Submitted:
18 January 2024
Posted:
19 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and methods
2.1. Experimental material
2.2. Experimental site & Design
| Sl. No. | Treatments | Details of Treatments |
|---|---|---|
| 1. | T1 | Borax @ 0.5% |
| 2. | T2 | Borax @ 0.3% |
| 3. | T3 | ZnSO4 @ 0.75% |
| 4. | T4 | ZnSO4 @ 0.5% |
| 5. | T5 | CaCl2 @ 0.5% |
| 6. | T6 | CaCl2 @ 0.1% |
| 7. | T7 | Humic acid @ 1.5% |
| 8. | T8 | Humic acid @ 1% |
| 9. | T9 | Seaweed extract @ 0.5% |
| 10. | T10 | Seaweed extract @ 0.1% |
| 11. | T11 | NAA @ 20 ppm |
| 12. | T12 | Control (Water Spray) |
2.3. Trait measurement
2.3.1. Determination of fruit and aril weight
2.3.2. Determination of fruit and nut size
2.3.3. Biochemical parameters
2.3.3.1. Determination of Total soluble solids (0Brix)
2.3.3.2. Determination of Titrable Acidity (%)
2.3.3.3. Determination of Total sugars (%)
2.3.3.4. Determination of Reducing sugar (%)
2.3.3.5. Determination of Non-reducing sugar (%)
2.3.3.6. Determination of TSS: acid ratio
2.3.3.7. Determination of Ascorbic acid content (mg/100g)
