Submitted:
16 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
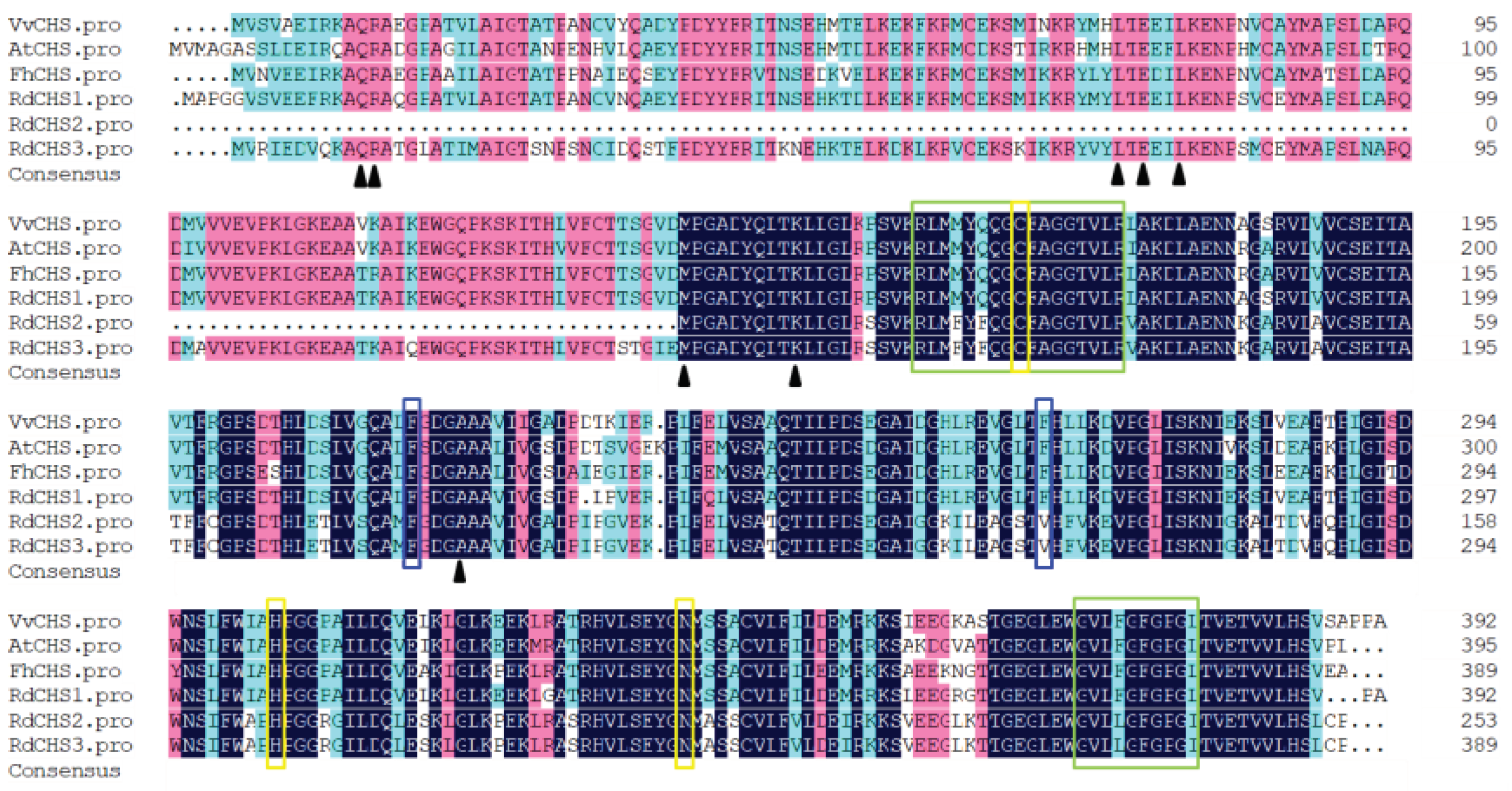
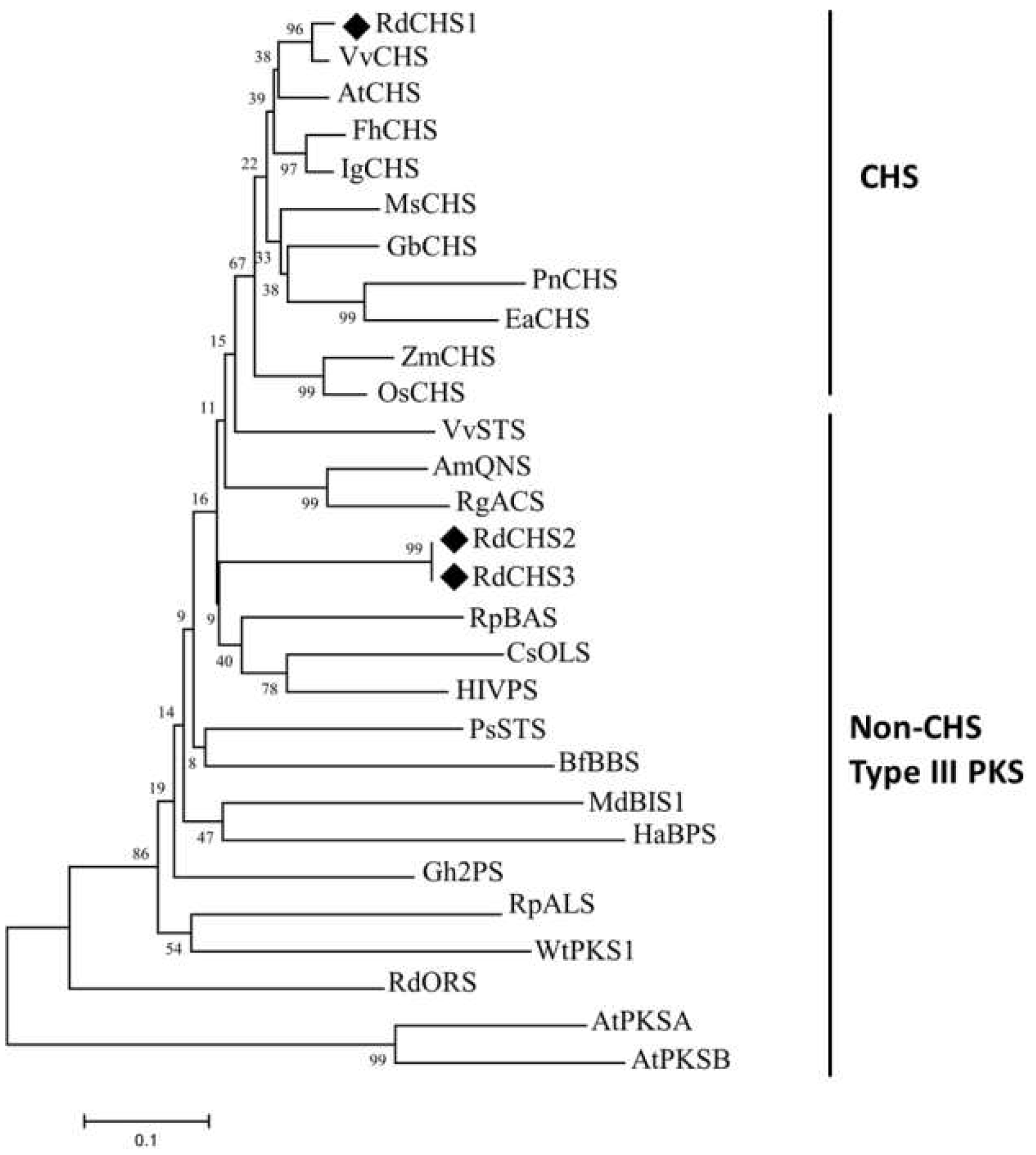
2.1. Cloning and sequence analysis of RdCHSs
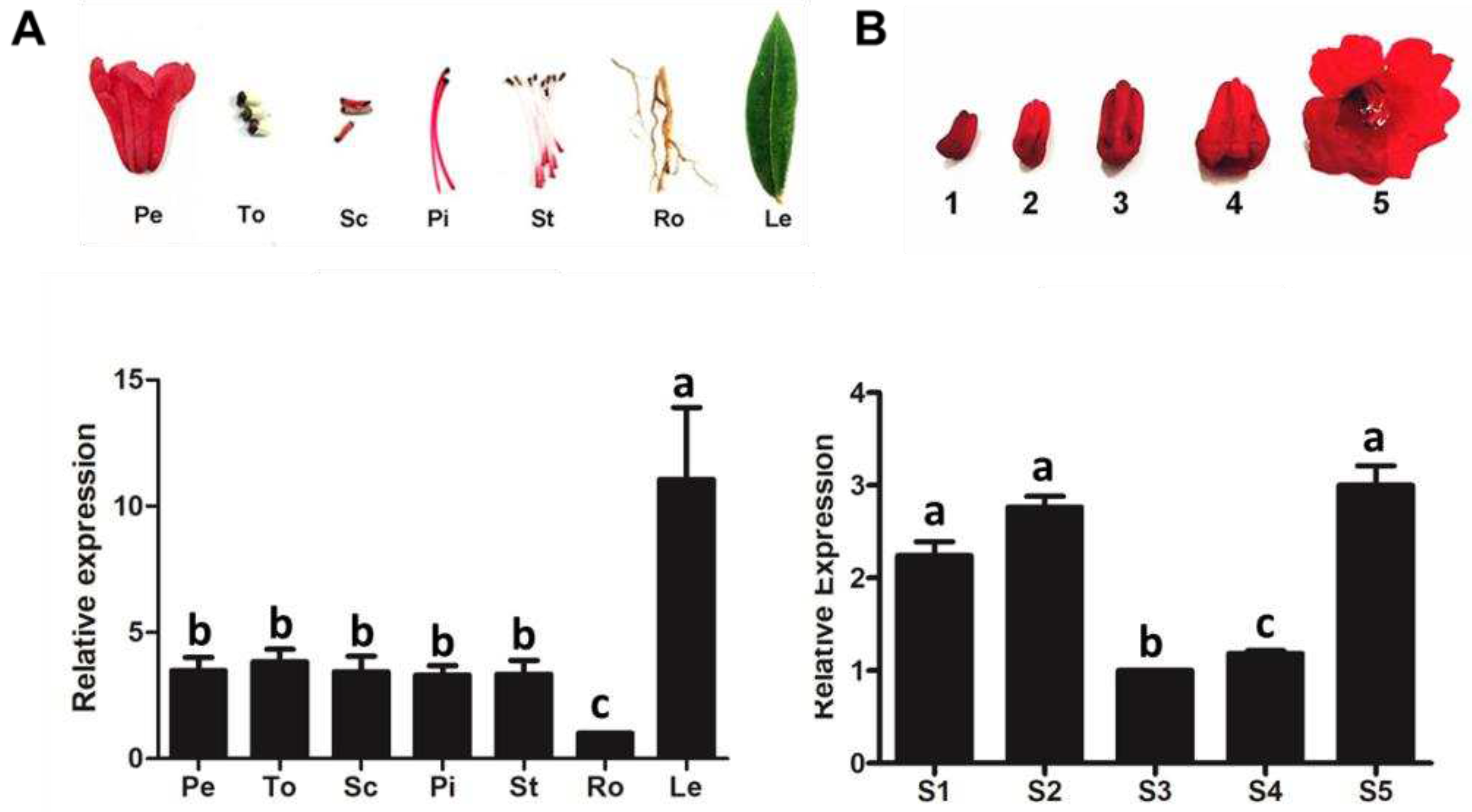
2.2. RdCHS1 expression patterns in developing flowers and different tissues
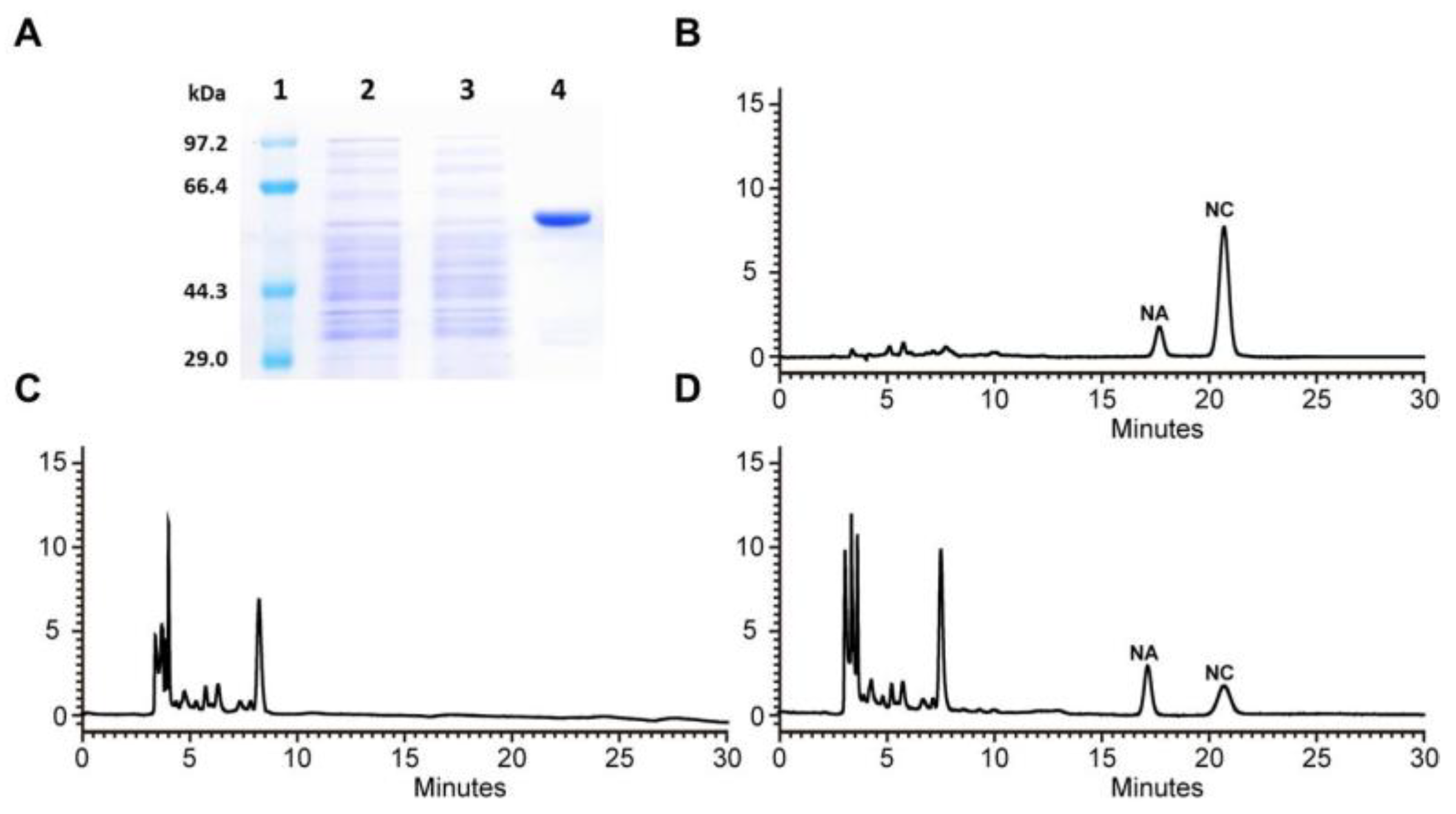
2.3. Biochemical characterization of RdCHS1
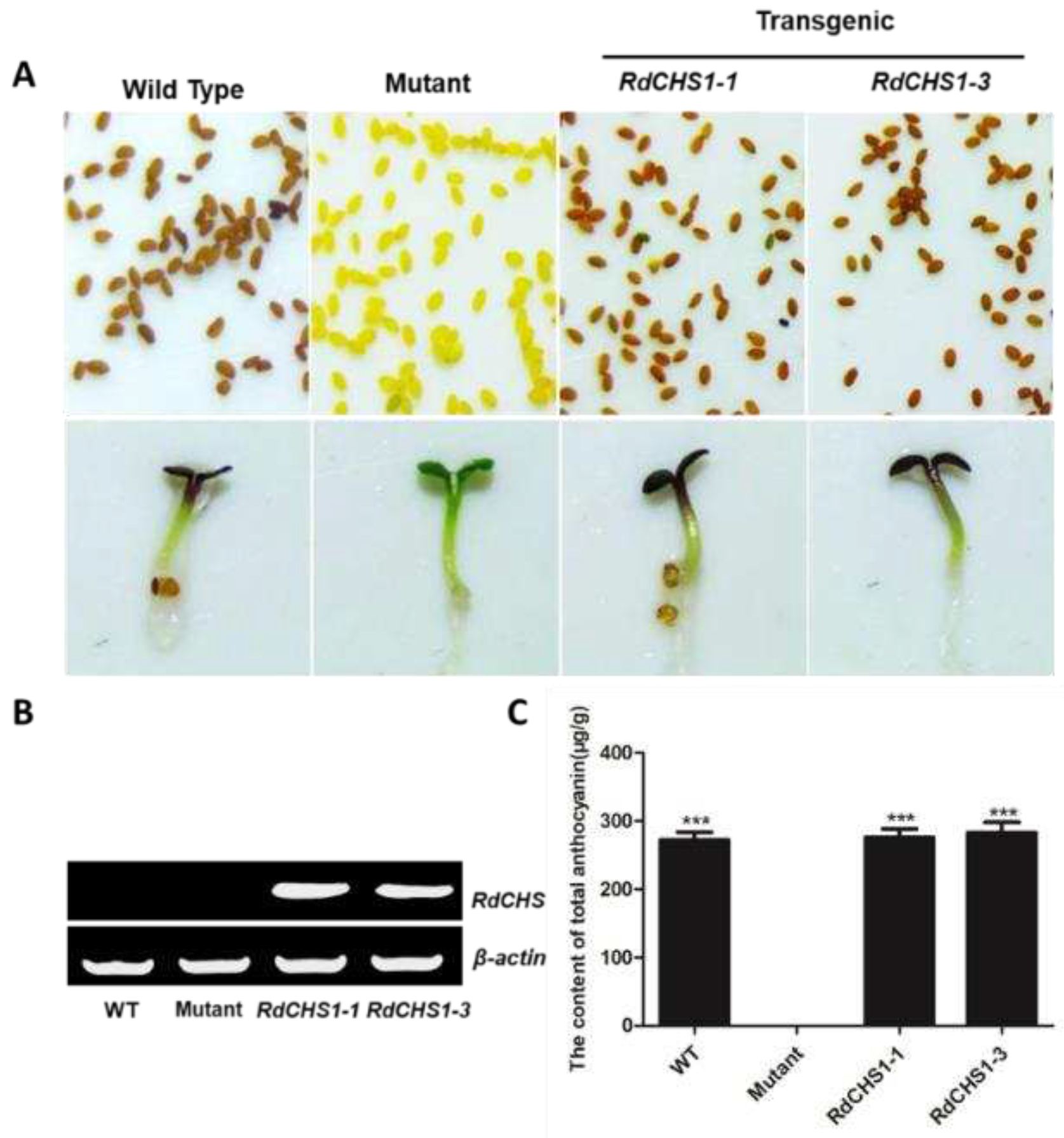
2.4. Complementation of the tt4 mutant with RdCHS1
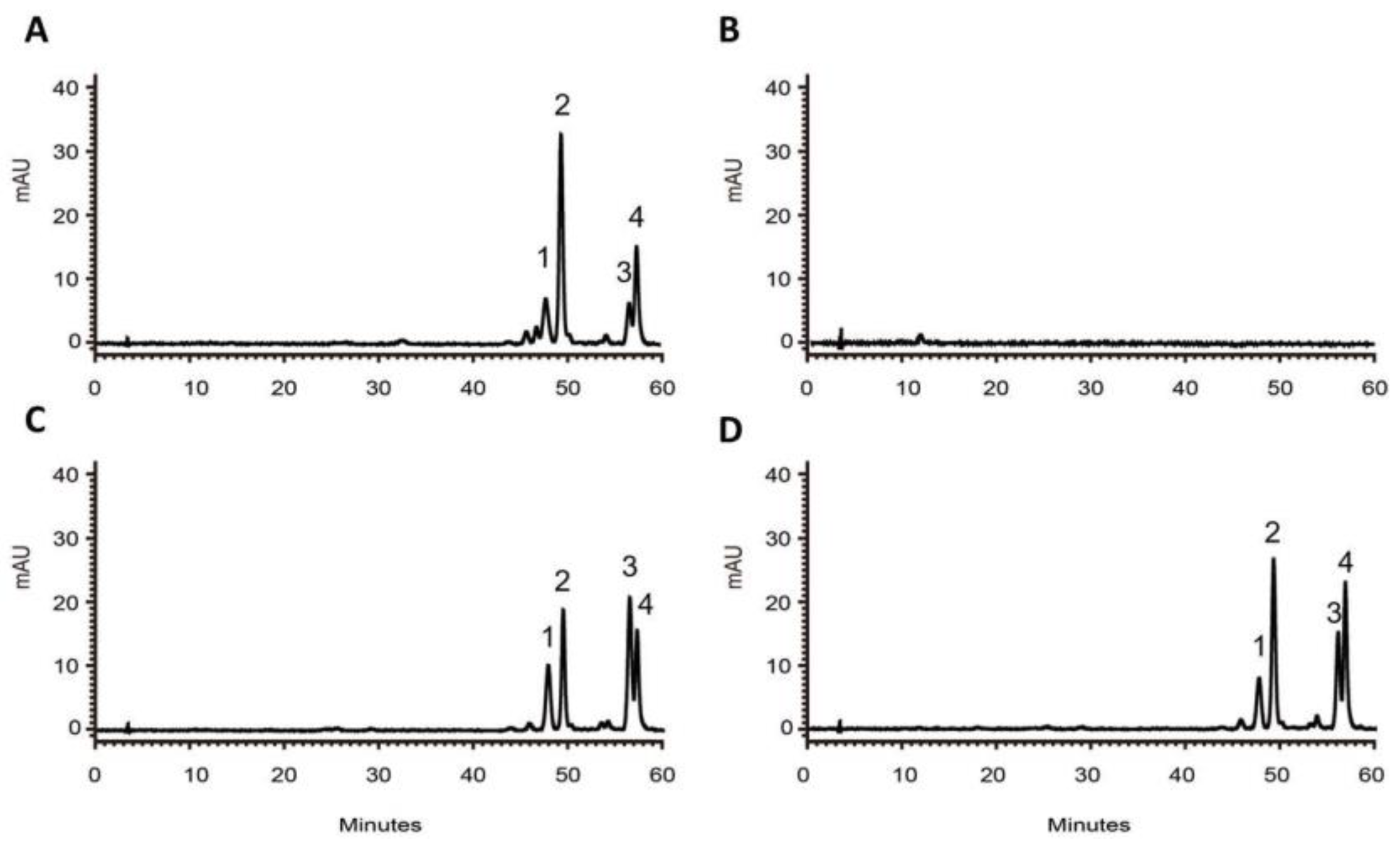
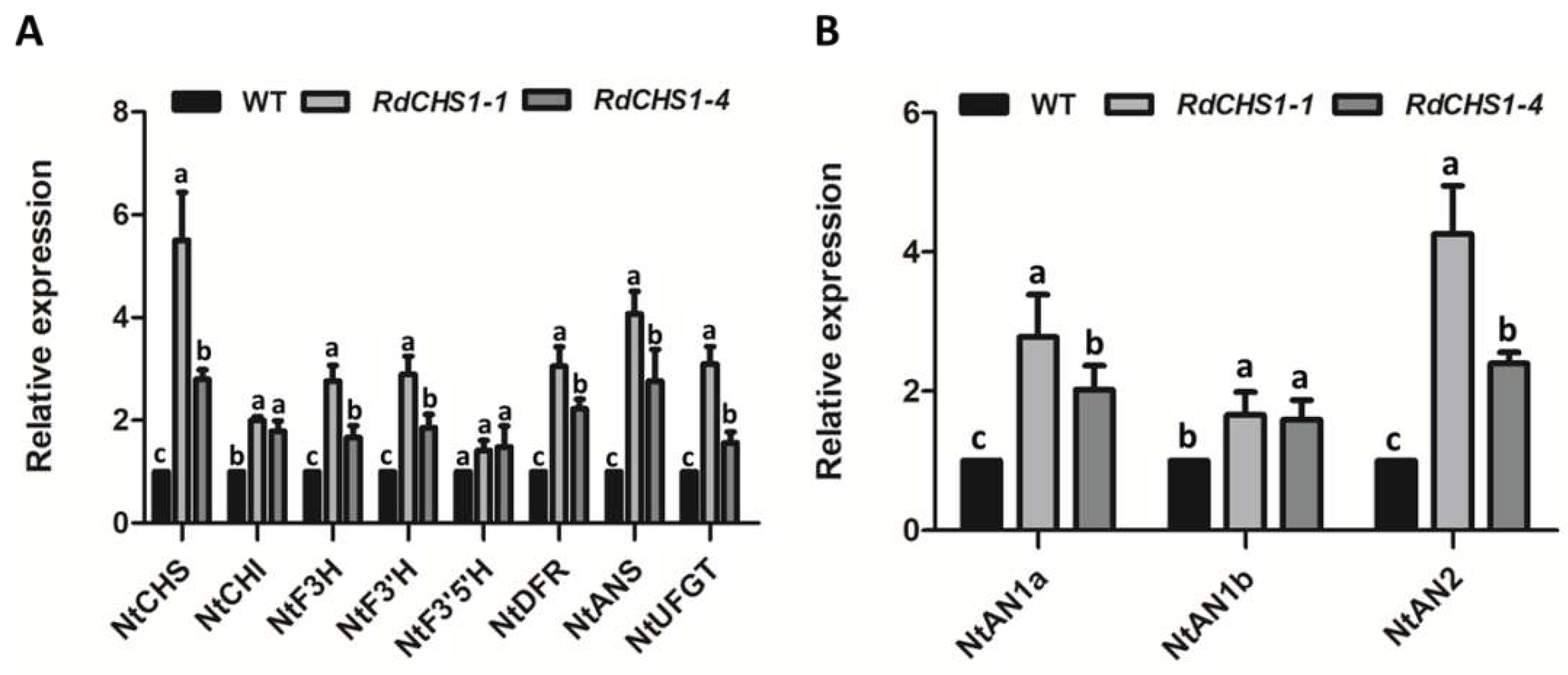
2.5. Overexpression of RdCHS1 in tobacco
3. Discussion
4. Materials and Methods
4.1. Plant materials
4.2. Chemicals
4.3. Full-length cDNA cloning of RdCHS1
4.4. Sequence alignment and phylogenetic analysis
4.5. Gene expression analysis
4.6. Soluble protein extraction and CHS enzyme assay
4.7. Expression vector construction and transformation of Arabidopsis and tobacco
4.8. Anthocyanin analysis of transgenic Arabidopsis seedlings and tobacco flowers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485-493. [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733-749. [CrossRef]
- Ferreyra, M.; Rius, S.; Casati, P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [CrossRef]
- Ma, M.; Zhong, M.; Zhang, Q.; Zhao, W.; Wang, M.; Luo, C. Phylogenetic Implications and Functional Disparity in the Chalcone synthase Gene Family of Common Sea grass Zostera marina. Front. Mar. Sci. 2021, 8, 760902. [CrossRef]
