Submitted:
17 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
2.1. Material
2.1.1. Plant material
2.1.2. Bacterial strains
2.1.3. Material for bacterial cell culture
2.2. Methods
2.2.1. Extraction of the essential oil
2.2.2. Phytochemical analysis
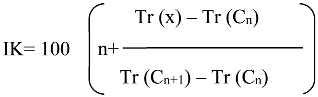
2.2.3. Antibacterial activity
2.3. Statistical analysis
3. Results and discussion
3.1. Results
3.1.1. Extraction of the essential oil
3.1.2. Chemical composition
3.1.3. Antibacterial activity
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorley, K.; Charles, H.; Greig, D.R.; Prochazka, M.; Mason, L.C.E.; Baker, K.S.; Godbole, G.; Sinka, K.; Jenkins, C. Emergence of extensively drug-resistant and multidrug-resistant Shigella flexneri serotype 2a associated with sexual transmission among gay, bisexual, and other men who have sex with men, in England: a descriptive epidemiological study. Lancet Infect. Dis. 2023, 23, 732–739. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Revelas, A. Healthcare-associated infections: A public health problem. Niger. Med. J. 2012, 53, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Emily, R.M.; Sydnor, T.M.P. Hospital epidemiology and infection control in acute-care settings. Clin. Microbiol. Rev. 2011, 24, 141–173. [Google Scholar]
- Anderson, D.J. Surgical site infections. Infect. Dis. Clin. North Am. 2011, 25, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; Dolati, Y.; Ahmadi, N.; Raoofi, N.; Sarhadi, Y.; Masoumi, M.; Sadat Hosseini, B.; Vali, N.; Gholamali, N.; Asadi, S.; Ahmadi, S.; Ahmadi, B.; Beiramy Chomalu, Z.; Asadollahi, E.; Rajabi, M.; Gharagozloo, D.; Nejatifar, Z.; Soheylirad, R.; Jalali, S.; Aghajani, F.; Navidriahy, M.; Deylami, S.; Nasiri, M.; Zareei, M.; Golmohammadi, Z.; Shabani, H.; Torabi, F.; Shabaninejad, H.; Nemati, A.; Amerzadeh, M.; Aryankhesal, A.; Ghashghaee, A. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS One 2023, 18, e0274248. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E. Risk factors in acquiring multidrug-resistant Klebsiella pneumoniae infections in a hospital setting in Saudi Arabia. Sci. Rep. 2023, 13, 11626. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices-an update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Wareth, G.; Neubauer, H. The Animal-foods-environment interface of Klebsiella pneumoniae in Germany: an observational study on pathogenicity, resistance development and the current situation. Vet. Res. 2021, 52, 16–52. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, L.; Hui, J.; Lu, J.; Yang, R.; He, Q.; Tian, N.; Bai, T.; Li, X. Relationship between antibiotic exposure and carbapenem-resistant Klebsiella pneumoniae infection within four types of control patients: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2023, 33, 137–151. [Google Scholar] [CrossRef]
- Moehring, R.; Mahlen, S. Infections due to Serratia species. https://www.uptodate.com/contents/infections-due-to-serratia-species, accessed on 03rd January 2024.
- Williams, D.J.; Grimont, P.A.D.; Cazares, A.; Grimont, F.; Ageron, E.; Pettigrew, K.A.; Cazares, D.; Njamkepo, E.; Weill, F.-X.; Heinz, E.; Holden, M.T.G.; Thomson, N.R.; Coulthurst, S.J. 2022. The genus Serratia revisited by genomics. Nat. Commun. 2022, 13, 5195, 1-18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, J.; Chen, X.; Ren, Y.; Zhao, L. Risk factors and molecular epidemiology of bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2023, 106, 115955. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics (Basel) 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO), 2024. Antimicrobial resistance. Facts Sheets. https://who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=As%20a%20result%20of%20drug,severe%20illness%2C%20disability%20and%20death., accessed 03rd Jan 2024.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–.Carbapenems. 2017 Jan 15.
