Submitted:
17 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
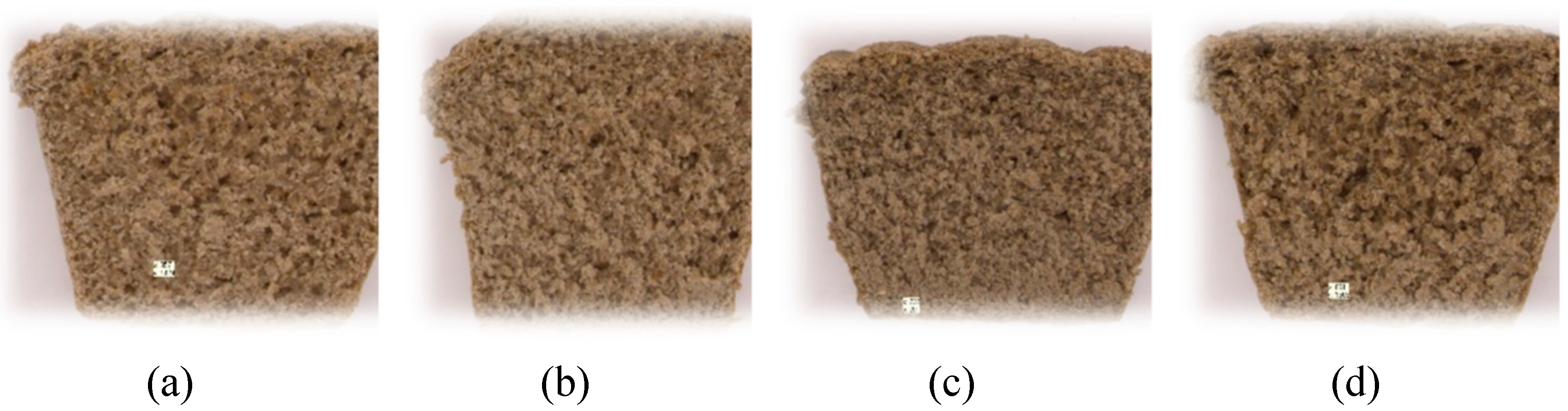
2.1. Characteristics of cricket powder and whole wheat flour
2.2. Assessment of quality parameters of designed cricket bread
2.2.1. Physicochemical properties
2.2.2. Nutritional value
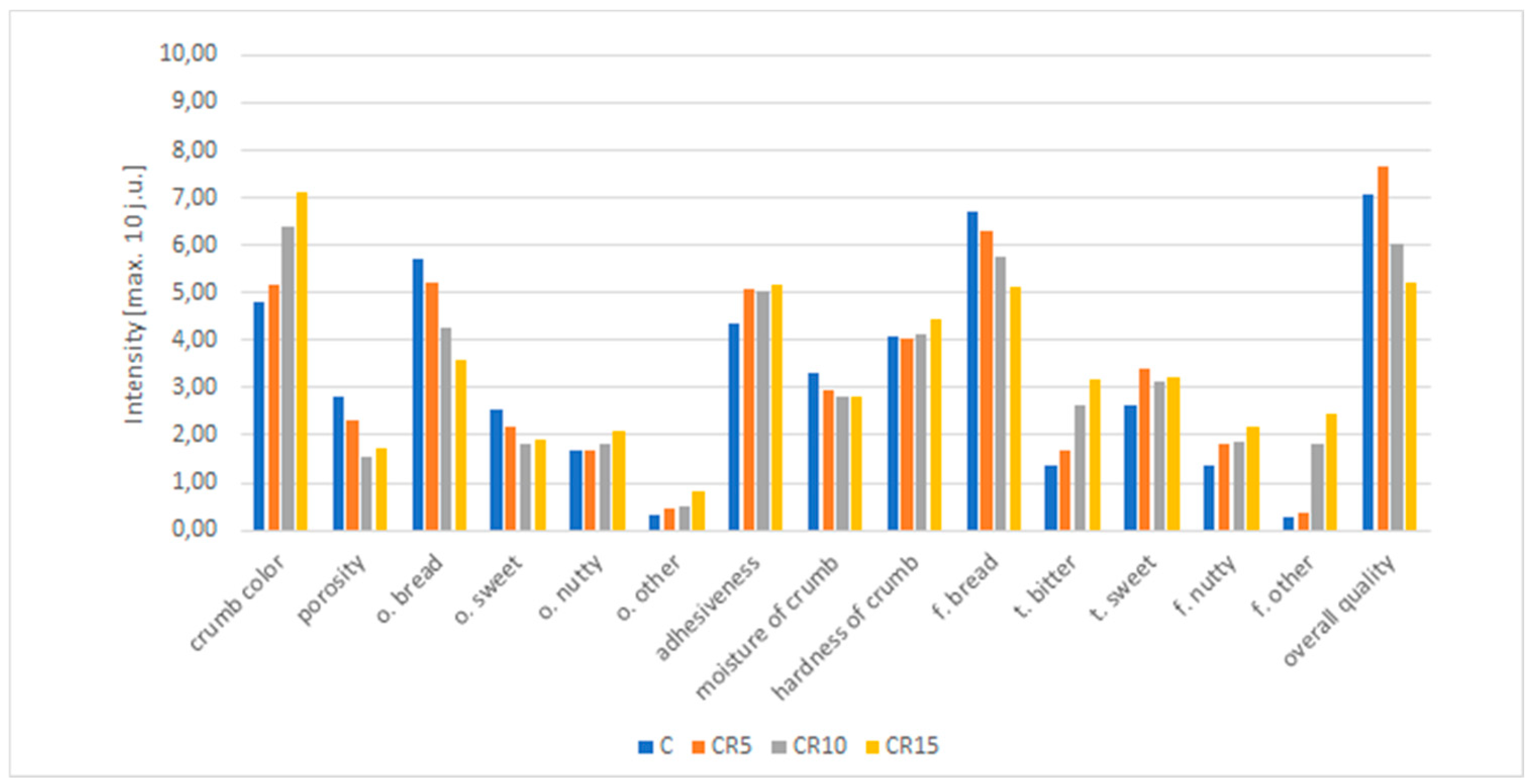
2.2.3. Sensory evaluation
2.2.4. Microbiological evaluation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Functional properties of cricket powder and whole wheat flour
3.2.1.1. The pH measurement
3.2.1.2. The water activity (aw) measurement
3.2.1.3. The water holding capacity (WHC) measurement
3.2.1.4. The fat absorption capacity (FAC) measurement
3.2.1.5. The Colour measurement
3.2.1.6. Chemical composition
3.2.2. Development of the composition of bread with the addition of cricket preparation
3.2.3. Physical parameters of designed breads
3.2.3.1. The density measurement of prepared breads
3.2.3.2. The hardness measurement of prepared breads
3.2.4. The nutritional value assessment of prepared breads
3.2.5. The microbiological evaluation of prepared breads
3.2.6. Sensory evaluation
3.2.7. Statistical analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible insects: future prospects for food and feed security. FAO Forestry Paper, Publisher: Food And Agriculture Organization of the United Nations, Rome, 2013.
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res. 2013, 5, 802-23.
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Research International 2015, 77, 460–46.
- Raheem, D.; Carrascosa, C.; Oluwole, O. B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169-2188.
- Sogari, G.; Bogueva, D.; Marinova, D. Australian consumers‘response to insects as food. Agric. 2019, 9, 1-15.
- Jongema, Y. Worldwide list of recorded edible insects. The Netherlands: Department of Entomology, Wageningen University & Research 2017, Available online: https://www.wur.nl/upload_mm/8/a/6/0fdfc700-3929-4a74-8b69-f02fd35a1696_Worldwide%20list%20of%20edible%20insects%202017.pdf (accessed on 28/08/2023).
