1. Introduction
The Covid-19 pandemic has been countered with a combination of specific and non-specific preventive strategies [
1]. Specific measures encompass the use of anti-viral monoclonal medications and active immune prophylaxis, which primarily involves vaccinations. On the other hand, non-specific prophylaxis revolves around individuals employing personal protective equipment and ensuring environments are properly sanitized. Each of these strategies works in tandem, complementing one another [
2]. However, the epidemiological landscape continues to shift due to the emergence of new mutations and variants of the SARS-CoV-2 virus. This evolving nature raises concerns over the potential development of escape mutants, which might not be detected or neutralized by the current immunization methods in place [
3].
The stability of the SARS-CoV-2 virus in the environment varies depending on the type of surface and surrounding conditions. Environmental factors like temperature, humidity, and sunlight also play a role in the virus's stability. Furthermore, another study in "The Lancet Microbe" showed that the virus's stability decreases with an increase in temperature. This variability highlights the importance of regular cleaning and disinfection, especially on frequently touched surfaces, to curb the transmission of the virus [
4].
Throughout the COVID-19 pandemic, households have been key transmission sites. SARS-CoV-2 has been identified on surfaces in homes, especially where prolonged contact occurs with infected individuals. Unlike hospitals, data on household SARS-CoV-2 contamination remains limited [5, 6].
Whereas research has shown widespread SARS-CoV-2 surface contamination in hospitals treating COVID-19 patients. In Singapore, over half of the isolation rooms had at least one contaminated surface, with a significant percentage of rooms showing contamination on frequently touched surfaces during a patient's initial illness week. A Toronto study found 26% of surface samples in over half of the patient rooms tested positive for the virus. Another study detected the virus in hospital air exhausts, indicating that virus-laden droplets might be carried by airflows onto equipment [
7].
Some COVID-19 patients seem to contaminate their surroundings more than others. Factors such as higher respiratory viral loads, the severity of hypoxia upon admission, comorbidity scores, and the duration since illness onset are linked to greater surface contamination [
7].
There are several considerable factors that affect the viability of SARS-CoV-2 in the environment. The persistence of SARS-CoV-2 varies depending on the type of surface. Generally, coronaviruses last longer on nonporous materials than porous ones. Surfaces like surgical masks, which are hydrophobic and synthetic, show longer virus persistence than absorbent materials like cotton [
8]. The virus's stability is also influenced by its surrounding medium. For instance, when proteins like bovine serum albumin or mucus are added, the virus remains more infective, suggesting airway secretions might enhance its longevity and transmission [9, 10]. SARS-CoV-2's stability decreases with increasing temperature. For instance, its half-life is 1.7–2.7 days at 20°C, but only a few hours at 40°C. At colder temperatures like 4°C, it can persist for up to 14 days, but at 56°C and 70°C, its survival time reduces to 10 minutes and 1 minute, respectively [
11]. Notably, the virus remains stable and more infectious at freezing temperatures (-20°C) and can last for 60 days on cold-chain food packaging kept below -18°C [
12]. The relationship between the virus's viability and relative humidity is intricate. Some findings indicate that SARS-CoV-2 decays fastest at 65% humidity and more slowly at both lower (40%) and higher (75%) levels. The rate of virus inactivation increases with temperature and is also influenced by relative humidity, creating a U-shaped relationship [
13].
The effectiveness of chemical disinfectants varies based on the virus structure and the environment. Inappropriate choice and incorrect usage can contribute to pathogen spread, raising public health issues. Therefore, thorough studies are essential to ensure the right selection and application of disinfectants [
14]. Alcohols, particularly ethanol and isopropanol, destroys microorganisms by dissolving lipid membranes and denaturing proteins, making them effective against a variety of pathogens, including SARS-CoV-2 [
15]. Quaternary ammonium compounds (QACs) are frequently used disinfectants in healthcare, food processing, and households. As cationic detergents [
16]. Recent studies confirm QACs' efficacy against SARS-CoV-2, making them a dominant disinfectant on the EPA's List N [
17]. Sodium hypochlorite, a key ingredient in household bleach and other commercial products, is recommended by health organizations for disinfecting surfaces, especially in COVID-19-exposed areas. A "strong chlorine solution" is notably effective against SARS-CoV-2 within minutes. Various studies confirm its efficacy against SARS-CoV-2, though results differ based on concentration and contact time. Notably, while hypochlorous acid is cost-effective and generally safe for many applications, including mouthwashes and sanitizers, excessive chlorine can have environmental and health implications [
18].
Hydrogen peroxide is a popular disinfectant known for its environmental safety, as it breaks down into water and oxygen. Its non-toxic nature makes it suitable for disinfecting medical equipment, surfaces, and even skin [
19]. Additionally, hydrogen peroxide can be vaporized for fumigation, which is particularly useful for decontaminating hard-to-reach areas, like entire rooms in hospitals. This gaseous form enhances its biocidal activity. Its disinfecting action arises from the generation of hydroxyl free radicals that damage microbial lipids, proteins, and DNA, with its small molecular size allowing it to penetrate microbial defenses without inducing lysis [
20]. While disinfectants like hydrogen peroxide, sodium hypochlorite, quaternary ammonium compounds (QACs), and alcohols are effective against pathogens like COVID-19, their widespread use in the environment raises concerns. These agents can have deleterious effects on surfaces, often leading to discoloration, corrosion, and degradation, especially of delicate or specialized materials [
19]. SARS-CoV-2 is highly sensitive to oxidizing agents due to the pronounced electrophilicity of its spike protein. Oxidizing agents, being electron donors with negative charges, can neutralize the virus. SARS-CoV-2's contagion levels peak during winter, with reduced air dilution and increased levels of pollutants like sulphur dioxide [
19]. In contrast, summer sees a drop in cases due to improved air dilution and prevalent oxidizing pollutants like ozone. Sanitizing confined areas with oxidizing agents could be beneficial. However, while the effectiveness of such disinfectants is acknowledged, specific research on their impact on SARS-CoV-2 is limited, as noted by Italian National Institute of Health. Nonetheless, treatments like gaseous ozone have shown promising results against Covid-19 infections [
20].
The aim of this study is to assess the efficacy and safety of Liquid hyperoxygen (IOL), an aqueous solution saturated with hydrophilic oxygen and oxidizing nitrogen species, as an antiviral agent against wild SARS-CoV-2 viruses.