- Dong, H.; Li, H.; Xue, Y.; Su, S.; Li, S.; Shan, X.; Liu, H.; Jiang, N.; Wu, X.; Zhang, Z.; Yuan, Y. E183K Mutation in Chalcone Synthase C2 Causes Protein Aggregation and Maize Colorless. Front. Plant Sci. 2021, 12:679654. [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226-1243. [CrossRef]
- Surangi, D.; David, H.; Vasantha, R. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [CrossRef]
- VanderMeer, I.M.; Spelt, C.E.; Mol, J.N.; Stuitje, A.R. Promoter analysis of the chalcone synthase (chsA) gene of Petunia hybrida: A 67 bp promoter region directs flower-specific expression. Plant Mol. Biol. 1990, 15, 95-109. [CrossRef]
- Hans, S.; Heinz, S. Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet. 1986, 202, 429434. [CrossRef]
- Feinbaum, R.; Ausubel, F. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol. Cell. Biol. 1988, 8, 1985-1992. [CrossRef]
- Colanero, S.; Perata, P.; Gonzali, S. What’s behind purple tomatoes? Insight into the mechanisms of anthocyanin synthesis in tomato fruits. Plant Physiol. 2020, 182, 01530.2019. [CrossRef]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.J. A Proteolytic Regulator Controlling Chalcone Synthase Stability and Flavonoid Biosynthesis in Arabidopsis. Plant Cell. 2017, 29, 1157-1174. [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [CrossRef]
- Ferrer, J.L.; Jez, J.M.; Bowman, M.E.; Dixon, R.A.; Noel, J.P. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol. 1999, 6, 775-784. [CrossRef]
