1. Introduction
Hair is a specialized derivative of the skin that is ideally organized to protect the human scalp. The well-known structure of hair consists of an external cuticle composed of overlapping scales that acts as a barrier to protect the inner structure. The central medulla is surrounded by the cortex, which is mainly composed of the protein keratin [
1]. This protein plays an important role in the physical and mechanical properties of hair. At the molecular level, keratin is a helical protein composed of two types of fibers: type I, with acidic amino acid residues, and type II, with basic amino acid residues. One strand of each type combines to form a coiled-coil dimer. These dimers coil together in antiparallel tetramers known as protofilaments. Finally, the protofilaments interact to form a single intermediate filament organized into micro-fibrils and larger macro-fibrils [
2]. The conformation of the keratin chain is controlled by hydrogen bonds, ionic forces, Van Der Waals interactions, and disulfide bonds. The disulfide bonds, originating from sulfur-containing cysteine residues, create strong crosslinks between adjacent chains, giving each individual’s hair its unique shape. Indeed, the more α-helical conformation the keratin has, the greater the curve in the protein chain, and the curlier the hair fiber. Conversely, a β-sheet conformation in the keratin decreases the formation of disulfide bonds and produces smoother hair fibers [
3]. In the haircare industry, many products have been developed to modify the disulfide bonds, with the aim of shaping or straightening the hair. These include chemical agents such as alkaline reducing agents, thioglycolates, or guanidine, which are used to break and rearrange bonds. Unfortunately, all these products damage the hair and reduce its strength [
4]. As a consequence, alternative, safe, active ingredients that smooth without inducing damage to the hair, the scalp, or the environment, are actively sought.
Hyaluronic acid (HA) is a key active ingredient in skincare products, but its benefits for hair have been little described. To date, interactions between HA and keratin have been described in the stratum corneum of the skin, where it is known to enhance skin hydration [
5]. In previous work, we first posed the hypothesis that HA can also interact with keratin in hair fiber, promoting keratin interconversion from α-helix to β-sheet conformation. Our data showed an increase of β-sheet conformation and a significant reduction in disulfide bonds after applying HA, thereby delivering an anti-frizz property [
3]. This interconversion was already observed during mechanical or chemical hair straightening, which leads to hair smoothing and ultimately produces an anti-frizz effect [
4]. Due to the cuticle on hair fibers, only low molecular weight molecules (˂ 10 kDa) have been found to penetrate the cortex [
6]. For molecules > 500 kDa, the penetration may only occur when the cuticle is damaged, e.g., after bleaching [
6]. In addition, no evidence of interaction of HA deep inside the hair has yet been established; exploring the penetration of such molecules into hair fibers presents several challenges. A range of techniques, including confocal fluorescence microscopy, have been used in attempts to examine the penetration of tagged molecules, but autofluorescence consistently compromises results [
7]. Raman spectroscopy is a promising method for this type of evaluation. This non-invasive technique detects the characteristic vibrational energy levels of molecules, and provides structural information. In confocal mode, it can gather information at various depths inside a tissue, and quantification is possible because inelastic Raman scattering correlates linearly with molecule concentration [
8]. In a previous study, we were able to track HA penetration ex vivo through the skin using this technique [
9]. Other studies performed on natural and intact human hair fibers demonstrated that confocal Raman spectroscopy can be used to investigate the influence of cosmetic ingredients on keratin structure. An example is the evaluation of a hair-smoothing or heat-protecting effect by analyzing α-helix and β-sheet keratin conformation and the S-S disulfide bridges [
10].
In the present study, we investigated whether Raman spectroscopy could be used to measure HA penetration in hair, and to assess the benefits of a blended and optimized HA formulation for use in haircare products.
2. Materials and Methods
2.1. Shampoo Composition for all the Tests Involving a Washing
The standard basic shampoo used contained water, sodium laurylether sulfate, sodium cocoamidopropylbethaine, sodium chlorhyde, phenoxyethanol, guar hydroxypropyl trimonium, and citric acid. This basic shampoo was blended with high molecular weight HA (HMW-HA; 1-1.4 MDa), low molecular weight HA (LMW-HA; 20-50 kDa) or a blend of both (HA-Blend; confidential ratio between LMW-HA and HMW-HA, commercially available under Resisthyal™ tradename supplied by Givaudan Active Beauty).
2.2. Measuring HA Penetration by Raman Spectroscopy
Hair Fiber Preparation and Treatment
One-gram samples (n = 10) of natural, undyed, light blond hair locks, measuring 10 cm in length, from a white European donor, were used to limit fluorescence interference during Raman spectroscopy measurements (INHIP, New York, USA). Five of the hair locks were irradiated at 20 J/cm² UVA and 0.6 J/cm² UVB in order to open the cuticle (Biosun, Vilber, Eberhardzell, Germany). Hair locks were then washed for 2 min with 0.5 g shampoo containing 1% (w/v) of LMW, HMW, or HA-Blend, rinsed 3 times with 200 mL distilled water, and dried for 3 min with a hair dryer kept 20 cm from the shaft. The washing, rinsing, drying, cycle was repeated 3 times before analysis. The same shampoo without additive was used as control. Untreated hair locks were washed with distilled water.
Raman Spectroscopy and Confocal Measurements on Hair Fibers
Raman spectra were recorded using a near-infrared, confocal, Raman micro-spectrometer (Labram, Horiba Jobin Yvon, Villeneuve d’Ascq, France). The unit comprised an optical microscope (Olympus, BX41, France) coupled to a dispersive Raman spectrometer (Horiba Jobin Yvon, Villeneuve d’Ascq, France) and a charge-coupled device (CCD) detector. Excitation was provided by a 50-mW titanium-sapphire laser (Model 3900S, Spectra-Physics, France) generating a 785-nm beam under the microscope objective. This irradiation is non-destructive for light blond hair samples and causes no thermal or photochemical degradation. Confocal Raman measurements were recorded using an optimized 100x infrared objective (Olympus, France) operating in air with a numerical aperture of 0.8. To obtain axial profiles, the objective was mounted on a high-precision piezoelectric device (Physik Instrumente, Germany) which allowed in-depth vertical scanning by focusing the laser at various depths within the hair fibers. Spectra were recorded over the length of the hair fiber with a 3-µm scanning step. For each spectrum, one 30-sec accumulation of laser exposure was retained. Data were acquired using LabSpec 5 software (Horiba Jobin Yvon, Villeneuve d’Ascq, France).