2. Materials and Methods
2.1. Liquid Hyperoxygen (LOH)
IOL is an aqueous electrolytic solution characterized by radical oxidative properties, attributable to the activity of single chemical species, present as solutes, while the solvent consists of pyrogenic bi-distilled water for liquid phase injections. The chemical-physical characteristics of the water used as a solvent to produce IOL solutions express a conductivity between 20 and 30 µS x cm-1 and with impurities that never exceed 1 µM/L, with the latent heat of vaporization equal to 9.780 kcal mol-1 and with 373.15 K as the boiling point. The solutes contained in the IOL-RONS® aqueous solution are represented by Highly Reactive Species of Oxygen and Nitrogen, the former universally known by the acronym ROS, while the latter by the acronym RNS, but when referring to mixtures containing both highly reactive species, the acronym RONS is used. The RNS, in IOL aqueous solutions, are dissolved as gaseous solutes in the aqueous solvent, reaching maximum concentration values close to 0.98 mg/ml, values which depend on both the temperatures and the pressures, however, the RONS concentration values never exceed 1 mg/ml. ROS, which make up most of the solutes in the IOL aqueous solution, all derive from molecular oxygen reduction intermediates and due to their outermost electron orbital, occupied by a single electron, are included in the group of molecules "one-electron oxidants" (OSE), also called in foreign Anglo-Saxon language "one-electron oxidant". Among the highly ROS, the most represented in the mixture besides dioxygen (O2), is the superoxide anion (O2-), while the hydroxyl radical (OH), singlet oxygen (O21∑g+) and hydrogen peroxide (H
2O
2), the latter molecule not included among the radical species. In addition to highly ROS, some highly RNS, such as nitric oxide (NO) and peroxynitrite (ONOO-) are also present in trace amounts [
21]. RNS, even if present in traces, represent a peculiar characteristic of IOL aqueous solutions and we owe them some biological activities of the solutions, first the antiviral ones, especially against SARS-CoV-2. The IOL stock solution (provided by Porti Verdi, Pisa, Italy) was diluted in physiological solution (0.9% NaCl in molecular grade bi-distilled water) 1/50, 1/100, 1/1000, 1/10000 vol/vol. Incubation times between IOL and throat samples containing wild SARS-CoV-2 were 1, 5, 10, 20, 30 min.
2.2. Evaluation of Liquid Hyperoxygen Efficacy. Challenge Assay with Sars-CoV-2 Virus
Ten throat swab samples containing viable SARS-CoV-2 virus were collected from COVID-19-affected patients 5 composed of omicron and 5 delta variants. Informed consent was obtained from all subjects at the San Martino Hospital, Genoa, Italy. The persistence of the virus and the ability of these samples to infect susceptible cells equipped with specific receptors for this virus were then verified. The presence or absence of the virus inside these cells was evaluated by qPCR. In particular, the biological test used was the 'challenge test' [19, 20]. This test uses kidney cells with high membrane expression of the ACE2 receptor which specifically binds the spike protein of SARS-CoV-2 and is therefore able to verify not only the presence of the virus but also above all its infective and pathogenetic capacity.
All the experiments were carried out in the BSL3 biosafety laboratory and carried out by personnel equipped with adequate personal protective equipment suitable for the COVID-19 biological risk (
Figure 1).
Cells expressing the high-affinity ACE2 receptor for the SARS-CoV-2 spike protein were incubated overnight at 37°C with the high viral load samples variously treated with IOL. At the end of the incubation, the plates were subjected to heating at 57°C for 30 min to inactivate the extracellular virus and to detach the cells from the adhesion plane. The cell suspension was then collected by scraping and aspiration with disposable Pasteur pipettes and then centrifuged for 15 min at 3000 g. The harvested cell pellet was resuspended and washed 2 times with phosphate-buffered saline (PBS) and re-centrifuged. The final collected pellet was then resuspended in sterile RNAse-free molecular grade bi-distilled water and frozen. Intracellular RNA extraction was performed by high-performance robotic equipment using the Janus G3 preparatory robot (Perkin Elmer) and extraction with magnetic beads using the automated Chemagic 360 D equipment (Perkin Elmer). The presence of SARS-CoV-2 viral RNA in the extracted intracellular RNA was verified by RNA-DNA reverse transcription and polymerization chain reaction (PCR) using a high-sensitivity Light Cycler II apparatus (Roche).
For the analysis of each sample, molecular fluorescent probes were used for the following genes: (a) Gene housekeeping house- Ribonuclease P/MRP Subunit P30 [RPP30] used as an internal positive control to verify the presence of RNA and the correct actuation of the PCR reaction; (b) SARS-CoV-2 Opening Reading Frame (Orf) viral gene Orf1ab labeled with Vic fluorescent probe; (c) SARS-CoV-2 N viral gene labeled with FAM fluorescent probe. The following PCR amplification time/temperature conditions were used: 50 °C x 15 min, 95 °C x 2 min, 45 cycles at 95 °C x 3 sec and 60 °C x 30 sec.
2.3. Evaluation of Liquid Hyperoxygen Efficacy in Oxidizing Biological Fluids
The ability of IOL to exert an oxidizing action on complex biological fluids and matrices was determined and quantified by the FRAS test (Free Radical Analytical System) on human plasma, using FRAS 5 EVOLVO (H&D Parma, Italy).
2.4. Evaluation of Liquid Hyperoxygen Safety
The safety of IOL was verified by: (a) in vitro evaluation of the absence of cellular cytotoxicity by comparison with the cytotoxic effects of other chemical disinfectants (benzalkonium chloride) evaluated with phase contrast optical microscopy; (b) the absence of in vivo cytotoxicity by human skin erythematogenesis test. To using IOL for environmental disinfection, the possibility of using oxidation-sensitive colorimetric indicators sensitive to oxidation was evaluated to determine the achievement of the effective dose of IOL suitable for neutralizing SARS-CoV-2. Each analysis was tested in triplicate in 3 independent experiments.
3. Results
3.1. Efficacy
The efficacy of IOL in neutralizing the ability of SARS-CoV-2 to infect sensitive cells was determined by comparing the amount of viral RNA present inside the cells after the incubation with samples containing the virus or with the same samples treated with IOL. The viral load was quantified by the PCR positivity cycle. The greater the amount of virus, the fewer amplification cycles needed to detect it. The raw data obtained by comparing the intracellular viral loads by PCR after incubation with untreated or IOL-treated samples for different times (1-30 min) are shown in
Table 1.
