Submitted:
18 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
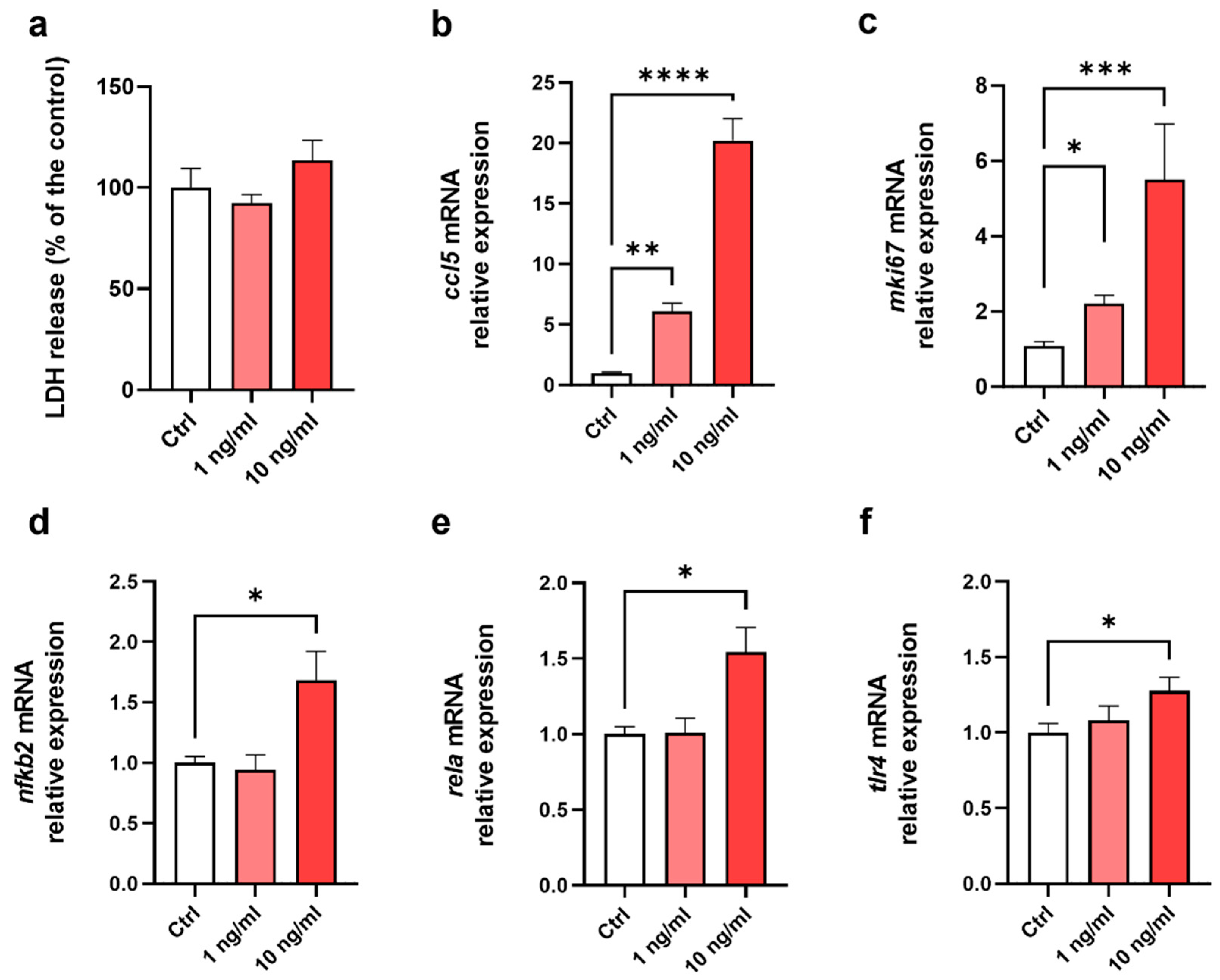
2.1. EDM model is functional and competent to respond to an inflammatory stress
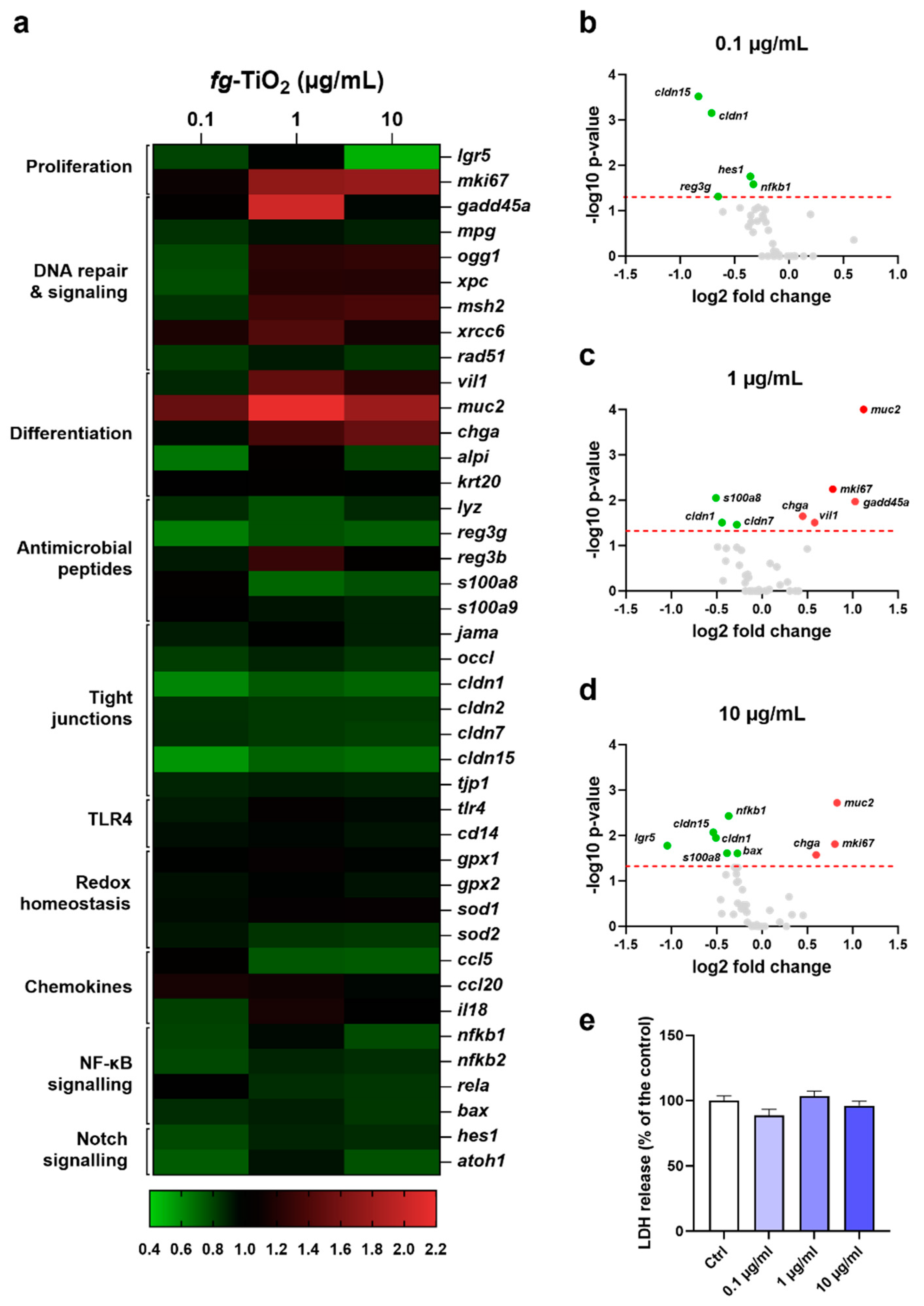
2.2. Fg-TiO2 dose-dependently modulated expression of genes regulating intestinal barrier function
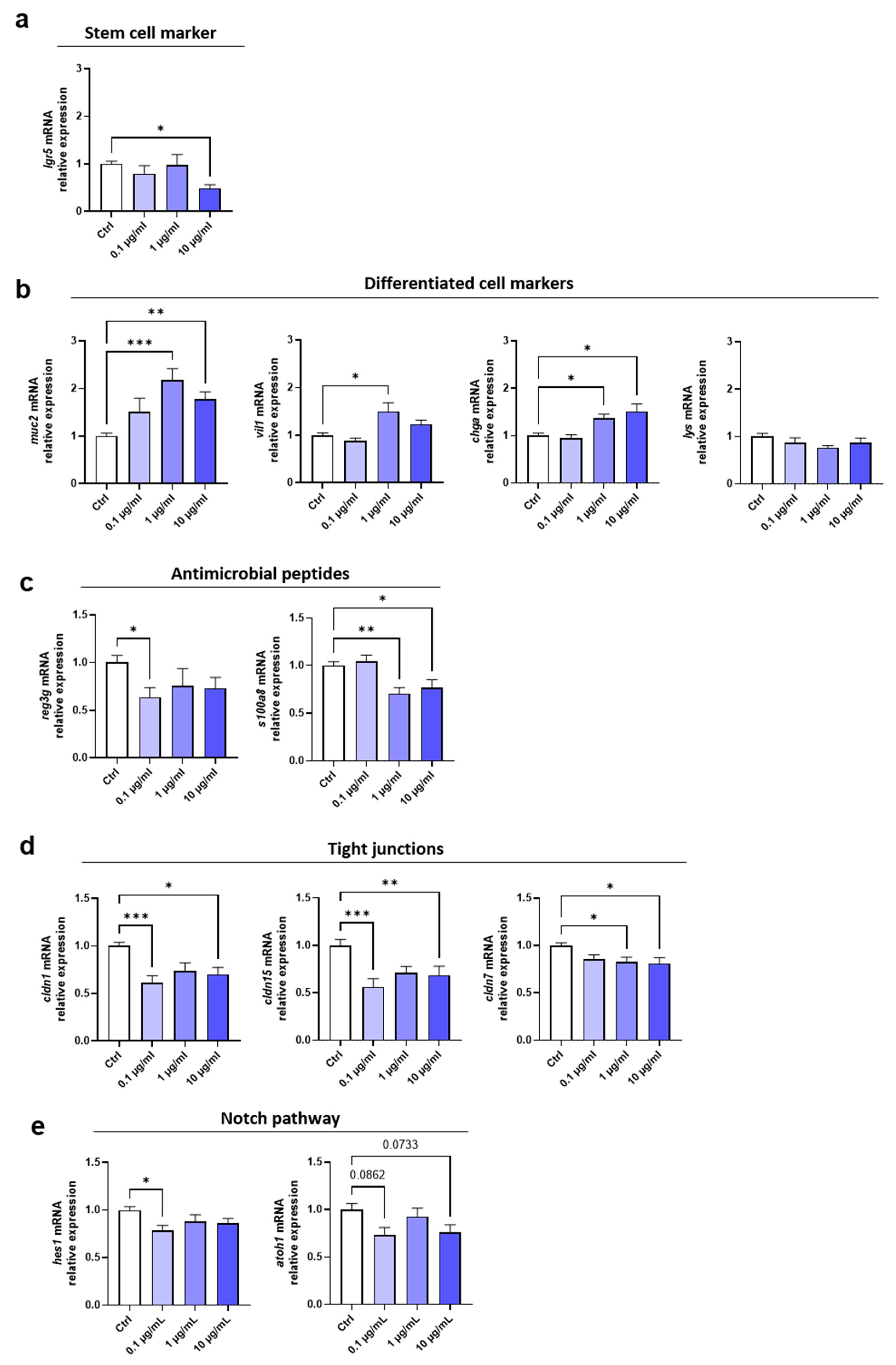
2.3. Fg-TiO2 altered gene expression involved in secretory cell differentiation, innate defences and epithelial TJ function of EDMs
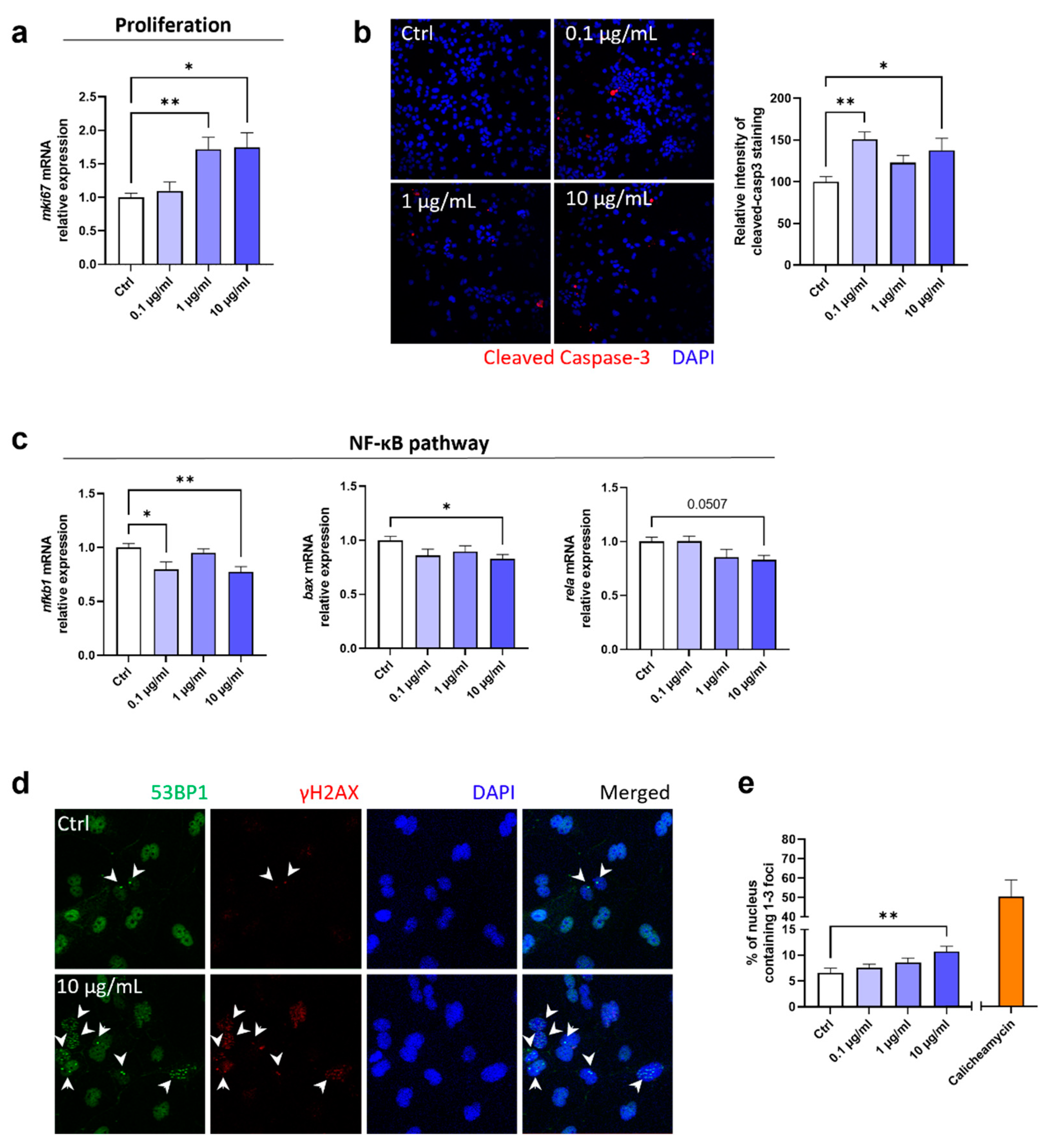
2.4. Fg-TiO2 increased apoptosis and induced genotoxicity
3. Discussion
4. Materials and Methods
4.1. Murine intestinal organoids
4.2. Preparation of enteroid-derived monolayers (EDMs) and treatments
4.3. Measurement of lactate dehydrogenase (LDH) release
4.4. Gene expression analysis
4.5. Immunofluorescence
4.6. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and Implications of Nanotechnologies for the Food Sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in Applications of Nanotechnology in Global Food Industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal Oxide-Based Nanocomposites in Food Packaging: Applications, Migration, and Regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety Assessment of Titanium Dioxide (E171) as a Food Additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-Evaluation of Silicon Dioxide (E 551) as a Food Additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal in Vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Cho, S.