Submitted:
16 January 2024
Posted:
17 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Research Methods
3. Results
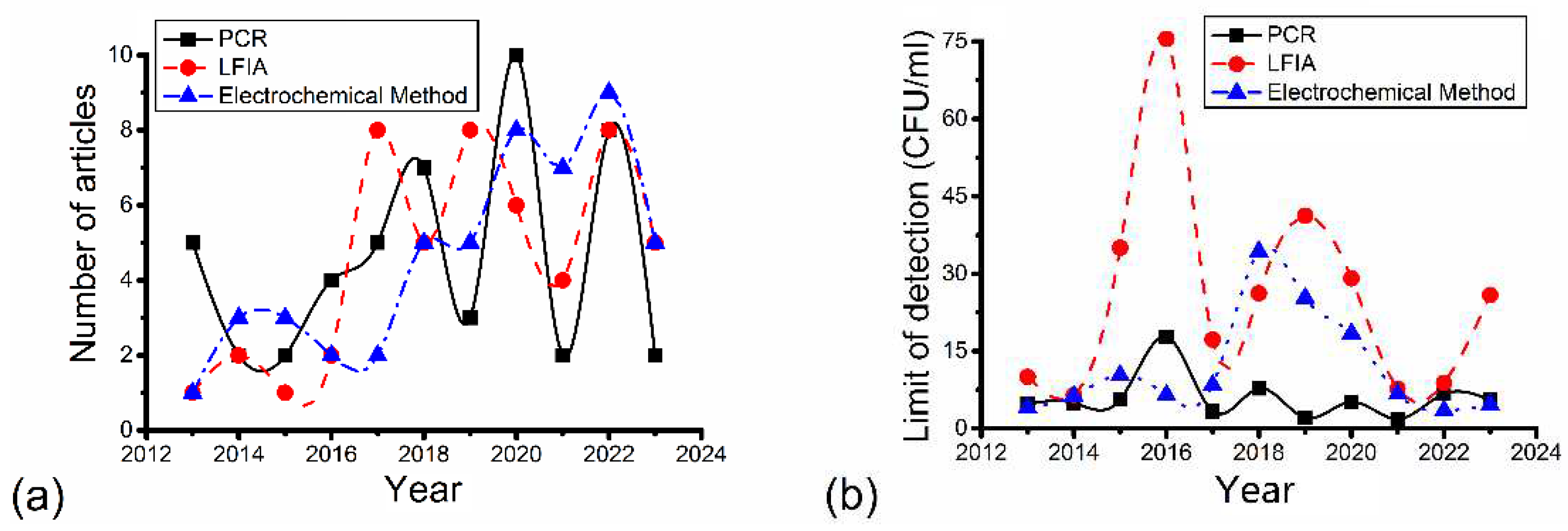
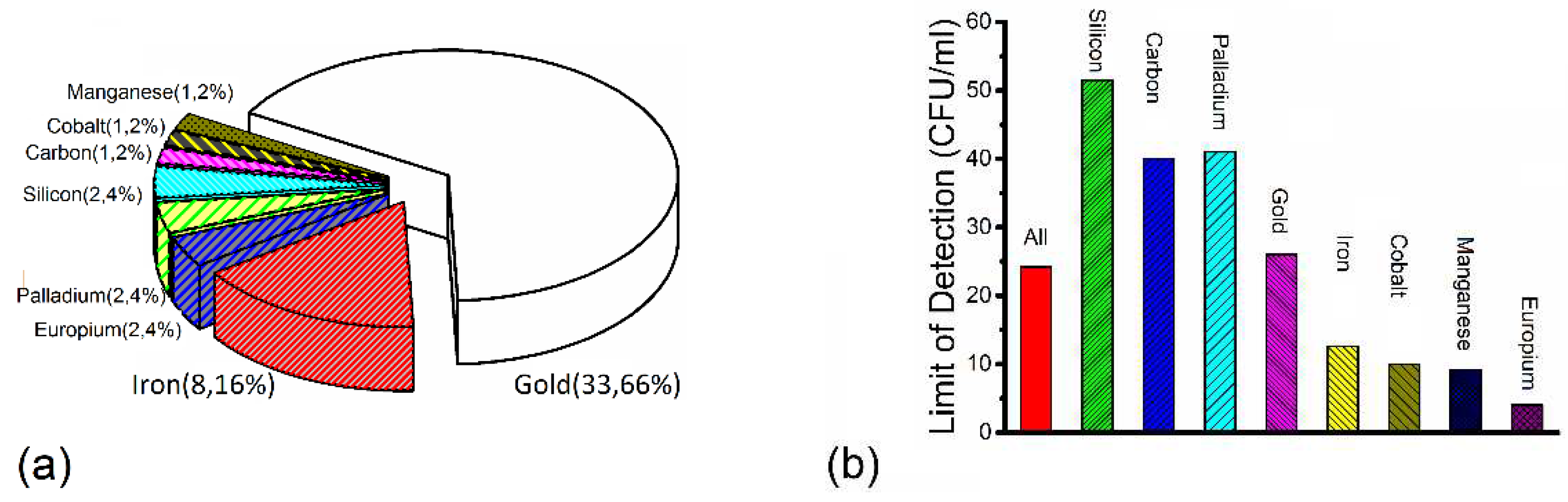
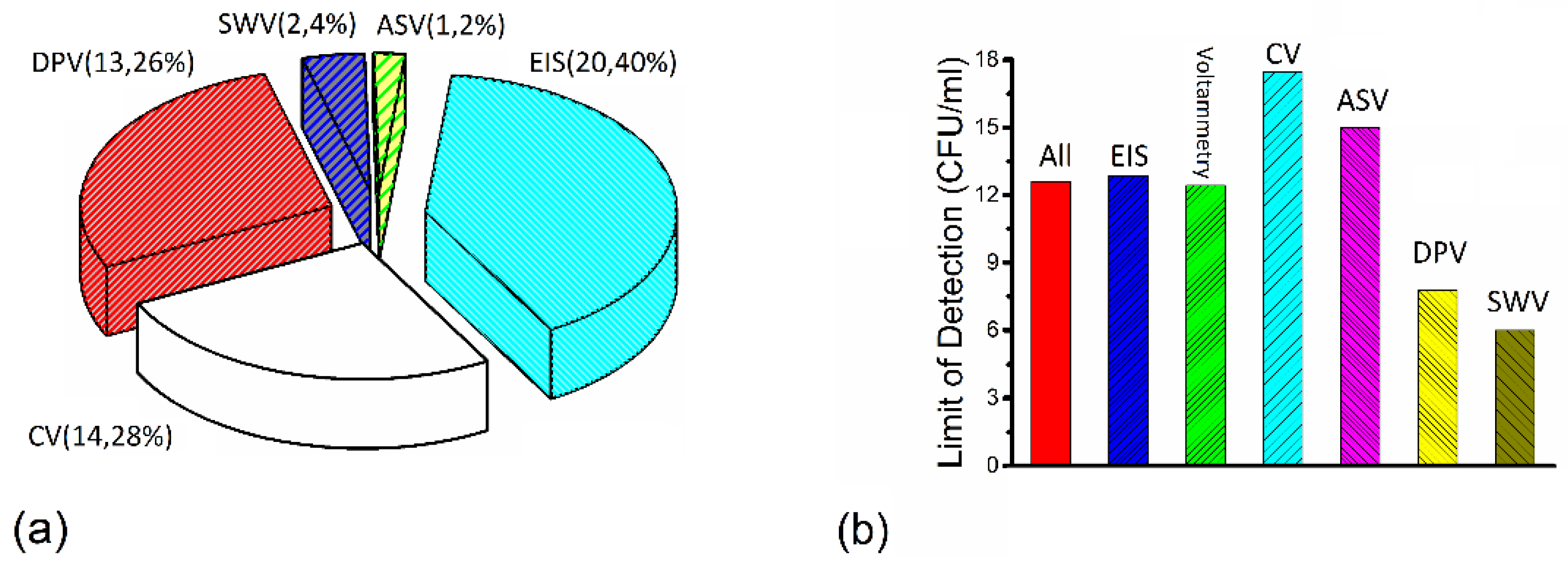

4. Discussion
Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roig-Sagués, A.X.; Trujillo-Mesa, A.J. Application of Emerging Non-Thermal Processing Technologies: Impact on Characteristics, Efficacy, and Safety of Foods. Foods 2023, 12, 4040. [Google Scholar] [CrossRef] [PubMed]
- Apaydın, D.; Tırpancı-Sivri, G.; Demirci, A.S. Modeling the γ-irradiation inactivation kinetics of foodborne pathogens Escherichia coli O157:H7, Salmonella, Staphylococcus aureus and Bacillus cereus in instant soup. Food Sci. Technol. Int. 2023, 10820132231210317. [Google Scholar] [CrossRef] [PubMed]
- Totsline, N.; Kniel, K.E.; Bais, H.P. Microgravity and evasion of plant innate immunity by human bacterial pathogens. npj Microgravity 2023, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.X.; de Santana, V.P.; Benevides, R.G.; Aliaga, M.T.A.; Billington, C.; Góes-Neto, A. Systematic review and meta-analysis: the efficiency of bacteriophages previously patented against pathogenic bacteria on food. BMC Syst. Rev. 2023, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Xu, D.; Wang, J.; Zaeim, D.; Han, J.; Qu, D. Isolation, antimicrobial resistance and virulence characterization of Salmonella spp. from fresh foods in retail markets in Hangzhou, China. PLoS ONE 2023, 18, e0292621. [Google Scholar] [CrossRef] [PubMed]
- Cookson, K.; Hofacre, C.; Da Costa, M.; Schaeffer, J.; Jones, M.; Baxter, J.; Berghaus, R.; Cosby, D.; Berrang, M.; McMillan, E. Live Salmonella Typhimurium vaccination of broilers results in lower Salmonella prevalence on carcasses at commercial processing. J. Appl. Poult. Res. 2024, 33. [Google Scholar] [CrossRef]
- Zhao, X.; Smith, G.; Javed, B.; Dee, G.; Gun’ko, Y.K.; Curtin, J.; Byrne, H.J.; O’connor, C.; Tian, F. Design and Development of Magnetic Iron Core Gold Nanoparticle-Based Fluorescent Multiplex Assay to Detect Salmonella. Nanomaterials 2022, 12, 3917. [Google Scholar] [CrossRef] [PubMed]
- Senturk, E.; Buzrul, S.; Sanlıbaba, P. Probabilistic modeling of the growth of Listeria monocytogenes: effect of nisin, temperature, and strain in the presence of potassium chloride or potassium sorbate. Int. J. Food Prop. 2023, 26, 3129–3137. [Google Scholar] [CrossRef]
- Holliday, M.; Uddipto, K.; Castillo, G.; Vera, L.E.; Quinlivan, J.A.; Mendz, G.L. Insights into the Genital Microbiota of Women Who Experienced Fetal Death in Utero. Microorganisms 2023, 11, 1877. [Google Scholar] [CrossRef]
- El-Sherbiny, M.M.; Devassy, R.P.; El-Hefnawy, M.E.; Al-Goul, S.T.; Orif, M.I.; El-Newehy, M.H. Facile Synthesis, Characterization, and Antimicrobial Assessment of a Silver/Montmorillonite Nanocomposite as an Effective Antiseptic against Foodborne Pathogens for Promising Food Protection. Molecules 2023, 28, 3699. [Google Scholar] [CrossRef]
- Rubinelli, P.M.; Liyanage, R.; Lay, J.; Acuff, J.C. The Bactericidal Activity of a Novel Aneurinibacillus aneurinilyticus Isolate Effectively Controls Foodborne Pathogens Campylobacter jejuni and Listeria monocytogenes. Appl. Sci. 2023, 13, 10257. [Google Scholar] [CrossRef]
- McAllister, J.; Gregory, J.; Adamopoulos, J.; Walsh, M.; Stylianopoulos, A.; Arnold, A.; Andersson, P.; Stewart, T. A food outbreak of Campylobacteriosis at a wedding-Melbourne, Australia, 2022. Int. J. Infect. Dis. 2023, 130 (Suppl. S2). [Google Scholar] [CrossRef]
- Bodie, A.R.; Rothrock, M.J.; Ricke, S.C. Comparison of optical density-based growth kinetics for pure culture Campylobacter jejuni, coli and lari grown in blood-free Bolton broth. J. Environ. Sci. Health B. 2023, 58, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, S.; Itamoto, C.; Hase, I. Pericarditis due to Campylobacter coli infection: a case report. BMC Infect. Dis. 2023, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- Qamar, K.M.; Nchasi, G.M.; Yousuf, J.M.; Mirha, H.T.M.; Kumar, P.M.; Islam, Z.M.; Malikzai, A.D. Food toxicity in Pakistan: a mini-review on foodborne diseases. Int. J. Surgery: Glob. Health 2023, 6. [Google Scholar] [CrossRef]
- Almousawi, A.E.; Alhatami, A.O.; Neama, N.A.; Baqir, A.M. Characterization and molecular evaluation of Staphylococcus aureus isolated from poultry and dairy cattle milk in Iraq. AIP Conf. Proc. 2022, 2386, 020014. [Google Scholar]
- Almuhayawi, M.S.; Alruhaili, M.H.; Gattan, H.S.; Alharbi, M.T.; Nagshabandi, M.; Al Jaouni, S.; Selim, S.; Alanazi, A.; Alruwaili, Y.; Faried, O.A.; et al. Staphylococcus aureus Induced Wound Infections Which Antimicrobial Resistance, Methicillin- and Vancomycin-Resistant: Assessment of Emergence and Cross Sectional Study. Infect. Drug Resist. 2023, ume 16, 5335–5346. [Google Scholar] [CrossRef]
- Afshari, A.; Taheri, S.; Hashemi, M.; Norouzy, A.; Nematy, M.; Mohamadi, S. Methicillin- and Vancomycin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci Isolated from Hospital Foods: Prevalence and Antimicrobial Resistance Patterns. Curr. Microbiol. 2022, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moufarrej, Y.; Patel, R. Pulmonary Hemorrhage in a 15-Year-Old Girl. Clin. Pediatr. 2023. [Google Scholar] [CrossRef]
- Del Giudice, P. Skin Infections Caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, adv00110. [Google Scholar] [CrossRef]
- European Commission. European Commission Regulation No 2073/2005 On microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 5R2073, 16–33.
- da Costa, M.R.; Pessoa, J.; Nesbakken, T.; Meemken, D. A systematic review to assess the effectiveness of pre-harvest meat safety interventions to control foodborne pathogens in beef. Food Control. 2023, 153. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Farid, W.A.A.; Al-Saad, D.S.H. Prevalence of Diarrheagenic Escherichia coli and its Relation to Household Factors and Symptoms Distribution among Children in Babylon Governorate Hospitals, Iraq. kufa J. Nurs. Sci. 2023, 13. [Google Scholar] [CrossRef]
- Abdullah, B.J.; Kadhim, K.S.; Al-Shemmari, I.G. Isolation and identification of non-O157 Shiga toxin Escherichia coli from humans and calves using conventional and molecular technique. Iran. J. Ichthyol. 2023, 10, 172–180. [Google Scholar]
- Shen, J.; Zhou, X.; Shan, Y.; Yue, H.; Huang, R.; Hu, J.; Xing, D. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nat. Commun. 2020, 11, 267. [Google Scholar] [CrossRef]
- Lee, K.-M.; Runyon, M.; Herrman, T.J.; Phillips, R.; Hsieh, J. Review of Salmonella detection and identification methods: Aspects of rapid emergency response and food safety. Food Control. 2015, 47, 264–276. [Google Scholar] [CrossRef]
- Imdahl, F.; Vafadarnejad, E.; Homberger, C.; Saliba, A.-E.; Vogel, J. Single-cell RNA-sequencing reports growth-condition-specific global transcriptomes of individual bacteria. Nat. Microbiol. 2020, 5, 1202–1206. [Google Scholar] [CrossRef]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Korean J. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.; Li, Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 149–197. [Google Scholar] [CrossRef]