Spectral-Data Processing
Spectral data were pre-processed using LabSpec 5 software (Horiba Jobin Yvon, Villeneuve d’Ascq, France). First, cosmic radiation was removed and all aberrant profiles were excluded from the database. The remaining raw spectral profiles were corrected in order to clean the Raman signal. The data were corrected for spectral shifts, noise was reduced using a 5-point-average Savitzky-Golay smoothing filter, and baseline was adjusted using a fifth-degree polynomial function to remove background fluorescence. Three independent axial profiles were recorded for each hair sample. The processing of corrected data maps was performed by using original software based on Least Squares fitting method that operates in the Matlab environment (The Math Works Inc., Natick, MA, USA). A thorough description of this statistical analysis has been published previously [
9]. Briefly, the method involves mathematical modeling of HA reference spectra in the overall spectral axial (Z) profiles to determine the contribution and distribution of these spectra within the measured profile.
2.3. Ex Vivo Analysis of Anti-Frizz Properties
Hair locks with various curl levels - from wavy to curly - were used (n = 10/condition). At Time 0, hair locks were divided into 3 equal parts (2 treated parts and one untreated part) in order to have an equal distribution of the different curl levels. Photographs were taken before washing. Then, hair locks were washed for 10 sec with shampoos (1 g shampoo/2 g hair) containing 1.5% (w/v) or 3% (w/v) HA-Blend, placebo shampoo, or water (for untreated locks). All locks were rinsed for 30 sec with distilled water and then air-dried. Photographs were taken after treatment and drying.
For the frizz analysis, hair locks were smoothed with a thermal hair straightener and photographed. Then they were placed in a room with extreme humidity (relative humidity 80% (SD = 10% RH) for 8 h and photographed again. Images were analyzed using Photoshop® to study the anti-frizz effect, by measuring hair-strand length before and after exposure to extreme humidity.
2.4. Ex Vivo Analysis of Restorative Properties by Tensile Test after Bleaching
Natural Caucasian brown hair locks (1 g each) were bleached twice using a Platifiz bleaching powder made of sodium persulfate, potassium persulfate, ammonium chloride, and sodium metasilicate (SP Equation). Then, locks were washed for 60 sec with shampoos containing 3% (w/v) HA-Blend, placebo shampoo, or plain water for non-treated hair locks, then rinsed for 20 sec. Treated locks were dried for 30 min at 40° C in a helmet hairdryer.