3.0. Results
3.1. Fruit set (%)
3.2. Fruit drop (%)
3.3. Fruit retention (%)
3.4. Fruit cracking (%)
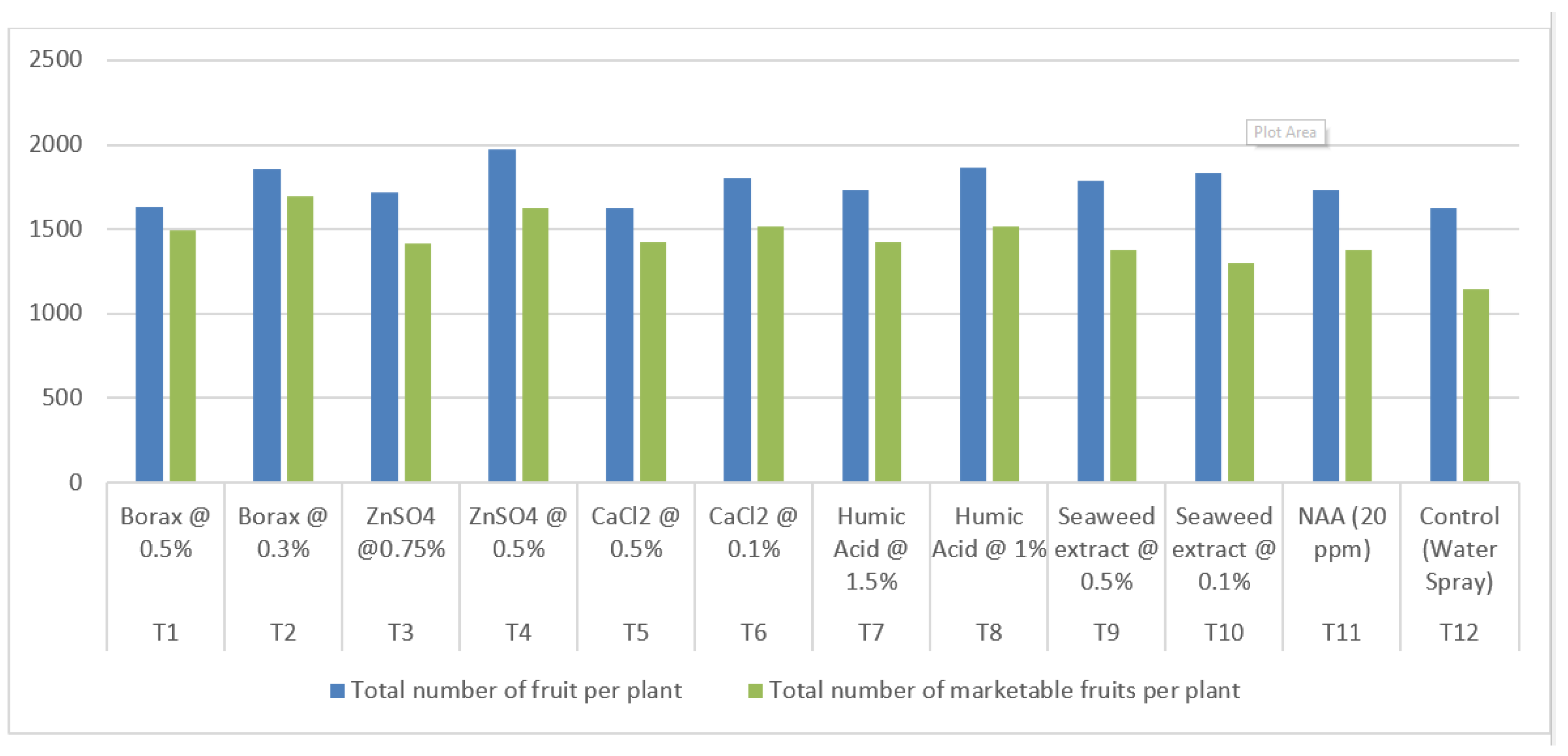
3.5. Total number of fruits per plant
3.6. Total number of marketable fruits per plant
3.7. Total yield per plant
3.8. Total marketable yield
3.9. Physico-Chemical parameters
3.9.1. Fruit length (cm)
3.9.2. Fruit breadth (cm)
3.9.3. Nut length (cm)
3.9.4. Nut breadth
3.9.5. Fruit weight
3.9.6. Aril weight
3.9.7. Seed weight
3.9.8. Pericarp weight
3.9.9. Aril: seed ratio
3.9.10. Aril: Pericarp ratio
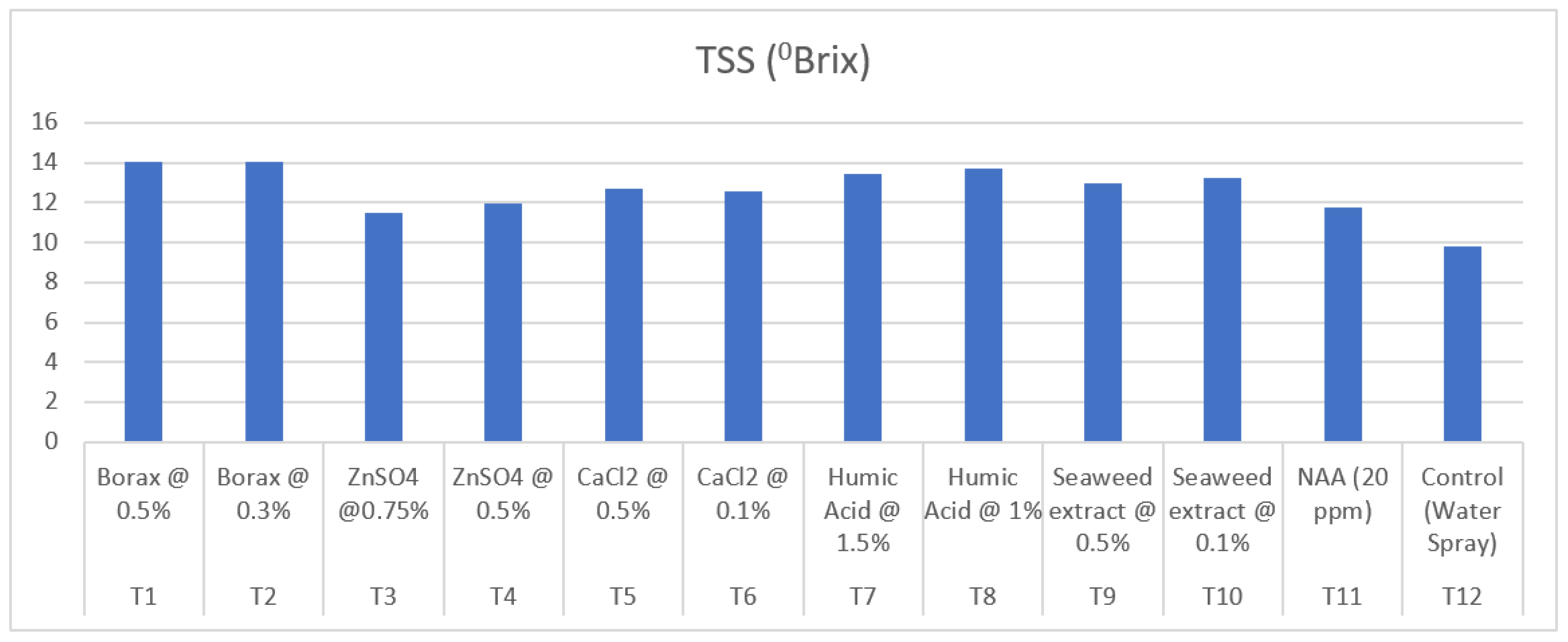
3.9.11. Total Soluble Solids (0Brix)
3.9.12. Titratable acidity (%)
3.9.13. Total sugar (%)
3.9.14. Reducing sugar (%)
3.9.15. Non-reducing sugar (%)
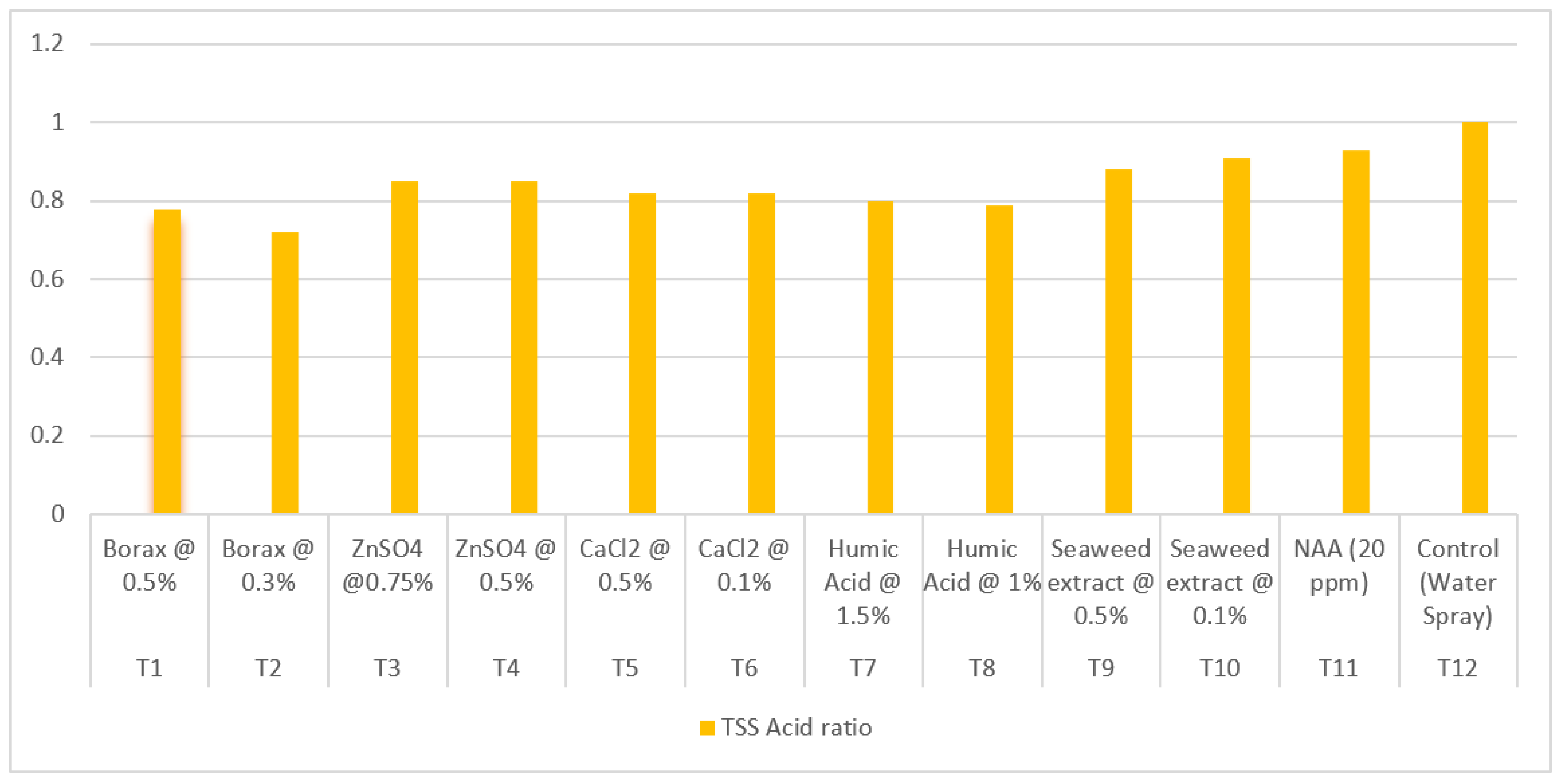
3.9.16. TSS: acidity ratio
3.9.17. Ascorbic content (mg/100 g)
4.0. Discussion
5.0. CONCLUSION
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menzel, C.M.. In: Plant resources of South East Asia. No.2. Edible Fruit and Nuts (Eds. Verheij EWM and Coronel RE), 1991, Pudoe, Wageningen.;191.