- Liu, X.J.; Chuang, Y.N.; Chiou, C.Y.; Chin, D.C.; Shen, F.Q.; Yeh, K.W. Methylation effect on chalcone synthase gene expression determines anthocyanin pigmentation in floral tissues of two on cidium orchid cultivars. Planta. 2012, 236, 401-409. [CrossRef]
- Dare, A.P.; Tomes, S.; Jones, M.; McGhie, T.K.; Stevenson, D.E.; Johnson, R.A.; Greenwood, D.R.; Hellens, R.P. Phenotypic changes associated with rna interference silencing of chalcone synthase in apple (Malus × domestica). Plant J. 2013, 74, 398-410. [CrossRef]
- Harris, N.; Luczo, J.; Robinson, S.; Walker, A. Transcriptional regulation of the three grapevine chalcone synthase genes and their role in flavonoid synthesis in Shiraz. Aust. J. Grape Wine Res. 2013, 19, 221-229. [CrossRef]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiäinen, M.; Laitinen, R.A.; Albert, V.A.; Valkonen, J.; Elomaa, P.; Teeri, T.H. Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of gerbera hybrida. New Phytol. 2014, 201, 1469-1483. [CrossRef]
- Liu, J.; Hao, X.L.; He, X.Q. Characterization of three chalcone synthase-like genes in Dianthus chinensis. Plant Cell Tiss Org. 2021, 146, 483-492. [CrossRef]
- Kuckuck, H. Ober vier neue Serien multipler Allele bei Antirrhinum majus. Z. Induct. Abstamm. Vererbgsl. 1936, 71, 429-440. [CrossRef]
- Koes, R.E.; Spelt, C.E;, Mol, J.N.M. The chalcone synthase multigene family of Petunia hybrid (V30): differential, light-regulated expression during flower development and UV light induction. Plant Mol Biol. 1989, 12, 213-225. [CrossRef]