A Mobile Tensile Tester (MTT) was used to evaluate hair damage. The system is based on a circular sample cassette, which allows for automatic measurement of up to 100 fiber samples. For this study, 75 hair fiber samples were mounted using brass crimps and placed on a rotary cassette. A Laser (Mitutoyo) measured the cross section of the hair fibers to normalize tensile-strength data. After this measurement, a pneumatically operated sample gripper picked up the sample. The gripper was mounted on a load cell which measures the force applied to the sample during elongation. The test was conducted with 100% relative humidity and ambient room temperature.
2.5. Ex Vivo Analysis of Keratin Integrity Following UV Irradiation
Hair Fiber Preparation and Treatment
One gram of natural, undyed, light-blond hair locks, 10 cm long, from a white European donor, were used to limit fluorescence interference during measurement (INHIP, USA). Locks were washed 3 times for 2 min with 500 µL of shampoo containing 3% (w/v) HA-Blend, rinsed 3 times with 200 mL distilled water, and dried for 3 min with a hair dryer placed 20 cm from the shaft. The locks were irradiated 7 times at 9 J/cm² UVA and 0.33 J/cm² UVB to open the cuticle (Biosun, Vilber, Germany). The same shampoo, without the active substance, was used as a placebo. Non irradiated hair locks were used as a negative control.
xPOLAR® Analysis for Keratin Integrity
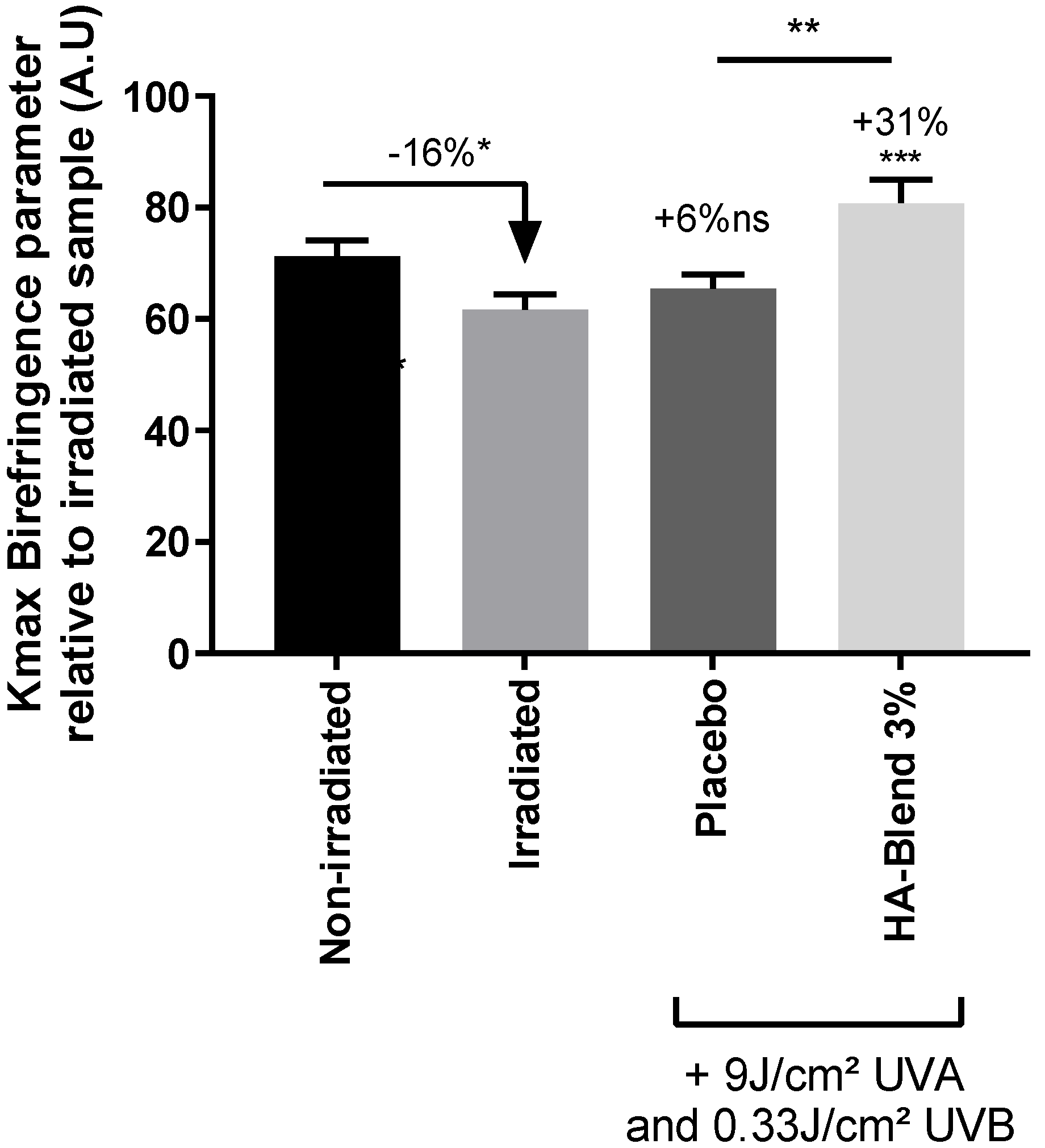
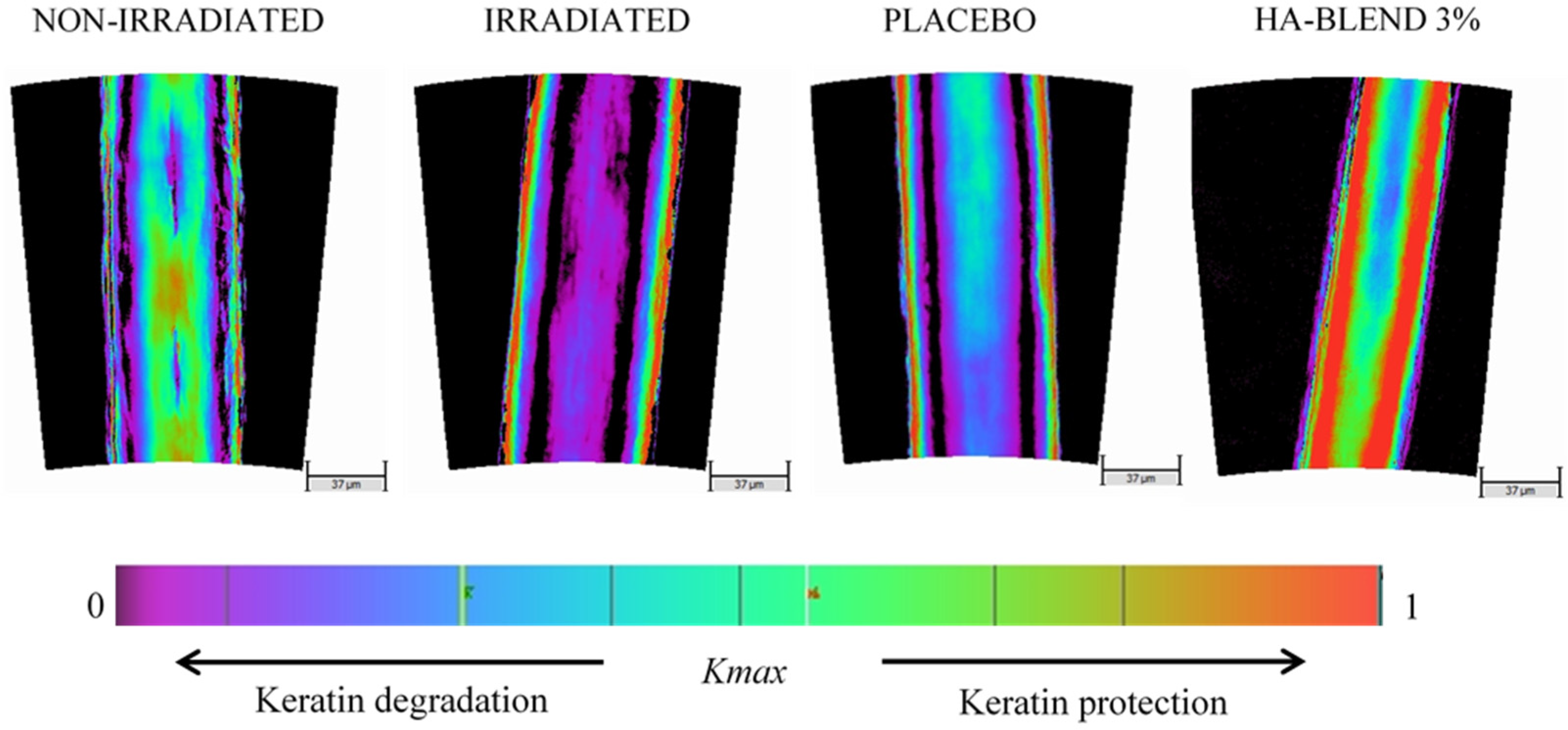
For each condition, 30 sections of 1-cm each, from different fibers, were mounted on a slide and covered with a coverslip. We performed 1 xPolar measurement on each segment of hair, so there were 30 measurement points per condition. In each pixel, a dimensionless value termed Kmax is dependent on the keratin birefringence (intrinsic physical parameter of the material with a numerical value that depends on the structural state of the keratin at the molecular level), and keratin thickness traversed by light. Oscillations in the Kmax parameter are caused by the irregularities in the thickness of the hair crossed.
2.6. Ex Vivo Analysis of Hydration
Fifteen natural, brown hair locks of 10 g each, and measuring 10cm, were used. Locks were washed using a neutral shampoo. After washing, the locks were slightly patted with a towel and then dried with a hairdryer. After drying, hair locks underwent product application as described above. Product (2 ml) was applied to wet hair locks, rubbed for 20 sec, and rinsed for 30 sec. After product application, locks were dried with a hairdryer, and then measured. Hair-moisture content was measured indirectly using a Tewameter TM 300 (Courage+Khazaka, electronic, GmbH). Tewameter is a probe that measures the density gradient of water evaporation. The measuring head of the probe is a narrow, hollow, cylinder (10-mm diameter, and 20-mm height), in order to minimize the influence of air turbulence inside the probe. Water loss from the hair was continuously measured for 1 h. A calibration curve was obtained using known amounts of water (0, 10, 50, 100, 200, 400, 600 µl).
