Submitted:
16 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
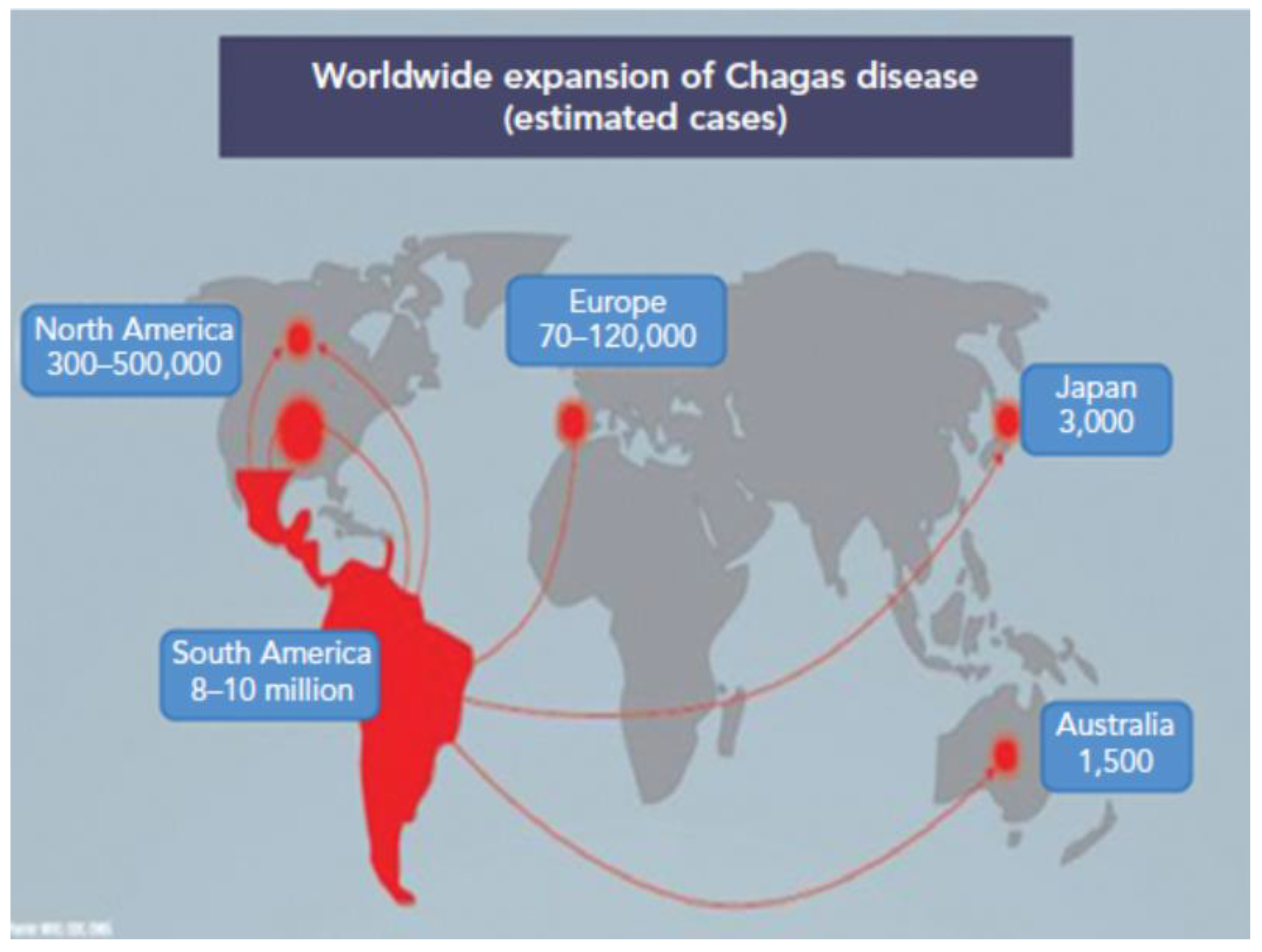
HERBAL MEDICINE
HERBAL MEDICINE COMPONENTS
PROBLEM STATEMENT
JUSTIFICATION
AIM
METHOD
STUDY SELECTION
DATA EXTRACTION
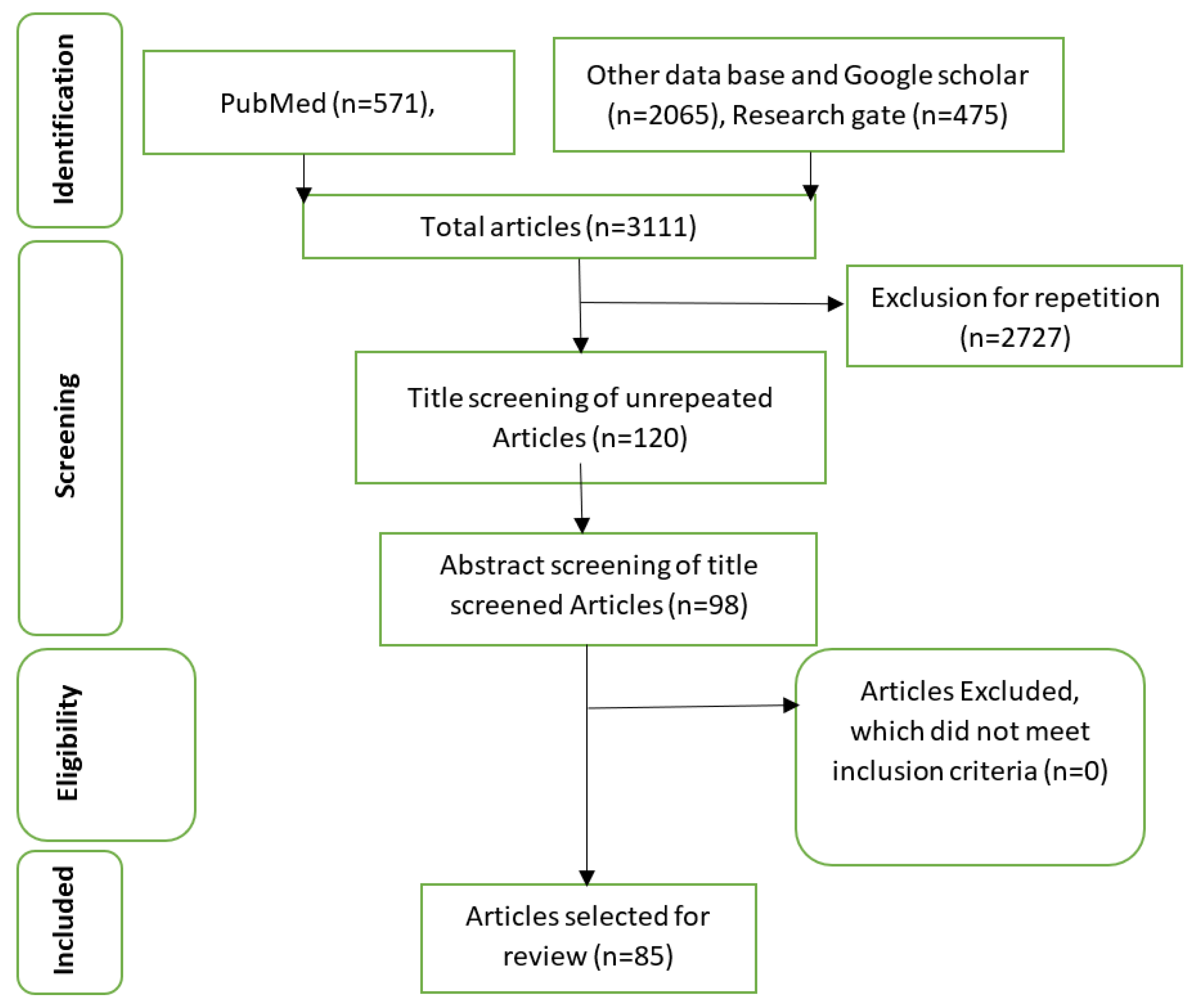
RESULT
| Table. | ||||||
| S/N | Plant | Aim | Chemicals/Compounds | Country | Result | Reference |
| 1 | Argemone ochroleuca, Capparis spinosa, Heliotropiumcurassavicum. | Test plant extracts against various diseases | Argemone ochroleuca (CHCl3 fraction), Capparis spinosa (EtOAc fraction), Heliotropiumcurassavicum (CHCl3 fraction) | Saudi Arabia | Ten crude methanolic extracts that demonstrated potent and adequately selective antiprotozoal activity were subjected to solvent fractionation using petroleum ether, ethyl acetate and chloroform1. Only three samples showed promising antiprotozoal activity. | [11] |
| 2 | Jacalin | Test the adjuvant effect of jacalin on the mouse humoral immune response | Trinitrophenyl and Trypanosoma cruzi | Brazil | Not specified | [12] |
| 3 | Meliaceae and Rutaceae | Test the trypanocidal activity of plant extracts | C. heterophyllus and Galipeacarinata | Brazil | The results obtained from the extracts and fractions revealed that the order Rutales is a promising source for the search of new drugs for Chagas disease | [13] |
| 4 | Terminalia catappa | Test the antioxidant and antitrypanosomal activities of extract and fractions | Ellagic acid, luteolin, apigenin, and quercetin | Brazil | The ethyl acetate fraction obtained from T. catappa leaves can be an effective alternative in the treatment and control of Chagas disease | [14] |
| 5 | plant-derived sulphur-containing amides | Test the antitrypanosomal activity of plant-derived sulphur-containing amides | Sulphur-containing amides | Australia | High antitrypanosomal activity | [15] |
| 6 | Stevia satureiifolia var. satureiifolia | Test the trypanocidal and leishmanicidal activities of flavonoids | Flavonoids | Argentina | Eupatorin and 5-desmethylsinensetin showed IC50 values of 0.2 and 0.4 μg/mL on T. cruziepimastigotes and 61.8 and 75.1 μg/mL on trypomastigotes, respectively. The flavonoid 5-desmethylsinensetin showed moderate activity against T. cruzi amastigotes (IC50 value = 78.7 μg/mL) and was the most active compound on L. braziliensis promastigotes (IC50 value = 37.0 μg/mL)1. Neither of the flavonoids showed cytotoxicity on Vero cells, up to a concentration of 500 μg/mL | [16] |
| 7 | Ophiocordyceps sp. | Identify antiparasitic agents against Trypanosoma cruzi | Leucinostatins | United states | Leucinostatin B also showed in vivo suppression of T. cruzi in a mouse model of Chagas disease | [17] |
| 8 | Lippiasidoides, Lippiaoriganoidesetc | Test the trypanocidal and cytotoxic activities of essential oilsLippiasidoides, Lippiaoriganoidesetc | Essential oils from Lippiasidoides, Lippiaoriganoidesetc | Brazil | The essential oil from Lippiasidoides was the most effective against trypomastigotes1Lippia origanoides essential oil was the most effective against amastigotes | [18] |
| 9 | Lonchocarpuscultratus | Determine the chemical structure and anti-Trypanosoma cruzi activity of extracts | chalcones 2’,4’-dihydroxy-5’-prenylchalcone and isocordoin, the flavanone 8-prenylpinocembrin, the alkaloid 4-hydroxy-N-methylproline, the triterpenes lupeol and lupenone | Saudi Arabia | Hexanic fraction and chalcone 2’,4’-dihydroxy-5’-prenylchalcone showed potent results against human cancer cell lines tested | [19] |
| 10 | Tomato | Test if Phytomonasserpens shares antigens with Trypanosoma cruzi | Antigens | Brazil | Antigens recognized by human sera and induce protective immunity in mice | [20] |
| 11 | Lycopodium clavatum | Test the effect of Lycopodium clavatum 13c treatment on Trypanosoma cruzi infection | Lycopodium clavatum 13c. |
Brazil | Beneficial immunomodulatory and neuro digestive effect | [21] |
| 12 | Lauraceae | Test the activity of neolignans against Trypanosoma cruzi | Neolignans | Brazil | A study on the trypanocidal activity of Brazilian plants against Trypanosoma cruzi showed potential effects of ethanol extracts obtained from Ocotea paranapiacabensis and Aegiphilalhotzkiana | [22] |
| 13 | Bertholletiaexcelsa | Test the trypanocidal activity of extracts and fractions | isolated Betulinic acid from the hexanic extract of Bertholletiaexcelsa |
Brazil | The crude extracts and fractions of Bertholletiaexcelsa were tested against Trypanosoma cruzi. The extract CE7 was the most effective, followed by fraction F2, which also showed higher trypomastigote lysis1 | [23] |
| 14 | Ageratinavacciniaefolia, Clethra fimbriata, Siparunasessiliflora, C. fimbriata | Characterize ethanolic extracts from Colombian plants with anti-Trypanosoma cruzi activity | Ethanolic extracts from Ageratinavacciniaefolia, Clethra fimbriata, Siparunasessiliflora, C. fimbriata | Colombia | The ethanolic extracts of Ageratinavacciniaefolia, Clethra fimbriata and Siparunasessiliflora showed antiprotozoal activity against epimastigotes and low cytotoxicity in mammalian cells. However, only the ethanolic extract of C. fimbriata showed activity against T. cruzi trypomastigotes, and it had low cytotoxicity in PBMCs. An analysis on the phytochemical composition of C. fimbriata extract showed5that its metabolites were primarily represented by two families of compounds: flavonoids and terpenoids. | [24] |
| 15 | Plant-Derived Alkaloids | Test the activity of plant-derived alkaloids against Trypanosoma cruzi | Alkaloids | Not specified | The study tested the activity of these alkaloids against Trypanosoma cruzi | [25] |
| 16 |
extracts obtained from eight different Cerrado plant species. |
Test the activity of Brazilian Cerrado plant extract against various parasites |
ethanolic extracts obtained from eight different Cerrado plant species. |
Brazil | The study found that 24 out of 37 extracts showed activities against parasites: 9 with anti-P. falciparum, 4 with anti-T. cruzi and 11 with anti-T. brucei gambiense activities. High anti-protozoal activity (IC50 values < 10 μg/mL) without obvious cytotoxicity to L6 cells was observed for eight extracts from plants. | [26] |
| 17 | Drimysbrasiliensis | Test the anti-leishmanial and anti-trypanosomal potential of polygodial | Polygodial | Brazil | The crude hexane extract and polygodial showed activity against Leishmania spp. Polygodial demonstrated high parasite selectivity towards Trypanosoma cruzi trypomastigotes, with a selectivity index of 19. It also showed a leishmanicidal effect, inducing intense ultrastructural damages in Leishmania in short-time incubation. | [27] |
| 18 | Excoecaria lucida | Test the anti-Trypanosoma cruzi activity of extracts and isolated compounds | EL1 and EL2. EL1 is ellagic acid, and EL2 is a 1:1 mixture of stigmasterol-3-O-β-D-glucopyranoside and sitosterol-3-O-β-D-glucopyranoside. | Not specified | The EL1 and EL2 samples were more active against bloodstream trypomastigote forms of Trypanosoma cruzi, with EC50 values of 53.0±3.6 and 58.2±29.0 μg/mL, respectively1. At 100 μg/mL, these samples also showed 70% inhibition of L929 cells infection. Toxicity assays demonstrated that after 96 h of treatment, only the fractions Hex and EA presented detectable cytotoxicity. | [28] |