- Koseki, M.; Goto, K.; Masuta, C.; Kanazawa, A. The star-type color pattern in Petunia hybrida “red Star” fowers is induced by the sequence-specifc degradation of the chalcone synthase RNA. Plant Cell Physiol. 2005, 46, 1879-1883. [CrossRef]
- Morita, Y.; Saito, R.; Ban, Y.; Tanikawa, N.; Kuchitsu, K.; Ando, T.; Yoshikawa, M.; Pollak, P.E.; Vogt, T.; Mo, Y.; Taylor, L.P. Chalcone synthase and flavonol accumulation in stigmas and anthers of Petunia hybrida. Plant Physiol. 1993, 102, 925-932. [CrossRef]
- Pollak, P.; Hansen, K.; Astwood, J.; Taylor, L. Conditional male fertility in maize. Sex. Plant Reprod. 1995, 8, 231-241. [CrossRef]
- Chang, C.; Bowman, J.L.; DeJohn, A.W.; Lander, E.S.; Meyerowitz, E.M. Restriction fragment length polymorphism linkage map for Arabidopsis thaliana. Proc. Natl Acad. Sci. 1988, 85, 6856-6860. [CrossRef]
- Buer, C.S.; Djordjevic, M.A. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 751-763. [CrossRef]
- Helariutta, Y.; Elomaa, P.; Kotilainen, M.; Griesbach, R.J.; SchrÖder, J.; Teeri, T.H. Chalcone synthase-like genes active during corolla development are diferentially expressed and encode enzymes with diferent catalytic properties in Gerbera hybrida (Aster aceae). Plant Mol Biol. 1995, 28, 47-60. [CrossRef]
- Nakatsuka, A.; Izumi, Y.; Yamagishi, M. Spatial and temporal expression of chalcone synthase and dihydrofavonol 4-reductase genes in the Asiatic hybrid lily. Plant Sci. 2003, 165, 759-767. [CrossRef]