- Schenk, F.; Weber, P.; Vogler, J.; Hecht, L.; Dietzel, A.; Gauglitz, G. Development of a paper-based lateral flow immunoassay for simultaneous detection of lipopolysaccharides of Salmonella serovars. Anal. Bioanal. Chem. 2017, 410, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current applications and future scope in food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Balivada, S.; Rachakatla, R.; Wang, H.; Samarakoon, T.; Dani, R.; Pyle, M.; Kroh, F.; Walker, B.; Leaym, X.; Koper, O.; Tamura, M.; Chikan, V.; Bossmann, S.; Troyer, D. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef]
- Liu, K.; He, Z.; Curtin, J.F.; Byrne, H.J.; Tian, F. A novel, rapid, seedless, in situ synthesis method of shape and size controllable gold nanoparticles using phosphates. Sci. Rep. 2019, 9, 7421. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Oh, S.-W. Rapid and sensitive detection of E. coli O157:H7 and S. Typhimurium in iceberg lettuce and cabbage using filtration, DNA concentration, and qPCR without enrichment. Food Chem. 2020, 327, 127036. [Google Scholar] [CrossRef] [PubMed]
- Wouters, Y.; Dalloyaux, D.; Christenhusz, A.; Roelofs, H.M.J.; Wertheim, H.F.; Bleeker-Rovers, C.P.; Morsche, R.H.T.; Wanten, G.J.A. Droplet digital polymerase chain reaction for rapid broad-spectrum detection of bloodstream infections. Microb. Biotechnol. 2020, 13, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. 2021, 882, 114989–114989. [Google Scholar] [CrossRef]
- McEachern, F.; Harvey, E.; Merle, G. Emerging Technologies for the Electrochemical Detection of Bacteria. Biotechnol. J. 2020, 15, e2000140. [Google Scholar] [CrossRef]
- Castaneda-Ruelas, G.M.; Guzman-Uriarte, J.R.; Valdez-Torres, J.B.; Leon-Felix, T. Evaluation of real-time polymerase chain reaction coupled to immunomagnetic separation (rtPCR-IMS) as an alternative method for the routine detection of Salmonella spp. in beef in Mexico. Rev. Mex. Cienc. Pecu. 2022, 13, 625–642. [Google Scholar]
- Hyeon, J.-Y.; Deng, X. Rapid detection of Salmonella in raw chicken breast using real-time PCR combined with immunomagnetic separation and whole genome amplification. Food Microbiol. 2017, 63, 111–116. [Google Scholar] [CrossRef]
- Garrido, A.; Chapela, M.-J.; Román, B.; Fajardo, P.; Lago, J.; Vieites, J.M.; Cabado, A.G. A new multiplex real-time PCR developed method for Salmonella spp. and Listeria monocytogenes detection in food and environmental samples. Food Control. 2013, 30, 76–85. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Ngo, T.A.; Kant, K.; Engelsmann, P.; Dave, V.P.; Shahbazi, M.-A.; Wolff, A.; Bang, D.D. Rapid detection of Salmonella enterica in food samples by a novel approach with combination of sample concentration and direct PCR. Biosens. Bioelectron. 2018, 129, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Hu, H.; Huang, Y.; Yang, X.; Wang, Q.; Chen, X. Improving the detection limit of Salmonella colorimetry using long ssDNA of asymmetric-PCR and non-functionalized AuNPs. Anal. Biochem. 2021, 626, 114229. [Google Scholar] [CrossRef]
- Ríos-Castillo, A.G.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Detection by real-time PCR and conventional culture of Salmonella Typhimurium and Listeria monocytogenes adhered to stainless steel surfaces under dry conditions. Food Control. 2022, 137, 108971. [Google Scholar] [CrossRef]
- Xie, G.; Yu, S.; Li, W.; Mu, D.; Aguilar, Z.P.; Xu, H. Simultaneous detection of Salmonella spp., Pseudomonas aeruginosa, Bacillus cereus, and Escherichia coli O157:H7 in environmental water using PMA combined with mPCR. J. Microbiol. 2020, 58, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Heymans, R.; Vila, A.; Heerwaarden, C.A.M.; Jansen, C.C.C.; Castelijn, G.A.A.; Voort, M.; Biesta-Peters, E.G. Rapid detection and differentiation of Salmonella species, Salmonella Typhimurium and Salmonella Enteritidis by multiplex quantitative PCR Raymond Heymans. PLoS One. 2018, 13, e0206316. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Mohler, V.L.; Gunn, A.A.; House, J.K. Development of a qcr for the detection and quantitifaication of salmonella spp. in sheep feces and tissues. J. Vet. Diagn. Invest. 2020, 32, 835–843. [Google Scholar] [CrossRef]
- Siala, M.; Barbana, A.; Smaoui, S.; Hachicha, S.; Marouane, C.; Kammoun, S.; Gdoura, R.; Messadi-Akrout, F. Screening and Detecting Salmonella in Different Food Matrices in Southern Tunisia Using a Combined Enrichment/Real-Time PCR Method: Correlation with Conventional Culture Method. Front. Microbiol. 2017, 8, 2416. [Google Scholar] [CrossRef]
- Azinheiro, S.; Ghimire, D.; Carvalho, J.; Prado, M.; Garrido-Maestu, A. Next-day detection of viable Listeria monocytogenes by multiplex reverse transcriptase real-time PCR. Food Control. 2022, 133, 108593. [Google Scholar] [CrossRef]
- Xiao. X.-L., Zhang, L.; Wu, H.; Yu, Y.-G.; Tang, Y.-Q.; Liu, D.-M.; Li, X.-F. Simultaneus detection of salmonella, listeria monocytogenes, and staphylococcus aureaus by multiplex real-time PCR Assays Using High-Resolution Melting. Food Anal. Methods 2014, 7, 1960–197. [CrossRef]
- Fan, W.; Gao, X.-Y.; Li, H.-N.; Guo, W.-P.; Li, Y.-Y.; Wang, S.-W. Rapid and simultaneous detection of Salmonella spp., Escherichia coli O157:H7, and Listeria monocytogenes in meat using multiplex immunomagnetic separation and multiplex real-time PCR. Eur. Food Res. Technol. 2022, 248, 869–879. [Google Scholar] [CrossRef]
- Wei, S.; Park, B.-J.; Kim, S.-H.; Seo, K.-H.; Jin, Y.-G.; Oh, D.-H. Detection of Listeria monocytogenes using Dynabeads® anti-Listeria combined with real-time PCR in soybean sprouts. LWT 2019, 99, 533–539. [Google Scholar] [CrossRef]
- M, S.M.; Nd, S. Pervasiveness of Listeria monocytogenes in Milk and Dairy Products. J. Food: Microbiol. Saf. Hyg. 2017, 2. [Google Scholar] [CrossRef]
- Heo, E.J.; Kim, H.-Y.; Suh, S.H.; Moon, J.S. Comparison of DNA Extraction Methods for the Quantification of Listeria monocytogenes in Dairy Products by Real-Time Quantitative PCR>. J. Food Prot. 2022, 85, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.P.; Jiang, Y.; Gao, F.; Zhang, L.; Zhou, G.H. Rapid and simultaneous analysis of five foodborne pathogenic bacteria using multiplex PCR. Eur. Food Res. Technol. 2013, 237, 627–637. [Google Scholar] [CrossRef]
- Milton, A.A.P.; Prasad, M.C.B.; Momin, K.M.; Priya, G.B.; Hussain, Z.; Das, S.; Ghatak, S.; Sen, A. Development of a novel single-tube SYBR Green real-time PCR assay for simultaneous detection of Brucella spp. and Listeria monocytogenes by melt curve analysis. Int. Dairy J. 2023, 145, 105737. [Google Scholar] [CrossRef]
- Xiao, F.; Bai, X.; Wang, K.; Sun, Y.; Xu, H. Rapid-Response Magnetic Enrichment Strategy for Significantly Improving Sensitivity of Multiplex PCR Analysis of Pathogenic Listeria Species. Appl. Sci. 2022, 12, 6415. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, X.; Xiong, S. Xu, H. Aguilar, Z.P. Xiong, Y. Large-volume immunomagnetic separation combined with multiplex pcf assay for simultaneous detection of Listeria monocytogens and Listeria ivanovii in lettuce. Food Control 2016, 59, 601–608. [CrossRef]
