Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Size Determination
2.3. Data Collection
2.3. Assessment of Plasmatic Aβ Peptides (Aβ-42, Aβ-40)
2.4. Statistical Methods
3. Results
3.1. Overall Patient Characteristics and Demographics
3.2. Aβ Peptides Correlation Analysis
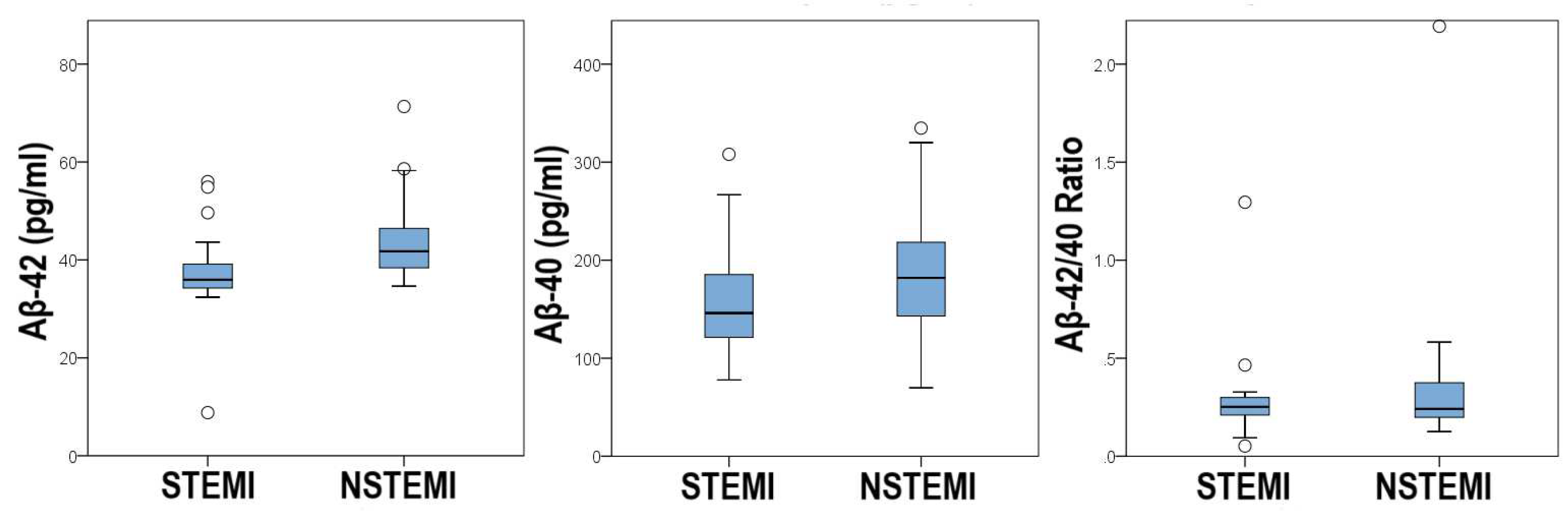
3.3. Comparison of Demographic, Clinical and Biochemical Characteristics Between Groups
3.4. Comparison of Aβ-42 and NT-proBNP with Cardiovascular Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G. A.; Turco, J. V.; Fuster, V.; Roth, G. A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol 2022, 80 (25), 2361–2371. [CrossRef]
- Lopez, A. D.; Mathers, C. D.; Ezzati, M.; Jamison, D. T.; Murray, C. J. L. Global and Regional Burden of Disease and Risk Factors, 2001: Systematic Analysis of Population Health Data. The Lancet 2006, 367 (9524), 1747–1757. [CrossRef]
- Bhatt, D. L.; Lopes, R. D.; Harrington, R. A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327 (7), 662–675. [CrossRef]
- Byrne, R. A.; Rossello, X.; Coughlan, J. J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M. J.; Dan, G. A.; Dweck, M. R.; Galbraith, M.; Gilard, M.; Hinterbuchner, L.; Jankowska, E. A.; Jüni, P.; Kimura, T.; Kunadian, V.; Leosdottir, M.; Lorusso, R.; Pedretti, R. F. E.; Rigopoulos, A. G.; Gimenez, M. R.; Thiele, H.; Vranckx, P.; Wassmann, S.; Wenger, N. K.; Ibanez, B. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur Heart J 2023, 44 (38), 3720–3826. [CrossRef]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I. K. Reassessing the Mechanisms of Acute Coronary Syndromes: The “Vulnerable Plaque” and Superficial Erosion. Circ Res 2019, 124 (1), 150–160. [CrossRef]
- Nardin, M.; Verdoia, M.; Laera, N.; Cao, D.; De Luca, G. New Insights into Pathophysiology and New Risk Factors for ACS. J Clin Med 2023, 12 (8), 2883. [CrossRef]
- Roher, A. E.; Kokjohn, T. A.; Clarke, S. G.; Sierks, M. R.; Maarouf, C. L.; Serrano, G. E.; Sabbagh, M. S.; Beach, T. G. APP/Aβ Structural Diversity and Alzheimer’s Disease Pathogenesis. Neurochem Int 2017, 110, 1–13. [CrossRef]
- Chen, G. F.; Xu, T. H.; Yan, Y.; Zhou, Y. R.; Jiang, Y.; Melcher, K.; Xu, H. E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol Sin 2017, 38 (9), 1205–1235. [CrossRef]
- Stakos, D. A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N. I.; Tual-Chalot, S.; Stellos, K. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease: JACC Focus Seminar. J Am Coll Cardiol 2020, 75 (8), 952–967. [CrossRef]
- Kitazume, S.; Yoshihisa, A.; Yamaki, T.; Oikawa, M.; Tachida, Y.; Ogawa, K.; Imamaki, R.; Takeishi, Y.; Yamamoto, N.; Taniguchi, N. Soluble Amyloid Precursor Protein 770 Is a Novel Biomarker Candidate for Acute Coronary Syndrome. Proteomics Clin Appl 2013, 7 (9–10), 657–663. [CrossRef]
