Submitted:
16 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1.0. Introduction
2.0. Materials and Methods
2.1. Soil and Biochar
2.2. Isolation of bacteria from diesel-contaminated soil
2.3. Identification of bacteria isolates
2.4. Assessment of the efficacy of bacterial isolates to remediate diesel-contaminated soil
2.5. Bacterial immobilisation on biochar
2.6. Bioremediation mesocosm experiment
2.7. Total petroleum hydrocarbon (TPH) analysis
2.8. Molecular microbiological analysis
2.8.1. Isolation of DNA from soil and bacteria samples
2.8.2. Quantitative PCR (qPCR) analysis
2.10. Fourier transform infrared (FTIR) analysis of the soil
2.11. Statistical and kinetic analysis
3.0. Results and Discussion
3.1. Isolation, identification, and screening of autochthonous hydrocarbonoclastic bacteria
3.2. Immobilisation of bacteria on biochar
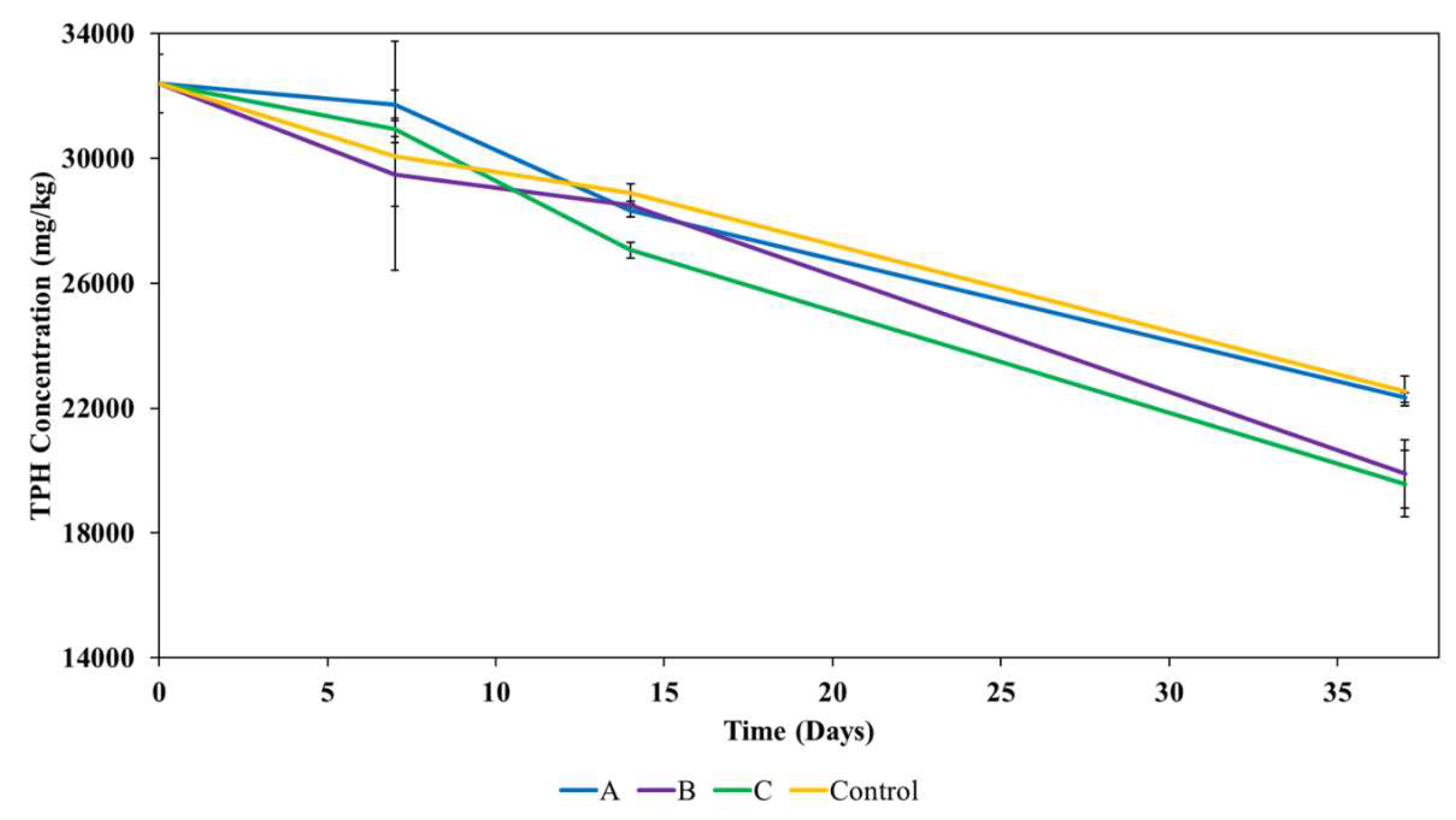