- Kumari, P.; Kumar,P.; Karuna, K.;Aftab A, Kumar A and Ahmad F.. Effect of Pre-harvest Salicylic Acid Spray on Shelf Life and Biochemical Changes of Litchi during Storage. Current Journal of Applied Science and Technology, 2023, 42 (7): 59-65.
- Marschner H.. Mineral nutrition of higher plants. Academic Press Limited Harcourt Brace and Company, Publishers, 2012,London, 347-364.
- Singh. O.P.;Phogat, K.P.S.Effect of plant growth regulators on fruit drop, size and quality of litchi cv. Calcuttia. Punjab Horticulture Journal. 1984. 24(1-4):83-88.
- Menzel, C.M.; Simpson, D.R. Lychee nutrition: A review. Scientia Horticulture, 1987, 31:195-224.
- Davarpanaha, S.; Tehranifara,A,;Abadíab,J,;Valb,J,;Davarynejada, G,;Aranc,M.; Khorassanid R..Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). 2018. Scientia Horticulturae,230, 86–91.
- Ram, R.A.;Bose,T.K.Effect of foliar application of of magnesium and micronutrients on growth, yield and fruit quality mandarin orange (Citrus reticulata Blanco.). Indian Journal of Horticulture. 2000. 57, 215-220.
- Priyadarshi,V.;Hota,D.; Karna,A.K.Effect of Growth Regulators and Micronutrients Spray on Chemical Parameters of Litchi (Litchi chinensis Sonn.) cv. Calcuttia, International Journal of Economic Plants, 2018. 5,:99-103.
- Nardi,S.; Schiavon, M.; Francioso, O.Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules. 2021. 26,2256.
- Yang, F.; Tang, C.; Antonietti, M.. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021. 50, 6221–6239.
- Begum, M.;Bordoloi,B.C.;Singha, D.D.; Ojha, N.J.. Role of seaweed extract on growth, yield and quality of some agricultural crops: A review. Agricultural Reviews, 2018. 39,321-326.
- Khan,A.S,;Ahmad, B,; Jaskani,M.J,; Ahmad,R.;Malik,A.U.Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physico-chemical properties of grapes. Int. J. Agric. Biol. 2012.14,383–388.
- Mosa, W.F.; Sas-Paszt, L.; Górnik, K.; Ali, H.M.; Salem, M.Z. Vegetative growth, yield, and fruit quality of guava (Psidium guajava L.) cv. maamoura as affected by some biostimulants. Bioresources, 2021. 16, 7379–7399.
- Ashour, M.; El-Shafei, A.A.; Khairy, H.M.; Abd-Elkader, D.Y.; Mattar, M.M.; Alataway, A.; Hassan, S.M. Effect of Pterocladia capillacea seaweed extracts on growth parameters and biochemical constituents of Jew’s Mallow. Agronomy, 2020.10,420.
- Sarkar,G.K.; Sinha,M.M.;Misra,R.S.Effect of NAA on fruit set, fruit drop, cracking, fruit size and quality in litchi cv. Rose Scented. Progressive Horticulture, 1984.16, 301-304.
- Bhat,S.K.;Raina,B.L.; Chogtu,S.K.; Muthoo,A.K..Effect of exogenous auxin application on fruit drop and cracking of litchi (Litchi chinensis Sonn.) cv. Dehradun. Advances in Plant Sciences. 1997.10, 83-86.
- Brahmachari,V.S.;Rani,R. Effect of growth substances on fruit drop, yield and physico-chemical composition of litchi fruits. Progressive Horticulture, 2001. 32: 50-55.
- Awasthi,R.P.;Tripathi,B.R.;Singh, A. Effect of foliar sprays of zinc on the fruit drop and quality of the litchi. Punjab Horticulture Journal. 1975. 15 :14-16.
- Menzel,C.M,;Simpson,D.R..Lychee cultivars: description and performance of major litchi cultivars in sub-tropical Queensland. Queensland Agricultural Journal, 1986. 112:126-136.
- Kanwar,J.S.;Njjar, G.S.Litchi cultivation in the Punjab problem and prospect. Punjab Horticulture Journal 1975.15:9-13.
- Ahmed,Y.M.; Shalaby, E.A.Effect of different seaweed extracts and compost on vegetative growth, yield and fruit quality of cucumber. J. Hortic. Sci. Ornam. Plants 2012. 4: 235–240.
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B.M.Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni Suef Univ. J. Basic Appl. Sci.2018.7, 104–110.
- AOAC International.A.O.A.C. Official methods of analysis. In Association of Official Analytical Chemists, 20th ed.; Benjamin Franklin Station: 2016.Washington, DC, USA,.
- Priyadarshi,V.;Mehta,K,; Hota, D,; Mishra, G.; Jogur,A,Effect of growth regulators and micronutrients spray on vegetative growth of litchi (Litchi chinensis Sonn.) cv.Calcuttia. Agriculture Update 2018.12: 707-712.
- Rangana, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products. II edn. Tata McGraw-Hill Publ. Co., 1986.New Delhi.
- Brijpal,B.;Tiwari,J.P.;Mishra,K.K.;Lal,S.Effect of Zinc Sulphate on Vegetative Growth, Yield and Leaf Nutrient Status of Guava (Psidium guajava L.), Int.J.Curr.Microbiol.App.Sci., 2020. 9:1265-1273.
- Sharma, R.K.; Thakur, S.;Kumar, R. A note on the effect of foliar feeding of zinc and GA on fruiting of guava trees (Psidium guajava L.). Haryana J. Hort. Sci. 1993. 22: 207 – 208.
- Nurbhanje,K.H.; Varu, D.K.. Effect of biostimulant and biofertilizers on flowering and fruiting of pomegranate (Punica granatumL.) cv.Bhagwa. International Journal of Chemical Studies, 2019. 7: 378-382.