- Zamolodchikov, D.; Renné, T.; Strickland, S. The Alzheimer’s Disease Peptide β-Amyloid Promotes Thrombin Generation through Activation of Coagulation Factor XII. Journal of Thrombosis and Haemostasis 2016, 14 (5), 995–1007. [CrossRef]
- Stamatelopoulos, K.; Sibbing, D.; Rallidis, L. S.; Georgiopoulos, G.; Stakos, D.; Braun, S.; Gatsiou, A.; Sopova, K.; Kotakos, C.; Varounis, C.; Tellis, C. C.; Kastritis, E.; Alevizaki, M.; Tselepis, A. D.; Alexopoulos, P.; Laske, C.; Keller, T.; Kastrati, A.; Dimmeler, S.; Zeiher, A. M.; Stellos, K. Amyloid-Beta (1-40) and the Risk of Death From Cardiovascular Causes in Patients With Coronary Heart Disease. J Am Coll Cardiol 2015, 65 (9), 904–916. [CrossRef]
- Herczenik, E.; Bouma, B.; Korporaal, S. J. A.; Strangi, R.; Zeng, Q.; Gros, P.; Van Eck, M.; Van Berkel, T. J. C.; Gebbink, M. F. B. G.; Akkerman, J. W. N. Activation of Human Platelets by Misfolded Proteins. Arterioscler Thromb Vasc Biol 2007, 27 (7), 1657–1665. [CrossRef]
- Shen, M. Y.; Hsiao, G.; Fong, T. H.; Chou, D. S.; Sheu, J. R. Expression of Amyloid Beta Peptide in Human Platelets: Pivotal Role of the Phospholipase Cγ2-Protein Kinase C Pathway in Platelet Activation. Pharmacol Res 2008, 57 (2), 151–158. [CrossRef]
- Gowert, N. S.; Donner, L.; Chatterjee, M.; Eisele, Y. S.; Towhid, S. T.; Münzer, P.; Walker, B.; Ogorek, I.; Borst, O.; Grandoch, M.; Schaller, M.; Fischer, J. W.; Gawaz, M.; Weggen, S.; Lang, F.; Jucker, M.; Elvers, M. Blood Platelets in the Progression of Alzheimer’s Disease. PLoS ONE 2014, 9 (2), e90523. [CrossRef]
- Canobbio, I.; Catricalà, S.; Di Pasqua, L. G.; Guidetti, G.; Consonni, A.; Manganaro, D.; Torti, M. Immobilized Amyloid Aβ Peptides Support Platelet Adhesion and Activation. FEBS Lett 2013, 587 (16), 2606–2611. [CrossRef]
- Chong, Y. H.; Sung, J. H.; Shin, S. A.; Chung, J. H.; Suh, Y. H. Effects of the β-Amyloid and Carboxyl-Terminal Fragment of Alzheimer’s Amyloid Precursor Protein on the Production of the Tumor Necrosis Factor-α and Matrix Metalloproteinase-9 by Human Monocytic THP-1. Journal of Biological Chemistry 2001, 276 (26), 23511–23517. [CrossRef]
- Jang, S.; Chapa-Dubocq, X. R.; Parodi-Rullán, R. M.; Fossati, S.; Javadov, S. Beta-Amyloid Instigates Dysfunction of Mitochondria in Cardiac Cells. Cells 2022, 11 (3), 373. [CrossRef]
- Mitsis, A.; Gragnano, F. Myocardial Infarction with and without ST-Segment Elevation: A Contemporary Reappraisal of Similarities and Differences. Curr Cardiol Rev 2020, 17 (4). [CrossRef]