| 19 | Ursolic extracts of several plants. | Test the effect of ursolic acid-rich extract on Trypanosoma cruzi infection | Ursolic acid | Not specified | The ursolic acid-rich extract showed trypanocidal action in vitro. However, it worsened the condition of mice under experimental acute Chagas disease | [29] |
| S/N | Plant | Aim | Chemicals/Compounds | Country | Result | Reference |
| 20 | Alpinia | Test essential oils against Rhodniusnasutus | Essential Oils | Not specified | The essential oils and their main constituets were topically applied on R. nasutus fifth-instar nymphs. In the first 10 minutes of application, OLALPVIT and OLALPZER at 125 μg/mL provoked 73.3% and 83.3% of mortality, respectively. Terpinen-4-ol at 25 μg/mL and β-pinene at 44 μg/mL provoked 100% of mortality1. The monitoring of resistant insects showed that both essential oils exhibited antifeedant activity | [30] |
| 21 | Argentinean Asteraceae | Test the trypanocidal activity of sesquiterpene lactones | Sesquiterpene Lactones | Argentina | The four sesquiterpene lactones displayed trypanocidal activity on the bloodstream form of Trypanosoma cruzi. The 50% inhibitory concentration (IC50) values were 7.2 ± 0.3 µg/mL for eupatoriopicrin, 28.9±4.1 µg/mL for estafietin, 11.9 ± 4.5 µg/mL for eupahakonenin B, and 7.7 ± 0.4 µg/mL for minimolide. | [31] |
| 22 | Caseariasylvestris var. lingua | Test the trypanocidal activity of a new diterpene | Diterpene | Not specified | The study tested the trypanocidal activity of this new diterpene. | [32] |
| 23 | Lippia alba | Test the immunomodulation and antioxidant activities of major terpenes | Terpenes | Columbia | The study demonstrated meaningful antioxidant and immunomodulatory activity on markers involved in Chronic Chagasic Cardiomyopathy (CCC) pathogenesis (IFN-γ, TNF-α, IL-4, IL-10, and iNOS), which could explain their significant trypanocidal properties and their noteworthy role in preventing, and even reversing, the progression of cardiac damage in chronic Chagas disease | [33] |
| 24 | Plants of Brazilian Restingas | Test plants with tripanocide activity against Trypanosoma cruzi strains | quercetin, myricetin, and ursolic acid | Brazil | All isolated substances were effective in reducing protozoal proliferation. Essentially, quercetin and myricetin did not cause mammalian cell toxicity1. In summary, myricetin and quercetin molecules can be used as a scaffold to develop new effective drugs against Chagas’s disease | [34] |
| 25 | Zanthoxylumchiloperone | Test the trypanocidal activity of plant extracts | limonene and caryophyllene oxide | Colombia | The study demonstrated meaningful antioxidant and immunomodulatory activity on markers involved in Chronic Chagasic Cardiomyopathy (CCC) pathogenesis (IFN-γ, TNF-α, IL-4, IL-10, and iNOS), which could explain their significant trypanocidal properties and their noteworthy role in preventing, and even reversing, the progression of cardiac damage in chronic Chagas disease. | [35] |
| 26 | medicinal plants of Bolivia | Test the leishmanicidal and trypanocidal activities of Bolivian medicinal plants | Not specified | Bolivia | The study tested the leishmanicidal and trypanocidal activities of these Bolivian medicinal plants. | [36] |
| 27 | Muña | Test the insecticidal properties of essential oils | Essential Oils | Bolivia | The study tested the insecticidal properties of these essential oils against vectors of Chagas’ disease. | [37] |
| 28 | Smallanthussonchifolius | Test the trypanocidal activity of sesquiterpene lactones | Sesquiterpene Lactones | Andes | These compounds showed a significant trypanocidal activity against the epimastigote forms of the parasite with IC50 values of 0.84 μM (enhydrin), 1.09 μM (uvedalin), and 4.90 μM (polymatin B). After a 24h treatment with 10 μg/mL of enhydrin or uvedalin, parasites were not able to recover their replication rate | [38] |
| 29 | Canavalia ensiformis | Test the effect of Jaburetox on Rhodniusprolixus | Jaburetox | Brazil | The study found that Jaburetox is toxic and lethal to insects belonging to different orders when administered orally or via injection. It was observed that Jaburetox triggers a decrease in the expression of mRNA of UDP-N-Acetylglucosamine pyrophosphorylase and chitin synthetase | [39] |
| 30 | Piper jericoense | Test the activity of a furofuran lignan against Trypanosoma cruzi | Furofuran Lignan | Colombia | The study found that the furofuran lignan showed activity both in vitro and in vivo against Trypanosoma cruzi. | [40] |
| 31 | Lychnophorapohlii | Test the trypanocidal activity of plant extracts and isolated compounds | sesquiterpene lactones lychnopholide, centratherin, goyazensolide and 15-desoxygoyazensolide, and caffeic acid and the flavonoids luteolin and vicenin-2, and one active caffeoyl quinic acid derivative. These compounds were isolated from Lychnophorapohlii. | Brazil | The study found that the dichloromethane and methanol crude extracts from leaves plus inflorescences of Lychnophorapohlii were found to have trypanocidal activity. The bioassay-guided fractionation of the extracts yielded seven active compounds | [41] |
| 32 | Lychnophoragranmongolense | Test the trypanocidal and analgesic properties of the plant | Lychnophoragranmongolense | Brazil | The study found that the compounds showed trypanocidal and analgesic properties | [42] |
| 33 | Nectandraleucantha | Test the antitrypanosomal activity of dehydrodieugenol | Dehydrodieugenol | Brazil | The study found that dehydrodieugenol and its methylated derivative showed antitrypanosomal activity | [43] |
| 34 | Lonchocarpuscultratus | Test the anti-Trypanosoma cruzi activity of plant extracts | Compounds used were isolated from the seed of Lonchocarpuscultratus | Brazil | The study found that the compounds showed anti-Trypanosoma cruzi activity and cytotoxicity | [44] |
| 35 | Senna plant | Test the trypanocidal activity of (8-hydroxymethylen)-trieicosanyl acetate | (8-hydroxymethylen)-trieicosanyl acetate | Mexico | The study found that (8-hydroxymethylen)-trieicosanyl acetate showed anti-trypanosomal activity | [45] |
| 36 | Carica papaya | Test the anti-protozoal activity of crude seed extract against Trypanosoma cruzi | Seed Extract | Mexico | The study found that two doses (50 and 75 mg/kg) of the extract showed in vivo activity against the protozoan Trypanosoma cruzi. A significant reduction in the number of blood trypomastigotes was observed in animals treated with the evaluated doses of the C. papaya extract. | [46] |