- Bybordi, A.; Ebrahimian, E.Growth, yield and quality components of canola fertilized with urea and zeolite. Commun. Soil Sci. Plant Anal. 2013. 44, 2896–2915.
- Rana, V.S.;Sharma,V.;Sharma,S.; Rana,N.;Kumar,V.;Sharma,U.;Almutairi,K.R.;Avila-Quezada, G.D.;Abd_Allah,E.F.; Gudeta, K.).Seaweed Extract as a biostimulant agent to enhance the fruit Growth, yield, and quality of Kiwifruit. Horticulturae, 2023, 9, 432.
- Margal, P.B.;Thakare, R.S.;Kamble,B.M.;Patil,V.S.;Patil, K.B.; Titirmare, N.S..Effect of Seaweed Extracts on Crop Growth and Soil: A Review. Journal of Experimental Agriculture International, 2023. 45, 9–19.
- El-Khawaga, A.S.Partial replacement of mineral N fertilizers by Using humic acid and Spirulina platensis algae biofertilizer in Florida Prince peach orchards. Middle East J. Appl. Sci., 2011. 1: 5-10.
- Fathi, M.A.; Eissa, F.M.; Yehia, M.M.. Improving growth, yield and fruit quality of Desert Red peachand Anna apple by using some biostimulants. Minia J. Agric. Res. Develop., 2002. 22,519-534.
- Kabeel, H.;Abd F.M. El- Atif,; Baza, M.S.M.Growth, fruiting and nutritional status of "Le-Conte"pear trees in response to mineral and humate fertilizers. Annals of Agric. Sci. Moshtohor, 2008. 46, 139-156.
- Parr,A.J.;Loughman,B.C.;Boron and membrane functions in plants. In :‘Metals and micronutrients: Uptake and utilization by plants’ (Annu. Proc. Phytochem. Soc. Eur. No. 21; D.A. Roff and W.S. Pirepoint, eds.).Academic press, 1983,London, 87-107.
- Rajput,C.B.S.;Chand, S.Significance of boron and zinc in guava (Psidium guajava L.). Bangladesh Hort. 1975. 3 : 22-27.
- Singh, S.; Singh, J.P. Effect of foliar sprays of NAA and boron on flowering, fruiting, fruit retention and yield of litchi (Litchi chinensis Sonn.). International Journal of Chemical Studies. 2019. 7:1995-1999.
- Russel,D.A.. Boron and Soil fertility. In the year book of Agriculture, U.S.D.A. 1957.Washington D.C.
- Banyal, A.K.; Rangra,A.K. Response of yield and quality attributes of litchi cv. Dehradun to soil and foliar application of boron. Journal of Hill Agriculture, 2011. 2:33-37.
- Dugger,W.M.Boron in plant metabolism In: Lalichli, A. and Bieleski, R.L. (eds.), Encyclopedia of Plant Physiology, 1968.Springer Verlag 5 B: 628-650.
- Sharma, N.; Belsare, C. Effect of Plant Bio-Regulators and Nutrients on Fruit Cracking and Quality in Pomegranate (Punica granatum L.) ‘G-137’ in Himachal Pradesh. Acta Horticulturae, 2011, 890, 347-352.
- Brahmachari, V.S. and Kumar, R. Effect of foliar sprays of mineral nutrients on fruit set retention and cracking in litchi (Litchi chinensis Sonn.) fruits. Haryana Journal of Horticultural Sciences, 1997, 26,177-180.
- Babu, N., Singh, A.R. and Babu, N.. Effect of micronutrients spray on fruit cracking and fruit maturity in litchi (Litchi chinensis Sonn.) fruits. Indian Agriculturist, 2002.46, 203-204.
- Khattab MM, Shaban AE, El-Shrief A, Mohamed A.. Effect of humic acid and amino acids on pomegranate trees under deficit irrigation. I: Growth, flowering and fruiting. Journal of Horticultural Science and Ornamental Plants. 2012. 4:253−59.
- Omar AH, Abdelall AH.. Influence of sulphuric acid, humic acid, sulphur and irrigation water on growth and productivity of Superior seedless vines grown under saline condition, Journal of Agricultural Science, Mansoura University. 2005.30:6951−61.
- Ismail, M.; Wanden, M.T.; EI-Sheikh, M..Response of ‘Thompson seedless’ and ‘Roomy Red’ grape cultivars to foliar sprays with yeast extract and GA. J. Agric. Sci. , 2003. 28, 6321–6334.
- Sanchez-Sanchez A, Sanchez-Andreu J, Jorda J, Bermudez D..Humic substances and amino acids improve effectiveness of chelate Fe EDDHA in lemon trees. Journal of Plant Nutrition. 2002, 25:2433−42.
- Kumar, M., Kumar, R. and Singh, R.P. Effect of micronutrients and plant growth regulators on fruiting of litchi. International Journal of Agricultural Sciences, 2009 5:521-524.
- Xu, W.P., Wang, L., Yang, Q., Wei, Y.H., Zhang, C.X. and Wang, S.P.Effect of Calcium and Boron on the Quality of Kiwifruit. Acta Horticulturae, 2015.1096,317-320.
- Gaur,B.;Beer,K.;Hada, T.S.;Kanth, N.;Syamal, M.M. Studies on the effect of foliar application of nutrients and GA3 on fruit yield and quality of winter Season Guava. The Ecoscan. 2014,6:479-483.
- Singh N, Kaur A, Gill BS.Effect of foliar application of zinc and boron on yield and fruit quality of litchi cv. Dehradun.. International Journal of Development Research, 2016, 6:8686-8688.
- Haq I, Rab A and Sajid M.Foliar application of calcium chloride and borax enhance the fruit quality of litchi cultivars. Animal and Plant science, 2013, 23: 1385-1390.
- 53. Li J G, Huang HB, Gao FF, Huang XM and Wang HC. An overview of litchi fruit cracking. Acta Horticulturae, 2008, 558, 205-208.
- Kazuhiro, I., Masashi, M. and Hiroyuki, F.The effect of spraying of calcium to the fruit quality the quality keeping period and the tree vigour of Kousui in green house. Bulletin Saga Prefectural Fruit Tree Exp. Sta., 2004, 15: 8-14.
- Singh, R., Godara, N.R., Singh, R., and Dahiya, S.S.Responses of foliar application of growth regulators and nutrients on ber cv. Umran. Haryana J. Hort. Sci., 2001, 30, 161-164.
- Bhusan,L.P.;Panda,C.;Dash,A.K.Effect of pre-harvest chemical treatments and mulching on marketability of mango (Mangifera indica L.) cv. Amrapali. Journal Crop and Weed, 2015.11: 216-219.