- Kokjohn, T. A.; Van Vickle, G. D.; Maarouf, C. L.; Kalback, W. M.; Hunter, J. M.; Daugs, I. D.; Luehrs, D. C.; Lopez, J.; Brune, D.; Sue, L. I.; Beach, T. G.; Castaño, E. M.; Roher, A. E. Chemical Characterization of Pro-Inflammatory Amyloid-Beta Peptides in Human Atherosclerotic Lesions and Platelets. Biochim Biophys Acta Mol Basis Dis 2011, 1812 (11), 1508–1514. [CrossRef]
- Troncone, L.; Luciani, M.; Coggins, M.; Wilker, E. H.; Ho, C.-Y.; Codispoti, K. E.; Frosch, M. P.; Kayed, R.; del Monte, F. Aβ Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J Am Coll Cardiol 2016, 68 (22), 2395–2407. [CrossRef]
- Stamatelopoulos, K.; Pol, C. J.; Ayers, C.; Georgiopoulos, G.; Gatsiou, A.; Brilakis, E. S.; Khera, A.; Drosatos, K.; de Lemos, J. A.; Stellos, K. Amyloid-Beta (1-40) Peptide and Subclinical Cardiovascular Disease. J Am Coll Cardiol 2018, 72 (9), 1060–1061. [CrossRef]
- Stellos, K.; Sibbing, D.; Stakos, D.; Braun, S.; Georgiopoulos, G.; Gatsiou, A.; Sopova, K.; Kastrati, A.; Stamatelopoulos, K. Association of Plasma Amyloid-Beta (1-40) Levels with Incident Coronary Artery Disease and Cardiovascular Mortality. Eur Heart J 2013, 34 (suppl 1), P3145–P3145. [CrossRef]
- Bayes-Genis, A.; Barallat, J.; de Antonio, M.; Domingo, M.; Zamora, E.; Vila, J.; Subirana, I.; Gastelurrutia, P.; Pastor, M. C.; Januzzi, J. L.; Lupón, J. Bloodstream Amyloid-Beta (1-40) Peptide, Cognition, and Outcomes in Heart Failure. Revista Española de Cardiología (English Edition) 2017, 70 (11), 924–932. [CrossRef]
- Stamatelopoulos, K.; Mueller-Hennessen, M.; Georgiopoulos, G.; Sachse, M.; Boeddinghaus, J.; Sopova, K.; Gatsiou, A.; Amrhein, C.; Biener, M.; Vafaie, M.; Athanasouli, F.; Stakos, D.; Pateras, K.; Twerenbold, R.; Badertscher, P.; Nestelberger, T.; Dimmeler, S.; Katus, H. A.; Zeiher, A. M.; Mueller, C.; Giannitsis, E.; Stellos, K. Amyloid- (1-40) and Mortality in Patients With Non–ST-Segment Elevation Acute Coronary Syndrome A Cohort Study. Ann Intern Med 2018, 168 (12), 855–865. [CrossRef]
- Rosengren, A.; Wallentin, L.; Simoons, M.; Gitt, A. K.; Behar, S.; Battler, A.; Hasdai, D. Cardiovascular Risk Factors and Clinical Presentation in Acute Coronary Syndromes. Heart 2005, 91 (9), 1141–1147. [CrossRef]
- Gandhi, S.; Garratt, K. N.; Li, S.; Wang, T. Y.; Bhatt, D. L.; Davis, L. L.; Zeitouni, M.; Kontos, M. C. Ten-Year Trends in Patient Characteristics, Treatments, and Outcomes in Myocardial Infarction from National Cardiovascular Data Registry Chest Pain-MI Registry. Circ Cardiovasc Qual Outcomes 2022, 15 (1), E008112. [CrossRef]
- Mandelzweig, L. The Second Euro Heart Survey on Acute Coronary Syndromes: Characteristics, Treatment, and Outcome of Patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J 2006, 27 (19), 2285–2293. [CrossRef]
- NHIS - Adult Tobacco Use - Glossary. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm.
