Submitted:
17 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Method
3. Results and Discussion
3.1. Material and Microstructure Characterization
3.2. Density and Hardness
3.3. Electrical conductivity
4. Conclusions
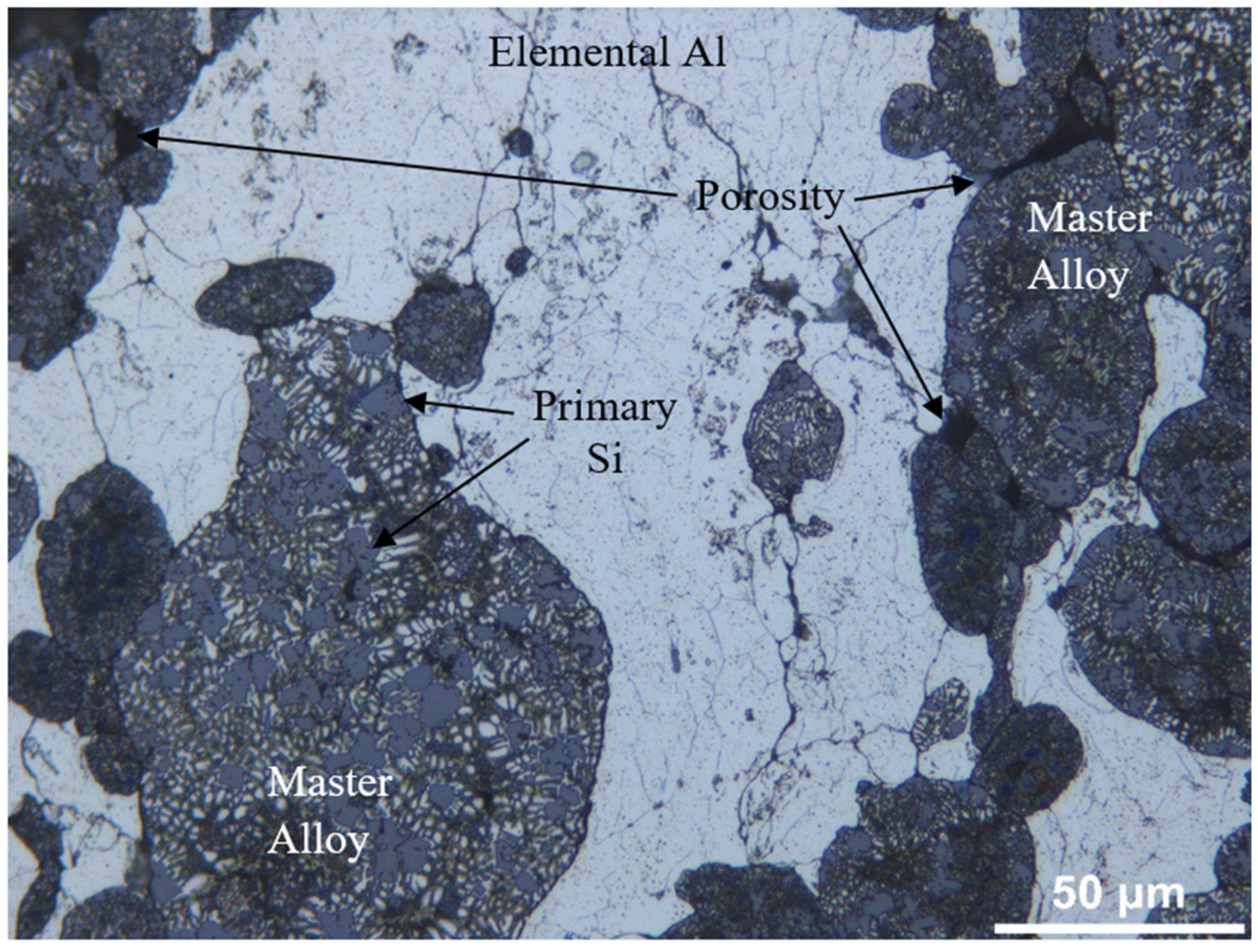
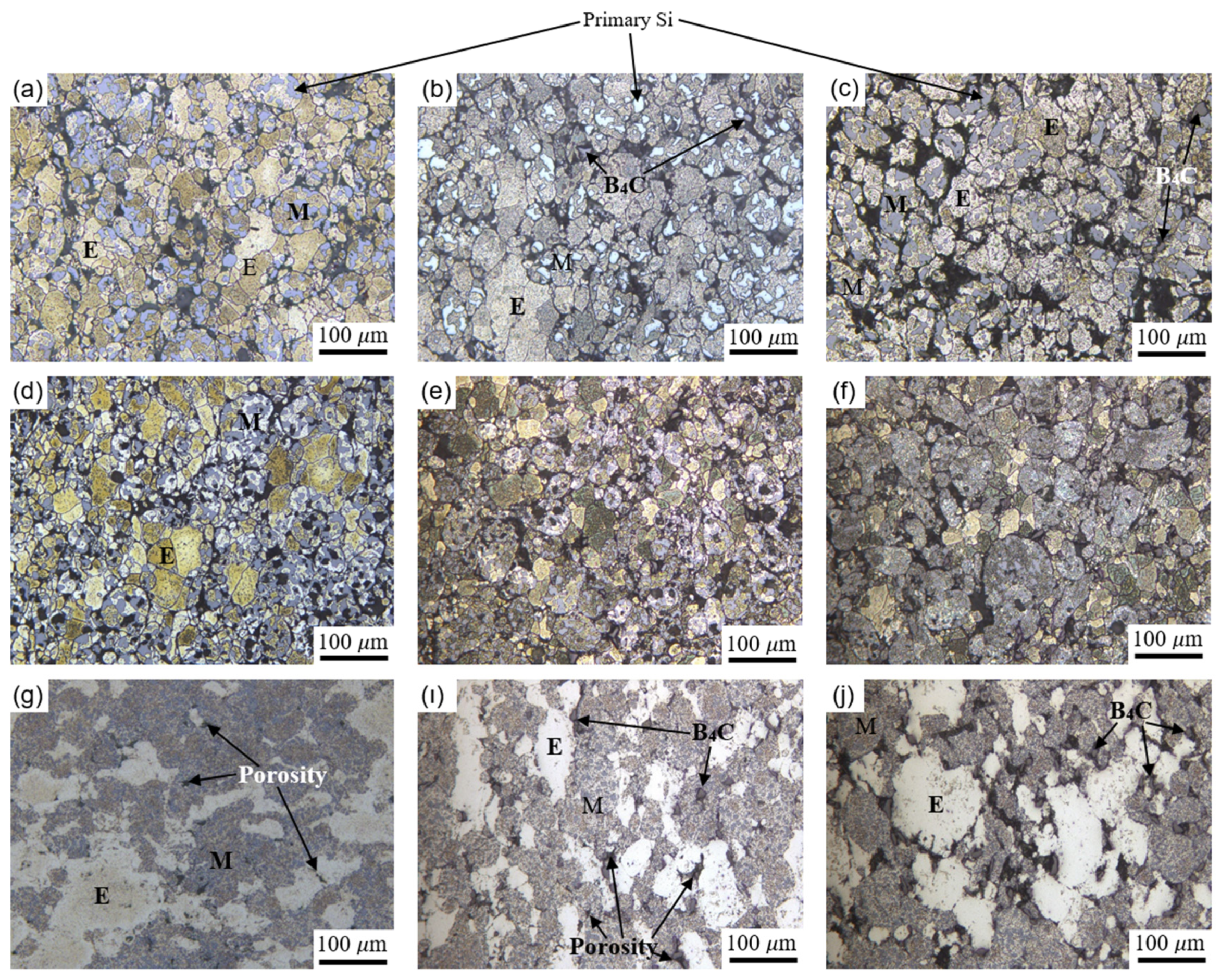
- Depending on the matrix material, the microstructures of all samples consist of elemental Al grains, master alloy grains and primary Si particles. CS and MWS samples gave porosity values close to each other. The superiority of the MWS technique over the CS technique is its high heating rate, short holding time and energy saving.