-W. Gastrointestinal Tract Modeling Using Organoids Engineered with Cellular and Microbiota Niches. Exp. Mol. Med. 2020, 52, 227–237. [Google Scholar] [CrossRef]
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent Advances in Three-Dimensional Stem Cell Culture Systems and Applications. Stem Cells Int. 2021, 2021, 9477332. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Breyner, N.M.; Su, H.-M.; Verdu, E.F.; Didar, T.F. Intestinal Organoids: A New Paradigm for Engineering Intestinal Epithelium in Vitro. Biomaterials 2019, 194, 195–214. [Google Scholar] [CrossRef]
- Leslie, J.L.; Young, V.B. A Whole New Ball Game: Stem Cell-Derived Epithelia in the Study of Host–Microbe Interactions. Anaerobe 2016, 37, 25–28. [Google Scholar] [CrossRef]
- d’Aldebert, E.; Quaranta, M.; Sébert, M.; Bonnet, D.; Kirzin, S.; Portier, G.; Duffas, J.-P.; Chabot, S.; Lluel, P.; Allart, S.; et al. Characterization of Human Colon Organoids From Inflammatory Bowel Disease Patients. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Zietek, T.; Giesbertz, P.; Ewers, M.; Reichart, F.; Weinmüller, M.; Urbauer, E.; Haller, D.; Demir, I.E.; Ceyhan, G.O.; Kessler, H.; et al. Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism – Update to the Human Model and Expansion of Applications. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Takahashi, Y.; Noguchi, M.; Inoue, Y.; Sato, S.; Shimizu, M.; Kojima, H.; Okabe, T.; Kiyono, H.; Yamauchi, Y.; Sato, R. Organoid-Derived Intestinal Epithelial Cells Are a Suitable Model for Preclinical Toxicology and Pharmacokinetic Studies. iScience 2022, 25, 104542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, J.; Fonseca, A.G.; Moshensky, A.; Kothari, T.; Sayed, I.M.; Ibeawuchi, S.-R.; Pranadinata, R.F.; Ear, J.; Sahoo, D.; et al. E-Cigarettes Compromise the Gut Barrier and Trigger Inflammation. iScience 2021, 24, 102035. [Google Scholar] [CrossRef] [PubMed]
- Altay, G.; Larrañaga, E.; Tosi, S.; Barriga, F.M.; Batlle, E.; Fernández-Majada, V.; Martínez, E. Self-Organized Intestinal Epithelial Monolayers in Crypt and Villus-like Domains Show Effective Barrier Function. Sci. Rep. 2019, 9, 10140. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, N.S.; de Kok, T.M.; Sijm, D.T.H.M.; van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.M.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D.; et al. Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System. Int. J. Mol. Sci. 2020, 22, 207. [Google Scholar] [CrossRef]
- Bischoff, N.S.; Proquin, H.; Jetten, M.J.; Schrooders, Y.; Jonkhout, M.C.M.; Briedé, J.J.; van Breda, S.G.; Jennen, D.G.J.; Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; et al. The Effects of the Food Additive Titanium Dioxide (E171) on Tumor Formation and Gene Expression in the Colon of a Transgenic Mouse Model for Colorectal Cancer. Nanomater. Basel Switz. 