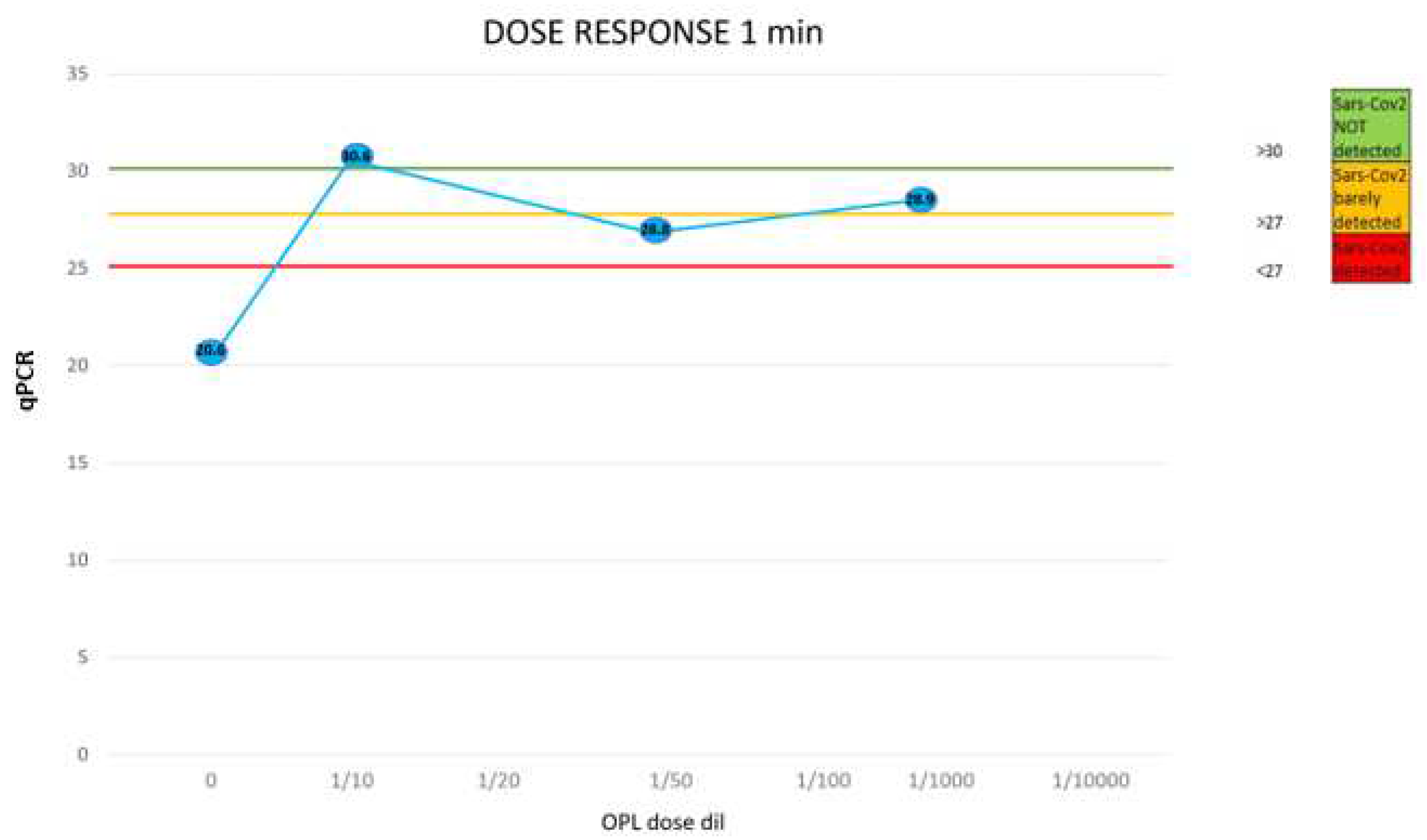
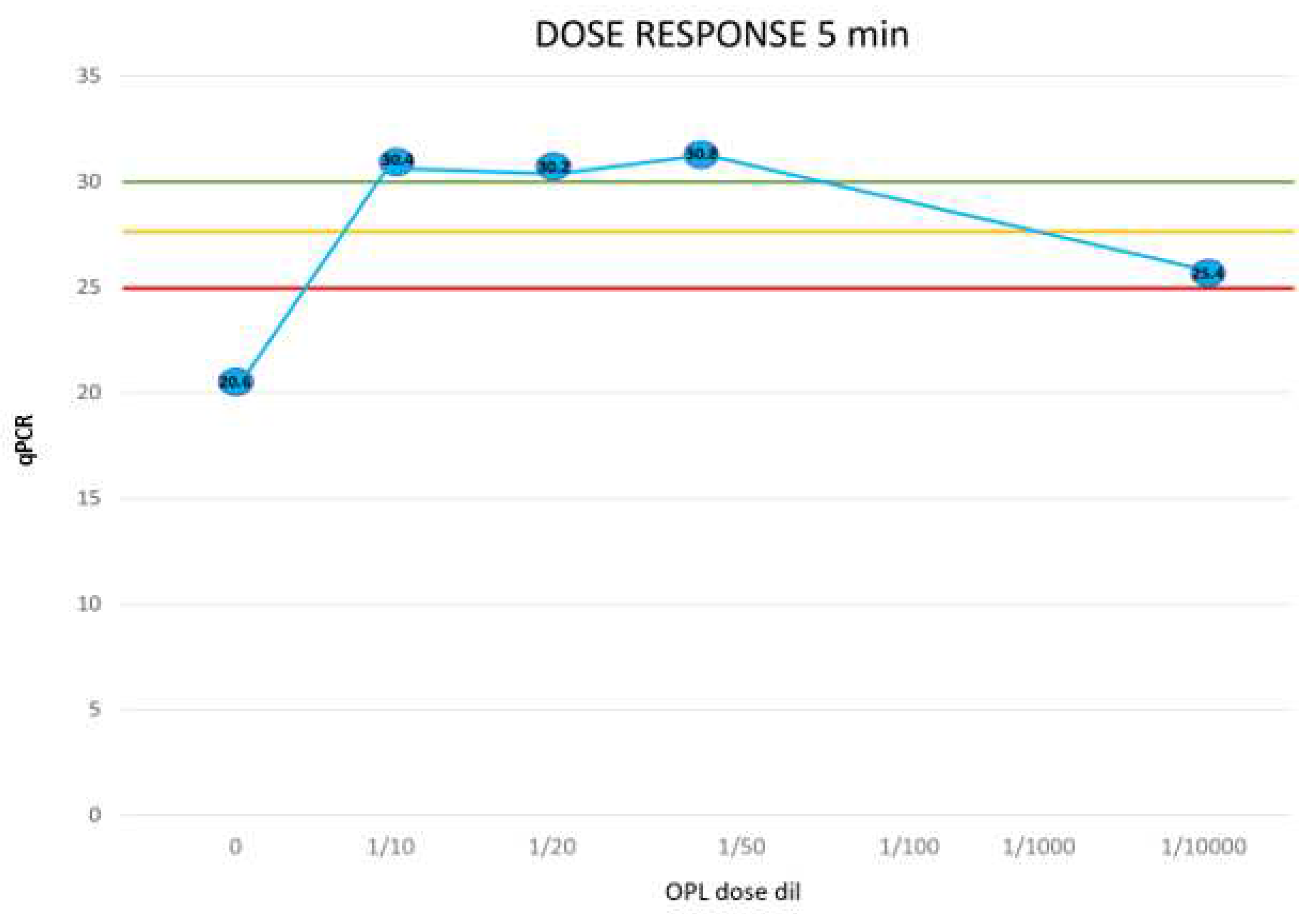
The level of viral load present in the sample analyzed is synthetically highlighted with a color code: green, virus not present; orange, virus present with low viral load (positivity ≥ 28th PCR cycle); red, virus present with high viral load (positivity ≤ 23rd PCR cycle). The analysis did not detect the presence of the virus in the negative controls (C-). Instead, the presence of high viral loads was tested in all positive controls (C+). Treatment with IOL significantly decreased the viral load present. A decrease (from red to orange) occurred in the case of incubations for very short periods (1 min). In this experimental condition, however, the viral load was reduced by as much as 10 orders of magnitude in the case of IOL diluted 1/50 (Exp 2 from 18.8 to 28.8) and by 8 orders of magnitude in the case of IOL diluted 1/1000 (Exp 3 from 21.3 to 28.9). For longer incubation times (5-30 min) the high viral load present (PCR cycle positivity 18- 21) was always completely inactivated. This result was obtained at all the dilutions evaluated (1/50, 1/100, 1/1000). In the sole case of the 1/10,000 dilution with incubation at 5 min, there was no neutralization of the virus but only when the viral load decreased by as much as 4 orders of magnitude (from 21 to 24) despite the high dilution and short incubation time.