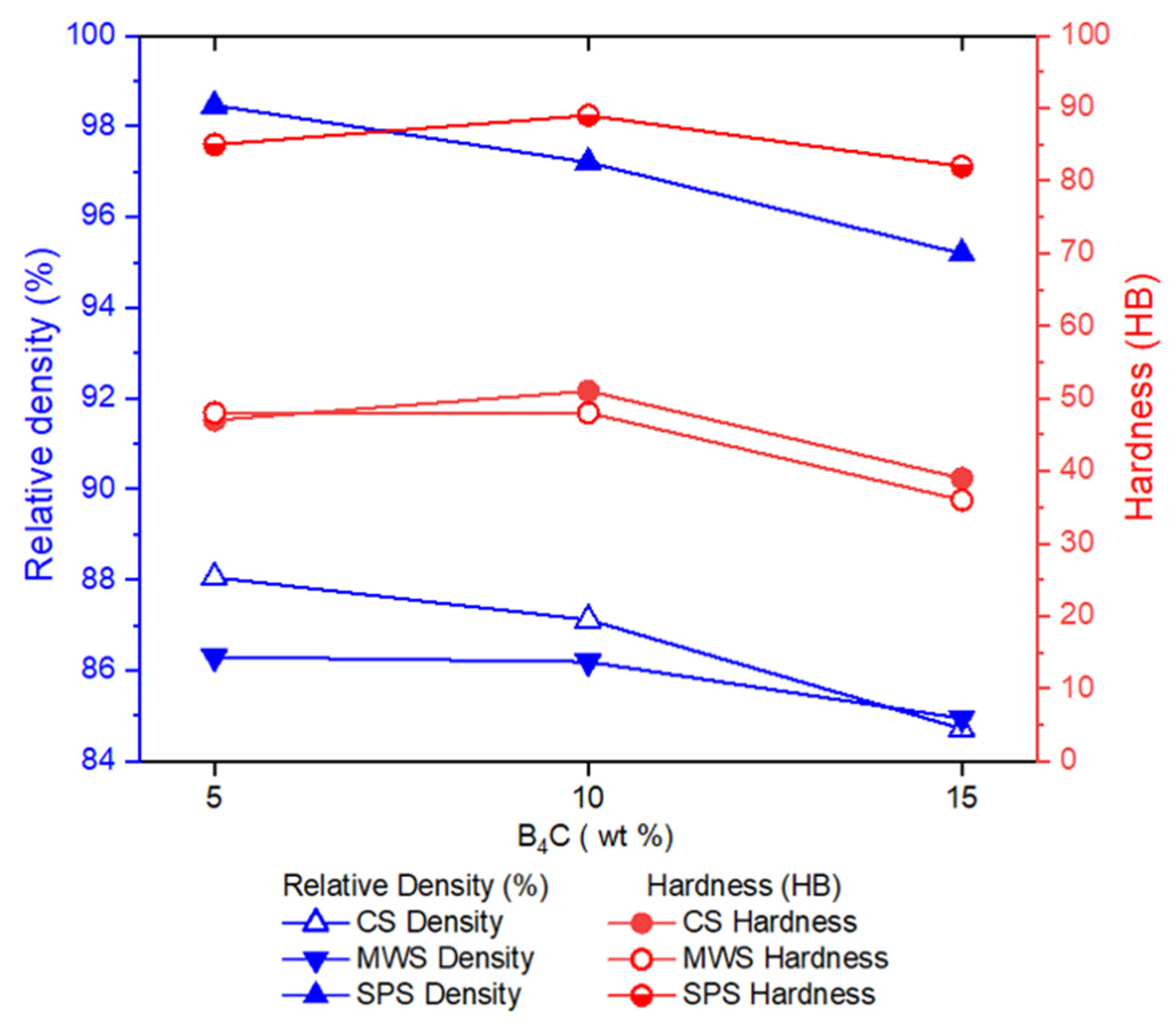
- The addition of 5 wt% B4C did not cause a significant change in the porosity ratio of the samples produced by CS and MWS techniques. The addition of 15 wt% B4C caused the porosity rate to increase in the samples. The samples with the lowest porosity rate, in other words, the samples with the highest density, are the samples produced with SPS. Densities over 95% were obtained in samples produced with SPS. Samples produced with CS and MWS techniques gave similar density values.