2.7. Statistical Analysis
Results that followed a normal distribution were analyzed statistically by applying a Student’s t-test. Non-normally distributed data were analyzed nonparametrically using the Mann–Whitney test. Significant p values are presented in figures as follows: #p value < 0.1; *p value < 0.05; **p value < 0.01; *** p value < 0.001.3.
3. Results
3.1. HA Penetration in Hair Fibers
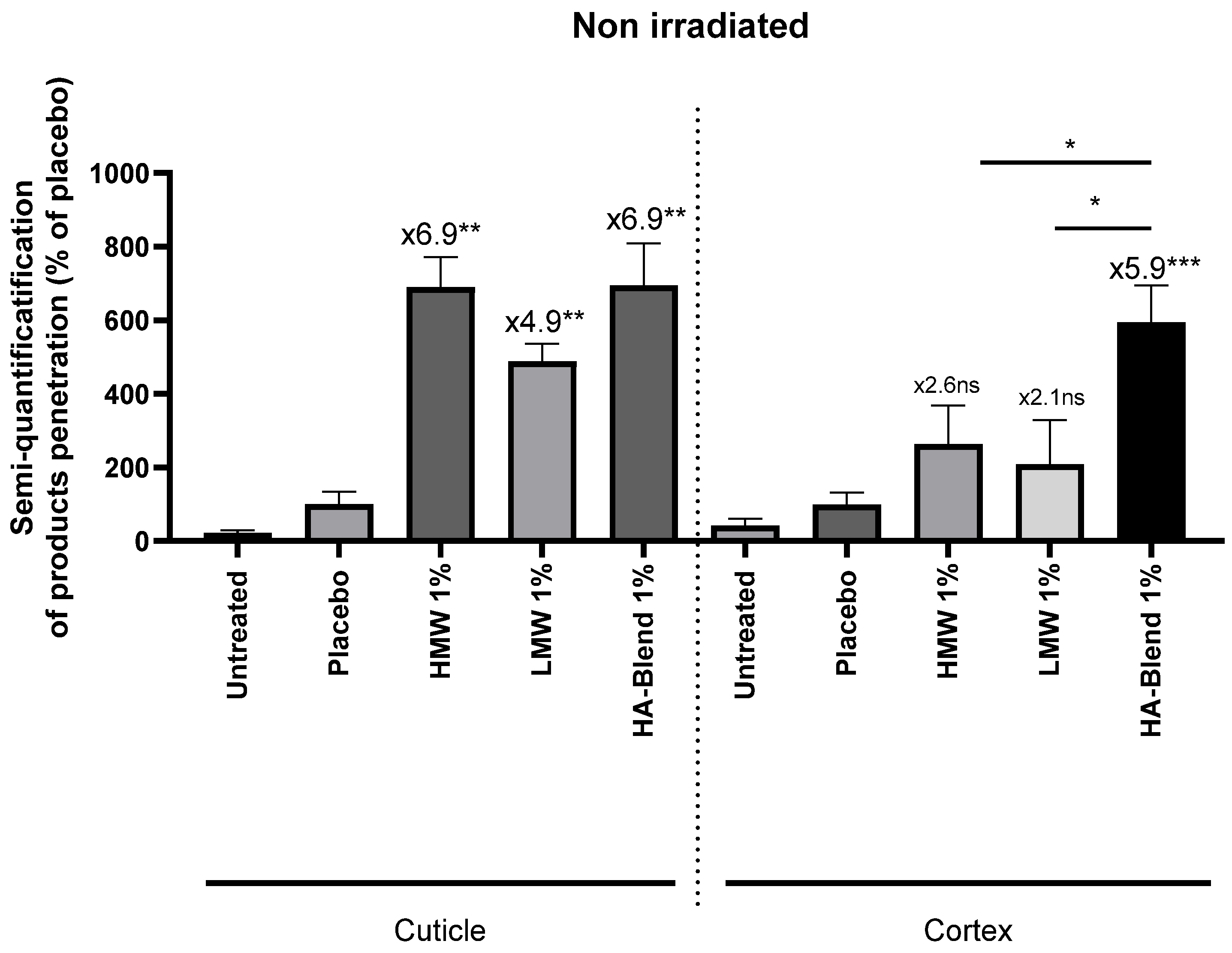
We used Raman spectroscopy to evaluate the penetration of hyaluronic acid through the hair fiber. Compared to the placebo shampoo (without any HA), we tracked the penetration of LMW, HMW and HA-Blend formula shampoos in irradiated and non-irradiated hair fibers using Raman spectroscopy. The cuticle was defined as a depth of 0 - 12 µm, and the cortex of 12 - 42 µm. With non-irradiated hair locks (
Figure 1), penetration of the active substances into the cuticle was significantly higher than that of the control, increasing by 6.9x, 4.9x, and 6.9x for HMW, LMW, and HA-Blend shampoos, respectively. Only the HA-Blend significantly penetrated the cortex (5.9-fold) more than did the placebo and significantly in comparison to the other HA shampoos.