In
Table 2, the left column indicates the IOL dilution used. Row '0' indicates the positive control not treated with IOL. Each row corresponds to a certain dilution. On the right, each column corresponds to a specific incubation time (0, no incubation, 1-30 min).
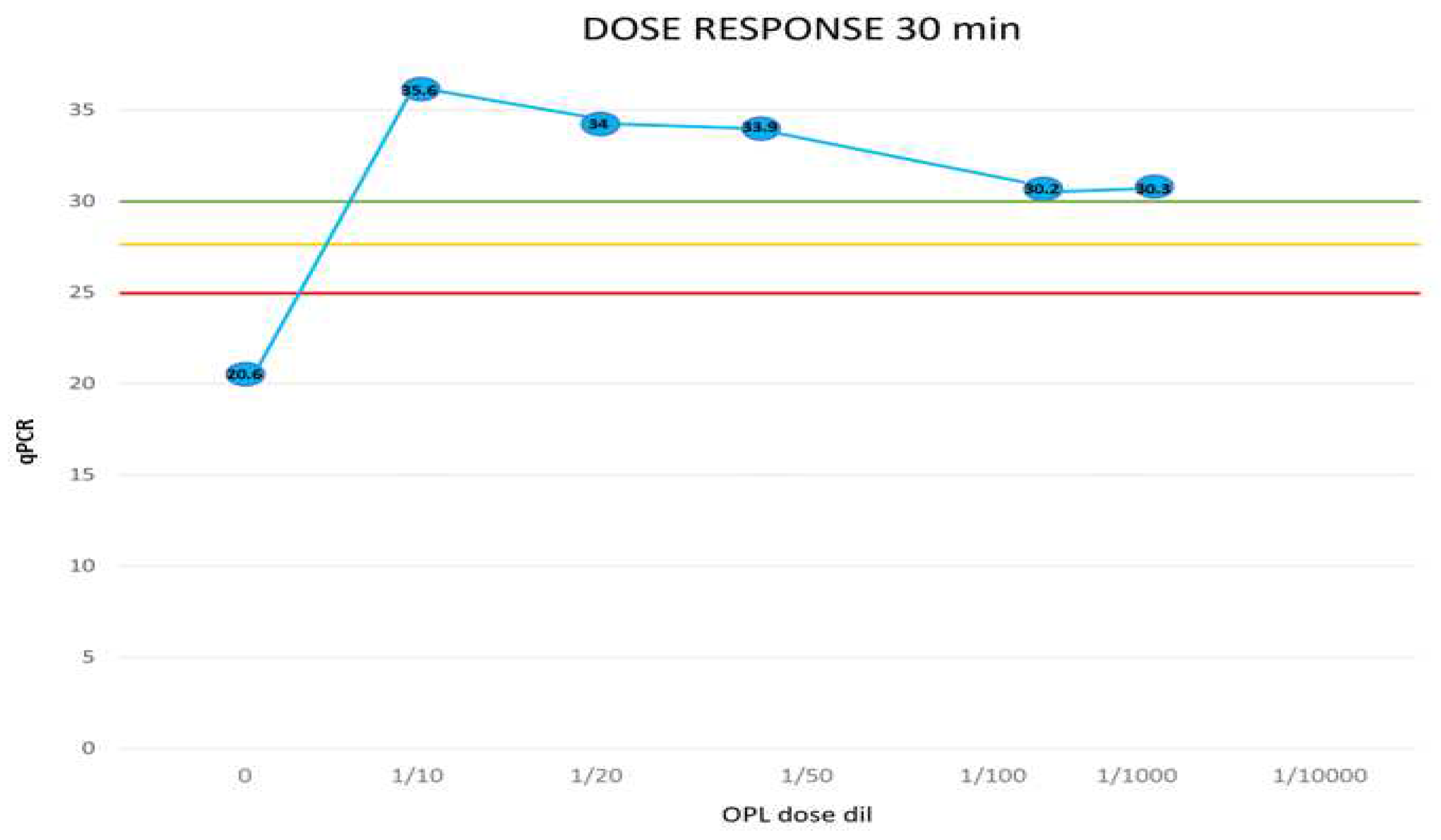
The sample not treated with IOL presented a high viral load with positivity at cycle 20.6. Treatment with IOL diluted 1/10 always completely inactivated the virus even for very short incubation periods (1 min). At the 1/50 dilution, at least 5 min of incubation is necessary to obtain the neutralization of the virus. At higher dilutions (1/100, 1/1000) neutralization is obtained after 30 min of incubation. At extreme dilutions (1/100009 after 5 min a decrease of the viral load of 5 orders of magnitude (from 20.6 to 25.4) is observed but not the complete inactivation of the virus.
All doses evaluated (dilutions 1/10-1/1000) decreased the viral load by 8 orders of magnitude. Achievement of the complete neutralization threshold was achieved for the 1/10 dose. The observed dose/response relationship substantiates the specificity of the antiviral effect of IOL on SARS-CoV-2 (
Figure 2).
All doses evaluated (1/10-1/1000 dilutions) neutralized SARS-CoV-2 with viral load decreases greater than 10 orders of magnitude. Only at the extreme dilution of 1/10,000 did 5 min of incubation cause a reduction of the viral load of 5 orders of magnitude but not the achievement of the threshold of complete viral inactivation. After 30 min of incubation, all doses evaluated (dilutions 1/10-1/1000) neutralized SARS-CoV-2 with decreases in viral load greater than 10 orders of magnitude (
Figure 3).
3.2. Quantification of the Oxidizing Capacity of IOL on Human Serum
Aliquots (500 ul) of human blood serum were collected from peripheral capillary blood by puncturing the fingertip with a lancet from a volunteer subject (58-year-old male). IOL was then added to the samples reaching maximum concentration values of RONS, close to 0.98 mg/ml (10 ul dilutions 1/10, 1/100, 1/1000. 1/10000). The evaluation of the total oxidative load was carried out by means of analysis of the oxidation capacity of the iron and photometric detection at 522 nm of the oxidized iron. The Fras 5 Evolvo system (H&D, Parma, Italy) was used, consisting of a centrifuge, thermostatic incubator at 37°C and a photometer. The determined oxidative load is expressed as equivalents of hydrogen peroxide (U Carr). The results obtained are shown in
Table 3.
The oxidative load value detected in the untreated sample was equal to 300 U Carr. At the 1/10 and 1/100 dilutions, the oxidation induced by IOL was such as to bring the values detected outside the instrumental reading scale (>5000 U Carr). The oxidative load increased in a detectable way from 300 to 4947 U Carr using IOL diluted 1/1000 and to 443 (therefore with an increase of 33%) using IOL diluted 1/10000. These results indicate that IOL can exert a powerful oxidative action even in the context of complex biological matrices. This result is relevant since viruses, including SARS-CoV-2 are always contained in complex biological fluids that can neutralize the action of disinfectants.