- The addition of 5 wt% B4C added to the samples did not cause a significant decrease in density. Compared to pure Alumix® 231 compacts, the highest density decrease was determined in the SPS sample with approximately 1.26% in the samples with 5 wt% B4C added.
- CS and MWS samples gave hardness values that were close to each other and had similar tendencies. The addition of 5 wt% B4C did not cause a significant change in hardness. The addition of 15 wt% B4C caused a 17% and 25% decrease in the hardness of the samples produced with CS and MWS, respectively. Samples produced with SPS technique gave ≈80% higher hardness values than samples produced with CS and MWS.
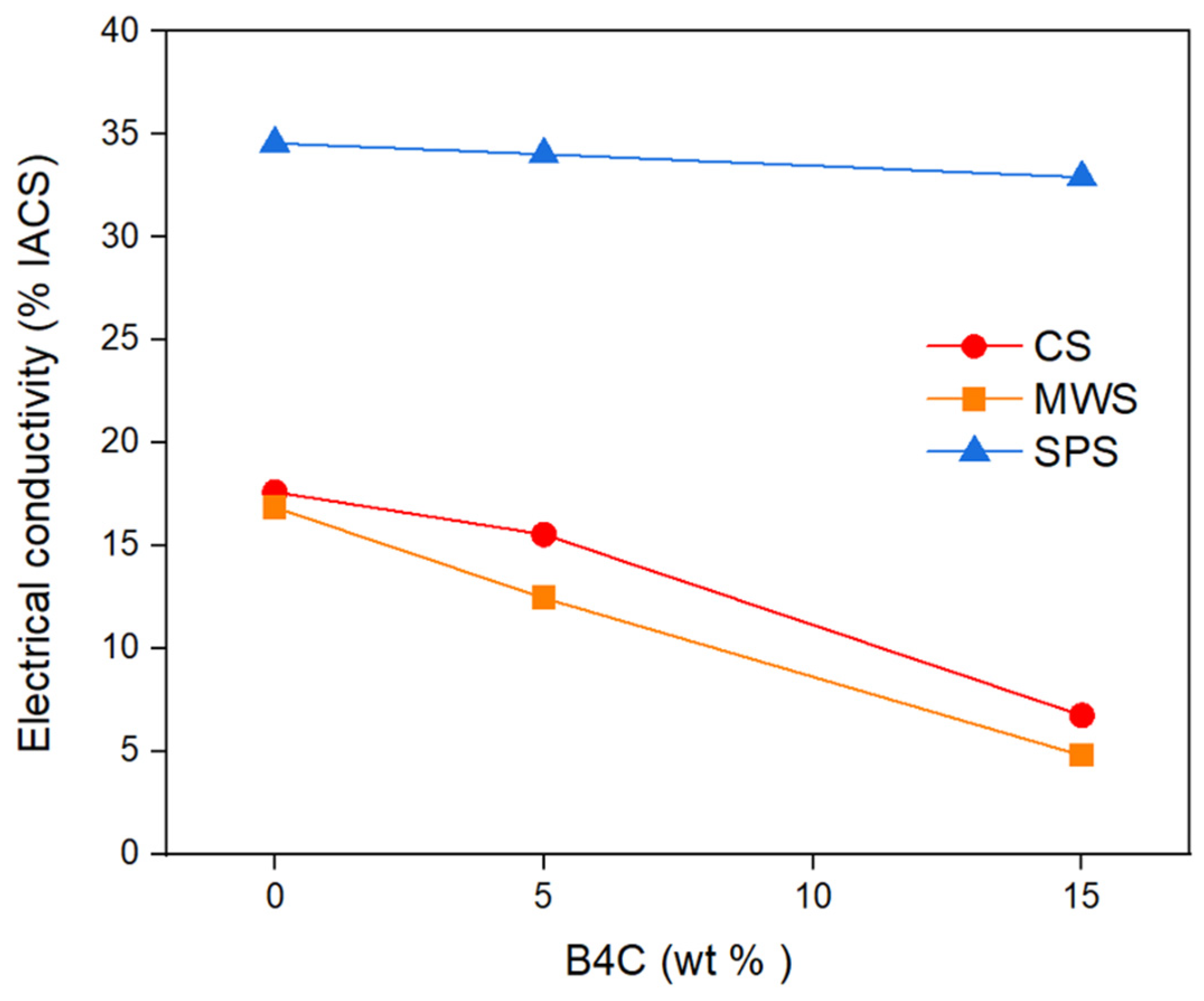
- Samples produced with SPS gave higher electrical conductivity values than samples produced with CS and MWS. Samples produced with CS and MWS gave similar electrical conductivity values due to the close porosity ratio. Porosity in samples produced with MWS is approximately 2% higher than CS coded samples, and electrical conductivity is approximately 4% lower. Electrical conductivities decreased as the weight percentage of B4C increased in all samples.