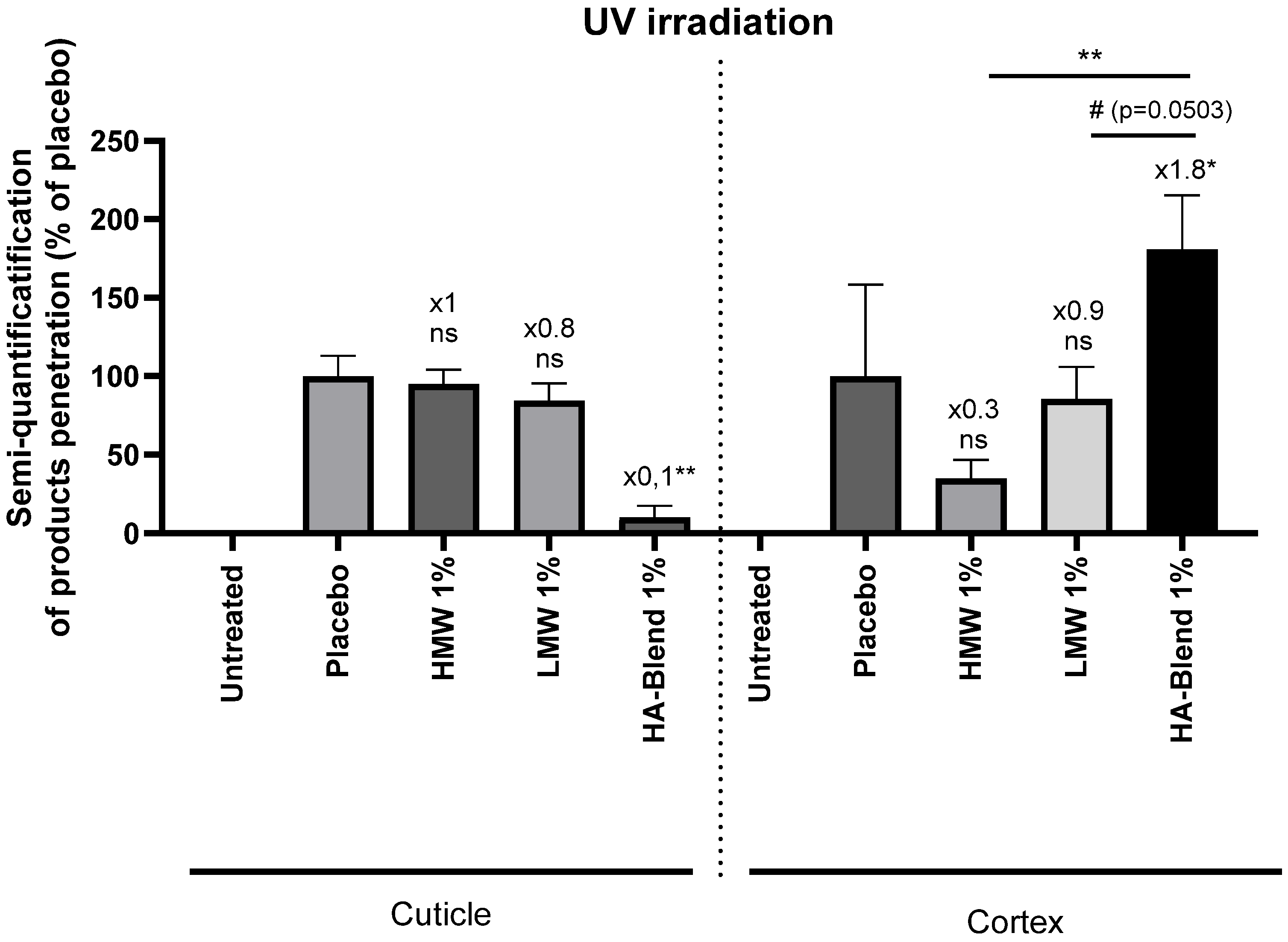
When hair fibers were irradiated with UVA and UVB, the difference in penetration into the cuticle was significantly reduced. Indeed, the HA-Blend showed only 0.1-fold penetration compared to the placebo. In contrast, the quantity penetrating the cortex was significantly increased to 1.8-fold of the placebo level. Once again, the amount of 1% (w/v) HA-Blend detected in the cortex of the irradiated hair fibers was significantly higher than the amount of 1% HMW (w/v) or 1% (w/v) LWM (
Figure 2).
3.2. Anti-Frizz Study
To assess a straightening effect, we worked at the ex vivo level on hair shafts. The smoothing effect was calculated based on variations in hair length in photos taken before and after exposure to humidity (
Table 1). The effect of HA-Blend at 3% (w/v) was significant, smoothing the hair 11% more than the placebo shampoo, and producing a visible effect (
Figure 3).
3.3. Reparation Study
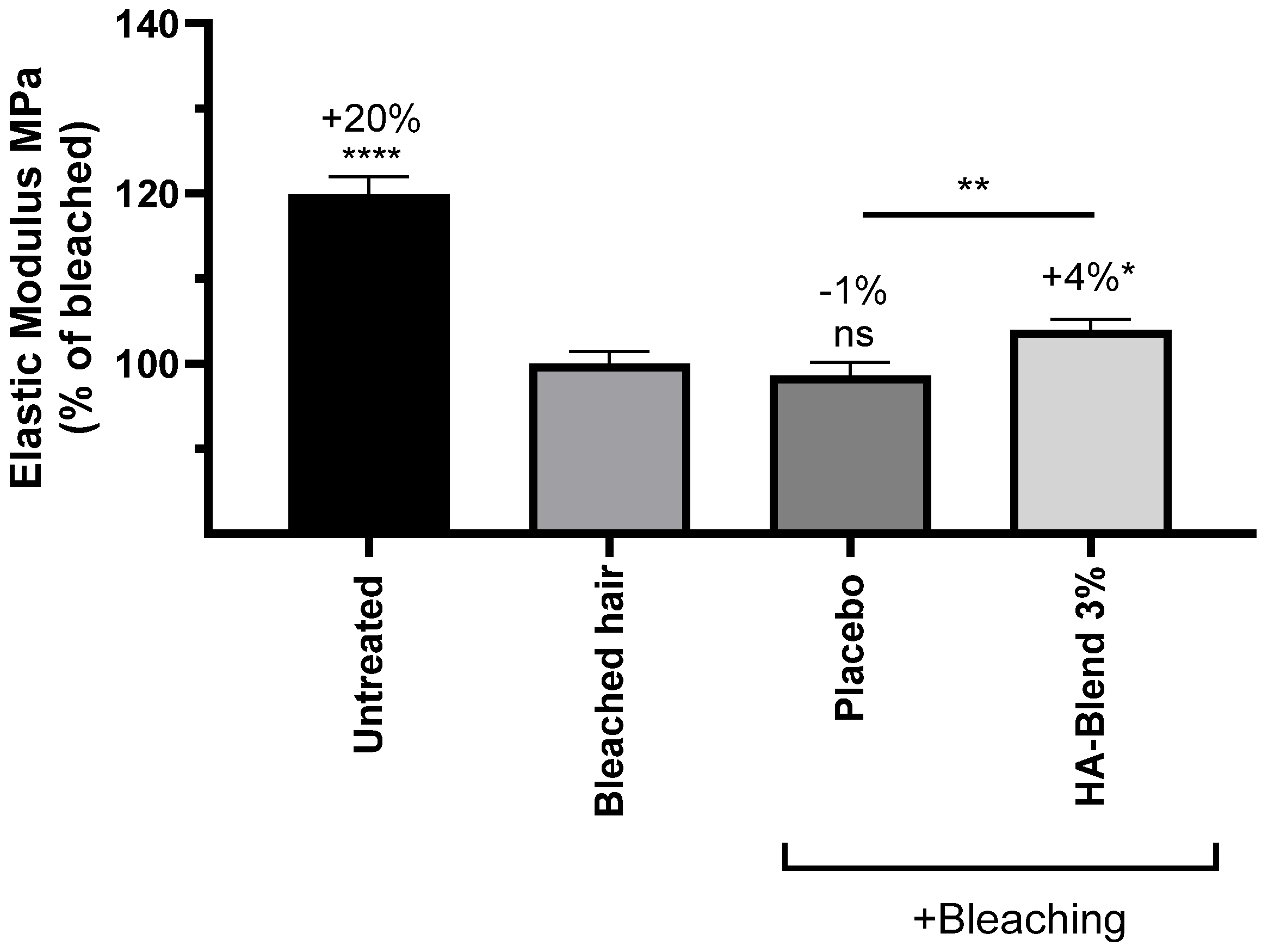
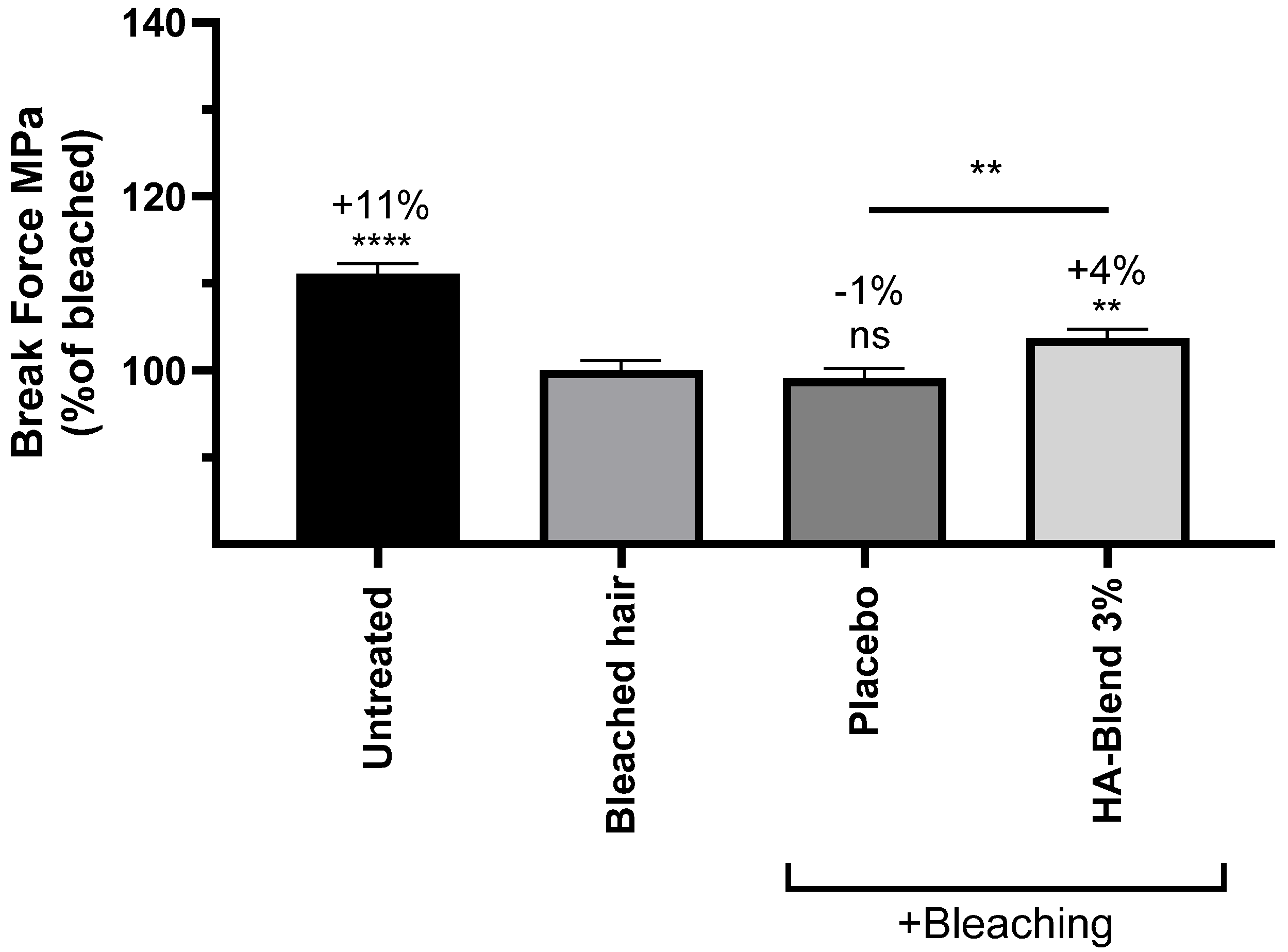
After bleaching with a chemical bleaching powder, the elastic modulus and the maximum force needed to break a fiber significantly decreased by 20% and 11%, respectively. The placebo did not shown any significant effect on the hair lock. After treatment with HA-Blend at 3% (w/v), the elastic modulus and the break force significantly increased by 4% for both parameters in comparison to the untreated damaged condition. The effect was also significant in comparison to the placebo (
Figure 4 and
Figure 5).
3.4. Keratin Integrity
The HA-Blend at 3% was able to repair damaged hair after bleaching. UV irradiation also damaged the hair and affected keratin structure. After irradiation, the keratin integrity was significantly reduced by 16%. The placebo did not produce any significant change. After treatment with HA-Blend at 3% (w/v), the keratin integrity was improved by 31% in comparison to the damaged condition. The effect was also significant in comparison to the placebo shampoo condition (
Figure 6).