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1-16. [CrossRef]
- Abro, Z.; Kassie, M.; Tanga, C.; Beesigamukama, D.; Diiro, G. Socio-economic and environmental implications of replacing conventional poultry feed with insect-based feed in Kenya. J. Clean. Prod. 2020, 265, 1-9. [CrossRef]
- Finke, M.D.; Oonincx, D.G.A.B. Nutrient content of insects. In: Insects as food and feed: from production to consumption. Van Huis, A.; Tomberlin, J.K., Wageningen Academic Publishers, Wageningen, the Netherlands 2017. pp. 290-317.
- Oonincx, D.G.A.B.; Dierenfeld, E.S. An investigation into the chemical composition of alternative invertebrate prey. Zoo Biology. 2012, 31, 40-54. [CrossRef]
- Oonincx, D.G.A.B.; Van der Poel, A.F. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biology 2011, 30, 9-16. [CrossRef]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biology 2013, 32, 27-36. [CrossRef]
- Cruz-López, S.O.; Escalona-Buendía, H.B.; Román-Guerrero, A.; Domínguez-Soberanes, J.; Alvarez-Cisneros Y.M. Charactezation of cooked meat models using grasshopper (sphenarium purpurascens) soluble protein extracted by alkalisation and ultrasound as meat-extender. Food Science of Animal Resources 2022, 42, 536-555. [CrossRef]
- van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional qualities and enhancement of edible insects. Annual Review of Nutrition 2021, 41, 551–576. [CrossRef]
- Megido, R.C; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, É.; Alabi,T.; Francis F. Consumer acceptance of insect-based alternative meat products in Western countries. Food Quality and Preference 2016, 52, 237-243. [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the development of edible insect-based foods in Europe. Foods 2021, 10, 1-22. [CrossRef]
- Regulation (EU) 2021/882. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/882/oj (accessed on 20 07 2023).
- Regulation (EU) 2021/1975. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/1975/oj (accessed on 20 07 2023).
- Regulation (EU) 2022/169. Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/169/oj (accessed on 20 07 2023).
- Regulation (EU) 2022/188. Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/188/oj (accessed on 20 07 2023).
- Regulation (EU) 2023/58. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/58/oj (accessed on 20 07 2023).
- Fernandez-Cassi, X.; Supeanu, A.; Vaga, M.; Jansson, A.; Boqvist, S.; Vagsholm, I. The house cricket (Acheta domesticus) as a novel food: a risk profile. Journal of Insects as Food and Feed 2019, 5, 137-157. [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food chemistry 2019, 272, 267-272. [CrossRef]
- Kouřimská, L.; Kotrbová, V.; Kulma, M.; Adámková, A.; Mlček, J.; Sabolová, M.; Homolková, D. Attitude of assessors in the Czech Republic to the consumption of house cricket Acheta domestica L.–A preliminary study. Czech Journal of Food Sciences 2020, 38, 72-76. [CrossRef]
- Tan, H. S. G.; Fischer, A. R.; Tinchan, P.; Stieger, M.; Steenbekkers, L. P. A.; van Trijp, H. C. Insects as food: Exploring cultural exposure and individual experience as determinants of acceptance. Food quality and preference 2015, 42, 78-89. [CrossRef]
- Hartmann, C.; Siegrist, M. Insects as food: Perception and acceptance. Findings from current research. Ernahrungs Umschau 2017, 64, 44-50. [CrossRef]