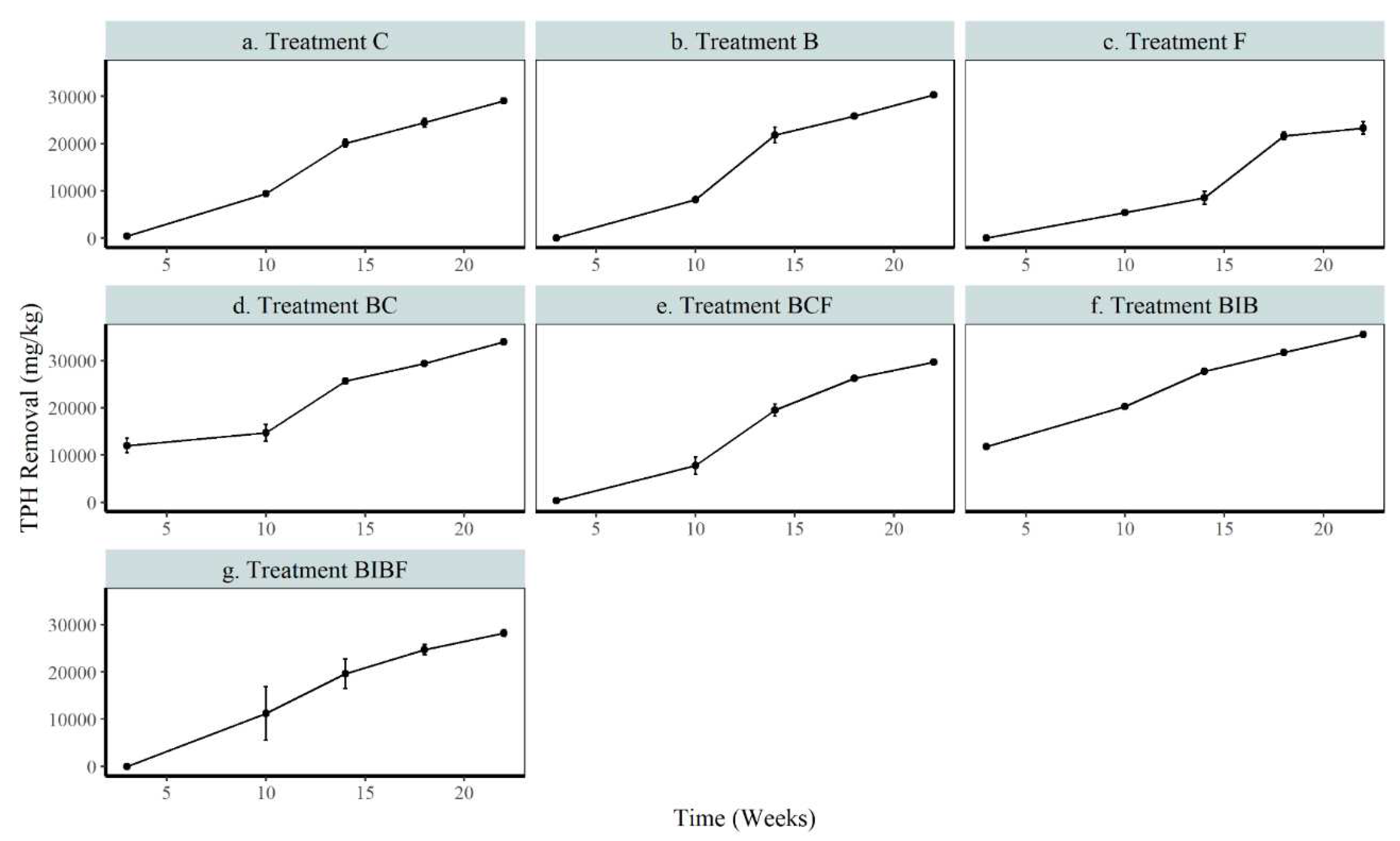
3.3. Remediation of contaminated soil
3.3.1. Impact of biochar on remediation of contaminated soil
3.3.2. Remediation kinetics and prediction
3.4. FTIR analysis of the soil
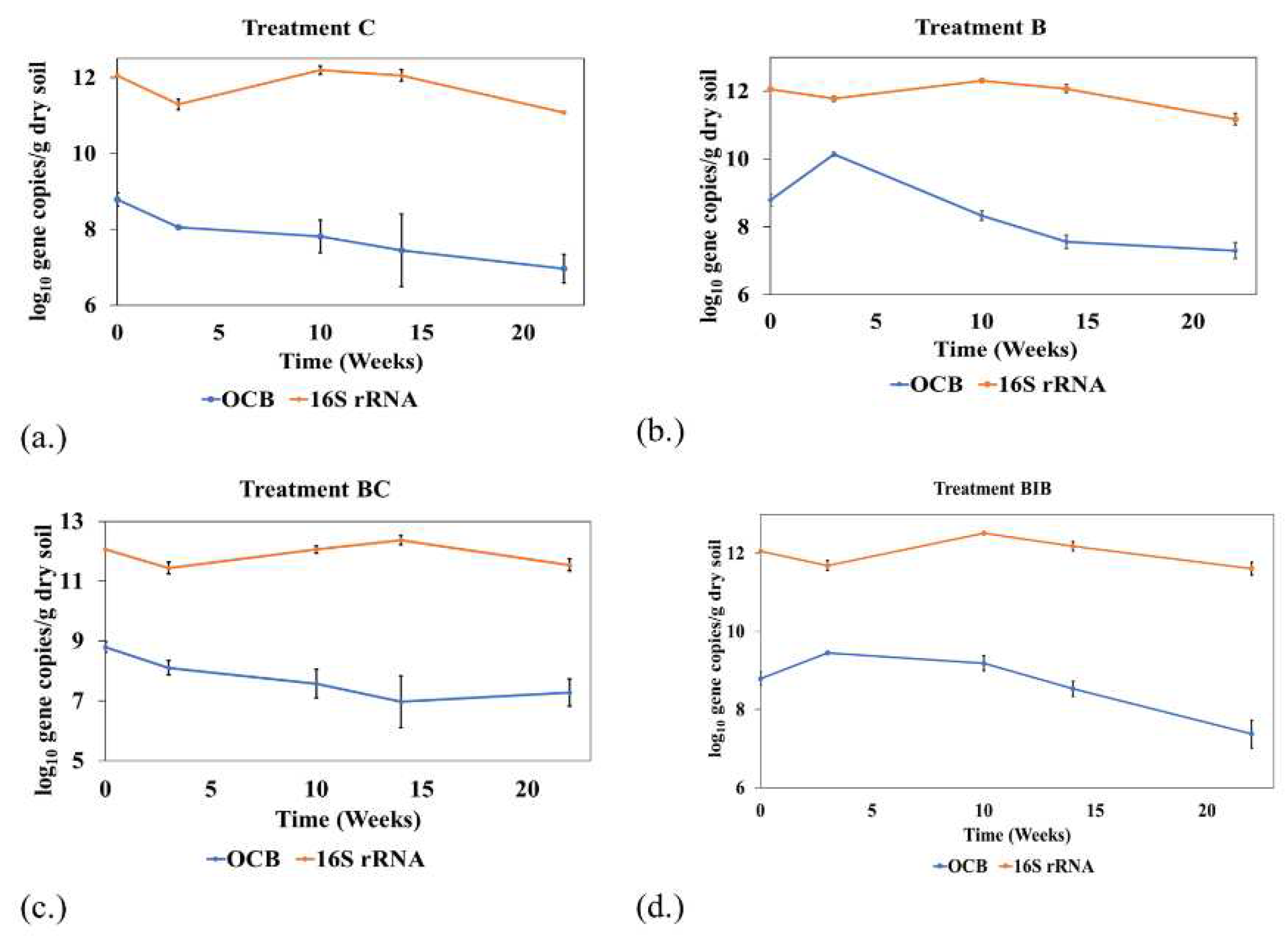
3.5. Quantification of functional genes
4.0. Conclusion
Funding
Acknowledgments
References
- BP, Statistical Review of World Energy – all data, 1965-2020, in, (2022).