3.5. Hydration Study
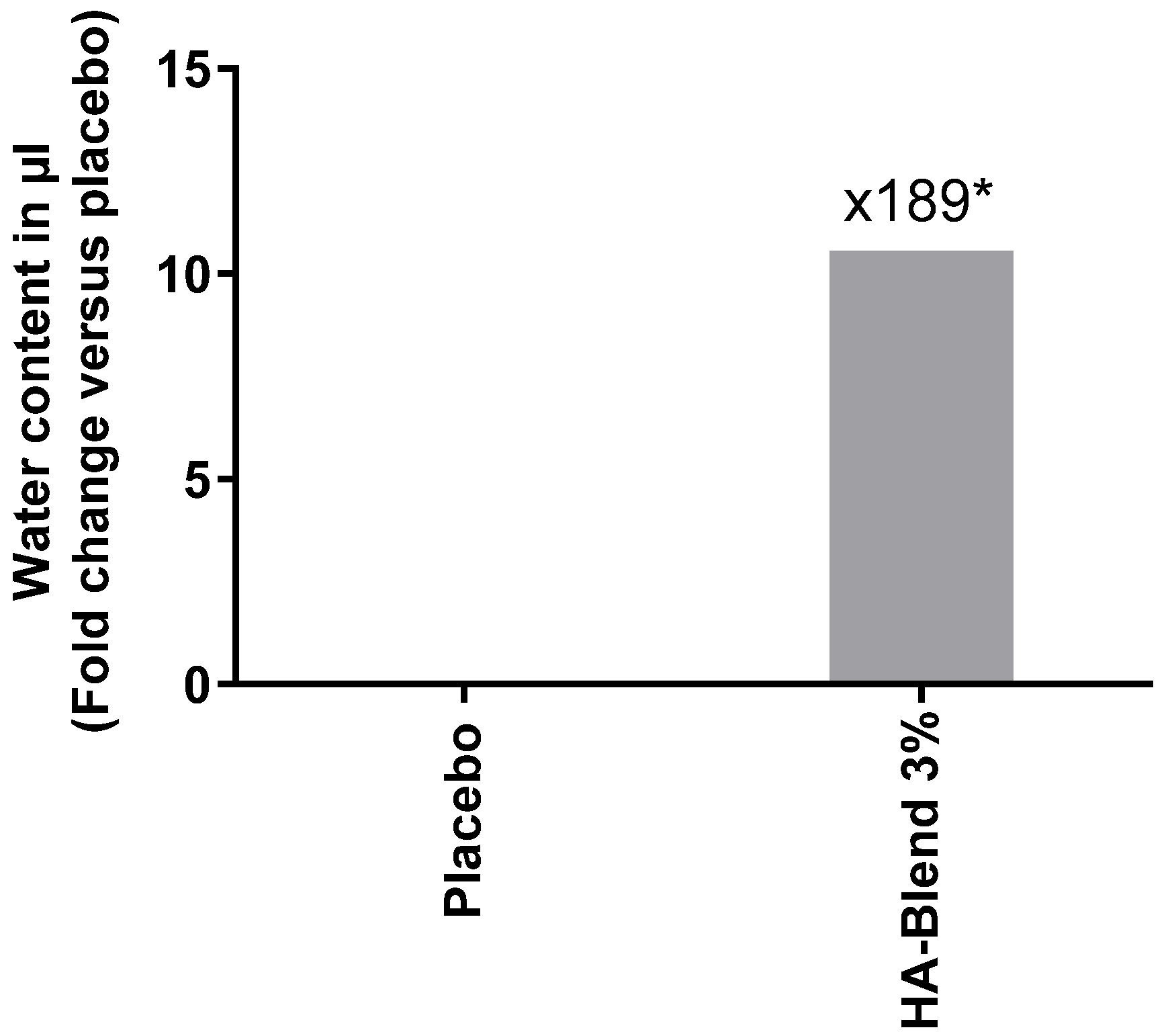
To evaluate whether HA is also able to moisturize hair, we measured the water evaporation using a Tewameter
®. Under the tested experimental conditions, HA-Blend 3% (w/v) increased the water content when compared to the same formulation without the active ingredient (placebo) by 189-times (
Figure 7). The increase in hair moisture content was described in the
Table 2 below.
4. Discussion
The aim of this study was to develop a method to measure HA penetration in hair, and to assess the benefits of a blended and optimized HA formulation for use in haircare products.
We established an innovative method to demonstrate the penetration of hyaluronic acid into the hair fiber in rinse-off conditions. UV irradiation damage is a leading cause of hair protein loss and color change [
11]. Using UVA and UVB irradiation, we damaged the hair to open the cuticle and enhance product penetration. The semi-quantification of the total Raman signal showed an increase in the quantity of LMW, HMW, and placebo (shampoo without the active substance) detected in damaged hair (over undamaged hair) (Data not shown). It is interesting to note that the total quantity of HA-Blend detected in irradiated hair fibers is the same but the penetration profile is different, with a lower quantity of HA-Blend in the cuticle and a higher amount in the cortex. That could suggest that the penetration of HA-Blend is already optimal in undamaged condition. We have thus compared the penetration of HA in normal or damaged hair locks to gain a greater understanding of how the product works. When using non-irradiated hair locks, the three active shampoos (LMW, HMW, and HA-Blend) had a similar ability to penetrate the cuticle; they were consistently better than the placebo shampoo. In contrast, when examining the cortex, the HA-Blend outperformed all the other formulations. With UV-damaged hair, penetration of the HA-Blend into the cuticle was significantly reduced, but penetration into the cortex was still significantly higher than that of the placebo or the two other active shampoos. This apparent discrepancy is due to the fact that when hair has undergone UV-induced damage, the placebo more readily penetrates the cuticle, whereas hydrophilic compounds such as HA bind more tightly to proteins such as keratin, which is in the cortex [
12]. In other words, once the HA-Blend penetrates the cuticle in damaged hair, this optimal formulation may be attracted to the keratin in the cortex, leaving only small amounts on the cuticle to repair the cortex. Even in the non-irradiated condition, only the HA-Blend significantly penetrated the cortex, due to its optimized ratio of low- to high-molecular weight HA formulation, and its capacity to bind to keratin. Besides, the optimal process behind the HA-Blend involved the addition of lactic acid to reduce the pH of the formula. It has been found that pH plays a critical role in molecule absorption and can have opposite effects depending on the initial ionization of the molecule (a cationic or anionic compound). Indeed, change in pH could lead to structural changes in keratin including changes in the binding affinity and the number of available binding sites [
12]. At pH > 6.0, hair keratin is negatively charged and attracts positively charged molecules. Inversely, at pH 2.0, cationic species showed a low level of binding to keratin due to the electrostatic repulsion [
12]. HA is an anionic compound and we hypothesize that acidification of the pH induced by lactic acid improved binding of HA-Blend to keratin.
By binding to the keratin, this optimal HA-Blend modified the keratin conformation, decreasing α-helices and promoting the formation of β-sheets, leading to smoothing of the hair. These data corroborated results from our previous study, in which analyses showed that this HA-Blend decreases the number of disulfide bonds in keratin and induces a change in the α-helix/β-sheet ratio [
3]. The results presented here establish that the change in keratin conformation is correlated to the effective penetration of the HA-Blend, allowing a direct interaction between HA and keratin. This anti-frizz potential was confirmed in ex vivo experiments, with the HA-Blend producing a significant smoothing effect 11% better than the placebo, thereby resulting in a visible benefit.