- Lal, R.L., Shukla, P. and Pandey, C.Response of plant growth regulators and mineral nutrients on fruit yield and quality of litchi (Litchi chinensis Sonn.) cv. Rose Scented. Progressive Horticulture, 2010.42: 217-219.
- Navjot Singh Amarjeet Kaur and Bikramjit Singh Gill.. Effect of foliar application of zinc and boron on yield and fruit quality of litchi cv. Dehradun, International Journal of Development Research, 2016,.6: 8686-8688.
- Brahmachari, V.S., Yadav, G.S. and Kumar, N.. Effect of foliar feeding of calcium, zinc and boron on field and quality attributes of litchi (Litchi chinensis Sonn.). The Orissa Journal of Horticulture, 1997, 25: 49-52.
- Alila, P. and Achumi, I.. Pre-harvest chemical treatments affect post-harvest quality of litchi fruit. Acta Horticulturae, 2012,934: 755-762.
- Misra, A.R. and Khan, I. Trichlorophenoxy acetic acid and micronutrient on fruit size, cracking, maturity quality of litchi cv. Rose Scented. Progressive Horticulture, 1981.13: 87-90.
- Soppelsa, S.; Kelserer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production. Effects on tree growth, yield and fruit quality at harvest and during storage. Front. Plant Sci., 2018. 9: 1342.
- Nath, S., Kumar, M., Ojha, O.K. and Jha, K.K.Yield and physico-chemical properties of litchi fruits as affected by different rates of pruning and chemical spray. Progressive Horticulture, 2012, 44: 166-169.
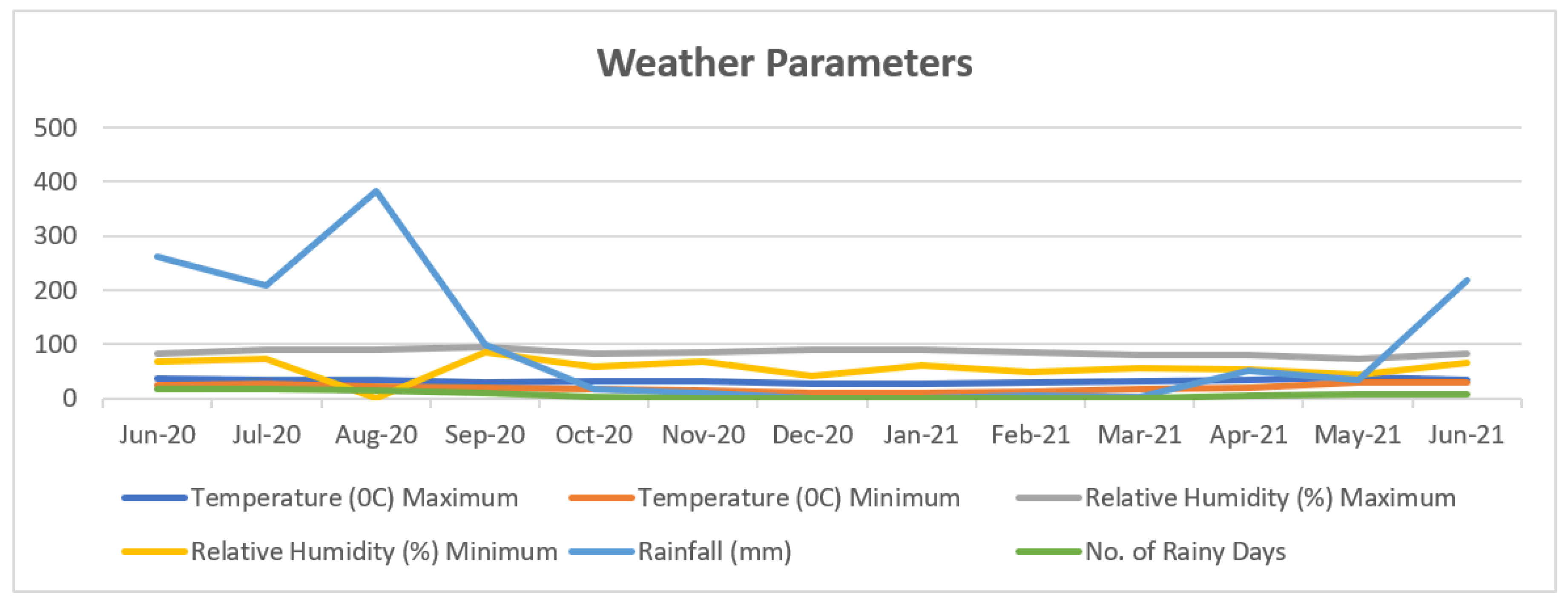
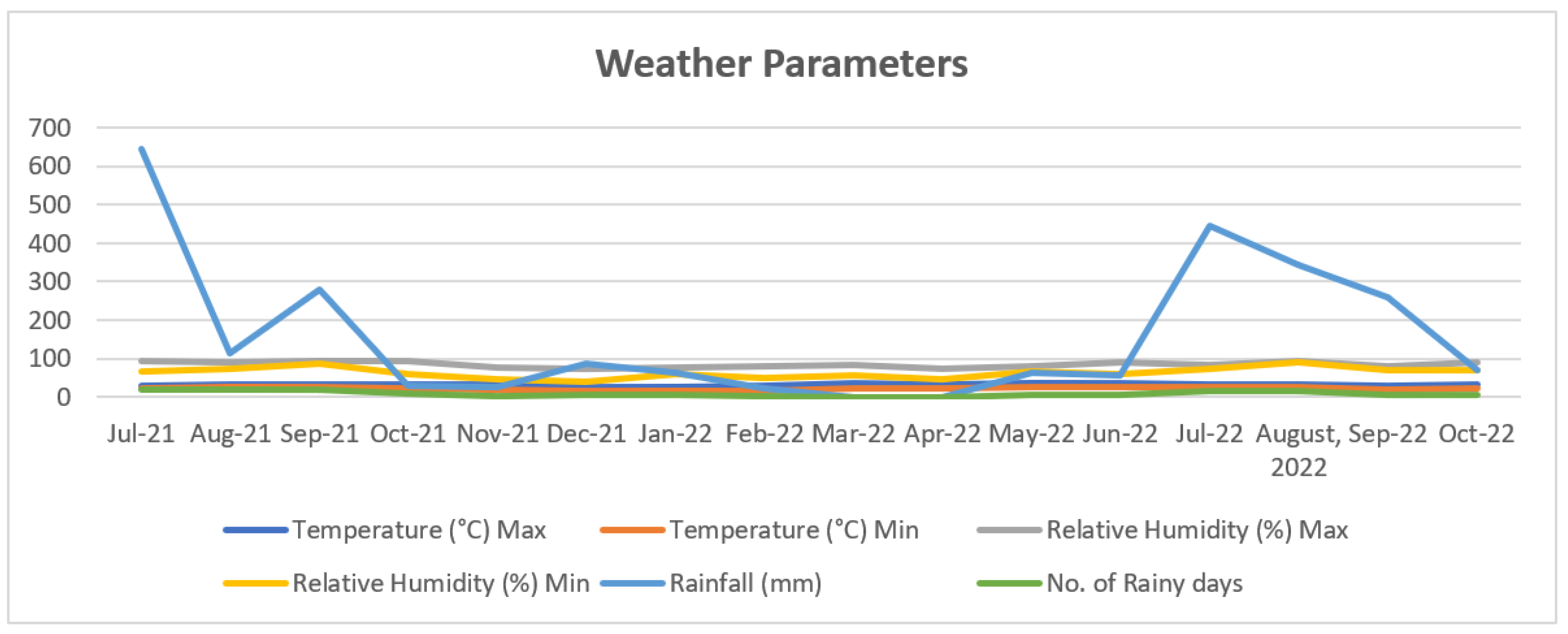
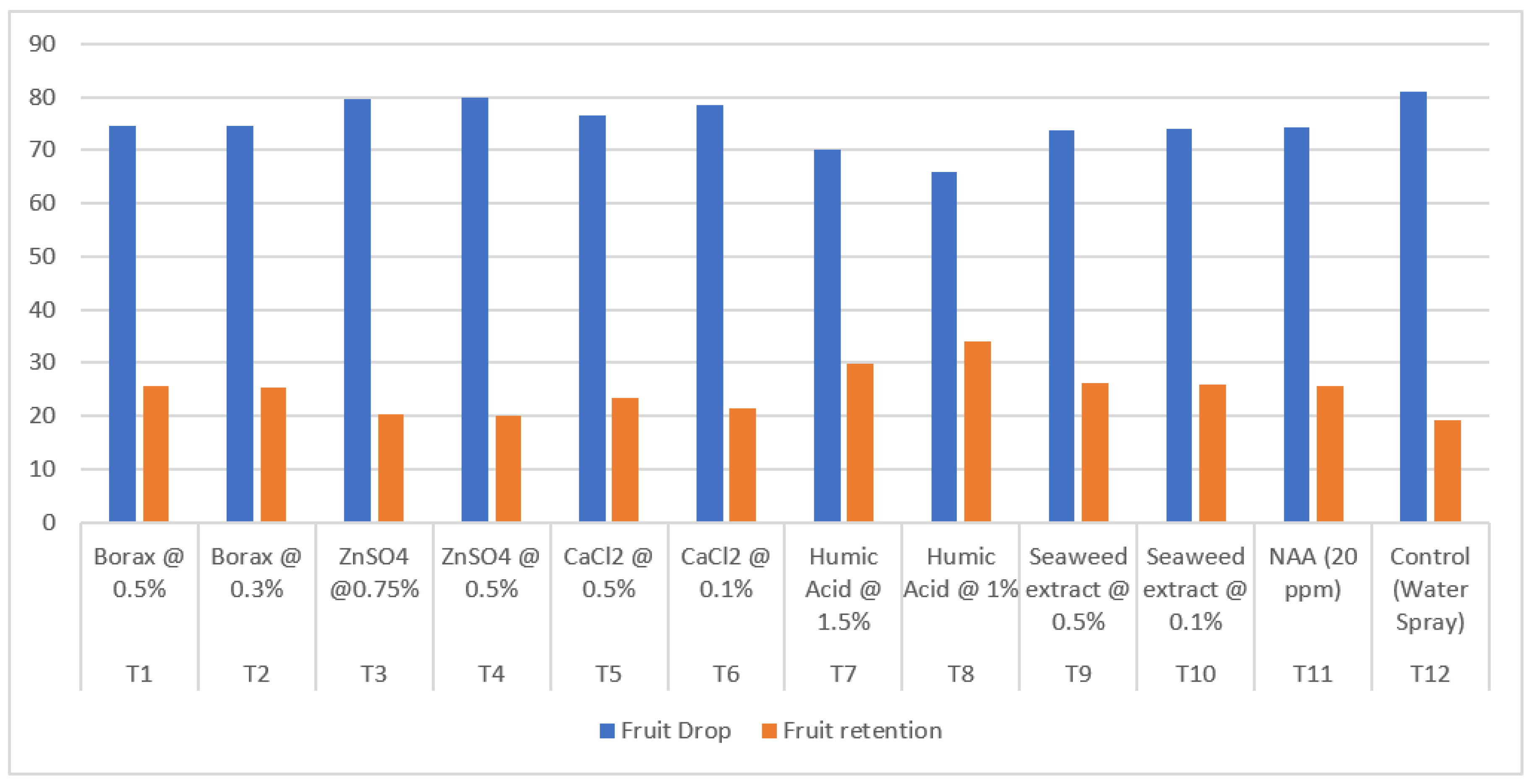
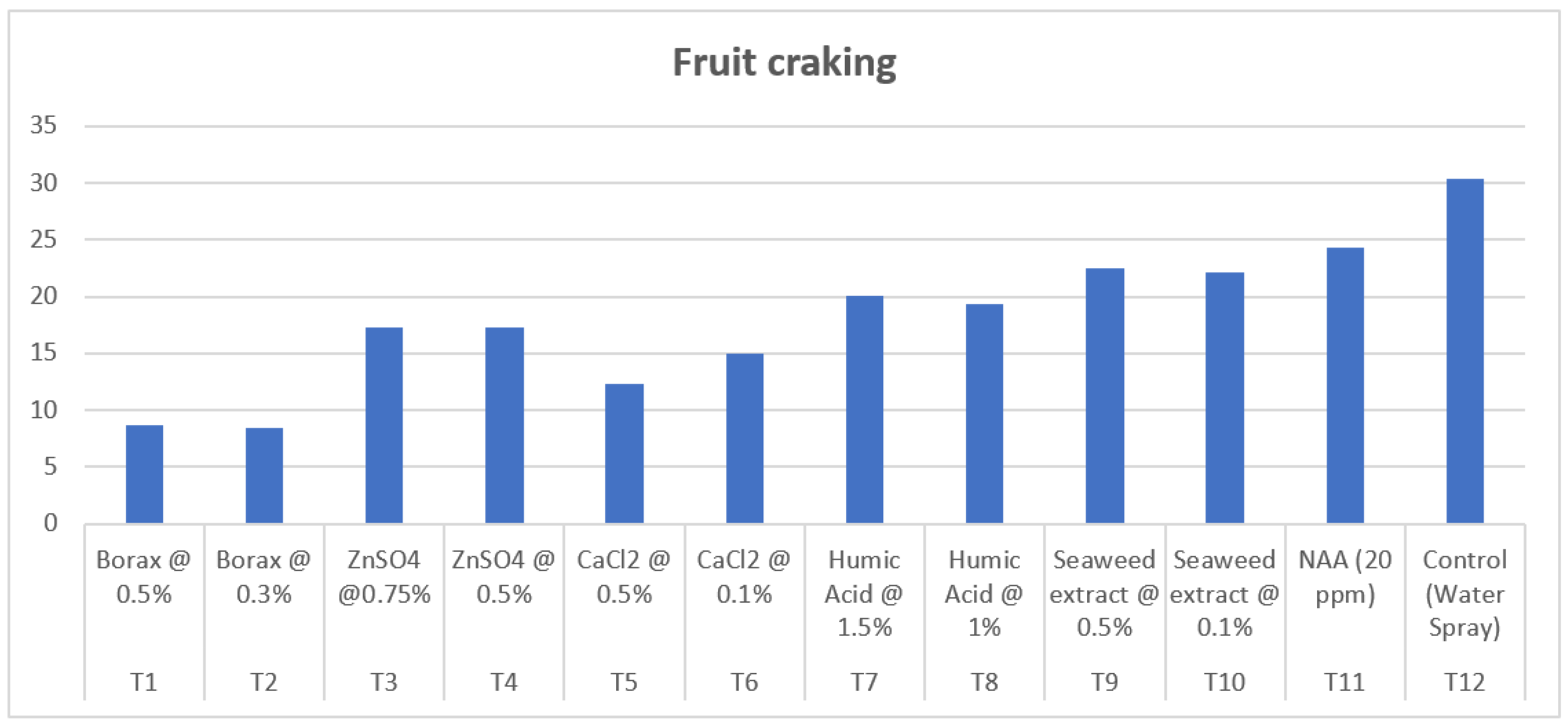
| Treatment | Treatment Details | Fruit set (%) |
Fruit drop (%) |
Fruit retention (%) | Fruit cracking (%) |
|---|---|---|---|---|---|
| T1 | Borax @ 0.5% | 53.07 | 74.47 | 25.53 | 8.73 |
| T2 | Borax @ 0.3% | 53.11 | 74.69 | 25.32 | 8.46 |
| T3 | ZnSO4 @0.75% | 52.10 | 79.59 | 20.41 | 17.25 |