- Suzuki, K.; Suzuki, T.; Nakatsuka, T.; Dohra, H.; Yamagishi, M.; Matsuyama, K.; Matsuura, H. RNA-seq-based evaluation of bicolor tepal pigmentation in Asiatic hybrid lilies (Lilium spp.). BMC Genom. 2016, 17, 611-629. [CrossRef]
- Zhang, L.; Xu, P.W.; Cai, Y.F.; Ma, L.L.; Li, S.F.; Xie, W.L. The draft genome assembly of Rhododendron delavayi Franch var. delavayi. GigaScience. 2017, 6, 1-11. [CrossRef]
- Sun, W.; Sun, S.; Xu, H.; Wang, Y.; Chen, Y.; Xu, X.; Yi, Y.; Ju, Z. Characterization of Two Key Flavonoid 3-O-Glycosyltransferases Involved in the Formation of Flower Color in Rhododendron Delavayi. Front. Plant Sci. 2022, 13, 863482. [CrossRef]
- Sun, W.; Meng, X.; Liang, L.; Jiang, W.; Huang, Y.; He, J. Molecular and Biochemical Analysis of Chalcone Synthase from Freesia hybrid in Flavonoid Biosynthetic Pathway. PLoS ONE. 2015, 10, e0119054. [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735-743. [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusionproteins in tobacco plants and generation of stably transformed plants. Nat Protoc. 2006, 4, 2019-2025. [CrossRef]
- Fanali, C.; Dugo, L.; D’Orazio, G.; Lirangi, M.; Dacha, M.; Dugo, P. Analysis of anthocyanins in commercial fruit juices by using nano-liquid chromatography-electrospray-mass spectrometry and high-performance liquid chromatography with UV-visdetector. J. Sep. Sci. 2011, 34, 150-159. [CrossRef]
