CrossRef]
- Tan, A. C., Konczak, I., Ramzan, I., & Sze, D. M. Y. (2011). Native Australian fruit polyphenols inhibit cell viability and induce apoptosis in human cancer cell lines. Nutrition and Cancer, 63(3), 444–455. [CrossRef]
- Tan, A. C., Konczak, I., Ramzan, I., Zabaras, D., & Sze, D. M. Y. (2011). Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of Kakadu plum and Illawarra plum polyphenolic fractions. Nutrition and Cancer, 63(7), 1074–1084. [CrossRef]
- Widyawati, T., Yusoff, N. A., Bello, I., Asmawi, M. Z., & Ahmad, M. (2022). Bioactivity-Guided Fractionation and Identification of Antidiabetic Compound of Syzygium polyanthum (Wight.)’s Leaf Extract in Streptozotocin-Induced Diabetic Rat Model. Molecules, 27(20). [CrossRef]
- Winnett, V., Sirdaarta, J., White, A., Clarke, F. M., & Cock, I. E. (2017). Inhibition of Klebsiella pneumoniae growth by selected Australian plants: natural approaches for the prevention and management of ankylosing spondylitis. Inflammopharmacology, 25(2), 223–235. [CrossRef]
- Zanatta, A. C., Vilegas, W., & Edrada-Ebel, R. (2021). UHPLC-(ESI)-HRMS and NMR-Based Metabolomics Approach to Access the Seasonality of Byrsonima intermedia and Serjania marginata From Brazilian Cerrado Flora Diversity. Frontiers in Chemistry, 9, 710025. [CrossRef]
Figure 1.
Bioassay guided fractionation protocol design (Mani, et al., 2022).
Figure 1.
Bioassay guided fractionation protocol design (Mani, et al., 2022).
Figure 2.
(A) Chromatogram of Pittosporum angustifolium extract showing retention times at which the five fractions were collected. (B) Elution gradient of P. angustifolium extract fractionation. (C) Chromatogram of P. angustifolium Fraction 1.
Figure 2.
(A) Chromatogram of Pittosporum angustifolium extract showing retention times at which the five fractions were collected. (B) Elution gradient of P. angustifolium extract fractionation. (C) Chromatogram of P. angustifolium Fraction 1.
Figure 3.
HPLC chromatogram of Pittosporum angustifolium Fraction 1 and the retention times (0-3 mins (Sub-fraction 1), 3-6 mins (Sub-fraction 2), and 6-11 mins (Sub-fraction 3)) at which the three subfractions fractions were collected.
Figure 3.
HPLC chromatogram of Pittosporum angustifolium Fraction 1 and the retention times (0-3 mins (Sub-fraction 1), 3-6 mins (Sub-fraction 2), and 6-11 mins (Sub-fraction 3)) at which the three subfractions fractions were collected.
Figure 4.
HPLC chromatogram of T. ferdinandiana extract and the retention times (0-6 mins (Fraction 1), 6-16 mins (Fraction 2), 16-30 mins (Fraction 3) and 30-40 mins (Fraction 4)) at which the four fractions were collected.
Figure 4.
HPLC chromatogram of T. ferdinandiana extract and the retention times (0-6 mins (Fraction 1), 6-16 mins (Fraction 2), 16-30 mins (Fraction 3) and 30-40 mins (Fraction 4)) at which the four fractions were collected.
Figure 6.
Percentage cell viability of cell lines treated with Pittosporum. angustifolium Fraction 1 subfractions 1, 2 and 3 of concentrations 16.55, 12.15 and 16.15 µg mL-1, respectively. One-way ANOVA test indicated no significant difference (p-value > 0.05) in cytotoxicity between the different subfractions for the same cell line, denoted by same letters on the respective bars, except in the case of HT29 cells. Negative control: cells without treatment, positive control: cell treated with 50 ug mL-1 cisplatin (chemotherapy drug).
Figure 6.
Percentage cell viability of cell lines treated with Pittosporum. angustifolium Fraction 1 subfractions 1, 2 and 3 of concentrations 16.55, 12.15 and 16.15 µg mL-1, respectively. One-way ANOVA test indicated no significant difference (p-value > 0.05) in cytotoxicity between the different subfractions for the same cell line, denoted by same letters on the respective bars, except in the case of HT29 cells. Negative control: cells without treatment, positive control: cell treated with 50 ug mL-1 cisplatin (chemotherapy drug).
Figure 7.
HPLC profiles of Pittosporum angustifolium subfractions 1 (A), subfraction 2 (B) and subfraction 3 (C).
Figure 7.
HPLC profiles of Pittosporum angustifolium subfractions 1 (A), subfraction 2 (B) and subfraction 3 (C).
Figure 8.
Chromatogram and retention times of selected phenolic standards.