- Luxton, T.N.; King, N.; Wälti, C.; Jeuken, L.J.C.; Sandoe, J.A.T. A Systematic review of the effect of therapeutic drug monitoring on patient health outcomes during treatment with carbapenems. Antibiotics (Basel) 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-lactams toxicity in the intensive care unit: An underestimated collateral damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: a systematic review. Crit. Care. 2017, 21, 276. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial activity of medicinal plants and their constituents in the context of skin and wound infections, considering European legislation and folk medicine-A review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef]
- Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multiple drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Aurelie, F.D.G.; Pierre, B.N.M.; Ascension, N.M.; Lebel, T.J. Chemical composition and biocide properties of Clausena anisata (Rutaceae) essential oil against developmental stages of the malaria vector Anopheles coluzzii. Am. J. Essent. Oils Nat. Products 2018, 6, 9–15. [Google Scholar]
- Senthilkumar, A.; Venkatesalu, V. Phytochemical analysis and antibacterial activity of essential oil of Clausena anisata (Wild). Hook. F. ex Benth. Int. J. Integr. Biol. 2009, 5, 116–120. [Google Scholar]
- Usman, L.A.; Hamid, A.A.; Olawore, N.O.; Fakunle, C.O.; Oladosu, I.A.; Zubair, M.F. Chemical composition of leaf essential oil of Clausena anisata growing in North Central Nigeria. JASR C. 2010, 6, 891–894. [Google Scholar]
- Makirita, W.E.; Chauka, L.; Chacha, M. Larvicidal activity of Clausena anisata fruits and leaves extracts against Anopheles gambiae Giless., Culex quinquefasciatus Say and Aedes egyptiae. Spatula DD - Peer Reviewed J. Complement. Med. Drug Discover. 2015, 5, 10–5455. [Google Scholar] [CrossRef]
- Sekar, D.K.; Kumar, G.; Karthik, L.; Bhaskara, K.V. A review on pharmacological and phytochemical properties of Aegle marmelos (L.) Corr. Serr. (Rutaceae). Asian J. Plant Sci. Res. 2011, 1, 8–17. [Google Scholar]
- Arbab, I.A.; Abdul, A.B.; Aspollah, M.; Abdullah, R.; Abdelwahab, S.I.; Ibrahim, M.Y.; Ali, L.Z. A review of traditional uses, phytochemical and pharmacological aspects of selected members of Clausena genus (Rutaceae). J. Med. Plant Res. 2012, 6, 5107–5118. [Google Scholar]
- Songue, J.L.; Kouam; Dongo, E.; Mpondo, T.N.; White, R.L. Chemical constituents from stem bark and roots of Clausena anisata. Molecules 2012, 17, 13673–13686. [Google Scholar] [CrossRef]
- Ojewole, J.A. Hypoglycaemic effect of Clausena anisata (Willd, Hook) methanolic root extract in rats. J. Ethnopharmacol. 2012, 81, 231–725. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standard Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition M07 A9. Clinical Laboratory Standard Institute. 2012, 29(2) 2012.
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial activities of the methanol extracts of Canarium schweinfurthii and four other Cameroonian dietary plants against multi-drug resistant Gram-negative bacteria. Saudi J. Biol. Sci. 2016, 23, 565–570. [Google Scholar] [CrossRef]
- Seukep, J.A.; Sandjo, L.P.; Ngadjui, B.T.; Kuete, V. 2016. Antibacterial activities of the methanol extracts and compounds from Uapaca togoensis against Gram-negative multi-drug resistant phenotypes. S. Afr. J. Bot. 2016, 103, 1–5. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef]
- Jung, I.G.; Jeong, J.Y.; Yum, S.H.; Hwang, Y.J. Inhibitory effects of selected medicinal plants on bacterial growth of methicillin-resistant Staphylococcus aureus. Molecules 2022, 27, 7780. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hikal, W.M.; Tkachenko, K.G. Essential oils with potential as insecticidal agents: A review. Int. J. Environ. Plan. Manag. 2017, 3, 23–33. [Google Scholar]
- Yaouba, A.; Tatsadjieu, N.L.; Jazet, P.M.; Etoa, F.X.; Mbofung, C.M. Antifungal properties of essential oils and some constituents to reduce foodborne pathogen. J. Yeast Fungal Res. 2010, 1, 001–008. [Google Scholar]
- Okokon, J.E.; Etebong, E.O.; Udobang, J.A.; Essien, G.E. Antiplasmodial and analgesic activities of Clausena anisata. Asian Pac. J. Trop. Med. 2012, 5, 214–219. [Google Scholar] [CrossRef]
- Njonkep, S.N.J. “Etude Phytochimique d’une Rutacee du Cameroun: Clausena anisata (Will). Hook f.ex.Benth.” [Thesis for Pharm.D] University of the Mountains; 2014”.