- de Oliveira, L. M.; da Silva Lucas, A. J.; Cadaval, C. L.; Mellado, M. S. Bread enriched with flour from cinereous cockroach (Nauphoeta cinerea). Innovative Food Science & Emerging Technologies 2017, 44, 30-35. [CrossRef]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Effect of drying processes in the chemical, physico-chemical, techno-functional and antioxidant properties of flours obtained from house cricket (Acheta domesticus). Eur. Food Res. Technol. 2019, 245, 1451–1458. [CrossRef]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schönlechner, R.; Rawdkuen, S. Whole Wheat Bread Enriched with Cricket Powder as an Alternative Protein. Foods 2022, 11, 1-13. [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects, LWT 2022, 159, 1-10. [CrossRef]
- Tiwari, A.; Jha, S.N. Extrusion cooking technology: Principal mechanism and effect on direct expanded snacks—An overview. Int. J. Food Stud. 2017, 6, 113–128. [CrossRef]
- Thu, T. N.; Dinh, K.; Virellia, To, M. Wes Schilling. Fatty Acid Composition of Meat Animals as Flavor Precursors. Meat and Muscle Biol. 2021, 5(1), 1–16. [CrossRef]
- Gantner, M.; Król, K.; Piotrowska, A.; Sionek, B.; Sadowska, A.; Kulik, K.; Wiącek, M. Adding Mealworm (Tenebrio molitor L.) Powder to Wheat Bread: Effects on Physicochemical, Sensory and Microbiological Qualities of the End-Product. Molecules 2022, 27, 1-13. [CrossRef]
- Khuenpet, K.; Pakasap, C.; Vatthanakul, S.; Kitthawee, S. Effect of larval-stage mealworm (Tenebrio molitor) powder on qualities of bread. International Journal of Agricultural Technology 2020, 16, 283-2.
- Bartkiene, E.; Starkute, V.; Katuskevicius, K.; Laukyte, N.; Fomkinas, M.; Vysniauskas, E.; Kasciukaityte, P.; Radvilavicius, E.; Rokaite, S.; Medonas, D.; Valantinaviciute, E.; Mockus, E.; Zokaityte, E. The contribution of edible cricket flour to quality parameters and sensory characteristics of wheat bread. Food Sci Nutr. 2022, 10, 4319-4330. [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [CrossRef]
- Bellary, A.N.; Indiramma, A.R.; Prakash, M.; Baskaran, R.; Rastogi, N.K. Anthocyanin infused watermelon rind and its stability during storage. Innov. Food Sci. Emerg. Technol. 2016, 33, 554–562.
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Physicochemical Properties and Consumer Acceptance of Bread Enriched with Alternative Proteins. Foods 2020, 9, 1-22. [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 1-13. [CrossRef]
- Regulation (EU) No. 1924/2006 of the European Parliament and of the Council of December, 20, 2011 on nutrition and health claims made on foods. Available online: (https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:En:PDF) (accessed on 10 January 2021).
- Wieczorek, M. N.; Kowalczewski, P. Ł.; Drabińska, N.; Różańska, M. B.; Jeleń, H. H. Effect of Cricket Powder Incorporation on the Profile of Volatile Organic Compounds, Free Amino Acids and Sensory Properties of Gluten-Free Bread. Polish Journal of Food and Nutrition Sciences 2022, 72, 431-442. [CrossRef]
- Borges, M. M.; da Costa, D. V.; Trombete, F. M.; Câmara, A. K. F. I. Edible insects as a sustainable alternative to food products: an insight into quality aspects of reformulated bakery and meat products. Current Opinion in Food Science 2022, 46, 100864. [CrossRef]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; Zamporlini, F.; Minazzato, G.; Trombetta, M. F.; Van Buitenen, A.; Van Campenhout, L.; Aquilanti, L. Protein fortification with mealworm (Tenebrio molitor L.) powder: Effect on textural, microbiological, nutritional and sensory features of bread. PLoS ONE, 2019, 14, 1–29. [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [CrossRef]
- Malomo, O.; Ogunmoyela, O.A.B.; Oluwajoba, S.O.; Dudu, O.E. Microbiological and nutritional quality of warankashi enriched bread. J Microbiol Biotechnol Food Sci. 2012, 2, 42-68.