- Suh, S.H.; Dwivedi, H.P.; Jaykus, L.-A. Development and evaluation of aptamer magnetic capture assay in conjunction with real-time PCR for detection of Campylobacter jejuni. LWT-Food Sci. Technol. 2014, 56, 256–260. [Google Scholar] [CrossRef]
- Knipper, A.; Plaza-Rodríguez, C.; Filter, M.; Wulsten, I.F.; Stingl, K.; Crease, T. Modeling the survival of Campylobacter jejuni in raw milk considering the viable but non-culturable cells (VBNC). J. Food Saf. 2023, e13077. [Google Scholar] [CrossRef]
- Wulsten, I.F.; Galeev, A.; Stingl, K. Underestimated Survival of Campylobacter in Raw Milk Highlighted by Viability Real-Time PCR and Growth Recovery. Front. Microbiol. 2020, 11, 1107. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-L. Development of a multiplex real-time recombinase polymerase amplification (RPA) assay for rapid quantitative detection of Campylobacter coli and jejuni from eggs and chicken products. Food Control. 2017, 73, 1247–1255. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Qiao, B.; Xu, X.-B.; Zhang, J.-Z. Development and application of a real-time polymerase chain reaction method for Campylobacter jejuni detection. World J. Gastroenterol. 2013, 19, 3090–3095. [Google Scholar]
- Bratz, K.; Gölz, G.; Riedel, C.; Janczyk, P.; Nöckler, K.; Alter, T. Inhibitory effect of high-dosage zinc oxide dietary supplementation on Campylobacter coli excretion in weaned piglets. J. Appl. Microbiol. 2013, 115, 1194–1202. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Proroga, Y.T.R.; Capuano, F.; Corrado, F.; Santonicola, S.; Medici, D.; Delibato, E.; Murru, N. Detection and quantification of Campylobacterin foods: Newanalytic approaches to detectand quantify Campylobacterspp. in food samples. Ital. J. Food Saf. 2020, 9, 8591. [Google Scholar] [PubMed]
- Wei, B.; Kang, M.; Jang, H.-K. Evaluation of potassium clavulanate supplementation of Bolton broth for enrichment and detection of Campylobacter from chicken. PLoS ONE 2018, 13, e0205324. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Kamara, J.N.; Vigre, H.; Hoorfar, J.; Josefsen, M.H. Direct Quantification of Campylobacter jejuni in Chicken Fecal Samples Using Real-Time PCR: Evaluation of Six Rapid DNA Extraction Methods. Food Anal. Methods 2013, 6, 1728–1738. [Google Scholar] [CrossRef]
- Chen, W.; Teng, J.; Yao, L.; Xu, J.; Liu, G. Selection of Specific DNA Aptamers for Hetero-Sandwich-Based Colorimetric Determination of Campylobacter jejuni in Food. J. Agric. Food Chem. 2020, 68, 8455–8461. [Google Scholar] [CrossRef]
- Yin, H.; Lin, Y.; Lin, C.; Tsai, W.; Wen, H. Rapid and sensitive detection of Staphylococcus aureus in processed foods using a field-deployed device to perform an insulated isothermal polymerase chain reaction-based assay. J. Food Saf. 2019, 39, e12690. [Google Scholar] [CrossRef]
- Ding, T.; Suo, Y.; Zhang, Z.; Liu, D.; Ye, X.; Chen, S.; Zhao, Y. A Multiplex RT-PCR Assay for S. aureus, L. monocytogenes, and Salmonella spp. Detection in Raw Milk with Pre-enrichment. Front. Microbiol. 2017, 8, 989–989. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-J.; Zang, Y.-X.; Liu, H.-B.; Li, P.; Wang, S. Rapid Detection of Staphylococcus aureus via Recombinase Polymerase Amplification Combined with Lateral Flow Strip. Food Anal. Methods 2018, 11, 2296–2306. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Ding, H.; Jiang, C.; Geng, Y.; Sun, X.; Jing, J.; Gao, H.; Wang, Z.; Dong, C. Recombinase polymerase amplification with polymer flocculation sedimentation for rapid detection of Staphylococcus aureus in food samples. Int. J. Food Microbiol. 2020, 331, 108691. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Deng, Y.; Bai, Y.; Xu, D.; Chen, E.; Wu, H.; Li, B.; Gao, L. Rapid and simultaneous detection of Salmonella, Shigella, and Staphylococcus aureus in fresh pork using a multiplex real-time PCR assay based on immunomagnetic separation. Food Control. 2014, 42, 87–93. [Google Scholar] [CrossRef]
- Bai, X.; Chen, G.; Wang, Z.; Xie, G.; Deng, M.; Xu, H. Simultaneous detection of Bacillus cereus and Staphylococcus aureus by teicoplanin functionalized magnetic beads combined with triplex PCR. Food Control. 2021, 132, 108531. [Google Scholar] [CrossRef]
- Li, F.; Xie, G.; Zhou, B.; Yu, P.; Yu, S.; Aguilar, Z.P.; Wei, H.; Xu, H. Rapid and simultaneous detection of viable Cronobacter sakazakii, Staphylococcus aureus, and Bacillus cereus in infant food products by PMA-mPCR assay with internal amplification control. LWT 2016, 74, 176–182. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, X.; Wang, Z.; Zhao, J.; Wang, X.; Yang, W.; Chen, Y. Multiplexed and DNA amplification-free detection of foodborne pathogens in egg samples: Combining electrical resistance-based microsphere counting and DNA hybridization reaction. Anal. Chim. Acta 2022, 1228, 340336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; He, P.; Zhang, Y.; Wang, Q. A multiplex PCR amplification strategy coupled with microchip electrophoresis for simultaneous and sensitive detection of three foodborne bacteria. J. Chromatogr. B 2018, 1093-1094, 141–146. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Liu, Z.-M.; Zhang, B.-Q.; Qu, M.; Mo, C.-S.; Luo, J.; Li, S.-L. Development of a novel target-enriched multiplex PCR (Tem-PCR) assay for simultaneous detection of five foodborne pathogens. Food Control. 2016, 64, 54–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.-q.; Guo, T.; Hong, L. simultaneous detection of five foodborne pathogens for pre-enrichment required in rapid Escherichia coli detection. Sci. Rep. 2018, 8, 17808. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; Nguyen, T.T.; Nguyen, T.K.O.; Le, T.T.H.; Nguyen, T.D. A novel multiplex PCR method for the detection of virulence-associated genes of Escherichia coli O157:H7 in food. 3 Biotech. 2016, 6, 5. [Google Scholar]
- Garrido-Maestu, A.; Azinheiro, S.; Carvalho, J.; Fuciños, P.; Prado, M. Optimized sample treatment, combined with real-time PCR, for same-day detection of E. coli O157 in ground beef and leafy greens. Food Control. 2020, 108, 106790. [Google Scholar] [CrossRef]
- Hu, J.; Huang, R.; Wang, Y. Wei, X.; Wang, Z.; Geng, Y.; Jing, J.; Gao, H. Sun, X.; Dong, C.; Jiang, C. Development of duplex pcr-elisa for simuultaneous detection of Salmonella spp. and Escherichia coli O15: H7 in food. J. Microbiol. Methods 2018, 154, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Wang, D.; Yao, S.; Ge, L. Wang, Y.; Zhao, Y.; Zhao, J. Song, X.; Zhao, C.; Li, J.; Liu, Y.; Jin, M.; Xu, K. A detection method of Escherichia coli O157:H7 based on immunomagnetic separation and aptamers-gold nanoparticle probe quenching Rhodamine B’s fluorescence. Food Sci. Biotechnol. 2021, 30, 1129–1138. [Google Scholar] [PubMed]
- Giovanni, M.; Setyawati, M.I.; Tay, C.Y.; Qian, H.; Kuan, W.S.; Leong, D.T. Electrochemical Quantification of Escherichia coli with DNA Nanostructure. Adv. Funct. Mater. 2015, 25, 3840–3846. [Google Scholar] [CrossRef]