| T4 | ZnSO4 @ 0.5% | 51.88 | 80.01 | 19.99 | 17.27 |
| T5 | CaCl2 @ 0.5% | 51.84 | 76.70 | 23.30 | 12.36 |
| T6 | CaCl2 @ 0.1% | 51.76 | 78.53 | 21.47 | 14.99 |
| T7 | Humic Acid @ 1.5% | 59.76 | 70.19 | 29.81 | 20.07 |
| T8 | Humic Acid @ 1% | 58.17 | 65.94 | 34.07 | 19.40 |
| T9 | Seaweed extract @ 0.5% | 51.60 | 73.72 | 26.28 | 22.54 |
| T10 | Seaweed extract @ 0.1% | 52.73 | 74.18 | 25.82 | 22.18 |
| T11 | NAA (20 ppm) | 53.53 | 74.29 | 25.71 | 24.31 |
| T12 | Control (Water Spray) | 46.49 | 80.91 | 19.10 | 30.36 |
| SE m (±) | 1.03 | 0.67 | 1.58 | 0.39 | |
| CD (0.05) | 2.08 | 1.36 | 3.18 | 0.78 | |
| CV (%) | 3.37 | 1.55 | 11.06 | 3.70 | |
| Treatment | Treatment Details | Total number of fruit per plant | Total number of marketable fruits per plant | Total yield per plant (Kg) | Total marketable yield per plant (Kg) |
|---|---|---|---|---|---|
| T1 | Borax @ 0.5% | 1636.31 | 1491.91 | 30.44 | 28.12 |
| T2 | Borax @ 0.3% | 1858.03 | 1695.05 | 38.69 | 35.34 |
| T3 | ZnSO4 @0.75% | 1717.80 | 1416.23 | 34.73 | 28.68 |
| T4 | ZnSO4 @ 0.5% | 1972.08 | 1622.14 | 35.85 | 29.56 |
| T5 | CaCl2 @ 0.5% | 1625.68 | 1425.05 | 31.10 | 28.29 |
| T6 | CaCl2 @ 0.1% | 1801.94 | 1518.85 | 32.97 | 28.11 |
| T7 | Humic Acid @ 1.5% | 1730.42 | 1421.51 | 32.10 | 26.33 |
| T8 | Humic Acid @ 1% | 1864.91 | 1519.29 | 34.93 | 28.39 |
| T9 | Seaweed extract @ 0.5% | 1785.04 | 1373.86 | 36.41 | 26.68 |
| T10 | Seaweed extract @ 0.1% | 1836.79 | 1297.67 | 36.68 | 25.92 |
| T11 | NAA (20 ppm) | 1735.27 | 1374.69 | 32.79 | 26.02 |
| T12 | Control (Water Spray) | 1627.77 | 1144.18 | 29.65 | 20.88 |
| SE m (±) | 69.74 | 56.57 | 1.53 | 1.09 | |
| CD (0.05) | 140.56 | 114.02 | 3.09 | 2.20 | |
| CV (%) | 6.84 | 8.80 | 7.84 | 6.83 | |
| Treatment | Treatment Details | Fruit length (cm) | Fruit breadth (cm) | Nut length (cm) | Nut breadth (cm) |
|---|---|---|---|---|---|