| 37 | Senna villosa | Test the trypanocidal activity of the plant in infected BALB/c mice | chloroform extract of Senna villosa leaves | Mexico | The study found that oral doses of 3.3, 6.6 and 13.2 μg/g were tested during 15 days on infected mice BALB/c, beginning treatment 40 days after infection to evaluate specifically the antitrypanosomal activity over the amastigote form of the parasite. In mice infected with 100 parasites, a significant reduction in the number of amastigote nests was observed in cardiac tissue of treated animals at all doses evaluated. An important reduction of amastigote nests was also observed in treated animals and infected with 500 parasites in comparison with untreated mice or mice treated with allopurinol | [47] |
| 38 | Mikania | Test the efficacy of sesquiterpene lactones against Trypanosoma cruzi and Leishmania sp. | Sesquiterpene Lactones | Argentina | The study found that mikanolide, deoxymikanolide and dihydromikanolide were active against Trypanosoma cruziepimastigotes, bloodstream trypomastigotes, and amastigotes. By contrast, scandenolide was not active on Trypanosoma cruzi. Besides, mikanolide and deoxymikanolide were also active on Leishmania braziliensis promastigotes. Deoxymikanolide presented the highest selectivity index for trypomastigotes (SI = 54) and amastigotes (SI = 12.5). In an in vivo model of Trypanosoma cruzi infection, deoxymikanolide was able to decrease the parasitemia and the weight loss associated with the acute phase of the parasite infection. More importantly, while 100% of control mice died by day 22 after receiving a lethal T. cruzi infection, 70% of deoxymikanolide-treated mice survived | [48] |
| 39 | Cedrelafissilis | Test the trypanocidal activity of limonoids and triterpenes | Limonoids and Triterpenes | Brazil | The study found that these compounds showed trypanocidal activity | [49] |
| S/N | Plant | Aim | Chemicals/Compounds | Country | Result | Reference |
| 40 | Arrabidaeatriplinervia | Test the trypanocidal activity of triterpenes | Triterpenes | Brazil | The study found that these compounds showed trypanocidal activity | [50] |
| 41 | Piper crassinervium | Test the in vitro activity of isolated compounds against Trypanosoma cruzi | The compounds studied in this research are isolated from Piper crassinervium | Brazil | The study found that these compounds showed activity against Trypanosoma cruzi | [51] |
| 42 | Piper regnellii | Test the activity of neolignans against Trypanosoma cruzi | Neolignans | Brazil | The study found that a series of 8.O.4′-neolignans (in their ketone, alcohol, and acetylated forms) showed interesting activities against T. cruziepimastigotes. These compounds also inhibited the in vitro differentiation of epimastigotes to trypomastigotes. When tested against HeLa cells, the same compounds did not affect the cell vitality and showed a low influence on cell viability, One of the tested compounds, compound 4 (3,4-methylenedioxi7-oxo-1′-allyl-3′,5′-dimethoxy-8.O.4′-neolignan) showed the best performance in both trypanocide and cytotoxic assays | [52] |
| 43 | Amaryllidaceae | Test the inhibitory potential of montanine against the Trypanosoma cruzi trans-sialidase enzyme | Montanine | Ghana | The study found that montanine is a potential inhibitor of the Trypanosoma cruzi trans-sialidase enzyme | [53] |
| 44 | Delphinium staphisagria | Test the in vitro and in vivo trypanocidal activity of flavonoids | Flavonoids | Spain | The study found that these compounds showed trypanocidal activity | [54] |
| 45 | Amaryllidaceae | Test the potential of Amaryllidaceae plants for the treatment of Chagas disease | The compounds studied in this research are from a collection of 79 extracts of Amaryllidaceae plants | Spain | The study found that two extracts, respectively from Crinum erubescens and Rhodophialaandicola, were identified as highly active and specific against Trypanosoma cruzi and its mammalian replicative form. The results retrieved in this study encourage further exploration of the chemical content of these extracts in search of new anti-Trypanosoma cruzi drug development starting points | [55] |
| 46 | Habranthusbrachyandrus | Test the anti-Trypanosoma cruzi activity of alkaloids | Alkaloids | Argentina | The study found that the alkaloid ismine was specifically active against the parasite and had low toxicity against HepG2 cells, but did not show anti-amastigote activity. The extract had specific anti-T. cruzi activity and the isolated alkaloid ismine was partially responsible for it. These results encourage further exploration of H. brachyandrus alkaloids in search of novel starting points for Chagas disease drug development | [56] |
| 47 | Anacardium occidentale | Test the inhibitory activity of compounds against Trypanosoma cruzisirtuins | Two derivates of cardol (1, 2), cardanol (3, 4), and anacardic acid (5, 6) isolated from cashew nut (Anacardium occidentale, L. Anacardiaceae) | Brazil | The study found that the two anacardic acids (5, 6) inhibited both TcSir2rp1 and TcSir2rp3, while the cardol compound (2) inhibited only TcSir2rp1. The most potent sirtuin inhibitor active against the parasite was the cardol compound (2), with an EC50 value of 12.25 µM, similar to that of benznidazole. Additionally, compounds (1, 4), which were inactive against the sirtuin targets, presented anti-T. cruzi effects1. In conclusion, the results showed the potential of Anacardium occidentale compounds for the development of potential sirtuin inhibitors and anti-Trypanosoma cruzi agents | [57] |
| 48 | Physalis angulata | Test the in vitro and in vivo antiparasitic activity of concentrated ethanolic extract | Ethanolic Extract | Brazil | The study found that the extract effectively inhibits the epimastigote growth and reduces bloodstream trypomastigote viability. It causes parasite cell death by necrosis. The extract impairs parasite infectivity as well as amastigote development in concentrations noncytotoxic to mammalian cells. In mice acutely-infected with T. cruzi, the extract reduced the blood parasitaemia. When combined with benznidazole, the extract showed a synergistic anti-T. cruzi activity | [58] |
| 49 | Pterodonpubescens seeds | Test the trypanocidal activity of geranylgeraniol | Geranylgeraniol | Brazil | The study found that GG-OH showed similar potency to PF1.2, while the oleaginous extract from P. pubescens seeds and the other fractions were about three times less active. | [59] |
| 50 | Arrabidaeachica | Test the photodynamic inactivation of Trypanosoma cruzi by pheophorbide a | Pheophorbide a | Brazil | Pheophorbide a compound had activity against the protozoan in the presence of light and caused morphological and ultrastructural changes, demonstrating its potential in photodynamic therapy. | [60] |
| 51 | Asteraceae | Test the potential of Asteraceae plants against Leishmaniasis and Chagas Disease | Ferulic acid, Rosmarinic acid, and Ursolic acid from Baccharisuncinella DC., Deoxymikanolide from Mikania micrantha, and (+)-15-hydroxy-labd-7-en-17-al from Aristeguietiaglutinosa Lam | Brazil | The study found that these compounds were effective in treating experimental leishmaniasis and showed in vivo anti-T. cruzi action. It was highlighted that these compounds may help in the development of new effective agents against these neglected diseases. | [61] |