- P/M samples produced with the SPS technique exhibited better microstructures than MWS and CS samples. Therefore, SPS samples provided very good data in terms of density, hardness and electrical conductivity. MWS and CS samples gave close values in terms of density, hardness and electrical conductivity. Therefore, the fact that the sintering temperature and holding time parameters in the SPS technique are much lower than in the MWS and CS techniques offers significant advantages in terms of energy and time savings. The MWS technique is also superior to the CS technique when the same parameters are taken into account.
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanna, V.; Kumar, V.; Bansal, S.A. Mechanical properties of aluminium-graphene/carbon nanotubes (CNTs) metal matrix composites: Advancement, opportunities and perspective. Mater. Res. Bull. 2021, 138, 111224. [Google Scholar] [CrossRef]
- Puchy, V.; Podobova, M.; Dzunda, R.; Hvizdos, P.; Velgosova, O.; Besterci, M.; Ballokova, B. Graphene nanoplatelets reinforced aluminum alloy matrix composites produced by spark plasma sintering. Kovove Mater. 2021, 59, 237–244. [Google Scholar] [CrossRef]
- Mosher, W.G.E.; Kipouros, G.J.; Caley, W.F.; Donaldson, I.W.; Bishop, D.P. On hot deformation of aluminium–silicon powder metallurgy alloys. Powder Met. 2011, 54, 366–375. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, C.; Wang, W.; Li, X.; Li, H. Evolution of primary Si phase, surface roughness and mechanical properties of hypereutectic Al-Si alloys with different Si contents and cooling rates. Phil. Mag. Lett. 2020, 100, 581–587. [Google Scholar] [CrossRef]
- Kumar, D.; Phanden, R.K.; Thakur, L. A review on environment friendly and lightweight magnesium-based metal matrix composites and alloys Mater. Today-Proc. 2021, 38, 359–364. [Google Scholar] [CrossRef]
- Altuntaş, G.; Altuntaş, O.; Bostan, B. Characterization of Al-7075/T651 alloy by RRA heat treatment and different pre-deformation effects. T. Indian I. Metals, 2021, 74, 3025–3033. [Google Scholar] [CrossRef]
- Altuntaş, G.; Bostan, B. Metallurgical characterization of natural aging effects on pre-deformed Al 7075/T651 alloy during retrogression and re-aging heat treatment. Kov. Mater. 2022, 60, 209–222. [Google Scholar] [CrossRef]
- Kareem, E.; Kudeiri, C.E.; Asarudheen, A.; Thanveer, A.; Ziout, A. A review on AA 6061 metal matrix composites produced by stir casting. Materials. 2021, 14, 175. [Google Scholar] [CrossRef]
- Ahamad, N.; Mohammad, A.; Rinawa, M.L.; Sadasivuni, K.K.; Gupta, P. Correlation of structural and mechanical properties for Al-Al2O3-SiC hybrid metal matrix composites. J. Compos. Mater. 2021, 55, 3267–3280. [Google Scholar] [CrossRef]
- Ozer, M.; Aydogan, S.I.; Cinici, H.; Ozer, A. Effects of sintering techniques and parameters on microstructure and mechanical properties of Al-15Si-2,5Cu-0.5Mg compacts and Al-15Si-2,5Cu-0.5Mg/B4C composites. Mater. Today Commun. 2022, 30, 103192. [Google Scholar] [CrossRef]
- Altuntaş, G.; Özdemir, A.T.; Bostan, B. A survey of the effect of cryogenic treatment and natural ageing on structural changes and second-phase precipitation in Al–Zn–Mg–Cu alloy. J. Therm. Anal. Calorim. 2023, 148, 10713–10725. [Google Scholar] [CrossRef]