- N. Das, P. Chandran, Microbial degradation of petroleum hydrocarbon contaminants: an overview, Biotechnology Research International, 2011 (2011) 941810. [CrossRef]
- Z. Wang, C. Yang, Z. Yang, B. Hollebone, C.E. Brown, M. Landriault, J. Sun, S.M. Mudge, F. Kelly-Hooper, D. Dixon, G., Fingerprinting of petroleum hydrocarbons (PHC) and other biogenic organic compounds (BOC) in oil-contaminated and background soil samples, Journal of Environmental Monitoring, 14 (2012) 2367-2381. [CrossRef]
- N.O.S.D.a.R.A. NOSDRA, Nigerian oil spill monitor, in, National Oil Spill Detection and Response Agency, 2022.
- J. Czarny, J. Staninska-Pięta, A. Piotrowska-Cyplik, W. Juzwa, A. Wolniewicz, R. Marecik, Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals, Journal of hazardous materials, 383 (2020) 121168. [CrossRef]
- F. Ahmed, A. Fakhruddin, A review on environmental contamination of petroleum hydrocarbons and its biodegradation, International Journal of Environmental Sciences & Natural Resources, 11 (2018) 1-7.
- C. Bona, I.M.d. Rezende, G.d.O. Santos, L.A.d. Souza, Effect of soil contaminated by diesel oil on the germination of seeds and the growth of Schinus terebinthifolius Raddi (Anacardiaceae) seedlings, Brazilian Archives of Biology and Technology, 54 (2011) 1379-1387. [CrossRef]
- R. Patowary, A. Devi, A.K. Mukherjee, Potential Application of Biochar for Efficient Restoration of Crude Oil-Contaminated Sites, in: Land Remediation and Management: Bioengineering Strategies, Springer, 2023, pp. 331-350.
- I.C. Ossai, A. Ahmed, A. Hassan, F.S. Hamid, Remediation of soil and water contaminated with petroleum hydrocarbon: A review, Environmental Technology and Innovation, 17 (2020) 100526. [CrossRef]
- E. Koshlaf, A.S. Ball, Soil bioremediation approaches for petroleum hydrocarbon polluted environments, AIMS Microbiology, 3 (2017) 25. [CrossRef]
- J. Matuštík, M. Pohořelý, V. Kočí, Is application of biochar to soil really carbon negative? The effect of methodological decisions in Life Cycle Assessment, Science of the Total Environment, 807 (2022) 151058. [CrossRef]
- S. Aziz, M.I. Ali, U. Farooq, A. Jamal, F.-J. Liu, H. He, H. Guo, M. Urynowicz, Z. Huang, Enhanced bioremediation of diesel range hydrocarbons in soil using biochar made from organic wastes, Environmental Monitoring and Assessment, 192 (2020) 569. [CrossRef]
- C.C. Dike, L.S. Khudur, I.G. Hakeem, A. Rani, E. Shahsavari, A. Surapaneni, K. Shah, A.S. Ball, Biosolids-derived biochar enhances the bioremediation of diesel-contaminated soil, Journal of Environmental Chemical Engineering, 10 (2022) 108633. [CrossRef]
- Y. Wang, F. Li, X. Rong, H. Song, J. Chen, Remediation of petroleum-contaminated soil using bulrush straw powder, biochar and nutrients, Bulletin of Environmental Contamination and Toxicology, 98 (2017) 690-697. [CrossRef]
- M. Couto, E. Monteiro, M. Vasconcelos, Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation, Environmental Science and Pollution Research, 17 (2010) 1339-1346. [CrossRef]
- C.C. Dike, I.G. Hakeem, A. Rani, A. Surapaneni, L. Khudur, K. Shah, A.S. Ball, The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil, Science of The Total Environment, 849 (2022) 157753. [CrossRef]
- A.S. Nwankwegu, C.O. Onwosi, Microbial cell immobilization: a renaissance to bioaugmentation inadequacies. A review, Environmental Technology Reviews, 6 (2017) 186-198. [CrossRef]
- S. Guo, X. Liu, J. Tang, Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil, Chemosphere, 286 (2022) 131663. [CrossRef]