| T1 | Borax @ 0.5% | 3.14 | 3.02 | 2.53 | 1.49 |
| T2 | Borax @ 0.3% | 3.66 | 3.12 | 2.49 | 1.70 |
| T3 | ZnSO4 @0.75% | 3.53 | 3.01 | 2.61 | 1.58 |
| T4 | ZnSO4 @ 0.5% | 3.33 | 2.95 | 2.31 | 1.64 |
| T5 | CaCl2 @ 0.5% | 3.56 | 3.08 | 2.38 | 1.53 |
| T6 | CaCl2 @ 0.1% | 3.41 | 2.91 | 2.43 | 1.44 |
| T7 | Humic Acid @ 1.5% | 3.03 | 2.95 | 2.33 | 1.61 |
| T8 | Humic Acid @ 1% | 2.83 | 2.61 | 2.26 | 1.56 |
| T9 | Seaweed extract @ 0.5% | 3.43 | 2.92 | 2.48 | 1.57 |
| T10 | Seaweed extract @ 0.1% | 3.45 | 3.02 | 2.50 | 1.65 |
| T11 | NAA (20 ppm) | 3.05 | 2.87 | 2.64 | 1.54 |
| T12 | Control (Water Spray) | 3.27 | 2.66 | 2.85 | 1.73 |
| SE m (±) | 0.12 | 0.12 | 0.14 | 0.06 | |
| CD (0.05) | 0.24 | 0.25 | 0.28 | 0.12 | |
| CV (%) | 6.36 | 7.28 | 9.86 | 6.41 | |
| Treatment | Treatment Details | Fruit weight (g) | Aril weight (g) | Seed weight (g) | Pericarp weight (g) | Aril :seed ratio | Aril: pericarp ratio |
|---|---|---|---|---|---|---|---|
| T1 | Borax @ 0.5% | 18.91 | 14.17 | 2.65 | 2.35 | 5.35 | 6.04 |
| T2 | Borax @ 0.3% | 20.85 | 16.01 | 2.19 | 2.23 | 7.31 | 7.18 |
| T3 | ZnSO4 @0.75% | 20.39 | 14.86 | 2.45 | 2.30 | 6.07 | 6.45 |
| T4 | ZnSO4 @ 0.5% | 18.30 | 13.07 | 2.92 | 2.28 | 4.47 | 5.75 |
| T5 | CaCl2 @ 0.5% | 19.80 | 15.70 | 2.38 | 2.33 | 6.62 | 6.77 |
| T6 | CaCl2 @ 0.1% | 18.59 | 14.04 | 2.81 | 2.35 | 5.07 | 5.98 |
| T7 | Humic Acid @ 1.5% | 18.63 | 13.50 | 2.82 | 2.43 | 4.82 | 5.56 |
| T8 | Humic Acid @ 1% | 18.84 | 13.86 | 2.65 | 2.37 | 5.27 | 5.84 |
| T9 | Seaweed extract @ 0.5% | 19.48 | 13.39 | 2.86 | 2.40 | 4.71 | 5.59 |
| T10 | Seaweed extract @ 0.1% | 20.02 | 14.40 | 2.57 | 2.30 | 5.61 | 6.26 |
| T11 | NAA (20 ppm) | 18.95 | 13.48 | 3.00 | 2.43 | 4.49 | 5.64 |
| T12 | Control (Water Spray) | 18.26 | 12.20 | 3.09 | 2.54 | 3.95 | 4.81 |
| SE m (±) | 0.51 | 0.35 | 0.08 | 0.08 | 0.27 | 0.25 | |