3.3. In Vitro Test
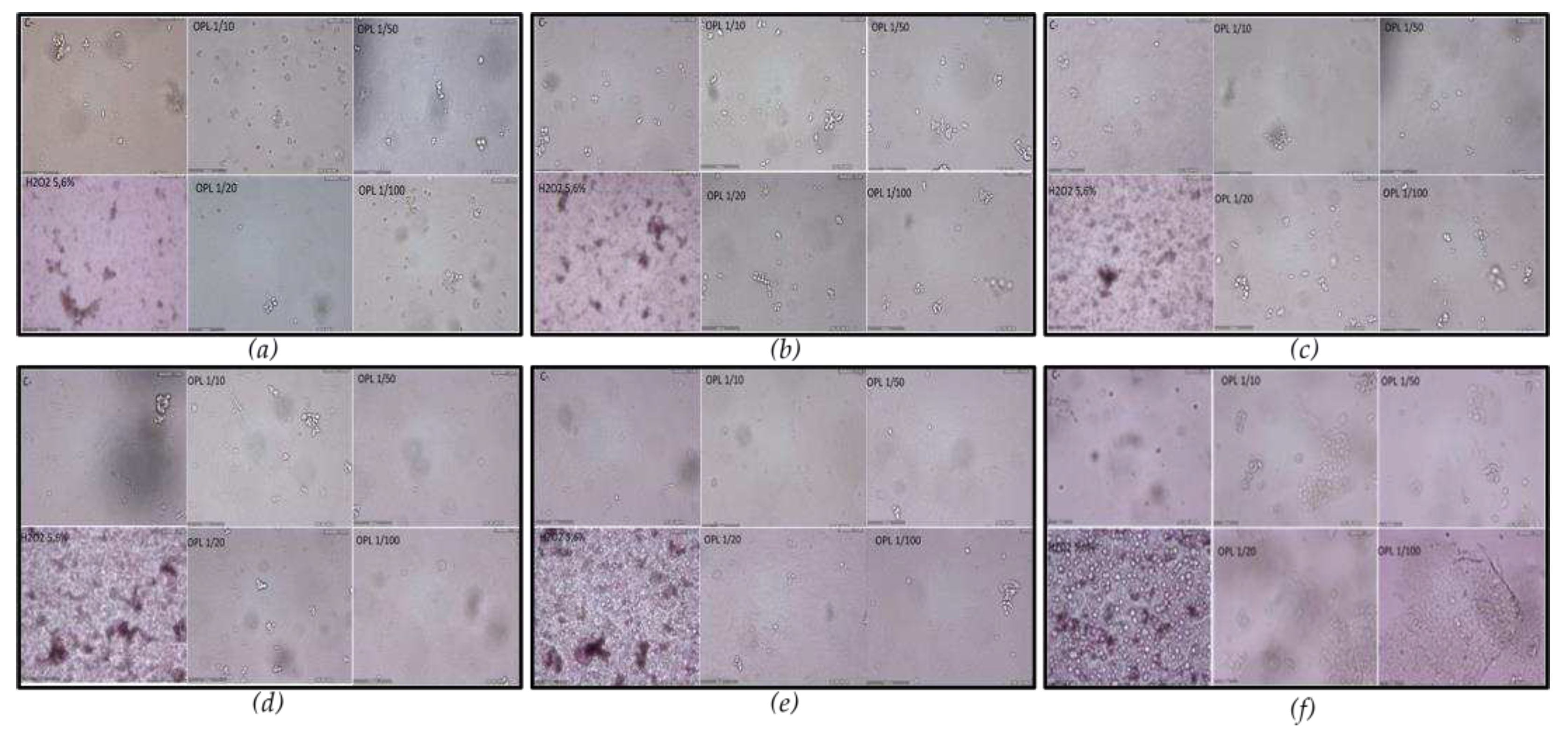
To verify the specificity and the action of IOL against SARS-CoV-2, neutralizing the pathogen without damaging human tissues, we will perform the in vitro test. Eukaryotic cells of the Vero line were incubated with IOL at different dilutions and for comparison with hydrogen peroxide. The cytotoxicity of IOL versus H
2O
2 5.6% vol/vol at 5, 10, 15, 30, 60, and 120 min was comparatively evaluated (
Figure 5).
3.4. In Vivo Test
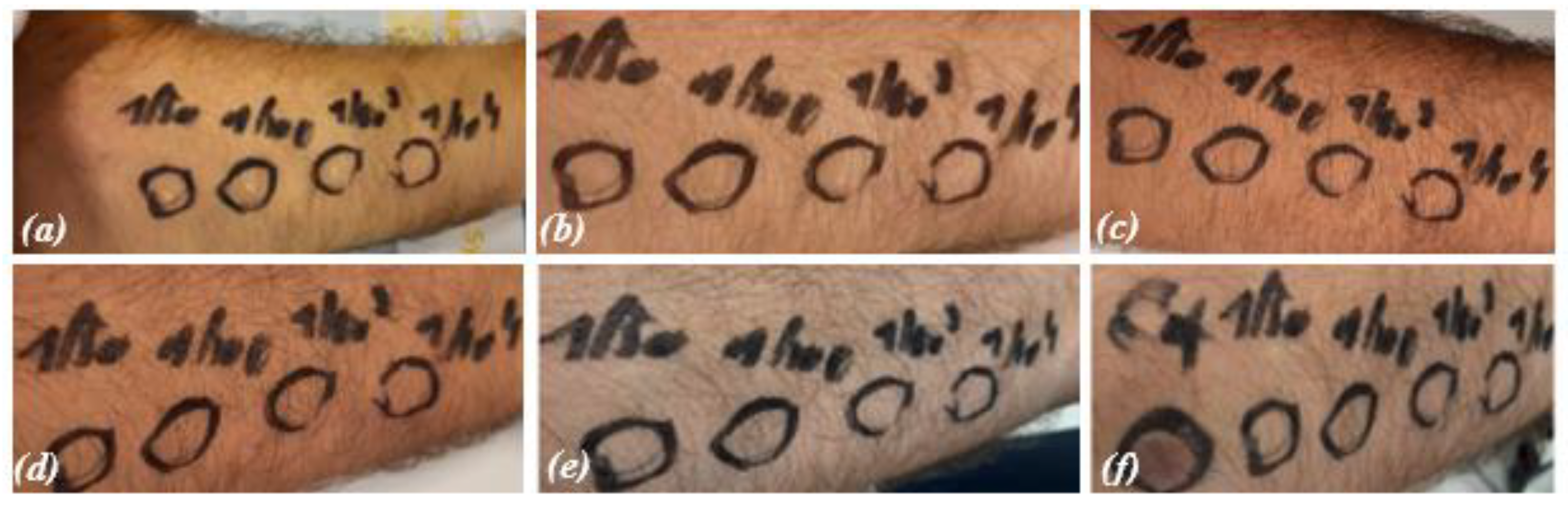
In the in vivo test, the erythematogenic capacity of IOL was evaluated compared with a disinfectant known for its cytotoxicity, including epithelial benzalkonium chloride used at 5% vol/vol [19, 21, 22]. IOL at different dilutions (1/10, 1/100, 1/1000, 1/10000) and benzalkonium chloride were applied on rounded skin areas located on the palmar face of the forearm delimited by a dermographic pen. Before application, the area was cleaned with saline solution. The solutions were left to act for 1, 5, 15, 30 and 60 min. The results obtained are shown in
Figure 6.