- Kang, N.; El Mansori, M. A new insight on induced-tribological behaviour of hypereutectic Al-Si alloys manufactured by selective laser melting. Tribol. International. 2020, 149, 105751. [Google Scholar] [CrossRef]
- Damavandi, E.; Nourouzi, S.; Rabiee, S.M.; Jamaati, R.; Tiamiyu, A.A.; Szpunar, J.A. Effects of prior ECAP process on the dynamic impact behaviors of hypereutectic Al-Si alloy. Mat Sci Eng A-Struct. 2020, 793, 139902. [Google Scholar] [CrossRef]
- Faraji, M. A new approach in numerical modeling of inoculation of primary silicon in a hypereutectic Al-Si alloy. Metall. Mater. Trans. B. 2021, 52, 778–791. [Google Scholar] [CrossRef]
- Saravanana, T.T.; Kamaraj, M.; Sharma, S.C.; Anoop, S.; Manwatkar, S.K.; Ravikanth, K.V.; Venugopal, A.; Kumaran, S. Influence of characteristic eutectic free microstructure on mechanical and corrosion response of spark plasma sintered hypereutectic Al-Si alloy. Mater. Lett. 2022, 308, 131104. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R Rep. 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Kaczmar, J.W.; Pietrzak, K.; Wlosinski, W.J. The production and application of metal matrix composite materials. Mater. Process. Tech. 2000, 106, 58–67. [Google Scholar] [CrossRef]
- German, R.M. Powder metallurgy and particulate materials processing, Metal Powder Industry, Princeton, New Jersey, USA, 2005; pp. 233–274. Princeton, New Jersey, USA.
- German, R.M. Sintering: From empirical observations to scientific principles, Butterwortf-Heinemann, Massachusetts, USA, 2014; pp. 387–408.
- Kang, S-J.L. Sintering densification, grain growth, and microstructure, Butterworth-Heinemann, Oxford, UK, 2005; pp. 197–205.
- Kaplan, Y.; Aksöz, S.; Ada, H.; İnce, E.; Özsoy, S. The effect of aging processes on tribo-metallurgy properties of al based ternary alloys product by P/M technique. Sci. Sinter. 2020, 52, 445–456. [Google Scholar] [CrossRef]
- Ozer, M.; Aydogan, S.I.; Ozer, A.; Cinici, H.; Ayas, E. Influence of spark plasma sintering and conventional sintering on microstructure and mechanical properties of hypereutectic Al-Si alloy and hypereutectic Al-Si/B4C composites. Kov. Mater. 2022, 60, 171–179. [Google Scholar] [CrossRef]
- Ozer, A.; Cetin, H. Correlation of microstructure, hardness, and electrical conductivity of hypereutectic Al-Si/B4C composites manufactured by hot pressing technique and subjected to hot extrusion. Can. Metall. Quart. 2023. [Google Scholar] [CrossRef]
- Pakiela, Z.; Ludwichowska, K.; Ferenc, J.; Kulczyk, M. Mechanical properties and electrical conductivity of Al 6101 and 6201 alloys processed by hydro-extrusion. IOP Conf. Ser-Mat. Sci. Eng. 2014, 63, 012120. [Google Scholar] [CrossRef]
- Allen, P.B.; Butler, W.H. Electrical conduction in metals. Phys. Today. 1978, 31, 44–49. [Google Scholar] [CrossRef]
- Heard, D.W.; Donaldson, I.W.; Bishop, D.P. Metalurgical assesment of a hypereutectic aluminum-silicon P/M alloy. J. Mater. Process. Tech. 2009, 209, 5902–5911. [Google Scholar] [CrossRef]
- Rudianto, H.; Yang, S.; Nam, K-W. ; Kim, Y-J. Mechanical properties of Al-14Si-2.5Cu-0.5Mg aluminum-silicon P/M alloy. Rev. Adv. Mater. Sci. 2011, 28, 145–149. [Google Scholar]