Current straightening methods, involving the thermal method (with an iron) or chemical agents, have been found to damage hair by reducing its physico-mechanical properties [
13]. Those treatments break and rearrange disulfide bonds, change the structure of keratin in favor of a β-sheet conformation, and disrupted cuticle [
14,
15]. We have shown that HA can straighten the hair and change the keratin conformation without damaging the fiber, as shown by the tensile-strength analysis. Indeed, we have demonstrated that HA treatment smoothed the hair while improving the elastic modulus and the maximum strength needed to break a fiber by filling damaged hair from the inside. We have demonstrated that the HA-Blend is also able to repair keratin integrity after UV irradiation.
Moreover, thermal straightening has been found to lead to a reduction of water content into the hair fiber [
16]. The authors of that paper explained that the changes in protein conformation and a reduction of the α-helix conformation may change the water accessibility. We have shown that even if HA interacts with keratin and changes its conformation, it is able to significantly increase water content in the fiber. This interaction between HA and keratin has already been described in the skin to provide hydration and maintain skin-barrier integrity [
5]. We hypothesize that the damage caused at the cuticle level by thermal straightening is mainly responsible for water loss, but using high molecular weight HA in the HA-Blend coats the fiber and improves hair hydration while smoothing the fiber.
5. Conclusions
Our results demonstrated that Confocal Raman Spectroscopy is a powerful, non-invasive technique to investigate the penetration of cosmetic ingredients into human hair fibers. To the best of our knowledge, this study is the first evidence of HA penetration into hair fibers, and the first comparison of the penetration of different molecular weights of HA. Our results show that an optimized formulation containing different molecular weight HA plus lactic acid penetrates deeply into the hair cortex to interact with keratin and exert a smoothing, hydrating, and reparative effect. This insight opens new doors for the haircare industry to understand the mechanisms of action of active molecules for applications in haircare.
6. Patents
This optimal blend is protected by the international patent application WO 2018/162604.
Author Contributions
Conceptualization, Cloe Boira, Marie Meunier, Amandine Scandolera and Romain Reynaud; methodology, Cloe Boira, Mohammed Essendoubi, Marie Meunier, Carole Lambert, Daniel Auriol, Michel Manfait, Olivier Piot, Amandine Scandolera and Romain Reynaud; software, Mohammed Essendoubi, Michel Manfait and Olivier Piot; validation, Cloe Boira, Mohammed Essendoubi, Marie Meunier, Carole Lambert, Daniel Auriol, Michel Manfait, Olivier Piot, Amandine Scandolera and Romain Reynaud; formal analysis, Cloe Boira, Mohammed Essendoubi, Marie Meunier and Carole Lambert; investigation, Cloe Boira, Mohammed Essendoubi, Marie Meunier and Carole Lambert; writing—original draft preparation, Cloe Boira; writing—review and editing, Cloe Boira, Mohammed Essendoubi, Marie Meunier, Carole Lambert, Daniel Auriol, Michel Manfait, Olivier Piot, Amandine Scandolera and Romain Reynaud. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Acknowledgments
The authors would like to thank Dermscan, Farcoderm, Kamax Innovative System and SP Equation for their participation in the studies presented.
Conflicts of Interest
The authors declare no conflict of interest.
References
- R.D. Sinclair, Healthy Hair: What Is it?, Journal of Investigative Dermatology Symposium Proceedings. 12 (2007) 2–5. [CrossRef]
- F.-C. Yang, Y. Zhang, M.C. Rheinstädter, The structure of people’s hair, PeerJ. 2 (2014) e619. [CrossRef]
- M. Essendoubi, M. Meunier, A. Scandolera, C. Gobinet, M. Manfait, C. Lambert, D. Auriol, R. Reynaud, O. Piot, Conformation changes in human hair keratin observed using confocal Raman spectroscopy after active ingredient application, Int J Cosmet Sci. 41 (2019) 203–212. [CrossRef]
- C.F. Cruz, M. Martins, J. Egipto, H. Osório, A. Ribeiro, A. Cavaco-Paulo, Changing the shape of hair with keratin peptides, RSC Adv. 7 (2017) 51581–51592. [CrossRef]
- M. Witting, A. Boreham, R. Brodwolf, K. Vávrová, U. Alexiev, W. Friess, S. Hedtrich, Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules, Mol. Pharmaceutics. 12 (2015) 1391–1401. [CrossRef]
- M.F. Gavazzoni Dias, Hair cosmetics: An overview, Int J Trichol. 7 (2015) 2. [CrossRef]
- E. Malinauskyte, R. Shrestha, P.A. Cornwell, S. Gourion-Arsiquaud, M. Hindley, Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair, Int. J. Cosmet. Sci. 43 (2021) 26–37. [CrossRef]
- R. Krombholz, D. Lunter, A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy, Molecules. 25 (2020) 4222. [CrossRef]
- M. Essendoubi, C. M. Essendoubi, C. Gobinet, R. Reynaud, J.F. Angiboust, M. Manfait, O. Piot, Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy, Skin Res Technol. 22 (2016) 55–62. [CrossRef]
- M. Essendoubi, N. Andre, B. Granger, C. Clave, M. Manfait, I. Thuillier, O. Piot, J. Ginestar, New approach for hair keratin characterization: Use of the confocal Raman spectroscopy to assess the effect of thermal stress on human hair fibre, Intern J of Cosmetic Sci. 44 (2022) 588–601. [CrossRef]
- A.C. Santos Nogueira, I. Joekes, Hair color changes and protein damage caused by ultraviolet radiation, Journal of Photochemistry and Photobiology B: Biology. 74 (2004) 109–117. [CrossRef]
- L. Li, S. Yang, T. Chen, L. Han, G. Lian, Investigation of pH effect on cationic solute binding to keratin and partition to hair, Int J Cosmet Sci. 40 (2018) 93–102. [CrossRef]
- C.F. Cruz, M. Martins, J. Egipto, H. Osório, A. Ribeiro, A. Cavaco-Paulo, Changing the shape of hair with keratin peptides, RSC Adv. 7 (2017) 51581–51592. [CrossRef]
- C. Kunchi, K. Venkateshan, Nvnd. Reddy, R. Adusumalli, Correlation between mechanical and thermal properties of human hair, Int J Trichol. 10 (2018) 204. [CrossRef]
- J.N. Hatsbach de Paula, F.M.A. Basílio, F.A. Mulinari-Brenner, Effects of chemical straighteners on the hair shaft and scalp, Anais Brasileiros de Dermatologia. 97 (2022) 193–203. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).