Figure 8.
Chromatogram and retention times of selected phenolic standards.
Figure 10.
Chromatogram and mass spectrum of compound 1 in Pittosporum angustifolium Fraction 1.
Figure 10.
Chromatogram and mass spectrum of compound 1 in Pittosporum angustifolium Fraction 1.
Figure 11.
Chromatogram and mass spectrum of compound 4 in Pittosporum angustifolium Fraction 1.
Figure 11.
Chromatogram and mass spectrum of compound 4 in Pittosporum angustifolium Fraction 1.
Figure 12.
Chromatogram and mass spectrum of compound 7 in Pittosporum angustifolium Fraction 1.
Figure 12.
Chromatogram and mass spectrum of compound 7 in Pittosporum angustifolium Fraction 1.
Figure 13.
Chromatogram and mass spectrum of compound 8 in Pittosporum angustifolium Fraction 1.
Figure 13.
Chromatogram and mass spectrum of compound 8 in Pittosporum angustifolium Fraction 1.
Figure 14.
Chromatogram and mass spectrum of compound 9 in Pittosporum angustifolium Fraction 1.
Figure 14.
Chromatogram and mass spectrum of compound 9 in Pittosporum angustifolium Fraction 1.
Figure 15.
Targeted GC-MS/MS compounds identified in Pittosporum angustifolium Fraction 1 and Fraction 1 sub-fractions 1,2 and 3.
Figure 15.
Targeted GC-MS/MS compounds identified in Pittosporum angustifolium Fraction 1 and Fraction 1 sub-fractions 1,2 and 3.
Figure 16.
Chemical structure of inositol.
Figure 16.
Chemical structure of inositol.
Figure 17.
Percentage cell viability of cell lines treated with Terminalia ferdinandiana Fractions. One-way ANOVA test indicated no significant difference (p > 0.05) in cytotoxicity between the different subfractions for the same cell line, denoted by same letters on the respective bars, except in the case of HT29 cells. Negative control: cells without treatment, positive control: cell treated with 50 ug mL-1 cisplatin (chemotherapy drug).
Figure 17.
Percentage cell viability of cell lines treated with Terminalia ferdinandiana Fractions. One-way ANOVA test indicated no significant difference (p > 0.05) in cytotoxicity between the different subfractions for the same cell line, denoted by same letters on the respective bars, except in the case of HT29 cells. Negative control: cells without treatment, positive control: cell treated with 50 ug mL-1 cisplatin (chemotherapy drug).
Table 3.
Tentative LC-MS characterization of compounds in Pittosporum angustifolium Fraction 1.
Table 3.
Tentative LC-MS characterization of compounds in Pittosporum angustifolium Fraction 1.
| Peak no. |
Proposed compound |
Molecular formula |
RT (min) |
Mode of ionisation |
Molecular Weight (g/mol) |
Observed Precursor mass (m/z) |
Theoretical mass (m/z) |
Product ions (MS/MS) |
Literature |
| 1 |
Chlorogenic acid |
C16H18O9
|
9.13 |
positive |
354.31 |
355.10 |
355.00* |
65.0, 188.0, 219.0, 275.8 |
|
| 1 |
Neochlorogenic acid |
C16H18O9
|
9.13 |
positive |
354.31 |
354.31 |
355.00 |
65.0, 188.0, 219.0, 275.8 |
Xiao et al 2016 |
| 2 |
Unidentified |
Unidentified |
23.29 |
negative |
Unidentified |
229.10 |
Unidentified |
157.1, 102.2 |
|
| 3 |
Unidentified |
Unidentified |
29.15 |
positive |
Unidentified |
313.10 |
Unidentified |
223.1, 158.2, 102.2 |
|
| 4 |
Berberine |
C20H18NO4+
|
30.65 |
positive |
336.4 |
336.20 |
336.12** |
287.2 |
Jiao et al 2018 |
| 5 |
Unidentified |
Unidentified |
33.09 |
positive |
Unidentified |
378.2 |
Unidentified |
102.1, 249.2 |
|
| 6 |
Unidentified |
Unidentified |
34.11 |
positive |
Unidentified |
326.9 |
Unidentified |
102.3, 185.1, 228.3 |
|
| 7 |
Bergapten |
C12H8O4
|
36.48 |
positive |
216.042 |
217.10 |
217.05* |
129.0, 202.0 |
|
| 8 |
Rosmarinic acid |
C18H16O8
|
44.44 |
positive |
360.3 |
361.20 |
361.09** |
181.05, 139.04 |
|
| 9 |
8'-epitanegool |
C20H24O7Na |
45.07 |
positive |
399.39 |
399.2 |
399.14 |
287.3, 304.2 |
Jiao et al 2018 |
| 10 |
Unidentified |
Unidentified |
46.07 |
positive |
Unidentified |
403.2 |
Unidentified |
102.2, 329.2, 361 |
|
Table 4.