- De Meyer, G. R. Y.; De Cleen, D. M. M.; Cooper, S.; Knaapen, M. W. M.; Jans, D. M.; Martinet, W.; Herman, A. G.; Bult, H.; Kockx, M. M. Platelet Phagocytosis and Processing of β-Amyloid Precursor Protein as a Mechanism of Macrophage Activation in Atherosclerosis. Circ Res 2002, 90 (11), 1197–1204. [CrossRef]
- Wang, H.; Kulas, J. A.; Wang, C.; Holtzman, D. M.; Ferris, H. A.; Hansen, S. B. Regulation of Beta-Amyloid Production in Neurons by Astrocyte-Derived Cholesterol. Proceedings of the National Academy of Sciences 2021, 118 (33), e2102191118. [CrossRef]
- Zhu, F.; Wolters, F. J.; Yaqub, A.; Leening, M. J. G.; Ghanbari, M.; Boersma, E.; Ikram, M. A.; Kavousi, M. Plasma Amyloid-β in Relation to Cardiac Function and Risk of Heart Failure in General Population. JACC Heart Fail 2023, 11 (1), 93–102. [CrossRef]
- Schmidt, R. A.; Morrell, C. N.; Ling, F. S.; Simlote, P.; Fernandez, G.; Rich, D. Q.; Adler, D.; Gervase, J.; Cameron, S. J. The Platelet Phenotype in Patients with ST-Segment Elevation Myocardial Infarction Is Different from Non–ST-Segment Elevation Myocardial Infarction. Translational Research 2018, 195, 1–12. [CrossRef]
- Wolska, N.; Celikag, M.; Failla, A. V.; Tarafdar, A.; Renné, T.; Torti, M.; Canobbio, I.; Pula, G. Human Platelets Release Amyloid Peptides Β1-40 and Β1-42 in Response to Hemostatic, Immune, and Hypoxic Stimuli. Res Pract Thromb Haemost 2023, 7 (4), 100154. [CrossRef]
- Bozkurt, B.; Nair, A. P.; Misra, A.; Scott, C. Z.; Mahar, J. H.; Fedson, S. Neprilysin Inhibitors in Heart Failure: The Science, Mechanism of Action, Clinical Studies, and Unanswered Questions. JACC Basic Transl Sci 2023, 8 (1), 88–105. [CrossRef]
- Burrinha, T.; Martinsson, I.; Gomes, R.; Terrasso, A. P.; Gouras, G. K.; Almeida, C. G. Upregulation of APP Endocytosis by Neuronal Aging Drives Amyloid-Dependent Synapse Loss. J Cell Sci 2021, 134 (9), jcs255752. [CrossRef]
- Zecca, C.; Pasculli, G.; Tortelli, R.; Dell’Abate, M. T.; Capozzo, R.; Barulli, M. R.; Barone, R.; Accogli, M.; Arima, S.; Pollice, A.; Brescia, V.; Logroscino, G. The Role of Age on Beta-Amyloid1–42 Plasma Levels in Healthy Subjects. Front Aging Neurosci 2021, 13, 698571. [CrossRef]