- Garcia, M.W.; Bregão, A.S., Parussolo, G.; Bernardi, A.O.; Stefanello, A.; Copetti, M.V. Incidence of spoilage fungi in the air of bakeries with different hygienic status. Int. J Food Microbiol. 2019, 290, 254-261. [CrossRef]
- Deschuyffeleer, N.; Audenaert, K.; Samapundo, S.; Ameye, S.; Eeckhout, M.; Devlieghere, F. Identification and characterization of yeasts causing chalk mould defects on par-baked bread. Food Microbiol. 2011, 28, 1019–1027. [CrossRef]
- Belz, M.C.E.; Mairinger, R.; Zannini, E. et al. The effect of sourdough and calcium propionate on the microbial shelf-life of salt reduced bread. Appl Microbiol Biotechnol. 2012, 96, 493–501. [CrossRef]
- Garofalo, C.; Zannini, E.; Aquilanti, L.; Silvestri, G.; Fierro, O.; Picariello, G.; Clementi, F. Selection of sourdough lactobacilli with antifungal activity for use as biopreservatives in bakery products. J. Agric. Food Chem. 2012, 60, 7719–7728. [CrossRef]
- Dagnas, S.; Membré, J-M. Predicting and Preventing Mold Spoilage of Food Products. J Food Prot. 2013, 76, 538-551. [CrossRef]
- Giannone, V.; Pitino, I.; Pecorino, B.; Todaro, A.; Spina, A.; Lauro, M.R.; Tomaselli, F.; Restuccia, C. Effects of innovative and conventional sanitizing treatments on the reduction of Saccharomycopsis fibuligera defects on industrial durum wheat bread. Int. J. Food Microbiol. 2016, 235, 71-76. [CrossRef]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.J. ;Villalobos, M.C.; Martis, A.; Córdoba, M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98-110.
- Quattrini, M.; Liang, N.; Fortin, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J of Food Microbiol. 2019, 302, 8-14. [CrossRef]
- El Houssni, I.; Khedid, K.; Zahidi, A.; Hassikou, R. The inhibitory effects of lactic acid bacteria isolated from sourdough on the mycotoxigenic fungi growth and mycotoxins from wheat bread. Biocat Agric Biotechnol. 2023, 102702. [CrossRef]
- Dymchenko, A.; Gerˇsl, M.; Gregor, T. Trends in bread waste utilization. Trends Food Sci Technol. 2023, 132, 93-102. [CrossRef]
- Garcia, M.W.; Bernardi, A.O.; Copetti, M.V. The fungal problem in bread production: insights of causes, consequences, and control methods. Curr Opin Food Sci. 2019, 29, 1–6. [CrossRef]
- Dos Santos, J.L.P.; Bernardi, A.O.; Pozza Morassi, L.L.; Silva, B.S.; Copetti, M.V.; Sant’Ana, A.S. Incidence, populations and diversity of fungi from raw materials, final products and air of processing environment of multigrain whole meal bread. Food Res Int, 2016, 87, 103-108.
- Garcia, M.W.; Sonnenstrahl A., Bregão, Parussolo, G.; Bernardi, A.O.; Stefanello, A.; Copetti, M.V. Incidence of spoilage fungi in the air of bakeries with different hygienic status. Int J Food Microbiol 2019, 290, 254-261.
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele składu i wartości odżywczej żywności, PZWL, 2023.
- ISO 4833-1:2013-12/A1:2022-06; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate Technique. Amendment 1: Clarification of Scope (ISO 4833-1:2013/Amd 1:2022). ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 05. 08.2022).
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38275.html (accessed on 5 08 2022).
- EN ISO 4121:2003; Sensory Analysis. Guidelines for the Use of Quantitative Response Scales. ISO: Geneva, Switzerland, 2003.
- EN ISO 8586:2012; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- EN ISO 8589:2010; Sensory Analysis. General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2010.