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.; Michaudel, C.; Guinot, M.; Grauso-Culetto, M.; Guillon, B.; Lecardonnel, J.; Jouneau, L.; Chapuis, C.; Bernard, H.; Hazebrouck, S.; et al. Long-Term Exposure from Perinatal Life to Food-Grade TiO2 Alters Intestinal Homeostasis and Predisposes to Food Allergy in Young Mice. Allergy 2023. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Chevalier, L.; Gaultier, E.; Cartier, C.; Weingarten, L.; Blanc, X.; Fisicaro, P.; Oster, C.; Noireaux, J.; Evariste, L.; et al. The Food Additive Titanium Dioxide Hinders Intestinal Production of TGF-β and IL-10 in Mice, and Long-Term Exposure in Adults or from Perinatal Life Blocks Oral Tolerance to Ovalbumin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 179, 113974. [Google Scholar] [CrossRef]
- Vignard, J.; Pettes-Duler, A.; Gaultier, E.; Cartier, C.; Weingarten, L.; Biesemeier, A.; Taubitz, T.; Pinton, P.; Bebeacua, C.; Devoille, L.; et al. Food-Grade Titanium Dioxide Translocates across the Buccal Mucosa in Pigs and Induces Genotoxicity in an in Vitro Model of Human Oral Epithelium. Nanotoxicology 2023, 17, 289–309. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-Grade TiO2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Radziwill-Bienkowska, J.M.; Talbot, P.; Kamphuis, J.B.J.; Robert, V.; Cartier, C.; Fourquaux, I.; Lentzen, E.; Audinot, J.