| 52 | Pentacaliadesiderabilis (Vell.) Cuatrec. (Asteraceae) | Test the anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone | Jacaranone | Brazil | The study found that jacaranone showed activity against promastigotes of Leishmania (L.) chagasi, Leishmania (V.) braziliensis, and Leishmania (L.). amazonensis showing an IC50 of 17.22, 12.93, and 11.86 μg/mL, respectively. Jacaranone was also tested in vitro against the Trypanosoma cruzi trypomastigotes and Plasmodium falciparum chloroquine-resistant parasites (K1 strain) showing an IC50 of 13 and 7.82 μg/mL, respectively, and was 3.5-fold more effective than benznidazole in anti- Trypanosoma cruzi assay. However, despite the potential against promastigotes forms, this compound was not effective against amastigotes of L. (L.) chagasi and T. cruzi. | [62] |
| 53 | Lippia alba | Test the anti-Trypanosoma cruzi activity of essential oils and their major terpenes | Essential Oils, Terpenes | Colombia | The study found that these oils or their bioactive terpenes (citral, caryophyllene oxide and limonene) could be inducing T. cruzi cell death by an apoptotic-like mechanism. Citral, caryophyllene oxide, and limonene showed a possible induction of an apoptotic-like phenotype | [63] |
| 54 | Anacardium occidentale | Test the trypanocidal activity of the chemical constituents of Anacardium occidentale | The compounds studied were essential/volatile oils (EOs) from various plants. The main constituents of EOS were: δ-cadinene (15.88%), trans-caryophyllene (9.77%) e α-Muurolol (9.42%) for EBEO; trans-caryophyllene (15.24%), bicyclogermacrene (7.33%) e cis-calamenene (7.15%) for HFEO; trans-caryophyllene (30.91%), caryophyllene oxide (13.19%) and spathulenol (5.68%) for HPEO; Xanthoxylin (17.20%) trans-caryophyllene (14.34%) and methyl-eugenol (5.60%) for HSEO; Thymol (49.81%), carvacrol (31.6%) and σ-cimene (10.27%) for LMEO and octanoic acid (38.83%) dodecanoic acid (38.45%) and decanoic acid (20.51%) for SCEO | Brazil | The study found that all the tested oils showed an inhibitory effect on the growth and survival of all forms of T. cruzi and moderate cytotoxicity towards the mammalian cells (100 < CC 50 < 9500 μg/mL) | [64] |
| 55 | Clethra fimbriata | Test the anti-Trypanosoma cruzi activity of a terpenoid-rich extract | Terpenoid-rich Extract | Heliyon | The study found that the Clethra fimbriata hexane extract exhibited the highest activity capable of inhibiting the three parasite developmental stages with an IC50 /EC50 of 153.9 ± 29.5 (epimastigotes), 39.3 ± 7.2 (trypomastigotes), and 45.6 ± 10.5 (amastigotes) μg/mL, presenting a low cytotoxicity in VERO cells with a selectivity index ranging from 6 | [65] |
| 56 | Camellia sinensis | Test the anti-Trypanosoma cruzi activity of catechins | Catechins | Not specified | The study found that the purified compounds lysed more than 50% of the parasites present in the blood of infected BALB/c mice at concentrations as low as 0.12 to 85 pM. The most active compounds were gallocatechin gallate and epigallocatechin gallate, with minimal bactericidal concentrations that inhibited 50% of isolates tested of 0.12 and 0.53 pM, respectively1. Country: The country where the research was conducted is not mentioned in the available snippets. |
[66] |
| S/N | Plant | Aim | Chemicals/Compounds | Country | Result | Reference |
| 57 | Cichoriumintybus | Test the anti-protozoal activity against Trypanosoma cruzi | The study revealed 11 compounds in C. intybus leaves strongly linked with activity against trypomastigotes, including the sesquiterpene lactone lactucin, and flavonoid- and fatty acid-derivatives. Furthermore, seven distinct C. intybus molecules (including two sesquiterpene lactone-derivatives) were predicted to be involved in reducing the number of mammalian cells infected with amastigotes | Not specified | All C. intybus extracts induced concentration-dependent effects against T. cruzi trypomastigotes. C. intybus leaf extracts had higher trypanocidal selectivity and lower cytotoxicity on mammalian cells than root extracts. The leaf extract of C. intybus cv. Goldine also significantly reduced the number of mammalian cells infected with T. cruzi amastigotes | [67] |
| 58 | Crithmummaritimum | Test the in vitro anti-Trypanosoma cruzi activity | The study involved testing decoctions, tinctures, and essential oils from everlasting’s flowers and sea fennel’s stems, leaves, and flowers against intracellular amastigotes of two T. cruzi strains | Portugal | The extract from the sea fennel flower decoction displayed significant anti-trypanosomal activity and no toxicity towards the host cell (EC50 = 17.7 µg/mL, selectivity index > 5.65). Subsequent fractionation of this extract afforded 5 fractions that were re-tested in the same model of anti-parasitic activity | [68] |
| 59 | Tabebuia | Study the chemical reactivity of naphthoquinones with anti-trypanosomal efficacy | Naphthoquinones | Not specified | The study found that among the cyclofunctionalised products the oxazolic and imidazolic derivatives showed ± 1.5 to 34.8 times higher activity than crystal violet, the standard drug for the sterilization of stored blood | [69] |
| 60 | Senna villosa, Serjaniayucatanensis, Byrsonimabucidaefolia, and Bourreria pulchra. | Test the in vitro and in vivo trypanocidal activity of medicinal plants | The compounds studied were ethanol extracts of the aforementioned plants | Mexico | The leaf extracts of S. yucatanensis and B. pulchra were the most active against epimastigotes (IC 100 = 100 μg/mL) and trypomastigotes of T. cruzi (95% or more reduction in the number of parasites at 100 and 50 μg/mL). However, only the leaf extract of S. yucatanensis showed significant trypanocidal activity when tested in vivo, reducing 75% of the parasitemia in infected mice at 100 mg/kg | [70] |
| 61 | Moringa oleifera | Test the cytotoxicity of a trypsin inhibitor from the flower extract against Trypanosoma cruzi | Trypsin Inhibitor | The research was conducted in affiliation with multiple institutions, including the Departamento de Bioquímica, Centro de Biociências, Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil; Departamento de Imunologia, Centro de PesquisasAggeuMagalhães, Fundação Oswaldo Cruz, Recife, Pernambuco, Brazil; and Departamento de Morfologia e Fisiologia Animal, Universidade Federal Rural de Pernambuco, Recife, Brazil. | Both the extract and the trypsin inhibitor caused lysis of T. cruzi trypomastigotes with LC50/24 h of 54.18 ± 6.62 and 41.20 ± 4.28 μg/mL, respectively. High selectivity indices (7.9 to >12) for T. cruzi cells over murine peritoneal macrophages and Vero cells were found for the extract and the trypsin inhibitor | [71] |