- He, L.; Simpson, D.; Ganzle, M.G. Detection of enterohaemorrhagic Escherichia coli in food by droplet digital pcr to detect simultaenous virulence factors in a single genome. Food Microbiol. 2020, 90, 103466. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Su, H.; Ding, H.; Sun, X.; Gao, H.; Geng, Y.; Wang, Z. Rapid analysis of Escherichia coli O157:H7 using isothermal recombinase polymerase amplification combined with triple-labeled nucleotide probes. Mol. Cell. Probes 2020, 50, 101501. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Lee, H.; Lee, S.; Kim, S.; Lee, J.; Ha, J. Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Rapid Detection of Escherichia coli in Fresh Foods Using a Combination of Enrichment and PCR Analysis. Food Sci. Anim. Resour. 2018, 38, 829–834. [Google Scholar]
- Bian, X.; Jing, F.; Li, G.; Fan, X.; Jia, C.; Zhou, H.; Jin, Q.; Zhao, J. A microfluidic droplet digital PCR for simultaneous detection of pathogenic Escherichia coli O157 and Listeria monocytogenes. Biosens. Bioelectron. 2015, 74, 770–777. [Google Scholar] [CrossRef]
- P. ; Panphut, W.; Lomae, A.; Wonsawat, W.; Citterio, D.; Ruecha, N. Dual Colorimetric/Electrochemical Detection of Salmonella typhimurium Using a Laser-Induced Graphene Integrated Lateral Flow Immunoassay Strip. Anal. Chem. 2023, 95, 13904–13912. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Liu, C.; Tian, Y.; Fang, S.; Yang, H.; Li, B.; Liu, Q. Colloidal gold immunochromatographic test strips for broad-spectrum detection of Salmonella. Food Control. 2021, 126, 108052. [Google Scholar] [CrossRef]
- Fulgione, A.; Cimafonte, M.; Della Ventura, B.; Iannaccone, M.; Ambrosino, C.; Capuano, F.; Proroga, Y.T.R.; Velotta, R.; Capparelli, R. QCM-based immunosensor for rapid detection of Salmonella Typhimurium in food. Sci. Rep. 2018, 31, 16137. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-B.; Zang, Y.-X.; Du, X.-J.; Li, P.; Wang, S. Development of an isothermal amplification-based assay for the rapid visual detection of Salmonella bacteria. J. Dairy Sci. 2017, 100, 7016–7025. [Google Scholar] [CrossRef]
- Du, X.-J.; Zhou, T.-J.; Li, P.; Wang, S. A rapid Salmonella detection method involving thermophilic helicase-dependent amplification and a lateral flow assay. Mol. Cell. Probes 2017, 34, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, X.; Liu, W.; Xie, J.; Zhang, H.; Du, S. A sensitive multimode dot fltration strip for the detection of Salmonella typhimurium using MoS2@Fe3O4. Microchim. Acta 2022, 189, 475. [Google Scholar] [CrossRef]
- Gao, P.; Wang, L.; He, Y.; Wang, Y.; Yang, X.; Fu, S.; Qin, X.; Chen, Q.; Man, C.; Jiang, Y. An Enhanced Lateral Flow Assay Based on Aptamer–Magnetic Separation and Multifold AuNPs for Ultrasensitive Detection of Salmonella Typhimurium in Milk. Foods 2021, 10, 1605. [Google Scholar] [CrossRef]
- Guo, R.; Wang, S.; Huang, F.; Chen, Q.; Li, Y.; Liao, M.; Lin, J. Rapid detection of Salmonella Typhimurium using magnetic nanoparticle immunoseparation, nanocluster signal amplification and smartphone image analysis. Sensors Actuators B: Chem. 2019, 284, 134–139. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Xu, J.; Qu, W.; Yao, L.; Yao, B.; Yan, C.; Chen, W. Filtration assisted pretreatment for rapid enrichment and accurate detection of Salmonella in vegetables. Food Sci. Hum. Wellness 2023, 12, 1167–1173. [Google Scholar] [CrossRef]
- Cheng, N.; Zhu, C.; Wang, Y.; Du, D.; Zhu, M.-J.; Luo, Y.; Xu, W.; Lin, Y. Nanozyme Enhanced Colorimetric Immunoassay for Naked-Eye Detection of Salmonella Enteritidis. J. Anal. Test. 2019, 3, 99–106. [Google Scholar] [CrossRef]
- Chen, K.; Ma, B.; Li, J.; Chen, E.; Xu, Y.; Yu, X.; Sun, C.; Zhang, M. A Rapid and Sensitive Europium Nanoparticle-Based Lateral Flow Immunoassay Combined with Recombinase Polymerase Amplification for Simultaneous Detection of Three Food-Borne Pathogens. Int. J. Environ. Res. Public Heal. 2021, 18, 4574. [Google Scholar] [CrossRef] [PubMed]
- Tu, J. Ting Wu, T.; Yu, Q. Li, J. Zheng, S. Qi, K.; Sun, G.; Xiao, R.; Chongwen Wang, C. Introduction of multilayered magnetic core–dual shell SERS tags into lateral flow immunoassay: A highly stable and sensitive method for the simultaneous detection of multiple veterinary drugs in complex samples, J. Hazard. Mater. 2023, 448, 130912. [Google Scholar]
- Wang, S.; Zhu, X.; Meng, Q.; Zheng, P.; Zhang, J.; He, Z.; Jiang, H. Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosens. Bioelectron. 2021, 183, 113186. [Google Scholar] [CrossRef]
- Shi, D.; Shi, H. Combining loop-mediated isothermal amplification and nanozyme-strip for ultrasensitive and rapid detection of viable Listeria monocytogenes cells and biofilms. LWT 2022, 154, 112641. [Google Scholar] [CrossRef]
- Morlay, A.; Roux, A.; Templier, V.; Piat, F.; Roupioz, Y. Label-free immuno-Biosensor for the fast detection of Listeria in food Label-Free Immuno-Sensors for the Fast Detection of Listeria in Food. Methods Mol. Biol. 2017. 1600, 49–59. [Google Scholar]
- Ngernpimai, S.; Srijampa, S.; Thongmee, P.; Teerasong, S.; Puangmali, T.; Maleewong, W.; Chompoosor, A.; Tippayawat, P. Insight into the Covalently Oriented Immobilization of Antibodies on Gold Nanoparticle Probes to Improve Sensitivity in the Colorimetric Detection of Listeria monocytogenes. Bioconjugate Chem. 2022, 33, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-B.; Du, X.-J.; Zang, Y.-X.; Li, P.; Wang, S. SERS-Based Lateral Flow Strip Biosensor for Simultaneous Detection of Listeria monocytogenes and Salmonella enterica Serotype Enteritidis. J. Agric. Food Chem. 2017, 65, 10290–10299. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.K.; Nayak, M.K.; Mehta, J.; Kim, K.-H.; Deep, A. Fluorescent nanobiosensors for the targeted detection of foodborne bacteria. TrAC Trends Anal. Chem. 2017, 97, 120–135. [Google Scholar] [CrossRef]
- Tominaga, T. Rapid quantification of coliforms in ready-to-eat foods using lateral-flow immunochromatographic assay. J. Food Saf. 2020, 40, e12835. [Google Scholar] [CrossRef]
- Wu, Z. Simultaneous Detection of Listeria monocytogenes and Salmonella typhimurium by a SERS-Based Lateral Flow Immunochromatographic Assay. Food Anal. Methods 2019, 12, 1086–1091. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, W.; Wang, Z.; Yang, R.; Yu, L.; Du, Q.; Ge, A.; Liu, C.; Chi, Z. Improved triple-module fluorescent biosensor for the rapid and ultrasensitive detection of Campylobacter jejuni in livestock and dairy. Food Control. 2022, 137, 108905. [Google Scholar] [CrossRef]
- Poonlapdecha, W.; Seetang-Nun, Y.; Wonglumsom, W.; Tuitemwong, K.; Erickson, L.E.; Hansen, R.R.; Tuitemwong, P. Antibody-conjugated ferromagnetic nanoparticles with lateral flow test strip assay for rapid detection of Campylobacter jejuni in poultry samples. Int. J. Food Microbiol. 2018, 286, 6–14. [Google Scholar] [CrossRef]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Herron, P.; Zourob, M. Rapid colorimetric lactoferrin-based sandwich immunoassay on cotton swabs for the detection of foodborne pathogenic bacteria. Talanta 2018, 185, 275–280. [Google Scholar] [CrossRef]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Zourob, M. A rapid colorimetric immunoassay for the detection of pathogenic bacteria on poultry processing plants using cotton swabs and nanobeads. Microchim. Acta 2018, 185, 164. [Google Scholar] [CrossRef]