- Heidenreich, P. A.; Bozkurt, B.; Aguilar, D.; Allen, L. A.; Byun, J. J.; Colvin, M. M.; Deswal, A.; Drazner, M. H.; Dunlay, S. M.; Evers, L. R.; Fang, J. C.; Fedson, S. E.; Fonarow, G. C.; Hayek, S. S.; Hernandez, A. F.; Khazanie, P.; Kittleson, M. M.; Lee, C. S.; Link, M. S.; Milano, C. A.; Nnacheta, L. C.; Sandhu, A. T.; Stevenson, L. W.; Vardeny, O.; Vest, A. R.; Yancy, C. W. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145 (18), e895–e1032. [CrossRef]
- Jering, K. S.; Claggett, B. L.; Pfeffer, M. A.; Granger, C. B.; Køber, L.; Lewis, E. F.; Maggioni, A. P.; Mann, D. L.; McMurray, J. J. V.; Prescott, M. F.; Rouleau, J. L.; Solomon, S. D.; Steg, P. G.; Von Lewinski, D.; Braunwald, E. Prognostic Importance of NT-ProBNP (N-Terminal Pro-B-Type Natriuretic Peptide) Following High-Risk Myocardial Infarction in the PARADISE-MI Trial. Circ Heart Fail 2023, 16 (5), e010259. [CrossRef]
- Kim, J. W.; Byun, M. S.; Lee, J. H.; Yi, D.; Jeon, S. Y.; Sohn, B. K.; Lee, J. Y.; A Shin, S.; Kim, Y. K.; Kang, K. M.; Sohn, C. H.; Lee, D. Y. Serum Albumin and Beta-Amyloid Deposition in the Human Brain. Neurology 2020, 95 (7), e815–e826. [CrossRef]
- Lazar, D. R.; Lazar, F. L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D. M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis Markers 2022, 2022, 9713326. [CrossRef]
- Kleemeier, S.; Abildgaard, A.; Ladefoged, S. A.; Thorsted Sørensen, J.; Stengaard, C.; Adelborg, K. High-Sensitivity Troponin T and I in Patients Suspected of Acute Myocardial Infarction. Scand J Clin Lab Invest 2022, 82 (2), 96–103. [CrossRef]
- Hilal, S.; Ikram, M. A.; Verbeek, M. M.; Franco, O. H.; Stoops, E.; Vanderstichele, H.; Niessen, W. J.; Vernooij, M. W. C-Reactive Protein, Plasma Amyloid-β Levels, and Their Interaction with Magnetic Resonance Imaging Markers. Stroke 2018, 49 (11), 2692–2698. [CrossRef]
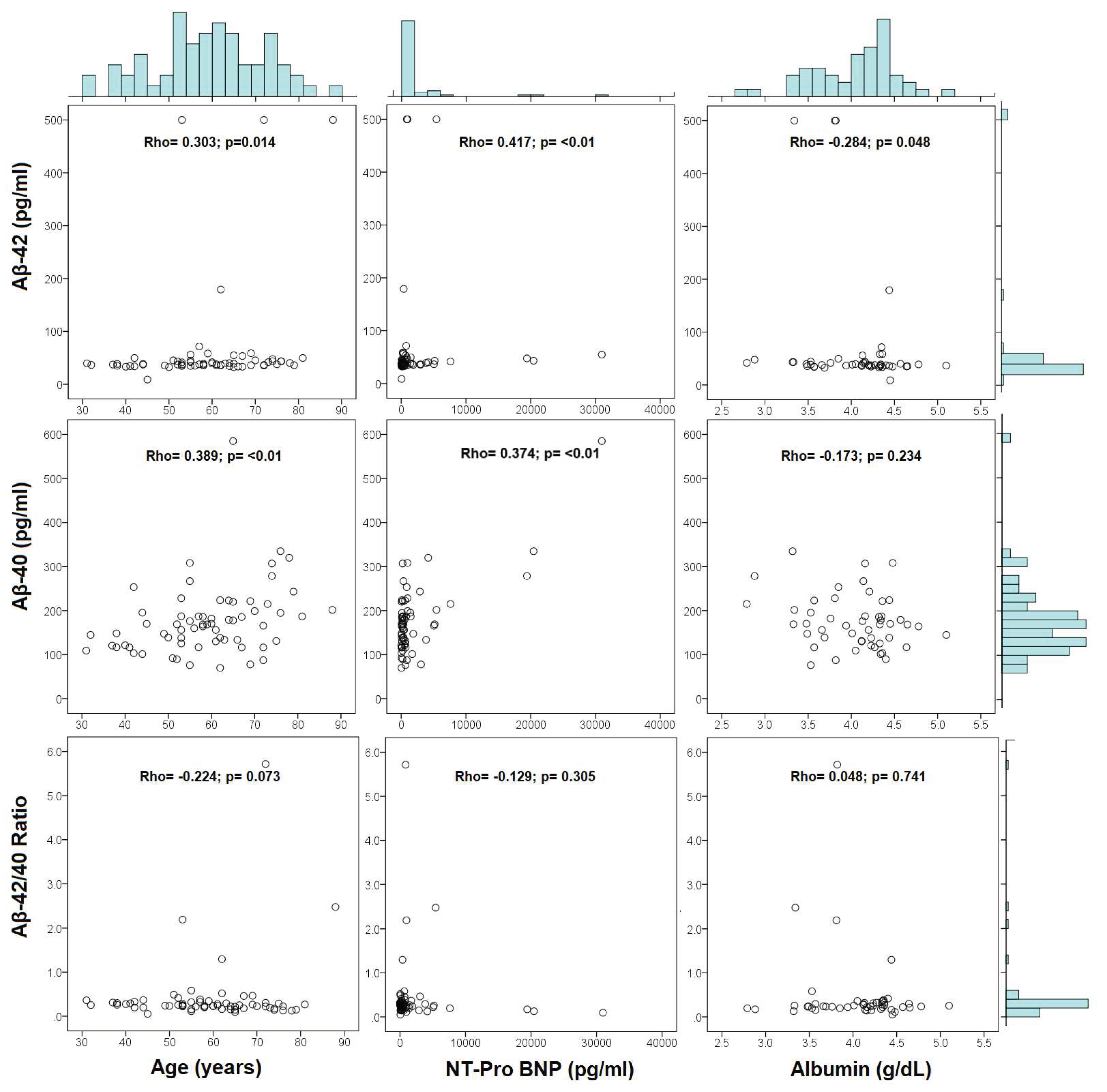
| Variable | Aβ-42 (pg/ml) | Aβ-40 (pg/ml) | Aβ-42/40 ratio | |||
|---|---|---|---|---|---|---|
| Rho | p-value | Rho | p-value | Rho | p-value | |