- Issakou, 2010. Determination des caractéristiques chimiques, des activités antiradicalaires, anti-inflammatoires et antifongiques des huiles essentielles de C. anisata et de Capsicum annum. [Mémoire de master 2]:, Université de Yaoundé I.; 2010.
- Delamare, A.P.L.; Moschen-Pistorello, I.T. Antibacterial activity of the essential oil of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D. antimicrobial and antioxidant activities of the essential oil and various extractes of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-Samseny, R.R.R.; Hilou, A.; Souza, A.; Dicko, M.H.; M’Batchi, B. Antibacterial activity against β-lactamase producing methicillin and ampicillin-resistants Staphylococcus aureus: fractional inhibitory concentration index (FICI) determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Kramkowski, K.; Ostaszewski, R. Evaluation of antibacterial activity against nosocomial pathogens of an enzymatically derived α-aminophosphonates possessing coumarin scaffold. Int. J. Mol. Sci. 2023, 24, 14886. [Google Scholar] [CrossRef] [PubMed]
- Kuaté Tokam, C.R.; Bisso Ndezo, B.; Boulens, N.; Allémann, E.; Delie, F.; Dzoyem, J.P. Antibiofilm activity and synergistic effects of thymol-loaded poly (Lactic-Co-Glycolic Acid) nanoparticles with amikacin against four Salmonella enterica Serovars. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 7274309. [Google Scholar] [CrossRef] [PubMed]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother. Res. 2006, 20, 371–373. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Eduardo, L.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial activity and time-kill kinetics of positive enantiomer of α-pinene against strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef]
- Brice, R.P.; Boniface, P.K.; Eutrophe Le Doux, K.; Vincent, N.; Yanick, K.M.D.; Paul, K.L.; Fabrice, F.B. Extracts from Cardiospermum grandiflorum and Blighia welwitschii (Sapindaceae) reveal antibacterial activity against Shigella species. S. Afr. J. Bot. 2024, 164, 419–428. [Google Scholar]