- Shan, S.; Lai, W.; Xiong, Y.; Wei, H.; Xu, H. Novel Strategies To Enhance Lateral Flow Immunoassay Sensitivity for Detecting Foodborne Pathogens. J. Agric. Food Chem. 2015, 63, 745–753. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, Z.; Cui, B.; Xu, E.; Jin, Z. Establishment of a dual mode immunochromatographic assay for Campylobacter jejuni detection. Food Chem. 2019, 289, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, Z.; Mohammadnejad, J.; Hosseini, M.; Bakhshi, B.; Rezayan, A.H. Whole cell FRET immunosensor based on graphene oxide and graphene dot for Campylobacter jejuni detection. Food Chem. 2020, 309, 125690. [Google Scholar] [CrossRef]
- Zang, X.; Kong, K.; Tang, H.; Tang, Y.; Tang, H.; Jiao, X.; Huang, J. A GICA strip for Campylobacter jejuni real-time monitoring at meat production site. LWT 2018, 98, 500–505. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Xu, E. Dual-Mode Aptasensor for SERS and Chiral Detection of Campylobacter jejuni. Food Anal. Methods 2019, 12, 2185–2193. [Google Scholar] [CrossRef]
- Masdor, N.A.; Altintas, Z.; Tothill, I.E. Sensitive detection of Campylobacter jejuni using nanoparticles enhanced QCM sensor. Biosens. Bioelectron. 2016, 78, 328–336. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Kim, S.; Park, J.S.; Cha, B.S.; Lee, E.S.; Han, J.; Shin, J.; Jang, Y.; Park, K.S. Loop-mediated isothermal amplification-based nucleic acid lateral flow assay for the specific and multiplex detection of genetic markers. Anal. Chim. Acta 2022, 1205, 339781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, L.; Ma, L.; Hua, M.Z.; Wang, S.; Lu, X. Rapid detection of methicillin-resistant Staphylococcus aureus in pork using a nucleic acid-based lateral flow immunoassay. Int. J. Food Microbiol. 2017, 243, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, M.; Xu, H.; Chen, F.; Ming, X.; Xu, H.; Wei, H.; Xu, F.; Liu, C. Development of a rapid and sensitive quantum dot-based immunochromatographic strip by double labeling PCR products for detection of Staphylococcus aureus in food. Food Control. 2014, 46, 225–232. [Google Scholar] [CrossRef]
- Ayamah, A.; Sylverken, A.A.; Ofori, L.A. Microbial Load and Antibiotic Resistance of Escherichia coli and Staphylococcus aureus Isolated from Ready-to-Eat (RTE) Khebab Sold on a University Campus and Its Environs in Ghana. J. Food Qual. 2021, 2021, 8622903. [Google Scholar] [CrossRef]
- Jin, B.; Ma, B.; Li, J.; Hong, Y.; Zhang, M. Simultaneous Detection of Five Foodborne Pathogens Using a Mini Automatic Nucleic Acid Extractor Combined with Recombinase Polymerase Amplification and Lateral Flow Immunoassay. Microorganisms 2022, 10, 1352. [Google Scholar] [CrossRef]
- Sung, Y.J.; Suk, H.-J.; Sung, H.Y.; Li, T.; Poo, H.; Kim, M.-G. Novel antibody/gold nanoparticle/magnetic nanoparticle nanocomposites for immunomagnetic separation and rapid colorimetric detection of Staphylococcus aureus in milk. Biosens. Bioelectron. 2013, 43, 432–439. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Du, J.; Liu, K.; Bai, Y. A highly sensitive multiplex lateral flow immunoassay for simultaneous detection of Listeria monocytogenes, Salmonella Typhimurium and Escherichia coli O157:H7. J. Food Meas. Charact. 2023, 17, 6577–6587. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Peters, S.; Eschbach, E.; Feßler, A.T.; Oberheitmann, B.; Schwarz, S. Development of a nucleic acid lateral flow immunoassay (NALFIA) for reliable, simple and rapid detection of the methicillin resistance genes mecA and mecC. Veter- Microbiol. 2017, 200, 101–106. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Qian, S.; Li, H.; Fu, P.; Zhou, H.; Zheng, J. An ultrasensitive lateral flow immunoassay platform for foodborne biotoxins and pathogenic bacteria based on carbon-dots embedded mesoporous silicon nanoparticles fluorescent reporter probes. Food Chem. 2023, 399, 133970. [Google Scholar] [CrossRef]
- Wang, Q.; Long, M.; Lv, C.; Xin, S.; Han, X.; Jiang, W. Lanthanide-labeled fluorescent-nanoparticle immunochromatographic strips enable rapid and quantitative detection of Escherichia coli O157:H7 in food samples. Food Control. 2020, 109, 106894. [Google Scholar] [CrossRef]
- Song, C.; Liu, J.; Li, J.; Liu, Q. Dual FITC lateral flow immunoassay for sensitive detection of Escherichia coli O157:H7 in food samples. Biosens. Bioelectron. 2016, 85, 734–739. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Dai, S.; Dou, M.; Jiao, S.; Yang, J.; Li, W.; Su, Y.; Li, Q.; Li, J. High-throughput, highly sensitive and rapid SERS detection of Escherichia coli O157:H7 using aptamer-modified Au@macroporous silica magnetic photonic microsphere array. Food Chem. 2023, 424, 136433. [Google Scholar] [CrossRef]
- Zhan, S.; Fang, H.; Fu, J.; Lai, W.; Leng, Y.; Huang, X.; Xiong, Y. Gold Nanoflower-Enhanced Dynamic Light Scattering Immunosensor for the Ultrasensitive No-Wash Detection of Escherichia coli O157:H7 in Milk. J. Agric. Food Chem. 2019, 67, 9104–9111. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, M.; Singh, S.; Deep, A.; Sharma, A.L. Highly Sensitive Optical Detection of Escherichia coli Using Terbium-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 48198–48205. [Google Scholar] [CrossRef]
- Dou, L.; Bai, Y.; Liu, M.; Shao, S.; Yang, H.; Yu, X.; Wen, K.; Wang, Z.; Shen, J.; Yu, W. ‘Three-To-One’ multi-functional nanocomposite-based lateral flow immunoassay for label-free and dual-readout detection of pathogenic bacteria. Biosens. Bioelectron. 2022, 204, 114093. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Liu, C.; Fang, S.; Ma, J.; Qiu, J.; Xu, D.; Li, L.; Yu, J.; Li, D.; Liu, Q. SERS-based lateral flow assay combined with machine learning for highly sensitive quantitative analysis of Escherichia coli O157:H7. Anal. Bioanal. Chem. 2020, 412, 7881–7890. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Y.; Huang, X.; Zhang, W.; Wu, Y.; Fang, H.; Zhang, C.; Xiong, Y. Dramatically Enhanced Immunochromatographic Assay Using Cascade Signal Amplification for Ultrasensitive Detection of Escherichia coli O157:H7 in Milk. J. Agric. Food Chem. 2020, 68, 1118–1125. [Google Scholar] [CrossRef]
- Ilhan, H.; Guven, B.; Dogan, U.; Torul, H.; Evran, S.; Çetin, D.; Suludere, Z.; Saglam, N.; Boyaci, I.H.; Tamer, U. The coupling of immunomagnetic enrichment of bacteria with paper-based platform. Talanta 2019, 201, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Song, Y.; Zeinhom, M.M.A.; Chang, Y.-C.; Sheng, L.; Li, H.; Du, D.; Li, L.; Zhu, M.-J.; Luo, Y.; et al. Nanozyme-Mediated Dual Immunoassay Integrated with Smartphone for Use in Simultaneous Detection of Pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef]
- Lou, Y.; Jia, Q.; Rong, F.; Zhang, S.; Zhang, Z.; Du, M. Universal biosensing platform based on polyMn-MOF nanosheets for efficient analysis of foodborne pathogens from diverse foodstuffs. Food Chem. 2022, 395, 133618. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, E.; Chamsaz, M.; Turner, A.; Jager, E.; Beni, V. Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens. Bioelectron. 2016, 80, 194–200. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tellez, J.; Sanchez-Ortega, I.; Hornung-Leoni, C.T.; Santos, E.M.; Miranda, J.M.; Rodriguez, J.A. Impedimetric Biosensor Based on a Hechtia argentea Lectin for the Detection of Salmonella spp. Chemosensors 2020, 8, 115. [Google Scholar] [CrossRef]