Benzalkonium chloride induced the formation of an erythematous halo over the entire area of skin where it was applied (positive control, C+, bottom right photo). At none of the concentrations used and not even for longer application times, IOL induced the formation of erythema. This result demonstrates the safety of IOL compared to other chemical disinfectants such as benzalkonium chloride.
3.5. Applications of Environmental Sensors to Detect the Achievement of the Disinfectant Charge of IOL
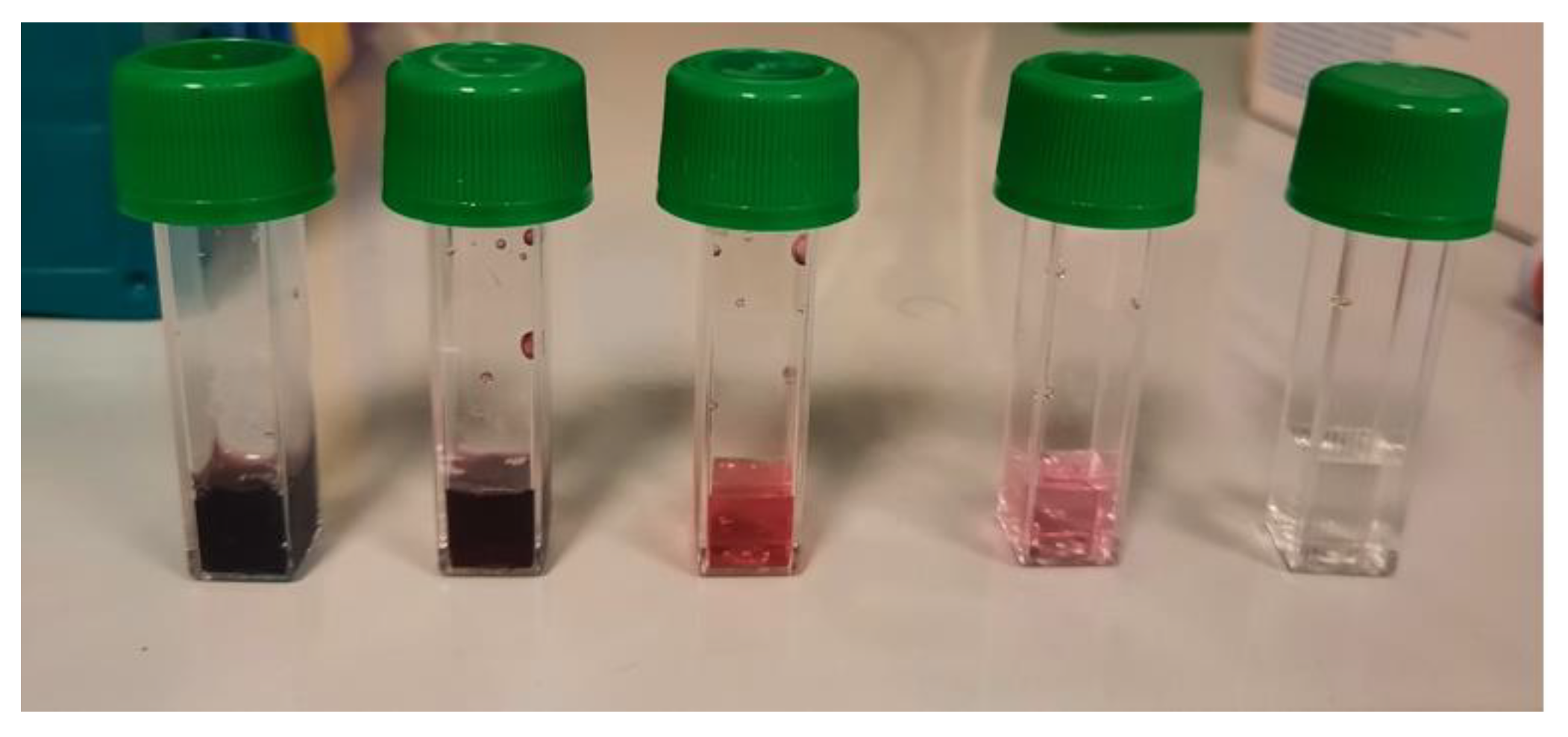
To establish whether a concentration of IOL is reached in the confined environment suitable for guaranteeing the successful neutralization of SARS-CoV-2, we evaluated the possibility of developing environmental sensors using the ability of chromogenic substrates to detect IOL-induced oxidation. The first sensor evaluated is based on a chromogenic liquid containing iron which is oxidized by the interaction with IOL changing its colour from transparent to pink to red to carmine red depending on the level of oxidation induced. An example of the application of this sensor is shown in
Figure 7.
The results obtained indicate that the colour of the liquid sensor becomes darker and more intense with increasing concentration of IOL used with dark red colours at concentrations of 1/10 and 1/100, dark pink at 1/1000, and light pink at 1 /10000.
Although quite sensitive, the liquid sensor presents some problems in the practical application in a confined environment: (a) its positioning does not appear simple due to the possibility of spilling the liquid since the test tube where the liquid is placed must be opened to interact with diffuse IOL in the air; (b) the interaction surface between the liquid sensor and the diffused IOL aerosol coincides with the liquid surface only and is therefore rather small; (c) the penetration capacity of the IOL aerosol inside the test tube with the cap present, even if not closed, appears at least questionable.
For all these reasons we thought it interesting to develop a solid rather than a liquid environmental sensor. The solid sensor consists of a colorimetric paper on which a chromogenic mixture sensitive to oxidation is adsorbed. We evaluated the ability of this sensor to colour shift proportionally based on the amount of IOL received. The results obtained, compared to what has already been done with the liquid sensor, are shown in
Figure 8.
The colorimetric paper was also very sensitive to IOL-induced oxidation. The map would have several advantages for use as an environmental sensor in particular: (a) it can be loaded on adhesive support for easy and immediate application to all environmental surfaces; (b) the interaction surface with the IOL aerosol is maximized and coincides with the entire sensor surface; (c) there are no sensor management problems or spillage of the liquids contained therein.
The findings suggest that at a concentration of RONS, approximately 0.98 mg/ml, IOL exhibits a significant ability to inhibit the SARS-CoV-2 virus. This virus's capacity to infect susceptible cells is swiftly neutralized, even at minimal concentrations (dilution 1/1000) and brief exposure times (5 min). This action is carried out selectively without inducing cytopathic effects on healthy cells or erythematogenic effects on the skin.
These results are due to the sensitivity of the SARS-CoV-2 virus towards highly reactive agents of oxygen and nitrogen. In fact, this virus, due to the distance between the trans-peri capsid spike protein, exposes large areas of its lipid mantle to peroxidation induced by oxidizing disinfectants. Furthermore, the viral spike protein is highly electrophilic (positive electrical charge) and therefore easily denatured by negatively charged oxidizing agents, such as superoxide anion (O2-) and peroxynitrite (ONOO-), even if in the radical aqueous mixture these the latter is present only in traces.