-N.; Jamme, F.; Réfrégiers, M.; et al. Toxicity of Food-Grade TiO2 to Commensal Intestinal and Transient Food-Borne Bacteria: New Insights Using Nano-SIMS and Synchrotron UV Fluorescence Imaging. Front. Microbiol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in Vitro Exposure of Intestinal Epithelial Cells to E171 Food Additive Causes Oxidative Stress, Inducing Oxidation of DNA Bases but No Endoplasmic Reticulum Stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Guillard, A.; Gaultier, E.; Cartier, C.; Devoille, L.; Noireaux, J.; Chevalier, L.; Morin, M.; Grandin, F.; Lacroix, M.Z.; Coméra, C.; et al. Basal Ti Level in the Human Placenta and Meconium and Evidence of a Materno-Foetal Transfer of Food-Grade TiO2 Nanoparticles in an Ex Vivo Placental Perfusion Model. Part. Fibre Toxicol. 2020, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.; Wiedey, R.; Kleinebudde, P. Alternatives to Titanium Dioxide in Tablet Coating. Pharm. Dev. Technol. 2021, 26, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Béchard, S.R.; Quraishi, O.; Kwong, E. Film Coating: Effect of Titanium Dioxide Concentration and Film Thickness on the Photostability of Nifedipine. Int. J. Pharm. 1992, 87, 133–139. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium Dioxide in Our Everyday Life; Is It Safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods To Define Nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171) 2022.