Target GC-MS/MS peak area and classification of compounds identified in Pittosporum angustifolium Fraction 1 and Fraction 1 sub-fractions 1, 2 and 3.
Table 4.
Target GC-MS/MS peak area and classification of compounds identified in Pittosporum angustifolium Fraction 1 and Fraction 1 sub-fractions 1, 2 and 3.
| |
Target |
Fraction 1 |
Sub-fraction 1 |
Subfraction 2 |
Subfraction 3 |
Compound Class |
| 1 |
Citric acid-4TMS |
2971610 |
87281 |
124060 |
23465 |
Carboxylic acids |
| 2 |
Glucose-meto-5TMS(1) |
2162365 |
230045 |
0 |
163277 |
Carbohydrate |
| 3 |
Inositol-6TMS(2) |
1194216 |
141218 |
1123 |
10389 |
Carbocyclic sugar |
| 4 |
2-Aminopimelic acid-3TMS |
1188393 |
35263 |
46722 |
10226 |
Amino acid |
| 5 |
Hydroxylamine-3TMS |
609044 |
0 |
574334 |
0 |
Hydroxylamine |
| 6 |
Glucose-meto-5TMS(2) |
423257 |
49736 |
3456 |
34808 |
Carbohydrate |
| 7 |
Galactose-meto-5TMS(2) |
422411 |
49066 |
3587 |
38804 |
Carbohydrate |
| 8 |
1,5-13C2-Citric acid |
412327 |
13144 |
16485 |
3281 |
Carboxylic acids |
| 9 |
Lactic acid-2TMS |
268289 |
437458 |
247071 |
444661 |
Carboxylic acids |
| 10 |
1,6-Anhydroglucose-3TMS |
228502 |
14470 |
1080 |
4143 |
Carbohydrate |
| 11 |
Alanine-2TMS |
225866 |
32076 |
8606 |
14237 |
Amino acid |
| 12 |
Xylose-meto-4TMS(1) |
178497 |
31305 |
4775 |
22749 |
Carbohydrates |
| 13 |
3-Aminoglutaric acid-2TMS |
169217 |
26046 |
4177 |
2165 |
Amino acid |
| 14 |
4-Aminobutyric acid-3TMS |
169048 |
17510 |
0 |
6009 |
Amino acid |
| 15 |
Arabinose-meto-4TMS |
165445 |
30249 |
4666 |
19117 |
Carbohydrates |
| 16 |
Palmitic acid-TMS |
154588 |
129131 |
126973 |
120981 |
Saturated fatty acid |
| 17 |
Sarcosine-d3-2TMS |
147242 |
130615 |
123520 |
94609 |
Amino acid |
| 18 |
Lyxose-meto-4TMS(2) |
146571 |
23892 |
3754 |
16430 |
Carbohydrates |
| 19 |
Proline-2TMS |
144359 |
37171 |
3022 |
0 |
Amino acid |
| 20 |
Malic acid-3TMS |
135932 |
12652 |
2318 |
3148 |
Carboxylic acids |
Table 5.
Terminalia ferdinandiana flesh (KPF) lyophilised fractions.
Table 5.
Terminalia ferdinandiana flesh (KPF) lyophilised fractions.
| Fractions |
Crystal product obtained (mg) |
Concentrations of fractions tested (mg/mL) |
| KPF1 |
38.10 |
0.095 |
| KPF 2 |
169.00 |
0.423 |
| KPF 3 |
77.40 |
0.194 |
| KPF 4 |
17.60 |
0.044 |
Table 6.
Average zone of inhibition (n=3) of Terminalia ferdinandiana flesh fractions.
Table 6.
Average zone of inhibition (n=3) of Terminalia ferdinandiana flesh fractions.
| Kakadu plum fractions |
Concentrations
µg/mL |
Bacterial strain zone of inhibition (mm) |
| Gram positive |
Gram negative |
| S. aureus |
E. coli |
P. aeruginosa |
S. typhimurium |
| 1 |
58.1 |
4.00 ± 0.71 |
0.00 |
0.00 |
0.00 |
| 2 |
169.0 |
4.30 ± 0.35 |
3.20 ± .10 |
2.10 ± 0.20 |
2.30 ± 0.40 |
| 3 |
77.9 |
0.00 |
0.00 |
0.00 |
0.00 |
| 4 |
17.6 |
4.20 ± 0.25 |
0.00 |
0.00 |
0.00 |
| Positive control (gentamicin) |
10 |
13.67 ± 0.58 |
16.33 ± 0.58 |
13.00± 0.10 |
12.67 ± 0.58 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).