4. Discussion
In this study, we investigated the antiviral efficacy and safety of IOL, an aqueous solution saturated with ROS and RNS, against the SARS-CoV-2 virus. Utilizing throat swab samples containing both omicron and delta variants of the virus, we exposed them to varying dilutions of IOL and assessed the remaining viral load using qPCR. Our findings reveal that IOL effectively neutralized SARS-CoV-2 in a dose and time-dependent manner, with notable reductions in viral load even at higher dilutions. Moreover, in safety assessments, IOL demonstrated no cytotoxic effects on Vero eukaryotic cells and induced no erythema when applied on human skin, in contrast to other disinfectants like hydrogen peroxide and benzalkonium chloride. This suggests IOL's potential as a specific and non-damaging disinfectant against SARS-CoV-2.
Our experimental procedures involved direct incubation of IOL with live SARS-CoV-2 virus obtained from patient throat swabs and subsequent evaluation of viral viability in cells expressing the ACE2 receptor. Our findings suggest that IOL, even at dilutions of 1/1000, was able to neutralize the infectivity of SARS-CoV-2 in a time-dependent manner, with considerable efficacy evident at shorter incubation times for higher concentrations. Furthermore, the development of a colorimetric paper sensor suggests the potential for real-time IOL monitoring in sanitized environments. Collectively, these results highlight the promising potential of IOL as an effective and safe antiviral agent against SARS-CoV-2.
In recent research on the antiviral effects of various agents on SARS-CoV-2, the use of IOL has emerged as a potential breakthrough. Leveraging its unique composition saturated with hydrophilic oxygen and oxidizing nitrogen species, IOL demonstrated a robust capacity to neutralize SARS-CoV-2 in vitro, which was assessed using a 'challenge test' with cells expressing the high-affinity ACE2 receptor, pivotal for the virus's entry. These findings highlighted a dose-dependent and time-sensitive efficacy of IOL, where even at extreme dilutions such as 1/10,000, there was a significant viral load reduction, with complete inactivation evident at lesser dilutions after extended incubation. The study also emphasized IOL's safety profile, evidenced by its non-cytotoxic nature in Vero cells and the absence of erythematous reactions on human skin, a stark contrast to agents like benzalkonium chloride. However, it is critical to compare these findings with previous research to determine the relative efficacy, specificity, and safety of the IOL compared to other potential antiviral agents.
The ongoing battle against the COVID-19 pandemic has seen numerous preventive strategies employed, both specific and non-specific [
23]. The significance of these preventive strategies is highlighted by the variable stability of the SARS-CoV-2 virus in different environments, as established by prior research [
24]. Studies have outlined the persistence of the virus on diverse surfaces and under different temperature conditions. It has been reported that SARS-CoV-2 remains viable on surfaces such as plastic and stainless steel for up to 2-3 days [
4]. Our current findings reveal a potent antiviral effect of IOL, an innovative solution that challenges the traditionally accepted duration of SARS-CoV-2 viability on surfaces.
While homes have been identified as significant transmission sites, with previous studies showing 46% of household surfaces testing positive for SARS-CoV-2 a month after symptoms receded [
25]. This suggests an enhanced protection level, especially when compared to the limited data on household SARS-CoV-2 contamination from earlier research [
26].
In outpatient settings, prior studies have identified contamination on diverse fixtures and equipment [
27]. The application of IOL in these settings, as per our results, offers promising evidence of reduced contamination, potentially ensuring safer patient interactions and reduced transmission rates. The high contamination rate in hospital settings, such as a study showing more than half of isolation rooms having at least one contaminated surface [
28], emphasizes the need for effective disinfection methods. In our research, when surfaces were treated with IOL, a notable decline in detectable SARS-CoV-2 was observed.
General observations from previous studies indicate that coronaviruses have a longer survival rate on nonporous materials like surgical masks than on absorbent ones like cotton [
28]. However, the substantial spacing between the trans-pericapsid spike proteins renders large sections of its lipid mantle susceptible to peroxidation by oxidizing agents. Moreover, the prominently electrophilic nature of the viral spike protein facilitates its denaturation by anionic oxidizing entities like superoxide anion (O2-) and peroxynitrite (ONOO-). Even though the latter appears minimally in the radical aqueous blend, their presence significantly influences the virus's susceptibility to IOL. Therefore, IOL's potential as a disinfectant in real-world settings such as households, hospitals, and clinics is accentuated, especially when integrated with colorimetric sensors to confirm adequate disinfection levels, ensuring both efficacy and safety [29, 30].
While our study demonstrates the antiviral efficacy of IOL against wild SARS-CoV-2 viruses, it is pertinent to acknowledge certain limitations. Firstly, our research relied on in vitro tests using the Vero cell line, which might not fully replicate the in vivo dynamics and interactions of the virus in human tissue. The study focused on two SARS-CoV-2 variants (omicron and delta) and may not represent the antiviral activity against other variants or future mutations. The use of PCR cycle thresholds as a surrogate measure for viral infectivity, while practical, may not capture the complete infectious potential of residual viral particles. Additionally, the oxidative action of IOL on complex biological fluids was mainly assessed on human plasma, which might not necessarily extrapolate its efficacy in other biological matrices. Lastly, the assessment of IOL's safety, although comprehensive, was restricted to short-term observations, leaving potential long-term effects unexplored.
Given the demonstrated efficacy of IOL in neutralizing the SARS-CoV-2 virus, even at significant dilutions, coupled with its noted safety profile in both in vitro and in vivo models, it becomes a promising candidate for environmental disinfection in confined spaces, in hospitals, clinics, and households to limit the spread of the virus. Furthermore, the development of solid colorimetric sensors to detect the presence of IOL ensures effective disinfection while negating any cytotoxic effects. It is noteworthy that liquid oxygen derivatives have been already proposed, because of their safety and efficacy, for medical treatments [
31].
5. Conclusions
The results obtained from the experimental study reported above indicate that the use of IOL, is a new powerful, safe, and effective tool for combating the pandemic COVID-19. IOL has shown itself to be particularly effective both in the asepsis of mucous membranes positive for COVID-19 and for topical cutaneous prevention treatment. Furthermore, the use of IOL has proved to be useful in the disinfection of confined spaces, intended for public use such as shops, restaurants, shopping centers, etc., and both to fragile subjects, such as protected residences, medical surgeries.
Author Contributions
Conceptualization, A.I. and G.B.; methodology, Z.K. and A.P.; validation, A.P., and Z.Z.; formal analysis, A.P.; investigation, A.I.; resources, A.I., data curation A.I,; C.A; U.B.; writing—original draft preparation, Z.K. and A.I.; writing—review and editing, A.P.; visualization, C.A; U.P; G.B.; supervision, A.I.; project administration, A.I. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (Liguria Region Ethics Committee, protocol registry number 163/2020). A subsequent protocol amendment, reviewed by the same local ethics committee before the conduction of the present study, was approved in May 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Acknowledgments
Authors want to thank Prof. Andrea Orsi and Dr. Bianca Bruzzone (IRCCS San Martino, Genoa, Italy) for providing Covid 19 swabs.
