Submitted:
18 January 2024
Posted:
19 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Spiniferites morphogroup: including all Spiniferites and Achomosphaera specimens;
- Batiacasphaera morphogroup: including B. sphaerica, B. sp. A, B. micropapillata, and B. hirsuta;
- Operculodinium morphogroup: including all Operculodinium specimens;
- Systematophora morphogroup: includes S. placacantha and S. ?ancyrea specimens. The latter differed from S. placacantha due to incomplete or absent proximal ridges;
- Lingulodinium morphogroup: including Lingulodinium machaerophorum and a single specimen of Lingulodinium sp. A;
- Nematosphaeropsis morphogroup: including N. labyrinthus;
- Impagidinium morphogroup: including Impagidinium sp. and I. strialatum specimens.
- Polysphaeridium morphogroup: including P. subtile and P. zoharyi;
- Black, opaque phytoclasts representing the most resistant, coalified, land plant remains;
- Dark brown phytoclasts, which are land plant remains, usually equidimensional, translucent (or with translucent edges), remaining in structureless wood or cortical tissue;
- Cuticle group, including structured land plant tissue remains with usually preserved cell structure; these are commonly the remains of the leaf epidermis;
- Pollen grains of angiosperms and gymnosperms; the most common are bisaccate pollen grains of gymnosperms;
- Pteridophyte spores;
- Dinoflagellate cysts;
- Algae and acritarch group, including algae other than dinoflagellate cysts, such as Prasinophyte (e.g., Tasmanites, Cymatiosphaera, and Leiosphaera), and incertae sedis microfossils (i.e., acritarchs);
- Foraminifera organic linings (zooclasts), which are the organic cells of foraminifera;