- Xiang, C.; Li, R.; Adhikari, B.; She, Z.; Li, Y.; Kraatz, H.-B. Sensitive electrochemical detection of Salmonella with chitosan–gold nanoparticles composite film. Talanta 2015, 140, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Feng, K. Li, T.; Ye, C.; Gao, X.; Yang, T.; Liang, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M.; Xiong, C.; Huang, G.; Zhang, J. A label-free electrochemical immunosensor for rapid detection of salmonella in milk by using CoFe-MOFs-graphene modified electrode. Food Control 2021, 130, 108357. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Y.; Lei, P.; Shen, B.; Cheng, W.; Ju, H.; Ding, S. A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA–AuNPs probe. Anal. Chim. Acta 2014, 846, 44–50. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Pulingam, T.; Thong, K.L.; Muniandy, S.; Ahmad, N.; Leo, B.F. Rapid and sensitive detection of Salmonella with reduced graphene oxide-carbon nanotube based electrochemical aptasensor. Anal. Biochem. 2020, 589, 113489. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, S.; Teh, S.J.; Appaturi, J.N.; Thong, K.L.; Lai, C.W.; Ibrahim, F.; Leo, B.F. A reduced graphene oxide-titanium dioxide nanocomposite based electrochemical aptasensor for rapid and sensitive detection of Salmonella enterica. Bioelectrochemistry 2019, 127, 136–144. [Google Scholar] [CrossRef]
- Bagheryan, Z.; Raoof, J.-B.; Golabi, M.; Turner, A.P.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef]
- Maciel, C.; Silva, N.F.D.; Teixeira, P.; Magalhães, J.M.C.S. Development of a Novel Phagomagnetic-Assisted Isothermal DNA Amplification System for Endpoint Electrochemical Detection of Listeria monocytogenes. Biosensors 2023, 13, 464. [Google Scholar] [CrossRef]
- Jiang, X.; Lv, Z.; Rao, C.; Chen, X.; Zhang, Y.; Lin, F. Simple and highly sensitive electrochemical detection of Listeria monocytogenes based on aptamer-regulated Pt nanoparticles/hollow carbon spheres nanozyme activity. Sensors Actuators B: Chem. 2023, 392, 133991. [Google Scholar] [CrossRef]
- Viswanath, K.B.; Suganya, K.; Krishnamoorthy, G.; Marudhamuthu, M.; Selvan, S.T.; Vasantha, V.S. Enzyme-Free Multiplex Detection of Foodborne Pathogens Using Au Nanoparticles-Decorated Multiwalled Carbon Nanotubes. ACS Food Sci. Technol. 2021, 1, 1236–1246. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.; Li, S.; Zhang, H.; Cui, L.; Liu, J.; Yao, W. Ultrasensitive detection of Listeria monocytogenes using solid-state electrochemiluminescence biosensing basedon the quenching effect of ferrocene on ruthenium pyridine. J. Food Saf. 2020, 41, e12868. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Jahne, M.; Rogers, S.; Suni, I.I. Detection of Listeria Monocytogenes by EIS. Electroanalysis 2013, 25, 2231–2237. [Google Scholar] [CrossRef]
- Mishra, A.; Pilloton, R.; Jain, S.; Roy, S.; Khanuja, M.; Mathur, A.; Narang, J. Paper-Based Electrodes Conjugated with Tungsten Disulfide Nanostructure and Aptamer for Impedimetric Detection of Listeria monocytogenes. Biosensors 2022, 12, 88. [Google Scholar] [CrossRef]
- Oliveira, D.A.; McLamore, E.S.; Gomes, C.L. Rapid and label-free Listeria monocytogenes detection based on stimuli-responsive alginate-platinum thiomer nanobrushes. Sci. Rep. 2022, 12, 21413. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef]
- Chen, W.; Cui, L. Li, C.; Su, Y.; Tong,; Y. Xu, W. A novel aptamer biosensor using ZnO-3DNGH for sensitive and selective detection of Listeria monocytogenes. Microchem. J. 2022, 179, 107414. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, C.; Yang, C.; Liu, Z.; Wan, S. Development of an in-situ signal amplified electrochemical assay for detection of Listeria monocytogenes with label-free strategy. Food Chem. 2021, 358, 129894. [Google Scholar] [CrossRef]
- Bonaldo, S.; Franchin, L.; Pasqualotto, E.; Cretaio, E.; Losasso, C.; Peruzzo, A.; Paccagnella, A. Influence of BSA Protein on Electrochemical Response of Genosensors. IEEE Sensors J. 2023, 23, 1786–1794. [Google Scholar] [CrossRef]
- Lyte, J.M.; Shrestha, S.; Wagle, B.R.; Liyanage, R.; Martinez, D.A.; Donoghue, A.M.; Daniels, K.M.; Lyte, M. Serotonin modulates Campylobacter jejuni physiology and in vitro interaction with the gut epithelium. Poult. Sci. 2021, 100, 100944. [Google Scholar] [CrossRef]
- Yadav, N.; Chhillar, A.K.; Rana, J.S. Detection of pathogenic bacteria with special emphasis to biosensors integrated with AuNPs. Sensors Int. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Morant-Miñana, M.C.; Elizalde, J. Microscale electrodes integrated on COP for real sample Campylobacter spp. detection. Biosens. Bioelectron. 2015, 70, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mintmier, B.; McGarry, J.M.; E Sparacino-Watkins, C.; Sallmen, J.; Fischer-Schrader, K.; Magalon, A.; McCormick, J.R.; Stolz, J.F.; Schwarz, G.; Bain, D.J.; et al. Molecular cloning, expression and biochemical characterization of periplasmic nitrate reductase from Campylobacter jejuni. FEMS Microbiol. Lett. 2018, 365, fny151. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Liu, Y.; Li, R.; Wu, C.; Zhu, L.; Zhang, J. Indirect electrochemical determination of ciprofloxacin by anodic stripping voltammetry of Cd(II) on graphene-modified electrode. J. Electroanal. Chem. 2015, 738, 123–129. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Nunez-Carmona, E.; Abbatangelo, M.; Zappa, D.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanostructured MOS Sensor for the Detection, Follow up, and Threshold Pursuing of Campylobacter Jejuni Development in Milk Samples. Sensors 2020, 20, 2009. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214–112214. [Google Scholar] [CrossRef]
- Shams, S.; Bakhshi, B.; Moghadam, T.T.; Behmanesh, M. A sensitive gold-nanorods-based nanobiosensor for specific detection of Campylobacter jejuni and Campylobacter coli. J. Nanobiotechnology 2019, 17, 43. [Google Scholar] [CrossRef]
- Sohouli, E.; Ghalkhani, M.; Zargar, T.; Joseph, Y.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Plonska-Brzezinska, M.E.; Ehrlich, H. A new electrochemical aptasensor based on gold/nitrogen-doped carbon nano-onions for the detection of Staphylococcus aureus. Electrochimica Acta 2021, 403, 139633. [Google Scholar] [CrossRef]
- El Wekil, M.M.; Halby, H.M.; Darweesh, M.; E. Ali, M.; Ali, R. An innovative dual recognition aptasensor for specifc detection of Staphylococcus aureus based on Au/Fe3O4 binary hybrid. Sci. Rep. 2022, 12, 12502. [Google Scholar] [CrossRef]