| 62 | Lippia alba | Test the immunomodulatory, trypanocide, and antioxidant properties of essential oil fractions | Essential Oil Fractions | The research was conducted in affiliation with multiple institutions, including the Departamento de Microbiologia, Instituto AggeuMagalhães IAM-FIOCRUZ/PE, Av. MoraesRego s/n, Campus da UFPE, 50670-420 Pernambuco, Brazil | The study found that limonene-enriched (ACT1) and citral/caryophyllene oxide-enriched (ACT2) essential oils fractions derived from Carvone and Citral- L. alba chemotypes, respectively, exhibit similar trypanocidal effects to Benznidazole (BZN), against amastigotes. Synergistic antiparasitic activity was observed when ACT1 was combined with BZN or ACT2. ACT1 also decreased the oxidative stress, mitochondrial metabolism, and genotoxicity of the therapies. The ACT1 + ACT2 and ACT1 + BZN experimental treatments reduced the pro-inflammatory cytokines (IFN-γ, IL-2, and TNF-α) and increased the anti-inflammatory cytokines (IL-4 and IL-10) | [72] |
| 63 | Castanedias antamartensis | Test the trypanocidal effect of alcoholic extract | Alcoholic Extract | Not specified | Not specified | [73] |
| 64 | Ambrosia species. | Test the in vitro and in vivo trypanocidal activity of four terpenoid derivatives | Terpenoid Derivatives | Not specified | The compounds cumanin and cordilin were found to be active against Trypanosoma cruziepimastigotes, showing 50% inhibition concentrations (IC 50) values of 12 µM and 26 µM, respectively. These compounds were also active against bloodstrean trypomastigotes, regardless of the T. cruzi strain tested. Psilostachyin and cumanin were also active against amastigote forms with IC 50 values of 21 µM and 8 µM, respectively | [74] |
| 65 | Calophyllumbrasiliense and Mammeaamericana (Clusiaceae) | Test the trypanocidal activity of mammea-type coumarins | Mammea-type Coumarins | Mexico | The most potent compounds were mammea A/BA, A/BB, A/AA, A/BD and B/BA, with MC100 values in the range of 15 to 90 g/ml. Coumarins with a cyclized ,-dimethylallyl substituent on C-6, such as mammea B/BA, cyclo F + B/BB cyclo F, and isomammeigin, showed MC100 values > 200 g/ml | [75] |
| 66 | Pinus oocarpa | Test the trypanocidal activity of oleoresin and terpenoids | Oleoresin, Terpenoids | Korea | Two hits satisfied the selection criteria and were escalated to molecular dynamics simulation and binding free energy calculations. These hits demonstrated higher dock scores, displayed interactions with the key residues portraying an ideal binding mode complemented by mapping to all the features of pharm 1 and pharm 2. Additionally, they rendered stable root mean square deviation (RMSD) and potential energy profiles, illuminating their potentiality as prospective antichagastic agents | [76] |
| 67 | The study involved numerous plants as sources of natural products | Review the potential of natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis | The compounds investigated were various plant-derived natural products from different structural classes, including various alkaloids, terpenoids, flavonoids, and quinonoids. | Not specified | Given the activities of these agents and their diverse range of effects on parasite biology, natural products are a potentially rich source of drug candidates and leads against leishmaniasis and trypanosomiasis | [77] |
| 68 | Quechua, Izoceño-Guaraní, Chiquitano, and Ayoreo | Review the phylobioactive hotspots in plant resources used to treat Chagas disease | The study does not mention the names of the specific compounds used. However, it mentions the bioprospecting of natural product classes effective against Trypanosoma cruzi | Bolivia |
Result: The study systematically uncovers antichagasic phylogenetic hotspots in the plant kingdom as a potential resource for drug discovery based on ethnopharmacological hypotheses |
[78] |
| 69 | Salvia gilliessi | Test the trypanocidal activity of a novel icetexane diterpene | Icetexane Diterpene | Not specified | The result of the research was that 5-epi-icetexone from Salvia gilliessi is active against Trypanosoma cruzi | [79] |
| S/N | Plant | Aim | Chemicals/Compounds | Country | Result | Reference |
| 70 | Eugenia uniflora | Test the anti-Trypanosoma cruzi activity | ethanolic extract from E. uniflora | Brazil | The results demonstrated that E. uniflora has anti-Trypanosoma activity. The concentration presenting 50% of activity (EC50) was 62.76 µg/mL, and the minimum inhibitory concentration (MIC) was ≤1024 µg/mL | [80] |
| 71 | Syzygiumaromaticum and Zingiber officinale | Test the anti-Trypanosoma cruzi activity of essential oils, alone or in combination with benznidazole | essential oils from Syzygiumaromaticum (clove) and Zingiber officinale (ginger), and benznidazole | Brazil | The results showed that clove and ginger essential oils, administered alone and in combination with benznidazole, promoted suppression of parasitemia (p < 0.0001), except for the animals treated with CEO alone, which presented a parasitemia curve similar to untreated animals | [81] |
| 72 | Pfaffiaglomerata | Test the in vitro activities of root extract and its hydrolyzed fractions | Root Extract, Hydrolyzed Fractions | Not Specified | The results showed that fractions F2 and F3 exhibited moderate activity, and pfaffic acid, one of the main chemical constituents of these fractions, displayed IC50 = 44.78 μm (21.06 μg/ml). On the other hand, the hydroalcoholic extract of P. glomerata roots, which is rich in pfaffosides, was inactive | [82] |
| 73 | South American Vernonieae | Test the trypanocidal activity of extracts and sesquiterpene lactones | Extracts, Sesquiterpene Lactones | The results showed that fractions F2 and F3 exhibited moderate activity, and pfaffic acid, one of the main chemical constituents of these fractions, displayed IC50 = 44.78 μm (21.06 μg/ml). On the other hand, the hydroalcoholic extract of P. glomerata roots, which is rich in pfaffosides, was inactive | [83] | |
| 74 | Genipaamericana | Test the trypanocidal activity of polysaccharide extract from leaves | Polysaccharide Extract | Brazil | The results showed that PFI had an effect against epimastigote forms (EC 50/24 h = 580 ± 0.17 μg/ml; EC 50/48 h = 530 ± 0.13 μg/ml; EC 50/72 h = 500 ± 0.14 μg/ml), while PFII did not show effect on any tested concentrations. In trypomastigotes, the PFI and PFII showed effect with EC 50 values of 100 ± 0.09 and 23 ± 0.06 μg/ml respectively. PFI and PFII were also able to decrease amastigotes/100 cells parameter. PFI and PFII were not cytotoxic in LLC-MK2 cells at the highest tested concentration, resulting in selectivity index (SI) higher than 15 for PFI and higher than 65 for PFII | [84] |
| 75 | Psilostachyin C that can be found in several plants | Test the trypanocidal activity of psilostachyin C | Psilostachyin C | Not specified | The results showed that Psilostachyin C may be considered a promising template for the design of novel trypanocidal agents. In addition, Psilostachyin C inhibited the growth of Leishmania mexicana and Leishmania amazonensis promastigotes. The IC(50) values were 1.2 μg/mL and 1.5 μg/mL, respectively | [85] |