- Amorphous organic matter (AOM), which represents structureless particles of uncertain origin (most likely marine in the case of the present study material) being a stage of bacterial decay of organic particles in oxygen–depleted environment.
3. Results
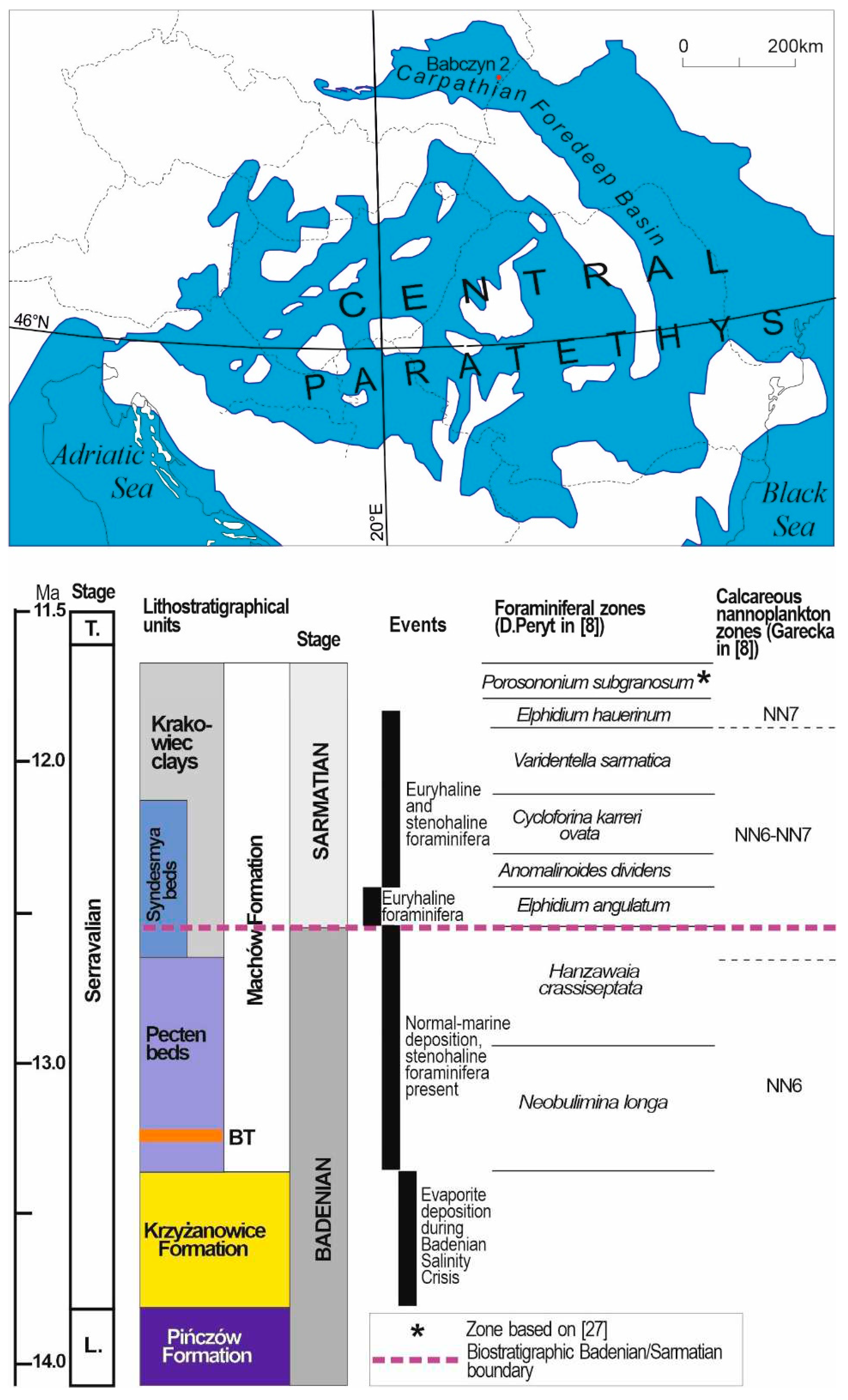
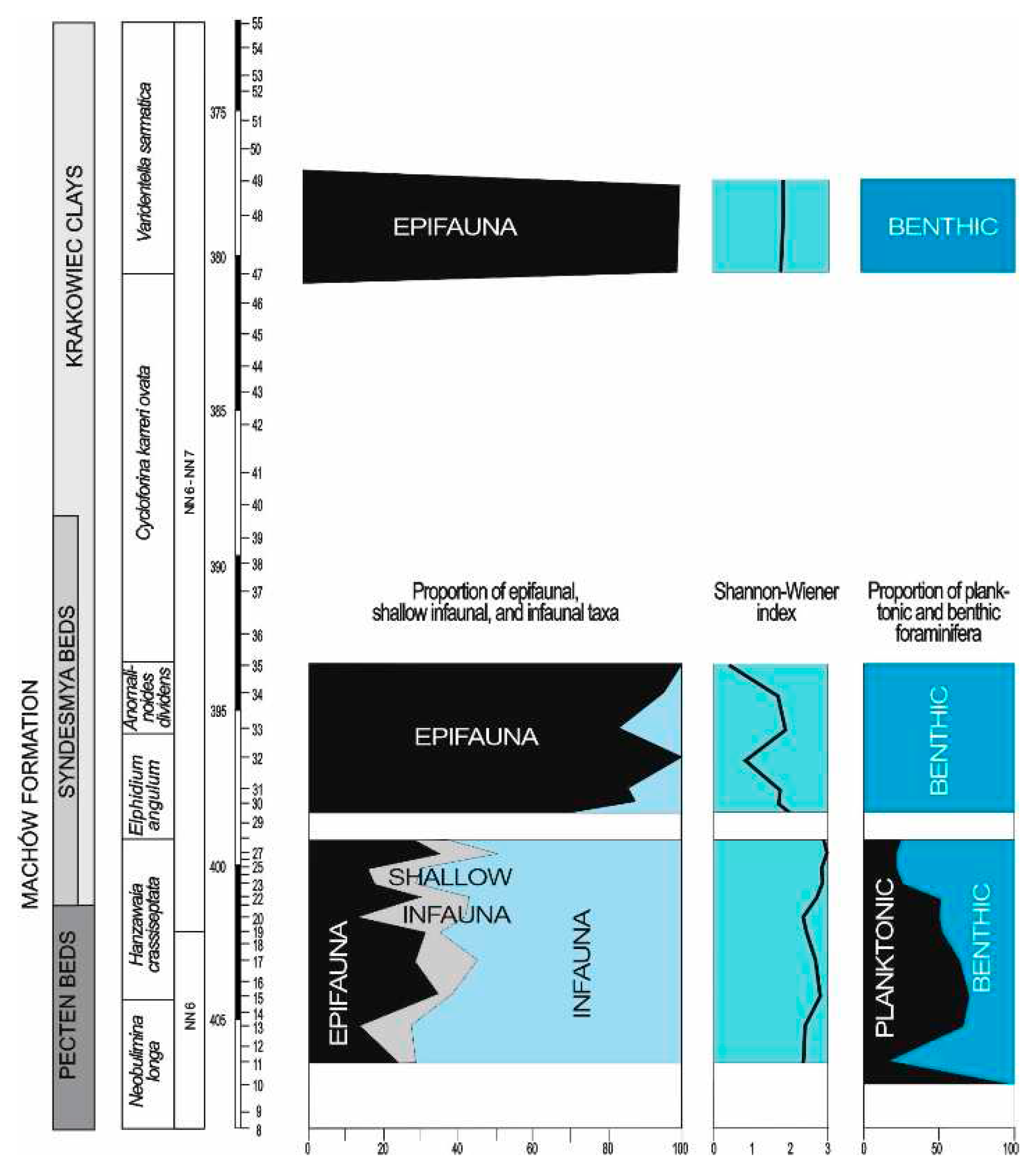
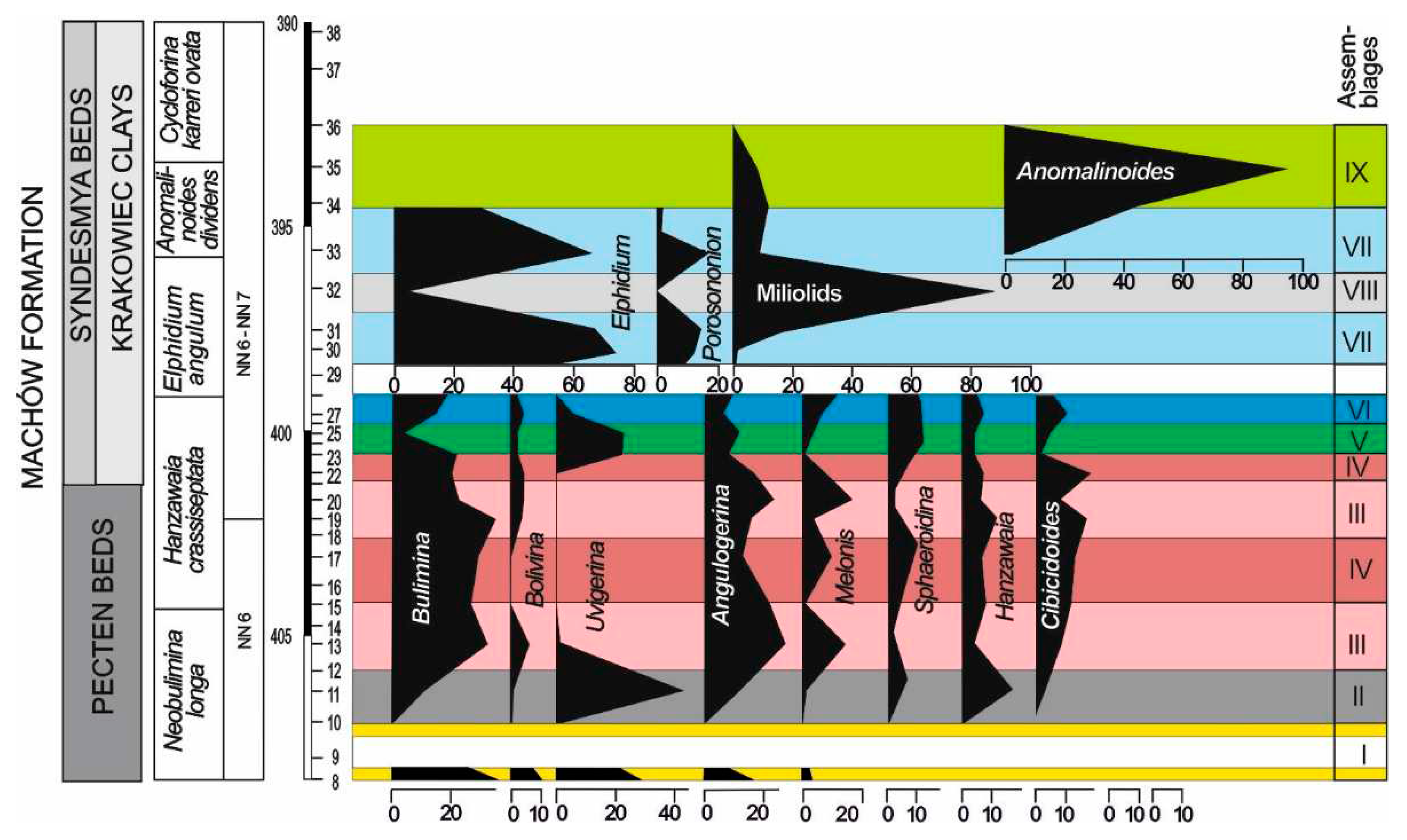
3.1. Foraminifera