| Nutrients content | Cricket protein powder | Whole wheat flour |
|---|---|---|
| Fat [g/100 g] | 16.29±3.10 | 1.16 ±0.23 |
| Protein [g/100 g] | 63.00±7.56 | 13.5 ±1.62 |
| Carbohydrates [g/100 g] | 9.83±0.95 | 66.04 ±1.43 |
| Fibre [g/100 g] | 7.80±1.20 | 9.5 ±1.5 |
| Physicochemical properties | Cricket powder | Whole wheat flour |
|---|---|---|
| pH | 6.90±0.02 | 6.77±0.13 |
| Fat absorption capacity [g oil/g powder] | 0.14±0.12 | 0.27±0.05 |
| Water holding capacity [g water/g powder] | 2.52±0.54 b | 1.52±0.14 a |
| aw | 0.18±0.00 a | 0.43±0.00 b |
| L* | 35.14±0.53 a | 81.58±1.34 b |
| a* | 4.56±0.10 a | 4.74±0.48 b |
| b* | 25.22±0.09 b | 21.57±0.98 a |
| Fatty acid profile | Cricket protein powder | Whole wheat flour |
|---|---|---|
| Saturated fatty acids [% of fat content] | ||
| (C14:0) myristic acid | 0.50±0.20* | 0.06 ±0.03 |
| (C15:0) pentadecanoic acid | 0.08±0.04 | 0.06 ±0.03 |
| (C16:0) palmitic acid | 23.98±4.80 | 14.80 ±2.96 |
| (C17:0) margaric acid | 0.15±0.06 | 0.07 ±0.03 |
| (C18:0) stearic acid | 9.66±1.94 | 0.97 ±0.30 |
| (C20:0) arachidic acid | 0.29±0.12 | 0.12 ±0.05 |
| (C22:0) behenic acid | <0.05 | 0.17 ±0.07 |
| (C24:0) lignoceric acid | <0.05 | 0.14 ±0.06 |
| Monounsaturated fatty acids [% of fat content] | ||
| (C16:1w7) palmitoleic acid | 0.67±0.21 | 0.12 ±0.05 |
| (C18:1w9) oleic acid | 24.64±4.93 | 12.99 ±2.60 |
| (C18:1w7) cis-11-vaccenic acid | 0.60±0.18 | 1.03 ±0.21 |
| (C18:1w9t) trans elaidic acid | 0.12±0.05 | <0.05 |
| Poliunsaturated fatty acids [% of fat content] | ||
| (C18:2w6) linoleic acid (LA) | 32.10±6.42 | 60.53 ±12.11 |
| (C18:2 ct) cis-9, trans-12 octadecadienoic acid | 0.58±0.18 | <0.05 |
| (C18:2w6t) trans linolelaidic acid | 0.13±0.06 | <0.05 |
| (C18:2 tc) trans-9, cis-12 octadecadienoic acid | 0.68±0.21 | <0.05 |
| (C18:3w3) cis-9, 12,15 alpha-linolenic acid (ALA) | 0.99±0.30 | 3.64 ±0.73 |
| (C20:2) cis-11,14- eicosadienoic acid | 0.43±0.18 | 0.08 ±0.04 |
| Contribution of individual fatty acid groups [% of fat content] | ||
| Saturated fatty acids | 34.66±6.94 | 16.39 ±3.28 |
| Monounsaturated fatty acids | 25.91±5.19 | 14.30 ±2.86 |
| Polyunsaturated fatty acids | 33.52±6.71 | 64.25 ±12.85 |
| Trans fatty acids | 1.51±0.31 | <0.55 ±0.17 |
| Omega 3 fatty acids (ALA, EPA, DHA, ETE, DPA)** | 0.99±0.30 | 3.64 ±0.73 |
| Omega 6 fatty acids (LA, GLA, ARA, DGLA)*** | 32.10±6.42 | 60.53 ±12.11 |
| Aminoacids profile | Cricket protein powder | Whole wheat flour |