- Okada, Y.; Sano, Y.; Kaneko, T.; Abe, I.; Noguchi, H.; Ito, K. Enzymatic reactions by five chalcone synthase homologs from Hop (Humulus lupulus L.). Biosci. Biotechnol. Biochem. 2004, 68, 1142-1145. [CrossRef]
- Ohno, S.; Hosokawa, M.; Kojima, M.; Kitamura, Y.; Hoshino, A.; Tatsuzawa, F.; Doi, M.; Yazawa, S. Simultaneous post-transcriptional gene silencing of two diferent chalcone synthase genes resulting in pure white flowers in the octoploid dahlia. Planta. 2011, 234, 945-958. [CrossRef]
- Yang, J.; Gu, H.; Yang, Z. Likelihood analysis of the chalcone synthase genes suggests the role of positive selection in morning glories (Ipomoea) J. Mol. Evol. 2004, 58, 54-63. [CrossRef]
- Koes, R.E.; Spelt, C.E.; vandenelzen, P.J.M.; Mol, J.N.M. Cloning and molecular characterization of the chalcone synthase multigene family of Petunia hybrida. Gene. 1989, 81, 245-257. [CrossRef]
- Wang, Z.; Yu, Q.; Shen, W.; ElMohtar, C.A.; Zhao, X.; Gmitter, F.G. Functional study of CHS gene family members in citrus revealed a novel CHS gene affecting the production of flavonoids. BMC Plant Biol. 2018, 18, 189. [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol Biochem. 2013, 72, 21-34. [CrossRef]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep. 2003, 20, 79-110. [CrossRef]
- Abe, I., Morita, H., Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep. 2010, 27, 809-838. [CrossRef]
- Schroder, J. A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci. 1997, 2, 373-378. [CrossRef]