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Assessment of the in Vitro Genotoxicity of TiO2 Nanoparticles in a Regulatory Context. Nanotoxicology 2018, 12, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, K.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles Transferred from Pregnant Mice to Their Offspring Can Damage the Genital and Cranial Nerve Systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef]
- Carlé, C.; Boucher, D.; Morelli, L.; Larue, C.; Ovtchinnikova, E.; Battut, L.; Boumessid, K.; Airaud, M.; Quaranta-Nicaise, M.; Ravanat, J.-L.; et al. Perinatal Foodborne Titanium Dioxide Exposure-Mediated Dysbiosis Predisposes Mice to Develop Colitis through Life. Part. Fibre Toxicol. 2023, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, L.; Hu, X.; Liu, C.; Shi, J.; Wang, H.; Zhan, L.; Song, H. Inhibition of Epithelial-Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium Dioxide Nanoparticle Ingestion Alters Nutrient Absorption in an In Vitro Model of the Small Intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ye, Y.; Wang, J.; Zhang, H.; Wu, Y.; Wang, Y.; Yan, L.; Zhang, Y.; Duan, S.; Lv, L.; et al. Effects of Titanium Dioxide Nanoparticles on Nutrient Absorption and Metabolism in Rats: Distinguishing the Susceptibility of Amino Acids, Metal Elements, and Glucose. Nanotoxicology 2020, 14, 1301–1323. [Google Scholar] [CrossRef] [PubMed]
- Pedata, P.; Ricci, G.; Malorni, L.; Venezia, A.; Cammarota, M.; Volpe, M.G.; Iannaccone, N.; Guida, V.; Schiraldi, C.; Romano, M.; et al. In Vitro Intestinal Epithelium Responses to Titanium Dioxide Nanoparticles. Food Res. Int. Ott. Ont 2019, 119, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium Dioxide Food Additive (E171) Induces ROS Formation and Genotoxicity: Contribution of Micro and Nano-Sized Fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium Dioxide Nanoparticles Exacerbate DSS-Induced Colitis: Role of the NLRP3 Inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Carolina Maciel Nogueira, W.M. de A.; rgio Hiroshi Toma, A.; Zonetti de Arruda Leite, M.L.L.; cia Ortiz-Agostinho, M.I.S.D. Titanium Dioxide Induced Inflammation in the Small Intestine. World J. Gastroenterol. 2012, 18, 4729–4735. [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public. Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Coméra, C.; Cartier, C.; Gaultier, E.; Catrice, O.; Panouille, Q.; El Hamdi, S.; Tirez, K.; Nelissen, I.; Théodorou, V.; Houdeau, E. Jejunal Villus Absorption and Paracellular Tight Junction Permeability Are Major Routes for Early Intestinal Uptake of Food-Grade TiO2 Particles: An in Vivo and Ex Vivo Study in Mice. Part. Fibre Toxicol. 2020, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium Dioxide Nanoparticle Impact and Translocation through Ex Vivo, in Vivo and in Vitro Gut Epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S.; et al. Food-Grade Titanium Dioxide Exposure Exacerbates Tumor Formation in Colitis Associated Cancer Model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Vital, N.; Rolo, D.; Roque, R.; Gonçalves, L.M.; Bettencourt, A.; Silva, M.J.; Louro, H. Investigation of the Genotoxicity of Digested Titanium Dioxide Nanomaterials in Human Intestinal Cells. Food Chem. Toxicol. 2022, 161, 112841. [Google Scholar] [CrossRef] [PubMed]
- Zijno, A.; De Angelis, I.; De Berardis, B.; Andreoli, C.; Russo, M.T.; Pietraforte, D.; Scorza, G.; Degan, P.; Ponti, J.; Rossi, F.; et al. Different Mechanisms Are Involved in Oxidative DNA Damage and Genotoxicity Induction by ZnO and TiO2 Nanoparticles in Human Colon Carcinoma Cells. Toxicol. In Vitro 2015, 29, 1503–1512. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Goyary, D.; Karmakar, S.; Chattopadhyay, P. Exploration of Cytotoxic and Genotoxic Endpoints Following Sub-Chronic Oral Exposure to Titanium Dioxide Nanoparticles. Toxicol. Ind. Health 2019, 35, 577–592. [Google Scholar] [CrossRef]
- Fujita, Y.; Khateb, A.; Li, Y.; Tinoco, R.; Zhang, T.; Bar-Yoseph, H.; Tam, M.A.; Chowers, Y.; Sabo, E.; Gerassy-Vainberg, S.; et al. Regulation of S100A8 Stability by RNF5 in Intestinal Epithelial Cells Determines Intestinal Inflammation and Severity of Colitis. Cell Rep. 2018, 24, 3296–3311.e6. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border Patrol: Regulation of Immunity, Inflammation and Tissue Homeostasis at Barrier Surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Pope, J.L.; Bhat, Ajaz.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 Regulates Intestinal Epithelial Homeostasis through the Modulation of Notch Signaling. Gut 2014, 63, 622–634. [CrossRef]