- Su, S.S.; Chang, I.T.H.; Kuo, W.C.H. Effects of processing conditions on the sintering response of hypereutectic Al-Si-Cu-Mg P/M alloy. Mater. Chem. Phys. 2013, 139, 775–782. [Google Scholar] [CrossRef]
- Ozer, A. The microstructures and mechanical properties of Al-15Si-2.5Cu-0.5Mg/(wt%)B4C composites produced through hot pressing technique and subjected to hot extrusion. Mater. Chem. Phys. 2016, 183, 288–296. [Google Scholar] [CrossRef]
- Arribas, I.; Martin, J.M.; Castro. F. The initial stage of liquid phase sintering for an Al–14Si–2.5Cu–0.5Mg (wt%) P/M alloy. Mat. Sci. Eng. A Struct. 2010, 527, 3949–3966. [Google Scholar] [CrossRef]
- Rudinsky, S.; Aguirre, J.M.; Sweet, G.; Milligan, J.; Bishop, D.P.; Brochu, M. Spark plasma sintering of an Al-based powder blend. Mat. Sci. Eng. A Struct. 2015, 621, 18–27. [Google Scholar] [CrossRef]
- Manonukul, A.; Salee, A. Relationship between atmospheric dew point and sinterability of Al-Si based alloy. J. Mater Sci. Technol. 2013, 29, 70–76. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Aqeeli, N.-A.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A review. J. Nanomater. 2012, 983470. [Google Scholar] [CrossRef]
- Shen, Z.; Johnsson, M.; Zhao, Z.; Nygren, M. Spark plasma sintering of alumina. J. Am. Ceram. Soc. 2002, 85, 1921–1927. [Google Scholar] [CrossRef]
- Ozer, M.; Kaplan, Y.; Ozer, A.; Aksoz, S. Influence of different sintering techniques on the wear properties of Al-15Si-2.5Cu-0.5Mg/B4C composites. Sci. Sinter. 2023. [Google Scholar] [CrossRef]
- Pal, H.; Sharma, V. Mechanical, electrical, and thermal expansion properties of carbon nanotube-based silver and silver-palladium alloy composites. Int. J. Min. Met. Mater. 2014, 21, 1132–1140. [Google Scholar] [CrossRef]
- Isfahani, M.J.; Payami, F.; Asadabad, M.A.; Shokri, A.A. Investigation of the effect of boron carbide nanoparticles on the structural, electrical, and mechanical properties of Al-B4C nanocomposites. J. Alloy. Compd. 2019, 797, 1348–1358. [Google Scholar] [CrossRef]
| Alumix® 231 | Al | Si | Cu | Mg | Lubr. Amidwax | |
| Nominal target: | Remainder | 14-16 | 2.4-2.8 | 0.5-0.8 | 1.5 | |
| Experimental: | Remainder | 15.10 | 2.75 | 0.60 | ||
| Compacting Pressure: 620 MPa | Sintering atmosphere: N2 | |||||
| Sintering temperature: 550-560 °C | Dewaxing: 380-420 °C | |||||
| Sintering time: approx. 60 min | ||||||
| Specimen Notation | Materials | Sintering | Sintering Temperature (°C) | Sintering Time (min) |
|---|---|---|---|---|
| CS-555/60 | Alumix 231® | Conventional | 555 | 60 |
| 5-CS-555/60 | Alumix 231® +5wt%B4C | Conventional | 555 | 60 |
| 15-CS-555/60 | Alumix 231® +15wt%B4C | Conventional | 555 | 60 |
| MWS-555/15 | Alumix 231® | Microwave | 555 | 15 |
| 5-MWS-555/15 | Alumix 231® +5wt%B4C | Microwave | 555 | 15 |
| 15-MWS-555/15 | Alumix 231® +15wt%B4C | Microwave | 555 | 15 |
| SPS-450/5 | Alumix 231® | Spark Plasma | 450 | 5 |
| 5-SPS-450/5 | Alumix 231® +5wt%B4C | Spark Plasma | 450 | 5 |
| 15-SPS-450/5 | Alumix 231® +15wt%B4C | Spark Plasma | 450 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