- Ayres, L.d.B.; Brooks, J.; Whitehead, K.; Garcia, C.D. Rapid Detection of Staphylococcus aureus Using Paper-Derived Electrochemical Biosensors. Anal. Chem. 2022, 94, 16847–16854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Lu, X. Molecular imprinting technology for sensing foodborne pathogenic bacteria. Anal. Bioanal. Chem. 2021, 413, 4581–4598. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Rahmati, Z.; Golchin, M.; Lotfi, Z.; Nemati, M. Electrochemical immunosensor for determination of Staphylococcus aureus bacteria by IgY immobilized on glassy carbon electrode with electrodeposited gold nanoparticles. Microchim. Acta 2020, 187, 567. [Google Scholar] [CrossRef] [PubMed]
- Ghalkhani, M.; Sohouli, E.; Khaloo, S.S.; Vaziri, M.H. Architecting of an aptasensor for the staphylococcus aureus analysis by modification of the screen-printed carbon electrode with aptamer/Ag–Cs-Gr QDs/NTiO2. Chemosphere 2022, 293, 133597. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Wang, Z.; Wei, X. Impedimetric aptasensor for Staphylococcus aureus based on nanocomposite prepared from reduced graphene oxide and gold nanoparticles. Microchim. Acta 2014, 181, 967–974. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sensors Actuators B: Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Svalova, T.S.; Medvedeva, M.V.; Kozitsina, A.N. A “Clickable” Electrodeposited Polymer Films Based on 3-Ethynylthiophene for the Covalent Immobilization of Proteins. Application to a Label-free Electrochemical Immunosensor for Escherichia Coli and Staphylococcus Aureus Determination. Electroanalysis 2021, 33, 2469–2475. [Google Scholar] [CrossRef]
- Khan, S.; Akrema, A.; Qazi, S.; Ahmad, R.; Raza, K.; Rahisuddin, R. In Silico and Electrochemical Studies for a ZnO−CuO-Based Immunosensor for Sensitive and Selective Detection of E. coli. ACS Omega 2021, 6, 16076–16085. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Yang, X.; Wu, J.; Liu, Q.; Li, D.; Huang, S.; Xie, H.; Yu, Z.; Gan, N. Reusable electrochemical biosensing platform based on egg yolk antibody-labeled magnetic covalent organic framework for on-site detection of Escherichia coli in foods. Sensors Actuators B: Chem. 2022, 369, 132320. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2018, 126, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Malvano, F.; Pilloton, R.; Albanese, D. Sensitive Detection of Escherichia coli O157:H7 in Food Products by Impedimetric Immunosensors. Sensors 2018, 18, 2168. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Zhao, G.; Dou, W. A Label-Free Electrochemical Immunosensor Modified with AuNPs for Quantitative Detection of Escherichia coli O157:H7. J. Electron. Mater. 2019, 48, 7960–7969. [Google Scholar] [CrossRef]
- Pandey, C.M.; Tiwari, I.; Singh, V.N.; Sood, K.; Sumana, G.; Malhotra, B.D. Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sensors Actuators B: Chem. 2017, 238, 1060–1069. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Qi, H.; Huang, X.; Shi, J.; Zou, X. A novel renewable electrochemical biosensor based on mussel-inspired adhesive protein for the detection of Escherichia coli O157:H7 in food. Sensors Actuators B: Chem. 2022, 372, 132601. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; You, H.; Li, D.; Yu, Z.; Gan, N. Tesla valve-assisted biosensor for dual-mode and dual-target simultaneous determination of foodborne pathogens based on phage/DNAzyme co-modified zeolitic imidazolate framework-encoded probes. Anal. Chim. Acta 2023, 1275, 341591. [Google Scholar] [CrossRef]
- Das, R.; Chaterjee, B.; Kapil, A.; Sharma, T.K. Aptamer-NanoZyme mediated sensing platform for the rapid detection of Escherichia coli in fruit juice. Sens. Bio-Sens. Res. 2020, 27, 100313. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Bie, S.; Suo, T.; Jia, G.; Liu, B.; Ye, R.; Li, Z. Development of an Electrochemical Biosensor for Rapid and Effective Detection of Pathogenic Escherichia coli in Licorice Extract. Appl. Sci. 2019, 9, 295. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Guo, J.; Ma, X. Nanomaterial Labels in Lateral Flow Immunoassays for Point-of-Care-Testing. J. mater. sci. technol. 2021, 60, 90–104. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, B.; Yue, L.; Miao, Y.; Hu, Y.; Ouyang, R. Application of Nanomaterials to Enhance Polymerase Chain Reaction. Molecules 2022, 27, 8854. [Google Scholar] [CrossRef] [PubMed]
- Suni, I.I. Impedance methods for electrochemical sensors using nanomaterials. TrAC Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, A.; Dutta, L.; Pati, K.R.; Korpan, Y.; Dahiya, I. Electrochemical Biosensors for the Detection of Antibiotics in Milk: Recent Trends and Future Perspectives. Biosensors 2023, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ma, S.; Hara, T.O.; Singh, S. Nanomaterials-Based Biosensors for the Detection of Prostate Cancer Biomarkers: Recent Trends and Future Perspective. Adv. Mater. Technol. 2023, 8, 2201860. [Google Scholar] [CrossRef]
- Kumar, H.; Kuča, K.; Bhatia, S.K.; Saini, K.; Kaushal, A.; Verma, R.; Bhalla, T.C.; Kumar, D. Applications of Nanotechnology in Sensor-Based Detection of Foodborne Pathogens. Sensors 2020, 20, 1966. [Google Scholar] [CrossRef]
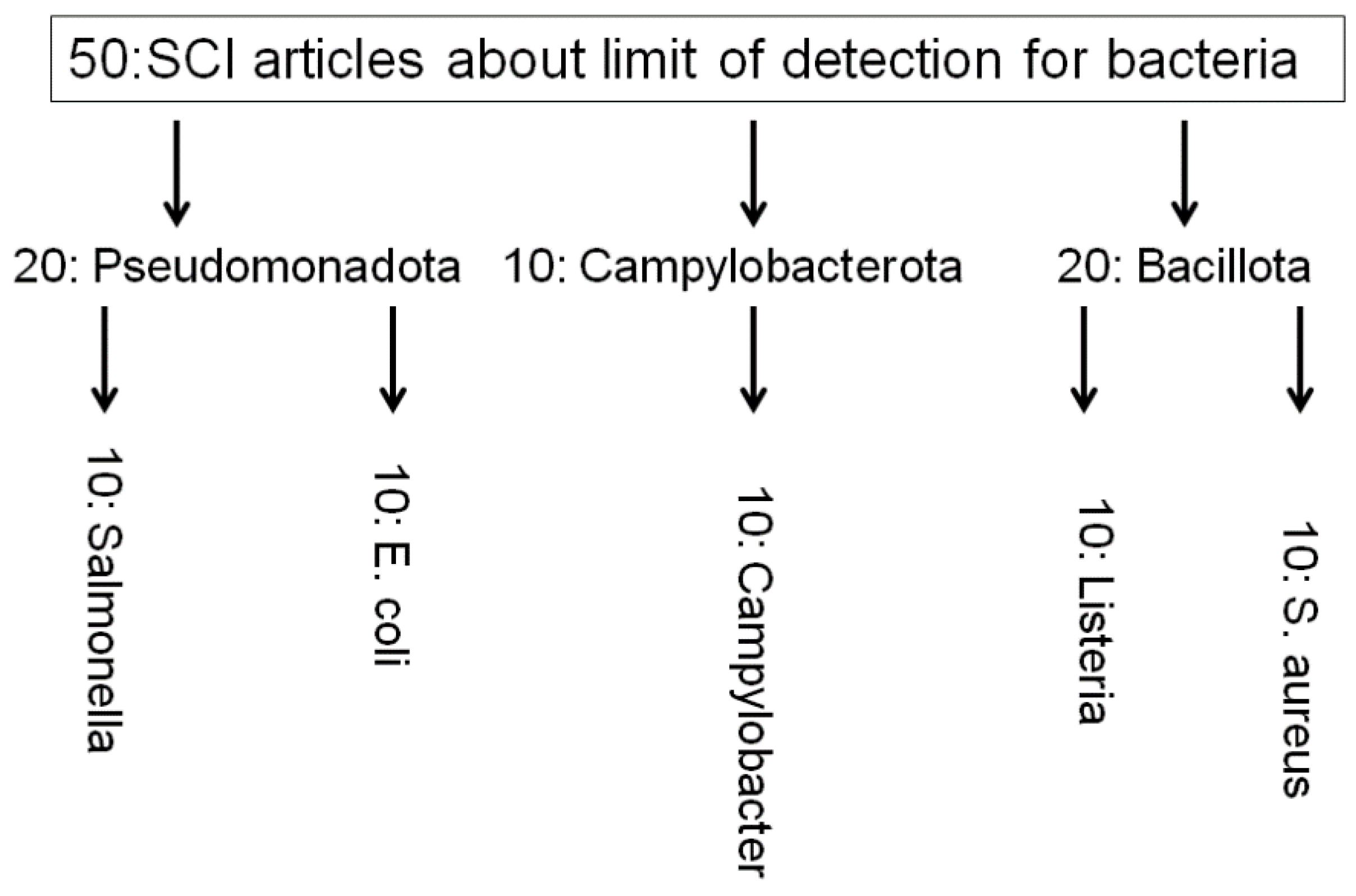
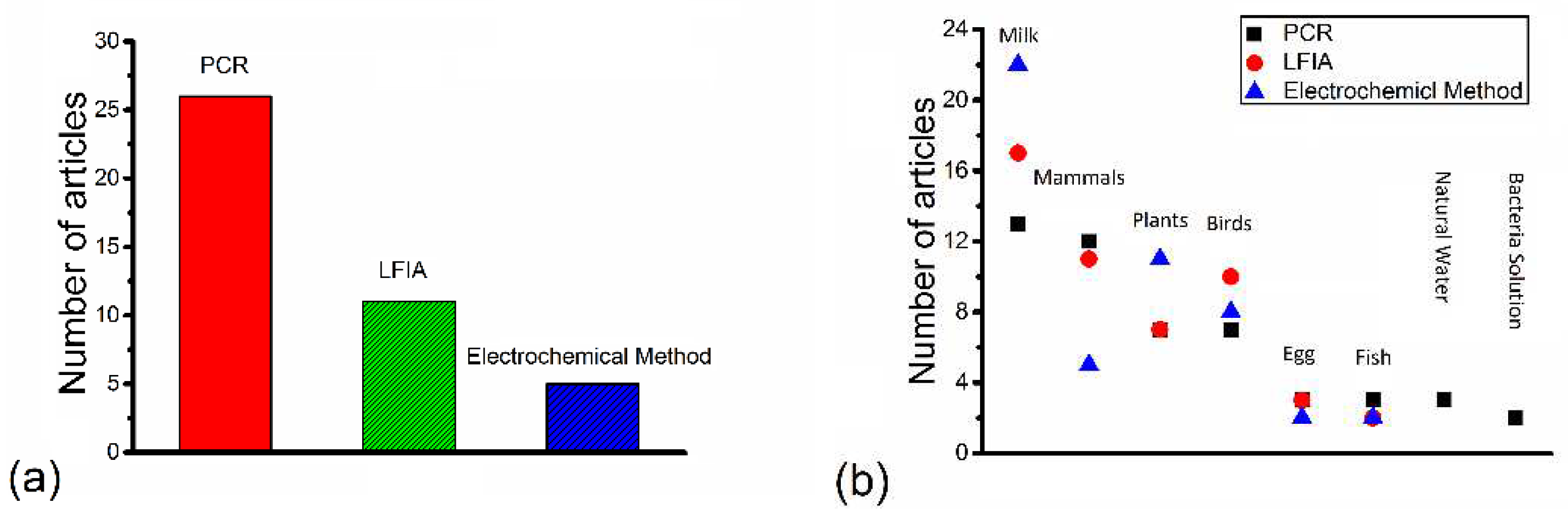
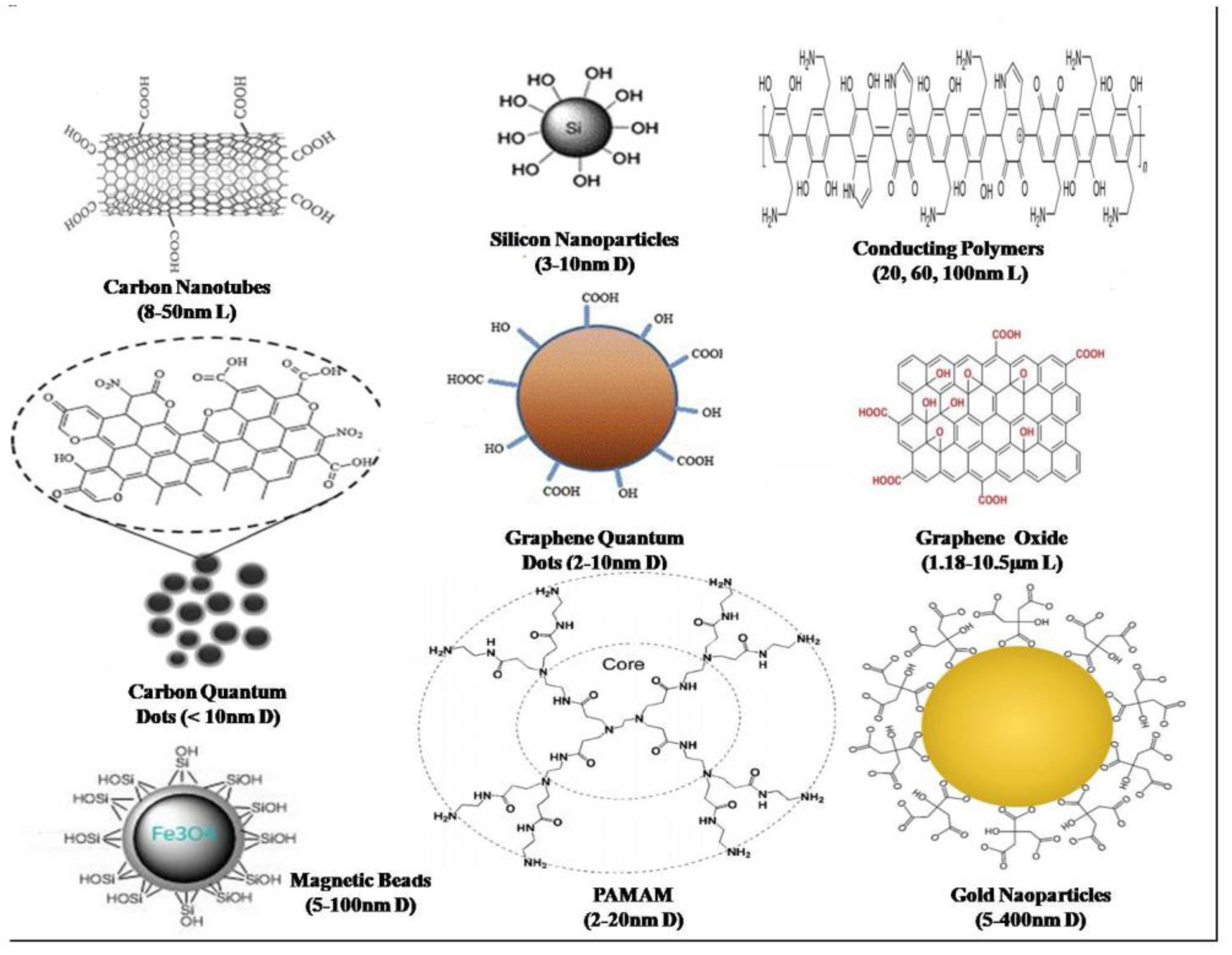
| Bacteria type | Multiplex? | Food sample | Sample number | LOD(CFU/ml) | Year | Reference |
|---|---|---|---|---|---|---|
| Salmonella | No | Beef | 10 | 0.04 | 2022 | [41] |
| No | Chicken | 10 | 0.1 | 2017 | [42] | |
| Yes | Bacteria Solution | 8 | 0.2 | 2013 | [43] | |
| Yes | Pork | 7 | 2 | 2019 | [44] | |
| No | Lettuce | 18 | 2.65 | 2021 | [45] | |
| Yes | Bacteria Solution | 6 | 3 | 2022 | [46] | |
| Yes | Natural Water | 8 | 3 | 2020 | [47] | |
| Yes | Chicken | 6 | 4 | 2018 | [48] | |
| No | Sheep | 7 | 9 | 2020 | [49] | |
| No | Chicken | 6 | 10 | 2017 | [50] | |
| Listeria | Yes | Fish | 9 | 0.2 | 2022 | [51] |
| Yes | Egg | 50 | 0.2 | 2014 | [52] | |
| Yes | Duck | 160 | 0.48 | 2022 | [53] | |
| No | Soybean | 20 | 4 | 2019 | [54] | |
| No | Milk | 35 | 5 | 2017 | [55] | |
| No | Milk | 6 | 5 | 2022 | [56] | |
| Yes | Pork | 5 | 9 | 2013 | [57] | |
| Yes | Milk | 13 | 10 | 2023 | [58] | |
| Yes | Lettuce | 14 | 10 | 2022 | [59] | |
| Yes | Lettuce | 21 | 10 | 2016 | [60] | |
| Campylobacter | No | Pork | 8 | 0.3 | 2014 | [61] |
| No | Milk | 5 | 1 | 2023 | [62] | |
| No | Milk | 8 | 1 | 2020 | [63] | |
| Yes | Chicken | 9 | 1 | 2017 | [64] | |
| No | Sheep | 41 | 4.3 | 2013 | [65] | |
| Yes | Pork | 30 | 10 | 2013 | [66] | |
| No | Pork | 54 | 10 | 2020 | [67] | |
| Yes | Chicken | 40 | 10 | 2018 | [68] | |
| No | Chicken | 6 | 10 | 2013 | [69] | |
| No | Milk | 12 | 13 | 2020 | [70] | |
| S. aureus | No | Milk | 24 | 0.25 | 2019 | [71] |
| Yes | Milk | 46 | 0.48 | 2017 | [72] | |
| No | Fish | 8 | 1.2 | 2018 | [73] | |
| No | Egg | 50 | 3.8 | 2020 | [74] | |
| Yes | Pork | 51 | 9.6 | 2014 | [75] | |
| Yes | Milk | 9 | 10 | 2022 | [76] | |
| Yes | Rice | 8 | 19 | 2016 | [77] | |
| Yes | Egg | 12 | 20 | 2022 | [78] | |
| No | Milk | 5 | 28 | 2018 | [79] | |
| Yes | Beef | 9 | 42 | 2016 | [80] | |
| E. coli | No | Natural Water | 6 | 0.04 | 2018 | [81] |
| Yes | Fish | 180 | 0.12 | 2016 | [82] | |
| Yes | Beef | 32 | 0.14 | 2020 | [83] | |
| Yes | Cabbage | 25 | 1 | 2018 | [84] | |
| No | Milk | 5 | 1.03 | 2021 | [85] | |
| No | Natural Water | 7 | 1.2 | 2015 | [86] | |
| Yes | Apple | 22 | 2 | 2020 | [87] | |