| Age (years) | 0.303 | 0.014 | 0.389 | 0.001 | -0.224 | 0.073 |
| BMI (Kg/m2) | -0.050 | 0.694 | -0.084 | 0.507 | -0.031 | 0.805 |
| One or more SMuRF | 0.167 | 0.184 | 0.298 | 0.016 | -0.251 | 0.044 |
| High sensitivity troponin I (pg/ml) | -0.021 | 0.869 | 0.063 | 0.617 | -0.142 | 0.260 |
| CRP (mg/dl) | 0.073 | 0.584 | 0.174 | 0.190 | -0.155 | 0.247 |
| NT-proBNP | 0.417 | 0.001 | 0.374 | 0.002 | -0.129 | 0.305 |
| Albumin (g/dl) | -0.284 | 0.048 | -0.173 | 0.234 | 0.048 | 0.741 |
| Total cholesterol (mg/dl) | -0.153 | 0.274 | -0.089 | 0.526 | 0.061 | 0.664 |
| HDL (mg/dl) | -0.005 | 0.972 | 0.061 | 0.657 | -0.043 | 0.757 |
| LDL (mg/dl) | -0.147 | 0.285 | -0.096 | 0.484 | 0.090 | 0.515 |
| Triglycerides | -0.074 | 0.611 | -0.051 | 0.726 | -0.051 | 0.725 |
| AIP | -0.073 | 0.617 | -0.079 | 0.585 | -0.024 | 0.869 |
| Symptom-to-blood sample† | 0.012 | 0.923 | -0.142 | 0.259 | 0.049 | 0.697 |
| Variable | STEMI (N = 34) |
NSTEMI (N = 31) |
p value |
|---|---|---|---|
| Age (years) | 58 ± 12 | 60 ± 12 | 0.503 |
| Sex Male Female |
29 (85.3%) 5 (14.7%) |
22 (71.1%) 9 (29.9%) |
0.135 |
| Body mass index (Kg/m2) Overweight Obesity Overweight/Obesity |
28.28 ± 4.15 14 (41.2%) 12 (35.3%) 26 (76.5%) |
27.83 ± 3.70 14 (45.2%) 9 (29.0%) 23 (74.0%) |
0.652 |
| Diabetes | 4 (11.8%) | 10 (32.3%) | 0.043 |
| Hypertension | 18 (52.9%) | 24 (77.4%) | 0.039 |
| Dyslipidemia | 6 (17.6%) | 6 (19.4%) | 0.859 |
| Previous myocardial infarction | 9 (26.5%) | 18 (58.1%) | 0.010 |
| Smoking status Current smoker Former smoker Nonsmoker |
16 (47.1%) 6 (17.6%) 12 (35.3%) |
5 (16.1%) 17 (54.8%) 9 (29.1%) |
0.003 |
| Number of SMuRFs One or more None |
29 (85.3%) 5 (14.7%) |
30 (96.3%) 1 (3.7%) |
0.121 |
| NYHA ≥2 Class |
5 (14.7) |
5 (16.1%) |
0.572 |
| Killip-Kimball ≥2 Class |
9 (26.5%) |
5 (16.1%) |
0.240 |
| GRACE Intermediate-High Risk |
22 (64.7%) |
18 (58.1%) |
0.583 |
| TIMI Intermediate-High Risk |
21 (61.8%) |
21 (67.7%) |
0.615 |
| CRUSADE Moderate-High Risk |
14 (41.2%) |
15 (48.4%) |
0.559 |
| LVEF Mid-range ejection fraction Reduced ejection fraction |
9 (27.3%) 11 (33.3%) |
6 (20.7%) 8 (27.6%) |
0.618 |