|---|---|---|
| Aspartic acid | 5.31±0.02* | 0.64±0.02 |
| Threonine | 2.37±0.02 | 0.36±0.02 |
| Serine | 2.69±0.02 | 0.63±0.02 |
| Glutamic acid | 6.98±0.02 | 3.91±0.02 |
| Proline | 3.66±0.02 | 1.31±0.02 |
| Glycine | 3.01±0.02 | 0.51±0.02 |
| Alanine | 5.32±0.02 | 0.45±0.02 |
| Valine | 3.57±0.02 | 0.54±0.02 |
| Methionine** | 1.08±0.02 | 0.19±0.02 |
| Isoleucine | 2.43±0.02 | 0.43±0.02 |
| Leucine | 4.69±0.02 | 0.89±0.02 |
| Tyrosine | 3.66±0.02 | 0.37±0.02 |
| Phenylalanine | 2.38±0.02 | 0.61±0.02 |
| Lysine | 3.57±0.02 | 0.34±0.02 |
| Histidine | 1.41±0.02 | 0.30±0.02 |
| Arginine | 3.90±0.02 | 0.63±0.02 |
| Taurine | 0.22±0.02 | < 0.02 |
| Hydroxyproline | 0.04±0.02 | < 0.02 |
| Cyst(e)ine, calc. from cysteic acid | 0.65±0.02 | 0.29±0.02 |
| Physicochemical properties. | C | CR5 | CR10 | CR15 |
|---|---|---|---|---|
| L* | 45.07±0.22 a | 44.24±0.68 a | 40.42±0.68 b | 40.65±0.56 b |
| a* | 6.20±0.07 c | 5.78±0.12 b | 5.11±0.06 a | 5.88±0.12 b |
| b* | 27.89±0.01 c | 27.11±0.09 b | 25.11±0.52 a | 26.78±0.10 b |
| ΔE | - | 1.03 | 6.27 | 4.15 |
| Density [g/cm3] | 13.91±0.18 b | 13.21±0.42 a | 13.90±0.18 a | 12.91±0.29 b |
| aw | 0.96±0.05 a | 0.95±0.03a | 0.96±0.00a | 0.96±0.00a |
| Hardness [N] | 30.08±4.17a | 31.93±4.99a | 55.89±5.31b | 39.94±4.41c |
| Nutritional value | C | CR5 | CR10 | CR15 |
|---|---|---|---|---|
| Energy value (kJ/kcal) | 877/211 | 890/213 | 904/216 | 916/219 |
| Fat (g) | 0.7 | 1.1 | 1.6 | 2.0 |
| Carbohydrates (g) | 39.8 | 38.1 | 36.5 | 34.8 |
| Fibre (g) | 5.8 | 5.7 | 5.7 | 5.6 |
| Protein (g) | 8.2 | 9.7 | 11.2 | 12.6 |
| % energy from protein | 15.7 | 18.2 | 20.7 | 23.0 |
| Samples | Days | TVC [log CFU g-1] | Y&M[log CFU g-1] |
|---|---|---|---|
| C | 0 | 3.71±0.28 a | nd |
| 2 | 7.10±0.01 bd | 3.67±0.02a | |
| 7 | 8.39±0.31 c | 4.33±0.07 b | |
| CR5 | 0 | 4.30±0.06 d | nd |
| 2 | 6.49±0.39 de | nd | |
| 7 | 7.37±0.34 b | 4.34±0.08 b | |
| CR10 | 0 | 4.52±0.16 d | nd |
| 2 | 7.19±0.20 b | 3.43±0.02 a | |
| 7 | 8.53±0.20 c | 4.40±0.11 b | |
| CR15 | 0 | 4.32±0.12 d | nd |
| 2 | 7.17±0.19 b | nd | |
| 7 | 9.44±0.29 df | 4.29±0.08 b |
| Samples | Wholemeal wheat flour | Cricket powder | Dried yeasts | Sugar | Salt | Water |
|---|---|---|---|---|---|---|
| C | 58.7 | 0.0 | 0.8 | 0.7 | 0.7 | 39.2 |
| CR5 | 55.8 | 2.9 | 0.8 | 0.7 | 0.7 | 39.2 |
| CR10 | 52.9 | 5.9 | 0.8 | 0.7 | 0.7 | 39.2 |
| CR15 | 49.9 | 8.8 | 0.8 | 0.7 | 0.7 | 39.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