- J. Guo, S. Yang, Q. He, Y. Chen, F. Zheng, H. Zhou, C. Hou, B. Du, S. Jiang, H. Li, Improving benzo (a) pyrene biodegradation in soil with wheat straw-derived biochar amendment: performance, microbial quantity, CO2 emission, and soil properties, Journal of Analytical and Applied Pyrolysis, 156 (2021) 105132. [CrossRef]
- L. Song, X. Niu, N. Zhang, T. Li, Effect of biochar-immobilized Sphingomonas sp. PJ2 on bioremediation of PAHs and bacterial community composition in saline soil, Chemosphere, 279 (2021) 130427. [CrossRef]
- M. Saeed, N. Ilyas, F. Bibi, S. Shabir, K. Jayachandran, R. Sayyed, A.A. Shati, M.Y. Alfaifi, P.L. Show, Z.F. Rizvi, Development of novel kinetic model based on microbiome and biochar for in-situ remediation of total petroleum hydrocarbons (TPHs) contaminated soil, Chemosphere, 324 (2023) 138311. [CrossRef]
- B. Chen, M. Yuan, L. Qian, Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers, Journal of Soils and Sediments, 12 (2012) 1350-1359. [CrossRef]
- X. Wei, P. Peng, Y. Meng, T. Li, Z. Fan, Q. Wang, J. Chen, Degradation Performance of Petroleum-Hydrocarbon-Degrading Bacteria and its Application in Remediation of Oil Contaminated Soil, in: IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2021, pp. 012096. [CrossRef]
- B. Zhang, L. Zhang, X. Zhang, Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar, RSC Advances, 9 (2019) 35304-35311. [CrossRef]
- P. Galitskaya, L. Akhmetzyanova, S. Selivanovskaya, Biochar-carrying hydrocarbon decomposers promote degradation during the early stage of bioremediation, Biogeosciences, 13 (2016) 5739-5752. [CrossRef]
- B. Xiong, Y. Zhang, Y. Hou, H.P.H. Arp, B.J. Reid, C.J.C. Cai, Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar, 182 (2017) 316-324. [CrossRef]
- H. Ren, Y. Deng, L. Ma, Z. Wei, L. Ma, D. Yang, B. Wang, Z.-Y. Luo, Enhanced biodegradation of oil-contaminated soil oil in shale gas exploitation by biochar immobilization, Biodegradation, 33 (2022) 621-639. [CrossRef]
- L. Spinosa, L. Molinari, Standardization: A Necessary Support for the Utilization of Sludge/Biosolids in Agriculture, Standards, 3 (2023) 385-399. [CrossRef]
- M. Yang, Z. Cao, Y. Zhang, H. Wu, W.S.a. Technology, Deciphering the biodegradation of petroleum hydrocarbons using FTIR spectroscopy: application to a contaminated site, 80 (2019) 1315-1325. [CrossRef]
- L.S. Khudur, E. Shahsavari, G.T. Webster, D. Nugegoda, A.S. Ball, The impact of lead co-contamination on ecotoxicity and the bacterial community during the bioremediation of total petroleum hydrocarbon-contaminated soils, Environmental Pollution, 253 (2019) 939-948. [CrossRef]
- L.S. Khudur, A.S. Ball, RemScan: A tool for monitoring the bioremediation of Total Petroleum Hydrocarbons in contaminated soil, MethodsX 5(2018) 705-709. [CrossRef]
- L.S. Khudur, E. Shahsavari, A.F. Miranda, P.D. Morrison, D. Nugegoda, A.S. Ball, Evaluating the efficacy of bioremediating a diesel-contaminated soil using ecotoxicological and bacterial community indices, Environmental Science and Pollution Research, 22 (2015) 14809-14819. [CrossRef]
- T. Sun, J. Miao, M. Saleem, H. Zhang, Y. Yang, Q. Zhang, Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning, Journal of Hazardous Materials, 398 (2020) 122941. [CrossRef]
- H.-Y. Ren, Z.-J. Wei, Y. Wang, Y.-P. Deng, M.-Y. Li, B. Wang, Effects of biochar properties on the bioremediation of the petroleum-contaminated soil from a shale-gas field, Environmental Science and Pollution Research, 27 (2020) 36427-36438. [CrossRef]
- Y.N. Çevik, H. Ogutcu, Identification of Bacteria in Soil by MALDI-TOF MS and Analysis of Bacillus spp., Paenibacillus spp. and Pseudomonas spp. with PCA, Analytical Chemistry Letters, 10 (2020) 784-797.