| Symptom-to-door time (minutes) | 392 ± 196 | 280 ± 171 | 0.018 |
| Symptom-to-blood sample time (minutes) | 418 ± 198 | 364 ± 193 | 0.221 |
| Door-to-electrocardiogram time (minutes) | 6 (5-8) | 5 (5-7) | 0.143 |
| Door-to-balloon time (minutes) |
508 (98-2377) (n = 31) |
2030 (1085-3576) (n = 16) |
0.001 |
| Symptom-to-catheter time (minutes) | 1700 ± 1889 | 3070 ± 2562 | 0.992 |
| Variable | STEMI (N = 34) |
NSTEMI (N = 31) |
p value |
|---|---|---|---|
| Aβ-42 (pg/ml) | 35.96 (34.27-39.14) | 41.76 (38.99-47.75) | 0.001 |
| Aβ-40 (pg/ml) | 169.38 ± 88.26 | 183.68 ± 68.96 | 0.472 |
| Aβ-42/40 ratio | 0.25 (0.21-0.30) | 0.24 (0.20-0.38) | 0.708 |
| High sensitivity troponin I (pg/ml) | 368.5 (67.1-4742.0) | 249 (65.3-1299.0) | 0.281 |
| NT-proBNP (pg/ml) | 257.5 (104.0-643.0) | 516.0 (199.0-643.0) | 0.097 |
| High sensitivity CRP (mg/dl) | 4.98 (2.47-9.50) | 4.46 (1.63-10.40) | 0.569 |
| Albumin (g/dl) | 4.14 ± 0.42 | 3.95 ± 0.52 | 0.149 |
| Total cholesterol (mg/dl) | 170.3 ± 43.7 | 157.5 ± 35.7 | 0.264 |
| Triglycerides (mg/dl) | 162.2 ± 65.8 | 149.4 ± 60.3 | 0.490 |
| HDL (mg/dl) | 38.65 ± 8.81 | 36.21 ± 7.65 | 0.285 |
| LDL (mg/dl) | 112.9 ± 41.4 | 98.1 ± 32.3 | 0.155 |
| AIP | 0.23 ± 0.23 | 0.23 ± 0.20 | 0.992 |
| Variable | All subjects (N=65) |
|||
|---|---|---|---|---|
| Aβ-42 (pg/ml) |
p value | NT-proBNP (pg/ml) | p value | |
| Diabetes Yes: No: |
42.50 (36.51-47.75) 37.64 (35.01-43.63) |
0.075 |
3978.0 (226.0-7611.0) 277.0 (110.0-690.0) |
0.004 |
| Hypertension Yes: No: |
39.70 (36.51-49.62) 36.14 (34.64-39.51) |
0.006 |
551.0 (181.0-1862.0) 231.0 (110.0-522.0) |
0.056 |
| Dyslipidemia Yes: No: |
37.07 (35.58-39.13) 38.76 (35.39-44.38) |
0.393 |
415.5 (76.7-1192.5) 342.0 (125.0-974.0) |
0.819 |
| Smoking Yes: No: |
38.39 (35.39-43.25) 38.57 (35.76-48.68) |
0.624 |
226.0 (99.0-975.0) 642.5 (257.5-1125.0) |
0.060 |
| Prior MI Yes: No: |
40.26 (36.14-53.37) 37.45 (35.01-41.76) |
0.066 |
516.0 (199.0-1862.0) 257.5 (110.0-766.0) |
0.197 |
| SMuRF ≥One: None: |
38.76 (35.76-45.13) 35.01 (33.52-37.64) |
0.039 |
315.0 (110.0-978.0) 470.0 (234.0-642.0) |
0.910 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