| 76 | Ambrosia spp | Test the mode of action of the sesquiterpene lactones psilostachyin and psilostachyin C | Sesquiterpene Lactones | Argentina | The results showed that both sesquiterpene lactones were capable of interacting with hemin1. Psilostachyin increased about 5 times the generation of reactive oxygen species in Trypanosoma cruzi after a 4h treatment, unlike psilostachyin C which induced an increase in reactive oxygen species levels of only 1.5 times | [86] |
| 77 | Lychnophorastaavioides | Test the trypanocidal activity of extracts | Extracts | Not specified | The result of the study was that Lychnophorastaavioides Mart. (Vernonieae, Asteraceae) showed trypanocidal activity | [87] |
| 78 | Not specified | Review the potential therapeutic use of herbal extracts in trypanosomiasis | Herbal Extracts | Not specified | Not specified | [88] |
| 79 | Smallanthus sonchifolius | Test the trypanocidal activity of germacranolide-type sesquiterpene lactones | Germacranolide-type Sesquiterpene Lactones | Argentina | The three compounds exhibited leishmanicidal activity on both parasite forms with IC 50 values of 0.42–0.54 μg/ml for promastigotes and 0.85–1.64 μg/ml for intracellular amastigotes. Similar results were observed on T. cruziepimastigotes (IC 50 0.35–0.60 μg/ml). The TEM evaluation showed marked ultrastructural alterations, such as an intense vacuolization and mitochondrial swelling in both L. mexicana promastigotes and T. cruziepimastigotes exposed to the STLs | [89] |
| 80 | cashew nut (Anacardium occidentale) | Study the differential lethal action of C17:2 and C17:0 anacardic acid derivatives in Trypanosoma cruzi | Anacardic Acid Derivatives | Not specified | Found a differential lethal action of the two anacardic acid derivatives in Trypanosoma cruzi | [90] |
| 81 | Garlic | Test the inhibition of phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruzi by ajoene | Ajoene | Not specified | The result of the research was that ajoene inhibited phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruzi | [91] |
| 82 | Extracts of Colombian plants | Test the trypanocidal activity of extracts of Colombian plants | Extracts | Colombia | Not specified | [92] |
| 83 | Aristeguietiaglutinosa | Test the in vitro and in vivo trypanocidal activity of extracts and active principles | Extracts, Active Principles | Uruguay | Both the hydro-ethanolic extract and the isolated active principle, (+)-15-hydroxy-7-labden-17-al, showed anti-Trypanosoma cruzi activity in vivo. They were well tolerated by mice and no secondary side effects were observed | [93] |
| 84 | Piper | Test the anti-Trypanosoma cruzi activity of piperovatine and piperlonguminine | Piperovatine, Piperlonguminine | Brazil | Both piperovatine and piperlonguminine showed antitrypanosomal activity against epimastigotes and intracellular amastigotes of Trypanosoma cruzi. They caused severe alterations in T. cruzi, mainly located in the plasma membrane and mitochondria, which might trigger biochemical alterations that lead to cell death | [94] |
| 85 | A. scabra & A. elatior | Test the brine shrimp lethality assay as a prescreening system for anti-Trypanosoma cruzi activity | The compounds investigated were the sesquiterpene lactone (STL) cumanin isolated from A. elatior and two other STLs, psilostachyin and cordilin, and one sterol glycoside, daucosterol, isolated from A. scabra | Not specified | The compounds cumanin and cordilin were found to be active against Trypanosoma cruziepimastigotes, showing 50% inhibition concentrations (IC 50) values of 12 µM and 26 µM, respectively. These compounds were also active against bloodstrean trypomastigotes, regardless of the T. cruzi strain tested. Psilostachyin and cumanin were also active against amastigote forms with IC 50 values of 21 µM and 8 µM, respectively | [95] |
DISCUSSION
Benefits, Drawbacks and Limitations.
Mechanistic Strategy
Conclusion
References
- Chagas disease - Diagnosis and treatment - Mayo Clinic. (2017). Mayoclinic.org. https://www.mayoclinic.org/diseases-conditions/chagas-disease/diagnosis-treatment/drc-20356218.
- Lee, J., Kim, S., & Park, Y. (2020). Research trends on Chagas disease: A bibliometric analysis. Journal of Health Sciences, 8(1), 67-78.
- Mintah, S. O., Asafo-Agyei, T., Archer, M.-A., Junior, P. A.-A., Boamah, D., Kumadoh, D., Appiah, A., Ocloo, A., Boakye, Y. D., & Agyare, C. (2019). Medicinal Plants for Treatment of Prevalent Diseases. In www.intechopen.com. IntechOpen. https://www.intechopen.com/chapters/64420.
- World. (2022, April 13). Chagas disease (also known as American trypanosomiasis). Who.int; World Health Organization: WHO. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-%28american-trypanosomiasis%29.
- Mayo Clinic. (2020, November 12). Chagas disease - Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/chagas-disease/symptoms-causes/syc-20356212.
- Centers for Disease Control and Prevention. (2019). CDC - DPDx - American Trypanosomiasis. Centers for Disease Control and Prevention. https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html.
- Better Health. 2021. “Digitalis from the foxglove plant”. Better Health.
- Martinez F, Perna E, Perrone SV, Liprandi AS. Chagas Disease and Heart Failure: An Expanding Issue Worldwide. Eur Cardiol. 2019 Jul 11;14(2):82-88. [CrossRef]
- Saleh M. A., Kamisah, Y. (2021). Use of Carica papaya and Euphorbia hirta in Chagas disease treatment. Journal of Herbal Medicine.
- WHO, 2022. “Herbal Medicine”. World Health Organization.
- Abdel-Sattar, E., et al. (2010). "In vitro activities of plant extracts from Saudi Arabia against malaria, leishmaniasis, sleeping sickness and Chagas disease." Phytother Res 24(9): 1322-1328.
- Albuquerque, D. A., et al. (1999). "The adjuvant effect of jacalin on the mouse humoral immune response to trinitrophenyl and Trypanosoma cruzi." Immunol Lett 68(2-3): 375-381.
- Ambrozin, A. R., et al. (2004). "Trypanocidal activity of Meliaceae and Rutaceae plant extracts." Mem Inst Oswaldo Cruz 99(2): 227-231.
- Araújo, S. A., et al. (2023). "In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa." Biology (Basel) 12(7).
- Astelbauer, F., et al. (2010). "High antitrypanosomal activity of plant-derived sulphur-containing amides." Int J Antimicrob Agents 36(6): 570-572.
- Beer, M. F., et al. (2016). "Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia." Pharm Biol 54(10): 2188-2195.
- Bernatchez, J. A., et al. (2022). "Identification of Leucinostatins from Ophiocordyceps sp. as Antiparasitic Agents against Trypanosoma cruzi." ACS Omega 7(9): 7675-7682.
- Borges, A. R., et al. (2012). "Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil." Exp Parasitol 132(2): 123-128.
- Bortoluzzi, A. A. M., et al. (2021). "Determination of chemical structure and anti-Trypanosoma cruzi activity of extracts from the roots of Lonchocarpuscultratus (Vell.) A.M.G. Azevedo & H.C. Lima." Saudi J Biol Sci 28(1): 99-108.
- Breganó, J. W., et al. (2003). "Phytomonasserpens, a tomato parasite, shares antigens with Trypanosoma cruzi that are recognized by human sera and induce protective immunity in mice." FEMS Immunol Med Microbiol 39(3): 257-264.