- L.S. Khudur, D.B. Gleeson, M.H. Ryan, E. Shahsavari, N. Haleyur, D. Nugegoda, A.S. Ball, Implications of co-contamination with aged heavy metals and total petroleum hydrocarbons on natural attenuation and ecotoxicity in Australian soils, Environmental Pollution, 243 (2018) 94-102. [CrossRef]
- H. Schafer, G. Muyzer, Denaturing gradient gel electrophoresis in marine microbial ecology, Methods in Microbiology, (2001) 425-468. [CrossRef]
- E. Shahsavari, A. Aburto-Medina, M. Taha, A.S. Ball, A quantitative PCR approach for quantification of functional genes involved in the degradation of polycyclic aromatic hydrocarbons in contaminated soils, MethodsX, 3 (2016) 205-211. [CrossRef]
- E. Koshlaf, E. Shahsavari, N. Haleyur, A.M. Osborn, A.S. Ball, Impact of necrophytoremediation on petroleum hydrocarbon degradation, ecotoxicity and soil bacterial community composition in diesel-contaminated soil, Environmental Science and Pollution Research, 27 (2020) 31171–31183. [CrossRef]
- S.H. Kim, H. Woo, S. An, J. Chung, S. Lee, S. Lee, What determines the efficacy of landfarming for petroleum-contaminated soils: Significance of contaminant characteristics, Chemosphere, 290 (2022) 133392. [CrossRef]
- L. Kachieng’a, M. Momba, Kinetics of petroleum oil biodegradation by a consortium of three protozoan isolates (Aspidisca sp., Trachelophyllum sp. and Peranema sp.), Biotechnology reports, 15 (2017) 125-131. [CrossRef]
- W. Sun, I. Ali, J. Liu, M. Dai, W. Cao, M. Jiang, G. Saren, X. Yu, C. Peng, I. Naz, Isolation, identification, and characterization of diesel-oil-degrading bacterial strains indigenous to Changqing oil field, China, Journal of basic microbiology, 59 (2019) 723-734. [CrossRef]
- S. Ferhat, S. Mnif, A. Badis, K. Eddouaouda, R. Alouaoui, A. Boucherit, N. Mhiri, N. Moulai-Mostefa, S. Sayadi, Screening and preliminary characterization of biosurfactants produced by Ochrobactrum sp. 1C and Brevibacterium sp. 7G isolated from hydrocarbon-contaminated soils, International Biodeterioration and Biodegradation, 65 (2011) 1182-1188. [CrossRef]
- S. Bhattacharya, A. Das, S. Srividya, P. Prakruti, N. Priyanka, B. Sushmitha, Prospects of Stenotrophomonas pavanii DB1 in diesel utilization and reduction of its phytotoxicity on Vigna radiata, International Journal of Environmental Science and Technology, 17 (2020) 445-454. [CrossRef]
- S. Haloi, T. Medhi, Optimization and characterization of a glycolipid produced by Achromobacter sp. to use in petroleum industries, Journal of basic microbiology, 59 (2019) 238-248. [CrossRef]
- M. Wu, W.A. Dick, W. Li, X. Wang, Q. Yang, T. Wang, L. Xu, M. Zhang, L. Chen, Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil, International Biodeterioration and Biodegradation, 107 (2016) 158-164. [CrossRef]
- D.K. Chaudhary, R. Bajagain, S.-W. Jeong, J. Kim, Insights into the biodegradation of diesel oil and changes in bacterial communities in diesel-contaminated soil as a consequence of various soil amendments, Chemosphere, 285 (2021) 131416. [CrossRef]
- P.V.O. Trindade, L.G. Sobral, A.C.L. Rizzo, S.G.F. Leite, A.U. Soriano, Bioremediation of a weathered and a recently oil-contaminated soils from Brazil: a comparison study, Chemosphere, 58 (2005) 515-522. [CrossRef]