| CD (0.05) | 1.04 | 0.71 | 0.16 | 0.15 | 0.54 | 0.51 | |
| CV (%) | 4.63 | 4.37 | 5.08 | 5.58 | 8.77 | 7.28 | |
| Treatment | Treatment Details | TSS (0Brix) | Titratable acidity (%) | Total sugar (%) | Reducing sugar (%) |
Non-reducing sugar (%) |
|---|---|---|---|---|---|---|
| T1 | Borax @ 0.5% | 14.02 | 0.78 | 10.12 | 7.93 | 2.07 |
| T2 | Borax @ 0.3% | 14.07 | 0.72 | 10.40 | 8.15 | 2.14 |
| T3 | ZnSO4 @0.75% | 11.47 | 0.85 | 8.88 | 7.01 | 1.78 |
| T4 | ZnSO4 @ 0.5% | 11.98 | 0.85 | 8.49 | 6.65 | 1.75 |
| T5 | CaCl2 @ 0.5% | 12.70 | 0.82 | 9.03 | 7.12 | 1.82 |
| T6 | CaCl2 @ 0.1% | 12.55 | 0.82 | 9.02 | 7.08 | 1.84 |
| T7 | Humic Acid @ 1.5% | 13.42 | 0.80 | 9.88 | 7.82 | 1.96 |
| T8 | Humic Acid @ 1% | 13.73 | 0.79 | 9.98 | 7.88 | 2.00 |
| T9 | Seaweed extract @ 0.5% | 12.95 | 0.88 | 9.28 | 7.27 | 1.92 |
| T10 | Seaweed extract @ 0.1% | 13.22 | 0.91 | 9.53 | 7.49 | 1.94 |
| T11 | NAA (20 ppm) | 11.78 | 0.93 | 8.63 | 6.78 | 1.76 |
| T12 | Control (Water Spray) | 9.82 | 1.00 | 7.85 | 6.08 | 1.69 |
| SE m (±) | 0.43 | 0.05 | 0.34 | 0.31 | 0.33 | |
| CD (0.05) | 0.86 | 0.09 | 0.68 | 0.62 | 0.66 | |
| CV (%) | 5.86 | 9.41 | 6.34 | 7.35 | 30.07 | |
| Treatment | Treatment Details | TSS :acid ratio | Ascorbic acid (mg/100g) |
|---|---|---|---|
| T1 | Borax @ 0.5% | 18.76 | 32.06 |
| T2 | Borax @ 0.3% | 20.33 | 32.22 |
| T3 | ZnSO4 @0.75% | 14.39 | 28.11 |
| T4 | ZnSO4 @ 0.5% | 14.74 | 28.50 |
| T5 | CaCl2 @ 0.5% | 16.35 | 29.61 |
| T6 | CaCl2 @ 0.1% | 16.00 | 28.72 |
| T7 | Humic Acid @ 1.5% | 17.25 | 30.67 |
| T8 | Humic Acid @ 1% | 17.96 | 30.56 |
| T9 | Seaweed extract @ 0.5% | 15.17 | 30.00 |
| T10 | Seaweed extract @ 0.1% | 14.98 | 30.11 |
| T11 | NAA (20 ppm) | 12.74 | 27.83 |
| T12 | Control (Water Spray) | 9.99 | 27.06 |
| SEm (±) | 1.25 | 0.33 | |
| CD (0.05) | 2.52 | 0.67 | |
| CV (%) | 13.75 | 1.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