- Brustolin Aleixo, C. F., et al. (2017). "Beneficial immunomodulatory and neuro digestive effect in Trypanosoma cruzi infection after Lycopodium clavatum 13c treatment." MicrobPathog 112: 1-4.
- Cabral, M. M., et al. (2010). "Neolignans from plants in northeastern Brazil (Lauraceae) with activity against Trypanosoma cruzi." Exp Parasitol 124(3): 319-324.
- Campos, F. R., et al. (2005). "Trypanocidal activity of extracts and fractions of Bertholletiaexcelsa." Fitoterapia 76(1): 26-29.
- Castañeda, J. S., et al. (2021). "Preliminary chemical characterization of ethanolic extracts from Colombian plants with promising anti - Trypanosoma cruzi activity." Exp Parasitol 223: 108079.
- Cavin, J. C., et al. (1987). "Plant-derived alkaloids active against Trypanosoma cruzi." J Ethnopharmacol 19(1): 89-94.
- Charneau, S., et al. (2016). "In vitro investigation of Brazilian Cerrado plant extract activity against Plasmodium falciparum, Trypanosoma cruzi and T. brucei gambiense." Nat Prod Res 30(11): 1320-1326.
- Corrêa, D. S., et al. (2011). "Anti-leishmanial and anti-trypanosomal potential of polygodial isolated from stem barks of DrimysbrasiliensisMiers (Winteraceae)." Parasitol Res 109(1): 231-236.
- da Silva, C. F., et al. (2018). "Anti-Trypanosoma cruzi Activity in vitro of Phases and Isolated Compounds from Excoecaria lucida Leaves." Med Chem 14(6): 556-562.
- Daga, M. A., et al. (2023). "Ursolic acid-rich extract presents trypanocidal action in vitro but worsens mice under experimental acute Chagas disease." Parasite Immunol 45(10): e13005.
- de Souza, T. A., et al. (2018). "Alpinia Essential Oils and Their Major Components against Rhodniusnasutus, a Vector of Chagas Disease." ScientificWorldJournal 2018: 2393858.
- Elso, O. G., et al. (2020). "Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species." Molecules 25(9).
- Espindola, L. S., et al. (2004). "Trypanocidal activity of a new diterpene from Caseariasylvestris var. lingua." Planta Med 70(11): 1093-1095.
- Espinel-Mesa, D. X., et al. (2021). "Immunomodulation and Antioxidant Activities as Possible Trypanocidal and Cardioprotective Mechanisms of Major Terpenes from Lippia alba Essential Oils in an Experimental Model of Chronic Chagas Disease." Antioxidants (Basel) 10(11).
- Faria, R. X., et al. (2017). "Plants of Brazilian restingas with tripanocide activity against Trypanosoma cruzi strains." J BioenergBiomembr 49(6): 473-483.
- Ferreira, M. E., et al. (2011). "Zanthoxylumchiloperone leaves extract: first sustainable Chagas disease treatment." J Ethnopharmacol 133(3): 986-993.
- Fournet, A., et al. (1994). "Leishmanicidal and trypanocidal activities of Bolivian medicinal plants." J Ethnopharmacol 41(1-2): 19-37.
- Fournet, A., et al. (1996). "Chemical constituents of essential oils of muña, Bolivian plants traditionally used as pesticides, and their insecticidal properties against Chagas' disease vectors." J Ethnopharmacol 52(3): 145-149.
- Frank, F. M., et al. (2013). "Trypanocidal Activity of Smallanthussonchifolius: Identification of Active Sesquiterpene Lactones by Bioassay-Guided Fractionation." Evid Based Complement Alternat Med 2013: 627898.
- Fruttero, L. L., et al. (2017). "Jaburetox affects gene expression and enzyme activities in Rhodniusprolixus, a Chagas' disease vector." Acta Trop 168: 54-63.
- García-Huertas, P., et al. (2018). "Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense." Exp Parasitol 189: 34-42.
- Grael, C. F., et al. (2005). "Chemical constituents of Lychnophorapohlii and trypanocidal activity of crude plant extracts and of isolated compounds." Fitoterapia 76(1): 73-82.
- Grael, C. F., et al. (2000). "A study of the trypanocidal and analgesic properties from Lychnophoragranmongolense (Duarte) Semir&Leitão Filho." Phytother Res 14(3): 203-206.
- Grecco, S. S., et al. (2017). "Antitrypanosomal activity and evaluation of the mechanism of action of dehydrodieugenol isolated from Nectandraleucantha (Lauraceae) and its methylated derivative against Trypanosoma cruzi." Phytomedicine 24: 62-67.
- Griebler, A., et al. (2021). "Anti-Trypanosoma cruzi activity, cytotoxicity, and chemical characterization of extracts from seeds of Lonchocarpuscultratus." J Infect Dev Ctries 15(2): 270-279.
- Jimenez-Coello, M., et al. (2010). "Anti-trypanosomal activity of (8-hydroxymethylen)-trieicosanyl acetate against infective forms of Trypanosoma cruzi." Pharm Biol 48(6): 666-671.
- Jiménez-Coello, M., et al. (2013). "Assessment of the anti-protozoal activity of crude Carica papaya seed extract against Trypanosoma cruzi." Molecules 18(10): 12621-12632.
- Jimenez-Coello, M., et al. (2011). "Antitrypanosomal activity of Senna villosa in infected BALB/c mice with Trypanosoma cruzi during the sub acute phase of infection." Afr J Tradit Complement Altern Med 8(5 Suppl): 164-169.
- Laurella, L. C., et al. (2017). "Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp." PLoSNegl Trop Dis 11(9): e0005929.
- Leite, A. C., et al. (2008). "Trypanocidal activity of limonoids and triterpenes from Cedrelafissilis." Planta Med 74(15): 1795-1799.
- Leite, J. P., et al. (2006). "Trypanocidal activity of triterpenes from Arrabidaeatriplinervia and derivatives." Biol Pharm Bull 29(11): 2307-2309.
- Lopes, A. A., et al. (2008). "In vitro activity of compounds isolated from Piper crassinervium against Trypanosoma cruzi." Nat Prod Res 22(12): 1040-1046.
- Luize, P. S., et al. (2006). "Activity of neolignans isolated from Piper regnellii (MIQ.) C. DC. var. pallescens (C. DC.) YUNCK against Trypanosoma cruzi." Biol Pharm Bull 29(10): 2126-2130.
- Manu, P., et al. (2023). "The Amaryllidaceae alkaloid, montanine, is a potential inhibitor of the Trypanosoma cruzi trans-sialidase enzyme." J Biomol Struct Dyn: 1-17.
- Marín, C., et al. (2011). "In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease." Journal of natural products 74(4): 744-750.
- Martínez-Peinado, N., et al. (2021). "Amaryllidaceae plants: a potential natural resource for the treatment of Chagas disease." Parasit Vectors 14(1): 337.
- Martinez-Peinado, N., et al. (2022). "Anti-Trypanosoma cruzi activity of alkaloids isolated from Habranthusbrachyandrus (Amaryllidaceae) from Argentina." Phytomedicine 101: 154126.
- Matutino Bastos, T., et al. (2019). "Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruziSirtuins." Molecules 24(7).
- Meira, C. S., et al. (2015). "In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi." Phytomedicine 22(11): 969-974.
- Menna-Barreto, R. F., et al. (2008). ″Anti-Trypanosoma cruzi activity of Pterodonpubescens seed oil: geranylgeraniol as the major bioactive component.″ Parasitol Res 103(1): 111-117.
- Miranda, N., et al. (2017). "Pheophorbide a, a compound isolated from the leaves of Arrabidaeachica, induces photodynamic inactivation of Trypanosoma cruzi." PhotodiagnosisPhotodynTher 19: 256-265.
- Moraes Neto, R. N., et al. (2019). "Asteraceae Plants as Sources of Compounds Against Leishmaniasis and Chagas Disease." Front Pharmacol 10: 477.
- Morais, T. R., et al. (2012). "Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacaliadesiderabilis (Vell.) Cuatrec. (Asteraceae)." Parasitol Res 110(1): 95-101.
- Moreno É, M., et al. (2018). "Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide)." BMC Complement Altern Med 18(1): 225.
- Oliveira de Souza, L. I., et al. (2017). "The chemical composition and trypanocidal activity of volatile oils from Brazilian Caatinga plants." Biomed Pharmacother 96: 1055-1064.