- AlBalawi, A.N.; Elmetwalli, A.; Baraka, D.M.; Alnagar, H.A.; Alamri, E.S.; Hassan, M.G. Chemical constituents, antioxidant potential, and antimicrobial efficacy of Pimpinella anisum extracts against multidrug-resistant bacteria. Microorganisms 2023, 11, 1024. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.-I.; Nihei, K.-I. Antimicrobial activity of anethole and related compounds from aniseed. J. Sci. Food Agric. 2008, 88, 242–247. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Masiuk, H.; Mnichowska-Polanowska, M.; Kaczmarek, M.; Giedrys-Kalemba, S.; Dołęgowska, B.; Zielińska-Bliźniewska, H.; Olszewski, J.; Sienkiewicz, M. The effect of fennel essential oil and trans-anethole on antibacterial activity of mupirocin against Staphylococcus aureus isolated from asymptomatic carriers. Postepy Dermatol. Alergol. 2019, 36, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.A.; Alves, M.S.; Santos, B.; Silva, E.V.A.; Araújo, F.S.M.; Bezerra, M.M.S.L.; Silva, P.O.A.; Rêgo, V.G.S.; Pessôa, H.L.F.; Oliveira Filho, A.A. Evaluation of the antibacterial activity of trans-anethole against Enterococcus cloacae and Enterococcus faecalis strains of food origin. Braz. J. Biol. 2023, 83, e269245. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Marei, G.I.K. ; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Phys. 2019, 158, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. 2014. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Roman, H.; Niculescu, A.G.; Lazăr, V.; Mitache, M.M. Antibacterial efficiency of Tanacetum vulgare essential oil against ESKAPE pathogens and synergisms with antibiotics. Antibiotics (Basel) 2023, 12, 1635. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Li, H.; Zhu, L.; Cao, S.; Liu, J. Sesquiterpenes and monoterpenes from the leaves and stems of Illicium simonsii and their antibacterial activity. Molecules 2022, 27, 1115. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef]
- da Silva, L.Y.S.; Paulo, C.L.R.; Moura, T.F.; Alves, D.S.; Pessoa, R.T.; Araújo, I.M.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Nonato, C.F.A.; da Costa, J.G.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Kowalska, G.; Mitura, P.; Bar, M.; Kowalski, R.; Menezes, I.R.A. Antibacterial activity of the essential oil of Piper tuberculatum Jacq. Fruits against multidrug-resistant strains: Inhibition of efflux pumps and β-Lactamase. Plants (Basel) 2023, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.G.; de Freitas, T.S.; Costa, M. do S.; da Silva, A.R.P.; Coutinho, H.D.M.; de Morais, S.M.; Marinho, E.S.; Teixeira, A.M.R.; dos Santos, H.S. Chemical composition, antibacterial and inhibitory activity of the efflux pump of essential oils from Croton piauhiensis Müll. Nutraceuticals 2023, 3, 591–604. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An overview of healthcare associated infections and their detection methods caused by pathogen bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef] [PubMed]

| Species | Strains |
| Bacillus spp. | 3C4 UR Mcc |
| Staphylococcus aureus | 00166 6/14 |
| Pseudomonas aeroginosa | 2’ |
| Klebsiella pneumoniae | Rea Ro2Mcc |
| Staphylococcus epidermis | 3 |
| Serratia spp. | 2C1 UR CB Mcc |
| Escherichia coli | 2.5922 |
| Salmonella typhimurium | O,6,7 HCN |
| Physical properties | Fresh leaves |
|---|---|
| Color | Yellowish brown |
| Consistence | Oily |
| Odor | Aniseed aroma |
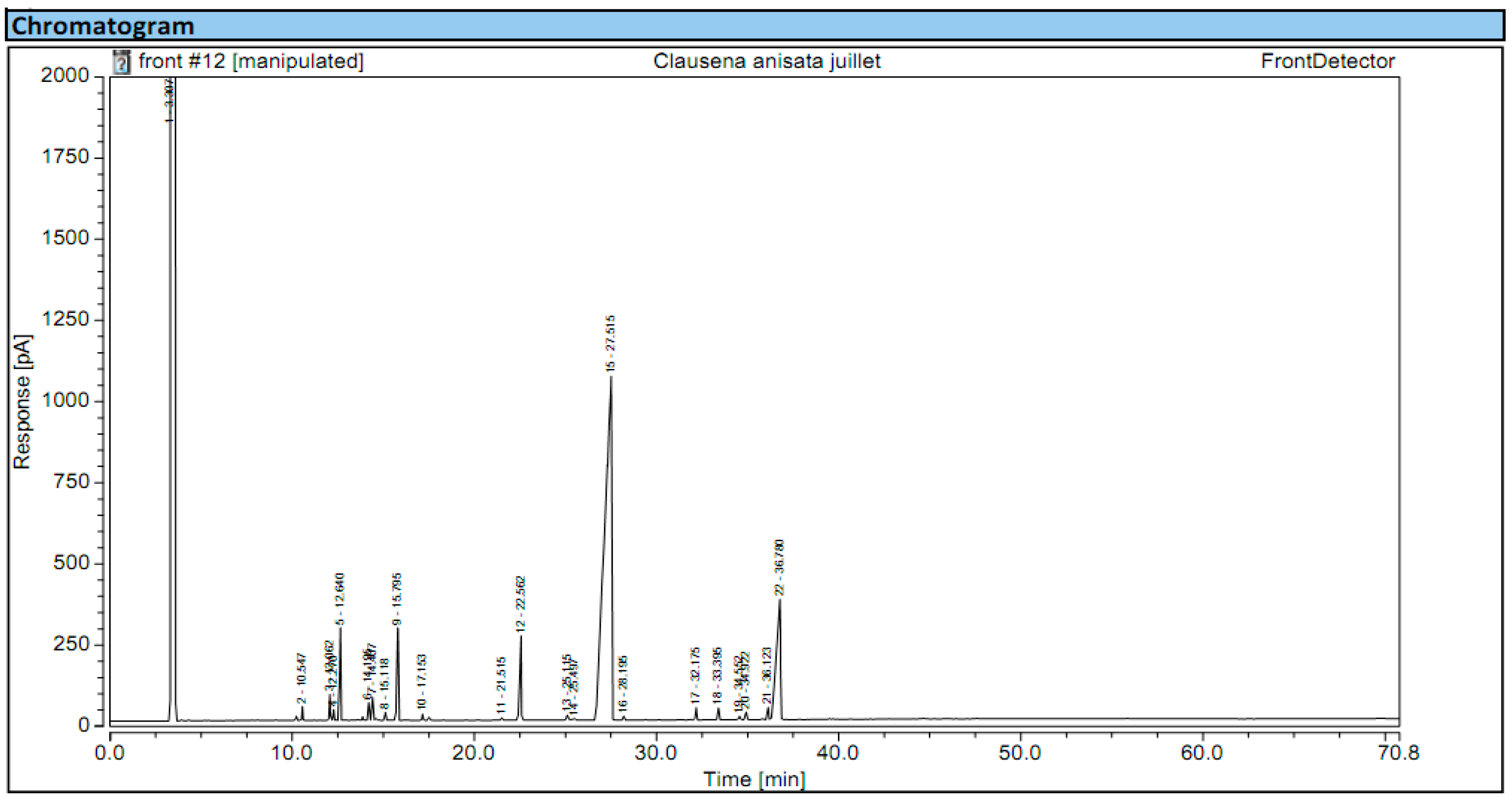
| Serial N° | RT | IK | Identified compound | Percentage (%) |
|---|---|---|---|---|
| 1 | 10.547 | 933 | α –Pinene | 0.26 |
| 2 | 12.062 | 971 | Sabinene | 0.77 |
| 3 | 12.270 | 976 | β –Pinene | 0.26 |
| 4 | 12.640 | 986 | Myrcene | 2.82 |
| 5 | 14.195 | 1021 | p-Cymene | 0.52 |
| 6 | 14.407 | 1025 | Limonene | 0.77 |
| 7 | 15.118 | 1041 | E-β –Ocimene | 0.26 |
| 8 | 15.795 | 1055 | γ –Terpinene | 3.33 |
| 9 | 17.153 | 1084 | Terpinolene | 0.25 |
| 10 | 21.515 | 1171 | α –Terpineol | Nd |
| 11 | 22.562 | 1191 | Estragole | 4.10 |
| 12 | 25.115 | 1242 | para anisaldehyde | 0.25 |
| 13 | 25.497 | 1250 | Z-Anethole | 0.25 |
| 14 | 27.515 | 1291 | E- Anethole | 70.77 |
| 15 | 28.195 | 1305 | Cinamyl alcool | Nd |
| 16 | 32.175 | 1387 | Cinamylacetate | 0.52 |
| 17 | 33.395 | 1413 | Methyleugenol | 0.52 |
| 18 | 34.552 | 1438 | β –Caryophyllene | Nd |
| 19 | 34.922 | 1447 | Isoeugenol | 0.25 |
| 20 | 36.123 | 1473 | γ –Gurjunene | 0.50 |
| 21 | 36.780 | 1487 | Methyl iso eugenol | 13.85 |
| Bacterial strains | MIC (µg/mL) | MBC (µg/mL) | MIC/MBC |
|---|---|---|---|
| Bacillus spp. | 31.25 | 31.25 | 1 |
| Staphylococcus aureus | 15.63 | 15.63 | 1 |
| Klebsiella pneumoniae | 3.91 | 15.63 | 4 |
| Pseudomonas aeroginosa | 62.5 | 62.5 | 1 |
| Staphylococcus epidermidis | 3.91 | 7.81 | 2 |
| Serratia spp. | 3.91 | / | / |
| Escherichia coli | 31.25 | 62.5 | 2 |
| Salmonella typhimurium | 31.25 | 125 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