| No | Milk | 7 | 4.4 | 2020 | [88] | |
| No | Beef | 12 | 10 | 2018 | [89] | |
| Yes | Milk | 8 | 10 | 2015 | [90] |
| Bacteria type | Multiplex? | Food sample | Sample number | Particle | Size(nm) | LOD(CFU/ml) | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Salmonella | No | Orange | 5 | Gold | 20 | 1 | 2023 | [91] |
| No | Chicken | 5 | Gold | 40 | 1 | 2019 | [92] | |
| No | Chicken | 6 | Gold | NA | 1 | 2018 | [93] | |
| No | Egg | 11 | Gold | 15 | 1.05 | 2017 | [94] | |
| No | Milk | 7 | Gold | 20 | 1.6 | 2017 | [95] | |
| Yes | Grape | 9 | Iron | 40 | 8 | 2022 | [96] | |
| No | Milk | 7 | Gold | 15 | 8.6 | 2021 | [97] | |
| No | Chicken | 5 | Iron | 150 | 16 | 2019 | [98] | |
| No | Lettuce | 6 | Gold | NA | 17 | 2023 | [99] | |
| No | Milk | 5 | Iron | NA | 34 | 2019 | [100] | |
| Listeria | Yes | Beef | 6 | Europium | NA | 7 | 2021 | [101] |
| No | Pork | 30 | Gold | 20 | 8 | 2023 | [102] | |
| No | Milk | 12 | Manganese | 200 | 9.2 | 2021 | [103] | |
| No | Lettuce | 5 | Iron | 189 | 10 | 2022 | [104] | |
| No | Milk | 8 | Gold | 50 | 10 | 2017 | [105] | |
| No | Pork | 6 | Gold | 28 | 11 | 2022 | [106] | |
| Yes | Egg | 9 | Gold | 10 | 19 | 2017 | [107] | |
| No | Lettuce | 6 | Gold | 10 | 30 | 2017 | [108] | |
| No | Lettuce | 5 | Palladium | NA | 48 | 2020 | [109] | |
| Yes | Milk | 6 | Gold | NA | 75 | 2019 | [110] | |
| Campylobacter | No | Milk | 7 | Iron | NA | 3 | 2022 | [111] |
| No | Poultry | 60 | Gold | 50 | 10 | 2018 | [112] | |
| Yes | Poultry | 9 | Iron | NA | 10 | 2018 | [113] | |
| Yes | Poultry | 8 | Cobalt | 50 | 10 | 2018 | [114] | |
| No | Fish | 105 | Iron | NA | 10 | 2014 | [115] | |
| No | Milk | 6 | Gold | 15 | 50 | 2019 | [116] | |
| No | Chicken | 6 | Gold | 2 | 100 | 2020 | [117] | |
| No | Pork | 112 | Gold | 30 | 100 | 2018 | [118] | |
| No | Chicken | 7 | Gold | 33 | 131 | 2019 | [119] | |
| No | Beef | 5 | Gold | 40 | 150 | 2016 | [120] | |
| S. aureus | No | Egg | 6 | Gold | 40 | 1.6 | 2022 | [121] |
| No | Pork | 9 | Gold | NA | 2 | 2017 | [122] | |
| No | Milk | 80 | Silicon | NA | 3 | 2014 | [123] | |
| No | Lamb | 36 | Gold | 40 | 5.96 | 2021 | [124] | |
| Yes | Fish | 80 | Gold | NA | 10 | 2022 | [125] | |
| No | Milk | 30 | Gold | 10 | 10 | 2013 | [126] | |
| Yes | Milk | 32 | Gold | 50 | 18 | 2023 | [127] | |
| Yes | Pork | 6 | Gold | 15 | 35 | 2015 | [128] | |
| No | Turkey | 6 | Carbon | NA | 40 | 2017 | [129] | |
| No | Milk | 6 | Silicon | NA | 100 | 2023 | [130] | |
| E. coli | No | Pork | 50 | Europium | NA | 1 | 2020 | [131] |
| No | Milk | 7 | Gold | NA | 1 | 2016 | [132] | |
| No | Pork | 8 | Gold | NA | 2.2 | 2023 | [133] | |
| No | Milk | 5 | Gold | NA | 2.7 | 2019 | [134] | |
| No | Apple | 7 | Gold | NA | 3 | 2020 | [135] | |
| No | Chicken | 7 | Iron | NA | 10 | 2022 | [136] | |
| No | Beef | 10 | Gold | 36 | 10 | 2020 | [137] | |
| No | Milk | 5 | Gold | 38 | 12.5 | 2020 | [138] | |
| Yes | Milk | 6 | Gold | 2 | 20 | 2019 | [139] | |
| Yes | Milk | 8 | Palladium | 35 | 34 | 2017 | [140] |
| Bacteria type | Multiplex? | Food sample | Sample number | Electrochemical technique | Sensor material | Linear range (CFU/ml) | LOD(CFU/ml) | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Salmonella | Yes | Milk | 8 | DPV | PolymerMn-MOF on gold | 10-108 | 2.6 | 2022 | [141] |
| No | Apple | 7 | EIS | screen- printed gold | 100-108 | 3 | 2016 | [142] | |
| No | Pork | 8 | CV | gold,graphene on glassy carbon | 24-2400 | 3 | 2014 | [143] | |
| No | Egg | 10 | EIS | Hechtia argentea lectin on gold | 15-2.57*107 | 5 | 2020 | [144] | |
| No | Milk | 5 | CV | chitosan hydrogel, and glassy carbon | 10-105 | 5 | 2015 | [145] | |
| No | Milk | 5 | DPV | CoFe-MOFs-graphene on gold | 10-105 | 6 | 2021 | [146] | |
| No | Milk | 9 | EIS | DNA-AuNPs | 24-2.4*108 | 6 | 2014 | [147] | |
| No | Chicken | 8 | DPV | ssDNA/rGO-CNT/GCE | 10-108 | 10 | 2020 | [148] | |
| No | Chicken | 7 | DPV | DNA/rGO-TiO2/GCE | 10-108 | 10 | 2019 | [149] | |
| No | Apple | 8 | EIS | diazonium layer onto SPEs | 10-108 | 10 | 2016 | [150] | |
| Listeria | No | Milk | 13 | SWV | Methylene blue | NA | 1 | 2023 | [151] |
| No | Lettuce | 12 | CV | Aptamer Pt/HCNs nanozyme | NA | 2 | 2023 | [152] | |
| Yes | Milk | 11 | EIS | AuNPs-MWCNTs | 10-107 | 3.22 | 2021 | [153] | |
| No | Pork | 6 | EIS | DNA modified ferrocene | 14-1.4*106 | 4 | 2020 | [154] | |
| No | Tomato | 6 | EIS | gold | NA | 4 | 2013 | [155] | |
| No | Milk | 8 | EIS | paper-based with tungsten disulfide | 10-108 | 4.5 | 2022 | [156] | |
| No | Chicken | 6 | CV | ALG-thiomer/Pt | 10-106 | 5 | 2022 | [157] | |
| No | Milk | 5 | EIS | gold interdigitated | 100-2200 | 5.5 | 2018 | [158] | |
| No | Pork | 25 | DPV | ZnO-3DNGH | 15-1.5*107 | 6.8 | 2022 | [159] | |
| No | Lettuce | 5 | DPV | Si@MB/AuNP modified glassy carbon | 100-107 | 10 | 2021 | [160] | |
| Campylobacter | No | Beef | 31 | EIS | DNA modified gold | NA | 8 | 2023 | [161] |
| No | Poultry | 118 | EIS | glassy carbon | NA | 10 | 2021 | [162] | |
| No | Chicken | 156 | DPV | DNA modified gold | 10-1000 | 10 | 2020 | [163] | |
| No | Poultry | 100 | SWV | thin-film gold | NA | 11 | 2015 | [164] | |
| Yes | Chicken | 36 | DPV | Ag/AgCl | NA | 13 | 2018 | [165] | |
| No | Poultry | 7 | ASV | graphene modified glassy carbon | 50-500 | 15 | 2015 | [166] | |
| No | Chicken | 50 | EIS | AuNPs on glassy carbon | NA | 50 | 2019 | [167] | |
| No | Milk | 6 | EIS | interdigited platinum | NA | 100 | 2020 | [168] | |
| No | Milk | 5 | CV | TiO2 | NA | 100 | 2020 | [169] | |
| No | Milk | 5 | CV | DNA modified gold | NA | 100 | 2019 | [170] | |
| S. aureus | No | Apple | 9 | CV | Au/nitrogen doped carbon | 10-108 | 1 | 2022 | [171] |
| No | Apple | 7 | CV | AuNPs@ Fe3O4/GCE | 10-107 | 1 | 2022 | [172] | |
| No | Milk | 6 | CV | Au modified paper- carbon | NA | 2 | 2022 | [173] | |
| No | Pork | 7 | EIS | Stamp imprinted gold | NA | 3 | 2021 | [174] | |
| No | Milk | 7 | EIS | AuNP modified GCE | 10-107 | 3.3 | 2020 | [175] | |
| No | Orange | 9 | CV | Ag-Cs-Gr QDs/NTiO2SPEs | 10-5*108 | 5 | 2022 | [176] | |
| No | Milk | 6 | DPV | carboxylated MWCNTs | NA | 5 | 2020 | [177] | |
| No | Fish | 7 | EIS | AuNPs- rGO-ssDNA | 10-106 | 10 | 2014 | [178] | |
| No | Milk | 7 | DPV | Ab-SWCNT | 10-107 | 13 | 2017 | [179] | |
| Yes | Milk | 7 | EIS | BSA on platinum | 100-106 | 15.9 | 2021 | [180] | |
| E. coli | No | Milk | 7 | DPV | ZnO-CuO on gold | 1000-106 | 2 | 2021 | [181] |
| No | Egg | 6 | CV | FerroceneSPCEs | 10-108 | 3 | 2022 | [182] | |
| No | Milk | 6 | DPV | CdS@ZIF-8 | 10-108 | 3 | 2019 | [183] | |
| No | Milk | 8 | EIS | cysteamine/ ferrocene- modified | NA | 3 | 2018 | [184] | |
| No | Milk | 5 | CV | AuNPs modified SPCEs | 7-7*106 | 3.5 | 2019 | [185] | |
| No | Milk | 9 | EIS | rGO-CysCu on gold | 10-108 | 3.8 | 2017 | [186] | |
| No | Fish | 5 | CV | AuNPs on glassy carbon | 15-1.5*108 | 4 | 2022 | [187] | |
| Yes | Lettuce | 12 | EIS | ZIF-8 decorated ferrocene | 10-107 | 5 | 2023 | [188] | |
| No | Apple | 9 | CV | silver | 10-108 | 10 | 2020 | [189] | |
| No | Milk | 5 | DPV | gold | 50-5*107 | 10 | 2019 | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