Conflicts of Interest
“The authors declare no conflict of interest.”.
References
- Reich, P., & Elward, A. (2022). Infection Prevention during the Coronavirus Disease 2019 Pandemic. Infectious disease clinics of North America, 36(1), 15–37. [CrossRef]
- Bellino S. (2022). COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients. Annals of medicine, 54(1), 2856–2860. [CrossRef]
- Harvey, W.T., Carabelli, A.M., Jackson, B. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19, 409–424 (2021). [CrossRef]
- Geng, Y., & Wang, Y. (2023). Stability and transmissibility of SARS-CoV-2 in the environment. Journal of Medical Virology, 95(1), e28103. [CrossRef]
- Marcenac, P., Park, G. W., Duca, L. M., Lewis, N. M., Dietrich, E. A., Barclay, L., ... & Pevzner, E. (2021). Detection of SARS-CoV-2 on Surfaces in Households of Persons with COVID-19. International journal of environmental research and public health, 18(15), 8184. [CrossRef]
- Maestre, J. P., Jarma, D., Jia-Rong, F. Y., Siegel, J. A., Horner, S. D., & Kinney, K. A. (2021). Distribution of SARS-CoV-2 RNA signal in a home with COVID-19 positive occupants. Science of the Total Environment, 778, 146201.
- Chia, P. Y., Coleman, K. K., Tan, Y. K., Ong, S. W. X., Gum, M., Lau, S. K., & Singapore 2019 Novel Coronavirus Outbreak Research Team. (2020). Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 11, 2800.
- Paton, S., Spencer, A., Garratt, I., Thompson, K. A., Dinesh, I., Aranega-Bou, P., & Pottage, T. (2021). Persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and viral RNA in relation to surface type and contamination concentration. Applied and Environmental Microbiology, 87(14), e00526-21. [CrossRef]
- Van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., ... & Munster, V. J. (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England journal of medicine, 382(16), 1564-1567.
- Pastorino, B., Touret, F., Gilles, M., de Lamballerie, X., & Charrel, R. N. (2020). Prolonged infectivity of SARS-CoV-2 in fomites. Emerging infectious diseases, 26(9), 2256.
- Riddell, S., Goldie, S., Hill, A., Eagles, D., & Drew, T. W. (2020). The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virology journal, 17(1), 1-7. [CrossRef]
- Liu, H., Fei, C., Chen, Y., Luo, S., Yang, T., Yang, L., & Song, J. (2021). Investigating SARS-CoV-2 persistent contamination in different indoor environments. Environmental research, 202, 111763.
- Morris, D. H., Yinda, K. C., Gamble, A., Rossine, F. W., Huang, Q., Bushmaker, T., & Lloyd-Smith, J. O. (2021). Mechanistic theory predicts the effects of temperature and humidity on inactivation of SARS-CoV-2 and other enveloped viruses. Elife, 10, e65902.
- Hirose, R., Nakaya, T., Naito, Y., Daidoji, T., Watanabe, Y., Yasuda, H., & Itoh, Y. (2017). Viscosity is an important factor of resistance to alcohol-based disinfectants by pathogens present in mucus. Scientific reports, 7(1), 13186. [CrossRef]
- Morton, H. E. (1950). The relationship of concentration and germicidal efficiency of ethyl alcohol. Annals of the New York Academy of Sciences, 53(1), 191-196. [CrossRef]
- Zinchenko, A. A., Sergeyev, V. G., Yamabe, K., Murata, S., & Yoshikawa, K. (2004). DNA compaction by divalent cations: structural specificity revealed by the potentiality of designed quaternary diammonium salts. ChemBioChem, 5(3), 360-368.
- Gerba, C. P. (2015). Quaternary ammonium biocides: efficacy in application. Applied and environmental microbiology, 81(2), 464-469.
- World Health Organization. (2020). Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts: interim guidance, 2020 (No. WHO/2019-nCov/IPC/HomeCare/2020.3). World Health Organization.
- Izzotti, A., Fracchia, E., Au, W., Colombo, M., Pfeffer, U., Emionite, L., & Pulliero, A. (2021). Prevention of COVID-19 infection and related complications by ozonized oils. Journal of Personalized Medicine, 11(3), 226. [CrossRef]
- Izzotti A, La Maestra S, Micale RT, Longobardi MG, Saccà SC. Genomic and post-genomic effects of anti-glaucoma drugs preservatives in trabecular meshwork. Mutat Res. 2015; 772:1-9. [CrossRef]
- Beckman, J. S., & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. AmericanJournal of Physiology-cell physiology, 271(5), C1424-C1437.
- Asif, M., Xu, Y., Xiao, F., & Sun, Y. (2021). Diagnosis of COVID-19, vitality of emerging technologies and preventive measures. Chemical Engineering Journal, 423, 130189. [CrossRef]
- Aboubakr, H. A., Sharafeldin, T. A., & Goyal, S. M. (2021). Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transboundary and emerging diseases, 68(2), 296-312. [CrossRef]
- Park, S. E. (2020). Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clinical and experimental pediatrics, 63(4), 119.
- Bedrosian, N., Mitchell, E., Rohm, E., Rothe, M., Kelly, C., String, G., & Lantagne, D. (2020). A systematic review of surface contamination, stability, and disinfection data on SARS-CoV-2 (through July 10, 2020). Environmental Science & Technology, 55(7), 4162-4173.
- Onakpoya, I. J., Heneghan, C. J., Spencer, E. A., Brassey, J., Plüddemann, A., Evans, D. H., ... & Jefferson, T. (2021). SARS-CoV-2 and the role of fomite transmission: a systematic review. F1000Research, 10.
- Montalli, V. A. M., Freitas, P. R. D., Torres, M. D. F., Torres Junior, O. D. F., Vilhena, D. H. M. D., Junqueira, J. L. C., & Napimoga, M. H. (2021). Biosafety devices to control the spread of potentially contaminated dispersion particles. New associated strategies for health environments. PloS one, 16(8), e0255533. [CrossRef]
- Owen, L., Shivkumar, M., Cross, R. B., & Laird, K. (2021). Porous surfaces: stability and recovery of coronaviruses. Interface Focus, 12(1), 20210039. [CrossRef]
- Rapporto ISS COVID-19 n. 25/2020 - Raccomandazioni ad interim sulla sanificazione di strutture non sanitarie nell’attuale emergenza COVID-19: superfici, ambienti interni e abbigliamento. Versione del 15 maggio 2020.
- Liu, Q., Brookbank, L., Ho, A., Coffey, J., Brennan, A. B., & Jones, C. J. (2020). Surface texture limits transfer of S. aureus, T4 bacteriophage, influenza B virus and human coronavirus. PLoS One, 15(12), e0244518. [CrossRef]
- Giovanni Barco, Emilia Bramanti, Massimo Onor, Edoardo Benedetti, Marina Mameli, Andrea Mangano, Alessandro Pascone, Ubaldo Prati; Polyatomic Liquid Oxygen (PLO®): A new methodology for the production in aqueous solution of reactive oxygen and nitrogen species (RONS) to be applied in medical treatments. AIP Advances 1 December 2021; 11 (12): 125218.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).