- Pardo-Rodriguez, D., et al. (2022). "A terpenoid-rich extract from Clethra fimbriata exhibits anti-Trypanosoma cru zi activity and induces T cell cytokine production." Heliyon 8(3): e09182.
- Paveto, C., et al. (2004). "Anti-Trypanosoma cruzi activity of green tea (Camellia sinensis) catechins." Antimicrob Agents Chemother 48(1): 69-74.
- Peña-Espinoza, M., et al. (2022). "Anti-protozoal activity and metabolomic analyses of Cichoriumintybus L. against Trypanosoma cruzi." Int J Parasitol Drugs Drug Resist 20: 43-53.
- Pereira, C. G., et al. (2021). "In Vitro Anti-Trypanosoma cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmummaritimum L.)." Plants (Basel) 10(11).
- Pinto, C. N., et al. (2000). "Chemical reactivity studies with naphthoquinones from Tabebuia with anti-trypanosomal efficacy." Arzneimittelforschung 50(12): 1120-1128.
- Polanco-Hernández, G., et al. (2012). "In vitro and in vivo trypanocidal activity of native plants from the Yucatan Peninsula." Parasitol Res 110(1): 31-35.
- Pontual, E. V., et al. (2018). "A trypsin inhibitor from Moringa oleifera flower extract is cytotoxic to Trypanosoma cruzi with high selectivity over mammalian cells." Nat Prod Res 32(24): 2940-2944.
- Quintero, W. L., et al. (2021). "Immunomodulatory, trypanocide, and antioxidant properties of essential oil fractions of Lippia alba (Verbenaceae)." BMC Complement Med Ther 21(1): 187.
- Quintero-Pertuz, H., et al. (2022). "Trypanocidal effect of alcoholic extract of Castanediasantamartensis (Asteraceae) leaves is based on altered mitochondrial function." Biomed Pharmacother 148: 112761.
- Ramírez-Macías, I., et al. (2012). "In vitro and in vivo studies of the trypanocidal activity of four terpenoid derivatives against Trypanosoma cruzi." Am J Trop Med Hyg 87(3): 481-488.
- Reyes-Chilpa, R., et al. (2008). "Trypanocidal constituents in plants: 7. Mammea-type coumarins." Mem Inst Oswaldo Cruz 103(5): 431-436.
- Rubio, J., et al. (2005). "Trypanocidal activity of oleoresin and terpenoids isolated from Pinus oocarpa." Z Naturforsch C J Biosci 60(9-10): 711-716.
- Salem, M. M. and K. A. Werbovetz (2006). "Natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis." Curr Med Chem 13(21): 2571-2598.
- Salm, A., et al. (2021). "Phylobioactive hotspots in plant resources used to treat Chagas disease." iScience 24(4): 102310.
- Sanchez, A. M., et al. (2006). "A novel icetexane diterpene, 5-epi-icetexone from Salvia gilliessi is active against Trypanosoma cruzi." Acta Trop 98(2): 118-124.
- Santos, K. K., et al. (2012). "Anti-Trypanosoma cruzi and cytotoxic activities of Eugenia uniflora L." Exp Parasitol 131(1): 130-132.
- Sarto, M. P. M., et al. (2021). "Essential oils from Syzygiumaromaticum and Zingiber officinale, administered alone or in combination with benznidazole, reduce the parasite load in mice orally inoculated with Trypanosoma cruzi II." BMC Complement Med Ther 21(1): 77.
- Silva, M. L., et al. (2017). "In vitro Activities of Pfaffiaglomerata Root Extract, Its Hydrolyzed Fractions and Pfaffic Acid Against Trypanosoma cruzi Trypomastigotes." Chem Biodivers 14(1).
- Sosa, A., et al. (2021). "Trypanocidal activity of South American Vernonieae (Asteraceae) extracts and its sesquiterpene lactones." Nat Prod Res 35(23): 5224-5228.
- Souza, R., et al. (2018). "Trypanocidal activity of polysaccharide extract from Genipaamericana leaves." J Ethnopharmacol 210: 311-317.
- Sülsen, V. P., et al. (2011). "Psilostachyin C: a natural compound with trypanocidal activity." Int J Antimicrob Agents 37(6): 536-543.
- Sülsen, V. P., et al. (2016). "Mode of Action of the Sesquiterpene Lactones Psilostachyin and Psilostachyin C on Trypanosoma cruzi." PLoS One 11(3): e0150526.
- Takeara, R., et al. (2003). "Trypanocidal activity of Lychnophorastaavioides Mart. (Vernonieae, Asteraceae)." Phytomedicine 10(6-7): 490-493.
- Teixeira, T. L., et al. (2014). "Potential therapeutic use of herbal extracts in trypanosomiasis." Pathog Glob Health 108(1): 30-36.
- Ulloa, J. L., et al. (2017). "Germacranolide-type sesquiterpene lactones from Smallanthussonchifolius with promising activity against Leishmania mexicana and Trypanosoma cruzi." Parasit Vectors 10(1): 567.
- Umehara, E., et al. (2020). "Differential lethal action of C17:2 and C17:0 anacardic acid derivatives in Trypanosoma cruzi - A mechanistic study." Bioorg Chem 102: 104068.
- Urbina, J. A., et al. (1993). "Inhibition of phosphatidylcholine biosynthesis and cell proliferation in Trypanosoma cruzi by ajoene, an antiplatelet compound isolated from garlic." Biochem Pharmacol 45(12): 2381-2387.
- Valencia, L., et al. (2011). Trypanocidal and cytotoxic activity of extracts of Colombian plants. Biomedica 31(4): 552-559.
- Varela, J., et al. (2014). "In vivo anti-Trypanosoma cruzi activity of hydro-ethanolic extract and isolated active principles from Aristeguietiaglutinosa and mechanism of action studies." Molecules 19(6): 8488-8502.
- Veiga-Santos, P., et al. (2013). The natural compounds piperovatine and piperlonguminine induce autophagic cell death on Trypanosoma cruzi. Acta Trop 125(3): 349-356.
- Zani, C. L., et al. (1995). Brine shrimp lethality assay as a prescreening system for anti-Trypanosoma cruzi activity. Phytomedicine 2(1): 47-50.
- Digital illustration of trypanosome parasites in blood which causing Chagas disease. — pathological, blood vessel - Stock Photo | #275201628. (n.d.). Focused Collection. Retrieved November 14, 2023, from https://focusedcollection.com/275201628/stock-photo-digital-illustration-trypanosome-parasites-blood.html.
- Gupta, R., & Sharma, A. (2021). Supportive therapy for Chagas disease: An overview. Indian Journal of Medical Sciences, 75(4), 234-245.
- PAHO. (n.d.). Chagas disease - PAHO/WHO | Pan American Health Organization. Www.paho.org. https://www.paho.org/en/topics/chagas-disease.
- Ramallo, I. A., et al. (2018). "A Bioactive Trypanosoma cruzi Bromodomain Inhibitor from Chemically Engineered Extracts." ACS Comb Sci 20(4): 220-228.
- Bosch-Navarrete, C., et al. (2023). Strasseriolides display in vitro and in vivo activity against trypanosomal parasites and cause morphological and size defects in Trypanosoma cruzi. PLoSNegl Trop Dis 17(9): e0011592.
- Canavaci, A. M., et al. (2010). In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoSNegl Trop Dis 4(7): e740.
- Herb-Drug Interactions. (2021, July). NCCIH. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions.
- Davey, M. (2019, April 10). Herbal medicines can have dangerous side effects, research reveals. The Guardian; The Guardian. https://www.theguardian.com/australia-news/2017/feb/06/herbal-medicines-can-have-dangerous-side-effects-research-reveals.
- NHS. (2019). Herbal Medicines. NHS. https://www.nhs.uk/conditions/herbal-medicines/.
- Chagas disease cases estimates worldwide 2019. (n.d.). Statista. https://www.statista.com/statistics/1200041/chagas-disease-estimated-cases-worldwide/.
- World Chagas Disease Day: Know About This Parasitic Ailment That Targets Society’s Most Vulnerable | Weather.com. (n.d.). The Weather Channel. Retrieved December 31, 2023, from https://weather.com/en-IN/india/news/news/2020-04-13-world-chagas-disease-day-parasitic-ailment-targets-society-most.
- Fernández, C. J., & García, B. A. (2022). Variation in the Mitochondrial Genome of the Chagas Disease Vector Triatoma infestans (Hemiptera: Reduviidae). Neotropical Entomology, 51(3), 483–492. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