- Bernal-Mizrachi, L.; Lovly, C.M.; Ratner, L. The Role of NF-ΚB-1 and NF-ΚB-2-Mediated Resistance to Apoptosis in Lymphomas. Proc. Natl. Acad. Sci. 2006, 103, 9220–9225. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vidal, A.; Vignard, J.; Mirey, G. Around and beyond 53BP1 Nuclear Bodies. Int. J. Mol. Sci. 2017, 18, 2611. [Google Scholar] [CrossRef] [PubMed]
- Smolkova, B.; El Yamani, N.; Collins, A.R.; Gutleb, A.C.; Dusinska, M. Nanoparticles in Food. Epigenetic Changes Induced by Nanomaterials and Possible Impact on Health. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 77, 64–73. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food Additives Containing Nanoparticles Induce Gastrotoxicity, Hepatotoxicity and Alterations in Animal Behavior: The Unknown Role of Oxidative Stress. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 146, 111814. [Google Scholar] [CrossRef] [PubMed]
- ANSES- French Agency for Food, Environmental and Occupational Health & Safety, France; Anastasi, E.; Riviere, G.; Teste, B. Nanomaterials in Food - Prioritisation & Assessment. EFSA J. Eur. Food Saf. Auth. 2019, 17, e170909. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The Current State of Animal Models in Research: A Review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Arnold, E.T.; Thomas, L.S.; Gonsky, R.; Zhou, Y.; Hu, B.; Arditi, M. TLR4 and MD-2 Expression Is Regulated by Immune-Mediated Signals in Human Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 20431–20437. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Fukata, M.; Thirunarayanan, N.; Martin, A.P.; Arnaboldi, P.; Maussang, D.; Berin, C.; Unkeless, J.C.; Mayer, L.; Abreu, M.T.; et al. Toll-Like Receptor Signaling in Small Intestinal Epithelium Promotes B-Cell Recruitment and IgA Production in Lamina Propria. Gastroenterology 2008, 135, 529–538.e1. [Google Scholar] [CrossRef]
- Daghero, H.; Doffe, F.; Varela, B.; Yozzi, V.; Verdes, J.M.; Crispo, M.; Bollati-Fogolín, M.; Pagotto, R. Jejunum-Derived NF-ΚB Reporter Organoids as 3D Models for the Study of TNF-Alpha-Induced Inflammation. Sci. Rep. 2022, 12, 14425. [Google Scholar] [CrossRef]
- Lee, J.W.; Wang, P.; Kattah, M.G.; Youssef, S.; Steinman, L.; DeFea, K.; Straus, D.S. Differential Regulation of Chemokines by IL-17 in Colonic Epithelial Cells1. J. Immunol. 2008, 181, 6536–6545. [Google Scholar] [CrossRef]
- Warhurst, A.C.; Hopkins, S.J.; Warhurst, G. Interferon γ Induces Differential Upregulation of α and β Chemokine Secretion in Colonic Epithelial Cell Lines. Gut 1998, 42, 208–213. [Google Scholar] [CrossRef]
- Bradford, E.M.; Ryu, S.H.; Singh, A.P.; Lee, G.; Goretsky, T.; Sinh, P.; Williams, D.B.; Cloud, A.L.; Gounaris, E.; Patel, V.; et al. Epithelial TNF Receptor Signaling Promotes Mucosal Repair in Inflammatory Bowel Disease. J. Immunol. 2017, 199, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vanuytsel, T.; Farré, R.; Verstockt, S.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Schuit, F.; Vermeire, S.; Arijs, I.; et al. Genetic and Transcriptomic Basis of Intestinal Epithelial Barrier Dysfunction in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like Receptors and Their Role in Gut Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Beaumont, M.; Lencina, C.; Painteaux, L.; Viémon-Desplanque, J.; Phornlaphat, O.; Lambert, W.; Chalvon-Demersay, T. A Mix of Functional Amino Acids and Grape Polyphenols Promotes the Growth of Piglets, Modulates the Gut Microbiota in Vivo and Regulates Epithelial Homeostasis in Intestinal Organoids. Amino Acids 2022, 54, 1357–1369. [Google Scholar] [CrossRef]