- Morita, H.; Wong, C.P.; Abe, I. How structural subtleties lead to molecular diversity for the type III polyketide synthases. J. Biol. Chem. 2019, 294, 15121-15136. [CrossRef]
- Park, H.L.; Yoo, Y.; Bhoo, S.H.; Lee, T.H.; Lee, S.W.; Cho, M.H. Two Chalcone Synthase Isozymes Participate Redundantly in UV-Induced Sakuranetin Synthesis in Rice. Int J Mol Sci. 2020, 21, 3777. [CrossRef]
- Oakley, T.H.; Østman, B.; Wilson, A.C. Repression and loss of gene expression outpaces activation and gainin recently duplicated fly genes. Proc. Natl. Acad. Sci. USA. 2006, 103, 11637-11641. [CrossRef]
- Gu, X. Statistical framework for phylogenomic analysis of gene family expression profiles. Genetics. 2004, 167, 531-542. [CrossRef]
- Jiang, C.; Schommer, C.K.; Kim, S.Y.; Suh, D.Y. Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry. 2006, 67, 2531-2540. [CrossRef]
- Abe, I.; Takahashi, Y.; Noguchi, H. Enzymatic formation of an unnatural C6-C5 aromatic polyketideby plant type III polyketide synthases. Org let. 2002, 4, 3623-3626. [CrossRef]
- Shirley, B.W.; Kubasek, W.L.; Storz, G.; Bruggemann, E.; Koornneef, M.; Ausubel, F.M.; Goodman, H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995, 8, 659-671. [CrossRef]
- Dong, X.; Braun, E.L.; Grotewold, E. Functional conservation of plant secondary metabolic enzymes revealed by complementation of Arabidopsis flavonoid mutants with maize genes. Plant Physiol. 2001, 127, 46-57. [CrossRef]