- A. Koolivand, H. Abtahi, M. Parhamfar, R. Saeedi, F. Coulon, V. Kumar, J. Villaseñor, M. Sartaj, N. Najarian, M. Shahsavari, P. Seyedmoradi, L. Rahimi, F. Bagheri, The effect of petroleum hydrocarbons concentration on competition between oil-degrading bacteria and indigenous compost microorganisms in petroleum sludge bioremediation, Environmental Technology & Innovation, 26 (2022) 102319. [CrossRef]
- F. Chaillan, A. Le Flèche, E. Bury, Y.-h. Phantavong, P. Grimont, A. Saliot, J. Oudot, Identification and biodegradation potential of tropical aerobic hydrocarbon-degrading microorganisms, Research in microbiology, 155 (2004) 587-595. [CrossRef]
- C.C. Dike, E. Shahsavari, A. Surapaneni, K. Shah, A.S. Ball, Can biochar be an effective and reliable biostimulating agent for the remediation of hydrocarbon-contaminated soils?, Environment International, 154 (2021) 106553. [CrossRef]
- U. Yousaf, A.H.A. Khan, A. Farooqi, Y.S. Muhammad, R. Barros, J.A. Tamayo-Ramos, M. Iqbal, S. Yousaf, Interactive effect of biochar and compost with Poaceae and Fabaceae plants on remediation of total petroleum hydrocarbons in crude oil contaminated soil, Chemosphere, 286 (2022) 131782. [CrossRef]
- R. Czajkowski, D. Krzyżanowska, J. Karczewska, S. Atkinson, J. Przysowa, E. Lojkowska, P. Williams, S. Jafra, Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase, Environmental microbiology reports, 3 (2011) 59-68. [CrossRef]
- C. Xu, W. Yang, L. Wei, Z. Huang, W. Wei, A. Lin, Enhanced phytoremediation of PAHs-contaminated soil from an industrial relocation site by Ochrobactrum sp, Environmental Science and Pollution Research, 27 (2020) 8991-8999. [CrossRef]
- C.C. Dike, I.G. Hakeem, A. Rani, A. Surapaneni, L. Khudur, K. Shah, A.S. Ball, The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil, Science of the Total Environment, (2022). [CrossRef]
- EPA Victoria, Waste disposal categories – characteristics and thresholds, in, (2021), pp. 1-10.
- M.H. Tazangi, S. Ebrahimi, R.G. Nasrabadi, S.A.M. Naeeni, Kinetic Monitoring of Bioremediators for Biodegradation of Gasoil-Polluted Soil, Water, Air, & Soil Pollution, 231 (2020) 418. [CrossRef]
- R. Li, Y. Liu, R. Mu, W. Cheng, S. Ognier, Evaluation of pulsed corona discharge plasma for the treatment of petroleum-contaminated soil, Environmental Science and Pollution Research, 24 (2017) 1450-1458. [CrossRef]
- B. Xiong, Y. Zhang, Y. Hou, H.P.H. Arp, B.J. Reid, C. Cai, Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar, Chemosphere, 182 (2017) 316-324. [CrossRef]
| Treatments | Description | Identification Code |
|---|---|---|
| Control | No amendment | C |
| Bacteria | 5 ml Bacteria suspension (6.5*109 CFU/ml) | B |
| Fertiliser* | 2% w/w of fertiliser | F |
| Biochar | 5% w/w of biochar | BC |
| Biochar with fertiliser* | 5% w/w of biochar + 2% w/w of fertiliser | BCF |
| Bacteria immobilised biochar | 5% w/w of biochar immobilised bacteria | BIB |
| Bacteria immobilised biochar co-application with fertiliser* | 5% w/w of biochar immobilised bacteria + 2% w/w of fertiliser | BIBF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