- Noah, T.K.; Donahue, B.; Shroyer, N.F. Intestinal Development and Differentiation. Exp. Cell Res. 2011, 317, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Dong, L.; Liu, C.; Su, L.; Guo, R.; Luo, Q.; Gan, B.; Cao, F.; Wang, Y.; et al. Perturbation of Intestinal Stem Cell Homeostasis and Radiation Enteritis Recovery via Dietary Titanium Dioxide Nanoparticles. Cell Prolif. n/a, e13427. [CrossRef]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Díaz-Urbina, D.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; González, M.I.; Reyes, J.L.; Villamar-Duque, T.E.; Flores-Sánchez, M.LO.; Hernández-Pando, R.; et al. Food-Grade Titanium Dioxide (E171) Induces Anxiety, Adenomas in Colon and Goblet Cells Hyperplasia in a Regular Diet Model and Microvesicular Steatosis in a High Fat Diet Model. Food Chem. Toxicol. 2020, 146, 111786. [Google Scholar] [CrossRef]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carriere, M. The Food Additive E171 and Titanium Dioxide Nanoparticles Indirectly Alter the Homeostasis of Human Intestinal Epithelial Cells in Vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic Effects of the Food Additives Titanium Dioxide and Silica on the Murine Intestinal Tract: Mechanisms Related to Intestinal Barrier Dysfunction Involved by Gut Microbiota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-Term Exposure to Titanium Dioxide Nanoparticles Promotes Diet-Induced Obesity through Exacerbating Intestinal Mucus Layer Damage and Microbiota Dysbiosis. Nano Res. 2021, 14, 1512–1522. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Løhr, M.; Sheykhzade, M.; Lykkesfeldt, J.; Wils, R.S.; Loft, S.; Møller, P. Telomere Length and Genotoxicity in the Lung of Rats Following Intragastric Exposure to Food-Grade Titanium Dioxide and Vegetable Carbon Particles. Mutagenesis 2019, 34, 203–214. [Google Scholar] [CrossRef]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol Decreases Colonic Permeability through Oestrogen Receptor β-Mediated up-Regulation of Occludin and Junctional Adhesion Molecule-A in Epithelial Cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef]
- Braniste, V.; Jouault, A.; Gaultier, E.; Polizzi, A.; Buisson-Brenac, C.; Leveque, M.; Martin, P.G.; Theodorou, V.; Fioramonti, J.; Houdeau, E. Impact of Oral Bisphenol A at Reference Doses on Intestinal Barrier Function and Sex Differences after Perinatal Exposure in Rats. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, Q.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Han, J.; Jia, R.; Liu, X.; Xi, Z. Intestinal Toxicity of Micro- and Nano-Particles of Foodborne Titanium Dioxide in Juvenile Mice: Disorders of Gut Microbiota–Host Co-Metabolites and Intestinal Barrier Damage. Sci. Total Environ. 2022, 821, 153279. [Google Scholar] [CrossRef] [PubMed]
- Paassen, N.B.; Loonen, L.M.P.; Witte-Bouma, J.; Male, A.M.K.; Bruijn, A.C.J.M. de; Sluis, M. van der; Lu, P.; Goudoever, J.B.V.; Wells, J.M.; Dekker, J.; et al. Mucin Muc2 Deficiency and Weaning Influences the Expression of the Innate Defense Genes Reg3β, Reg3γ and Angiogenin-4. PLOS ONE 2012, 7, e38798. [Google Scholar] [CrossRef] [PubMed]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.-H.; Walker, V.K. Impact of Food Grade and Nano-TiO2 Particles on a Human Intestinal Community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of Foodborne Inorganic Nanoparticles on the Gut Microbiota-Immune Axis: Potential Consequences for Host Health. Part. Fibre Toxicol. 2020, 17, 19. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-Mediated Genotoxicity Induced by Titanium Dioxide Nanoparticles in Human Epidermal Cells. Toxicol. In Vitro 2011, 25, 231–241. [Google Scholar] [CrossRef]
- Dorier, M.; Tisseyre, C.; Dussert, F.; Béal, D.; Arnal, M.-E.; Douki, T.; Valdiglesias, V.; Laffon, B.; Fraga, S.; Brandão, F.; et al. Toxicological Impact of Acute Exposure to E171 Food Additive and TiO2 Nanoparticles on a Co-Culture of Caco-2 and HT29-MTX Intestinal Cells. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 845, 402980. [Google Scholar] [CrossRef]
- Kaina, B. DNA Damage-Triggered Apoptosis: Critical Role of DNA Repair, Double-Strand Breaks, Cell Proliferation and Signaling. Biochem. Pharmacol. 2003, 66, 1547–1554. [Google Scholar] [CrossRef]
- Ruiz-Losada, M.; González, R.; Peropadre, A.; Gil-Gálvez, A.; Tena, J.J.; Baonza, A.; Estella, C. Coordination between Cell Proliferation and Apoptosis after DNA Damage in Drosophila. Cell Death Differ. 2022, 29, 832–845. [Google Scholar] [CrossRef]
- Park, S.B.; Jung, W.H.; Kim, K.Y.; Koh, B. Toxicity Assessment of SiO2 and TiO2 in Normal Colon Cells, In Vivo and in Human Colon Organoids. Molecules 2020, 25, 3594. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