- Sun, W.; Shen, H.; Xu, H.; Tang, X.; Tang, M.; Ju, Z.; Yi, Y. Chalcone Isomerase a Key Enzyme for Anthocyanin Biosynthesis in Ophiorrhiza japonica. Front. Plant Sci. 2019, 10, 865. [CrossRef]
- Chen, X.; Liu, W.; Huang, X.; Fu, H.; Wang, Q.; Wang, Y. Arg-type dihydroflavonol 4-reductase genes from the fern Dryopteris erythrosora play important roles in the biosynthesis of anthocyanins. PLoS ONE. 2020, 15, e0232090. [CrossRef]
- Schijlen, E.G.; RicdeVos, C.H.; vanTunen, A.J.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry. 2004, 65, 2631-2648. [CrossRef]
- Pourcel, L.; Irani, N.G.; Koo, A.J.; Bohorquez-Restrepo, A.; Howe, G.A.; Grotewold, E.A. chemical complementation approach reveals genes and interactions of flavonoids with other pathways. Plant J. 2013, 74(3):383-397. [CrossRef]
- Sitakanta, P.; Que, K.; David, Z.; Werkman, J.R.; Xie, C.H.; Barunava, P.; Ling, Y. Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta. 2010, 231, 1061-1076. [CrossRef]
- Yanhong, B.; Sitakanta, P.; Barunava, P.; Werkman, J.R.; Xie, C.H.; Ling, Y. Flavonoid-related basichelix-loop-helix regulators, NtAn1a and NtAn1b, of tobacco have originated from two ancestors and are functionally active. Planta. 2011, 234, 363-375. [CrossRef]
- Liu, H.; Lou, Q.; Ma, J. Cloning and functional characterization of dihydroflavonol 4-reductase gene involved in anthocyanidin biosynthesis of grape hyacinth. Int. J. Mol. Sci. 2019, 20, 4743. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