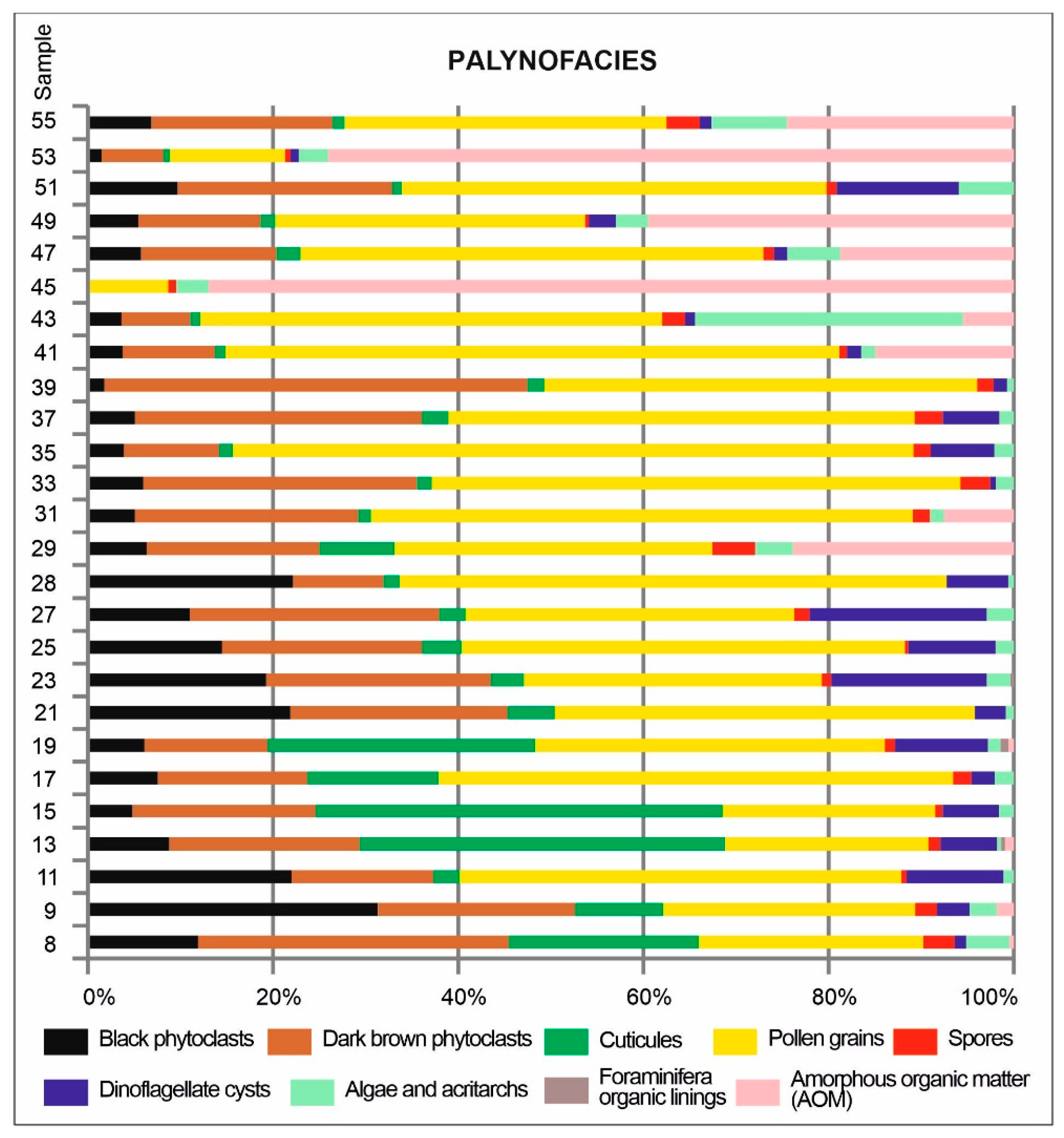
3.2. Palynofacies
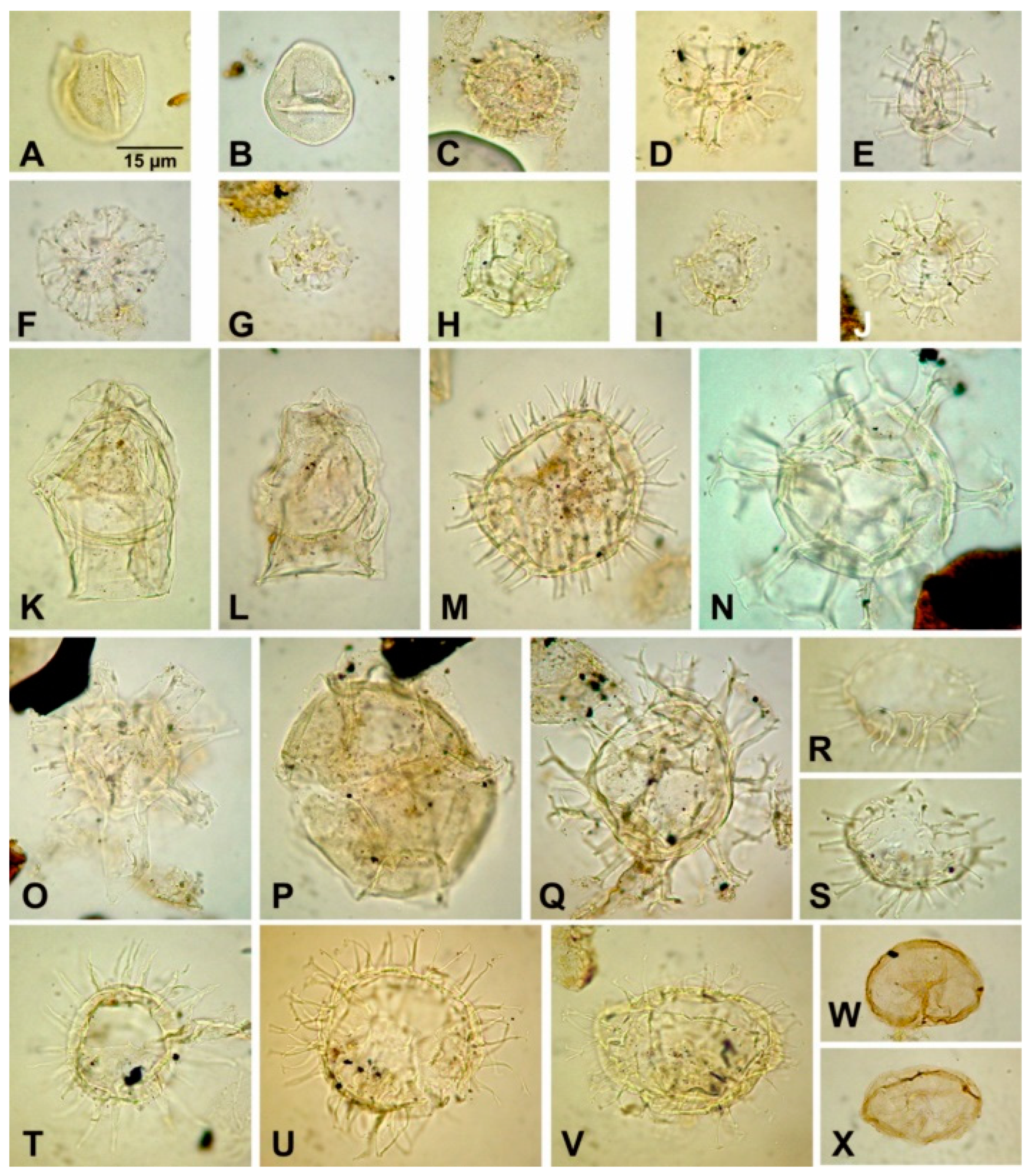
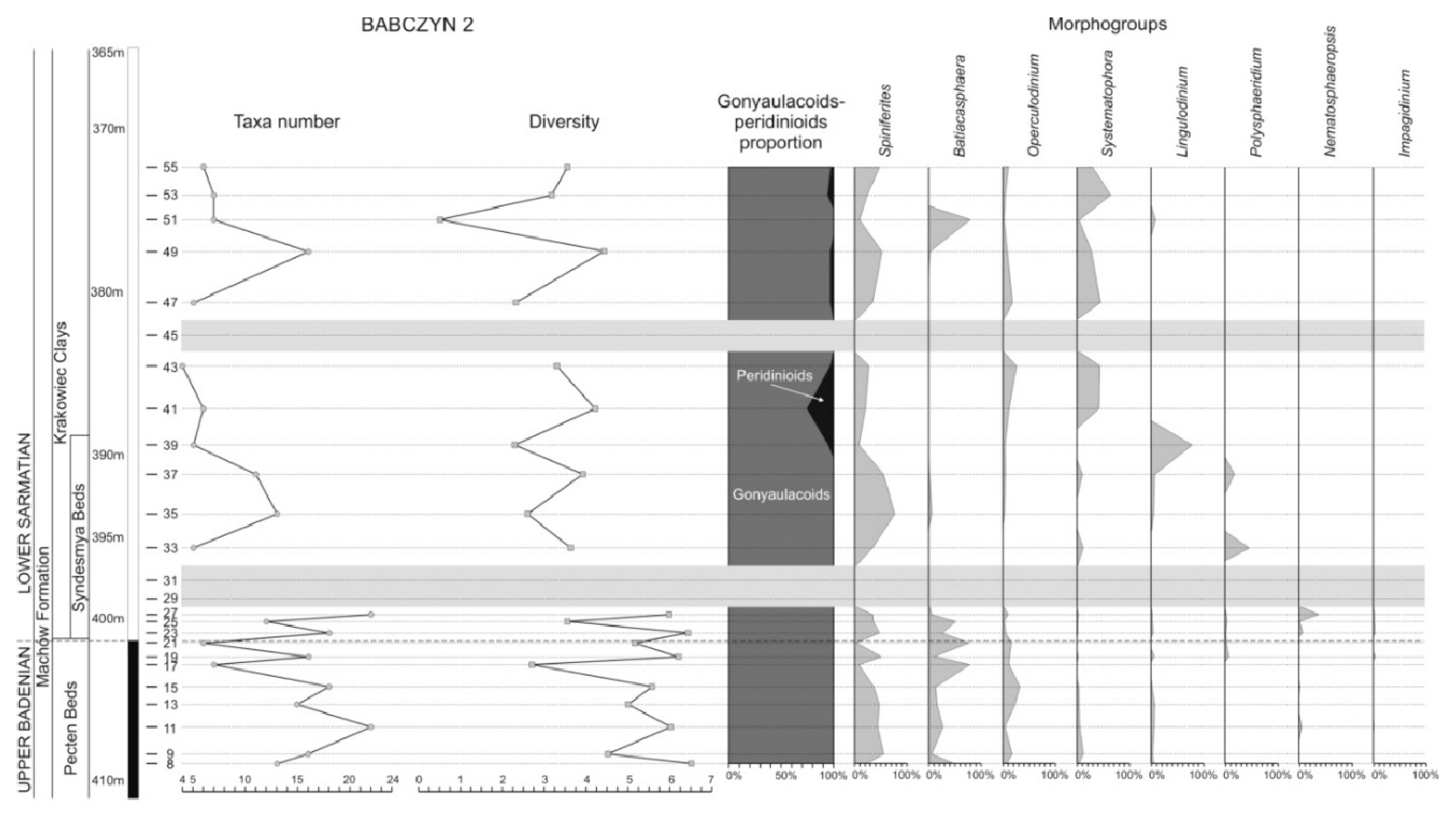
3.3. Dinoflagellate cysts
| Age | Upper Badenian | ||||||||||||
| Lithostratigraphy | Machów Formation | ||||||||||||
| Pecten Beds | Syndesmya Beds | ||||||||||||
| Sample | 8 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 | 25 | 27 | 28 | |
| DINOFLAGELLATE CYSTS | |||||||||||||
| 1. | Pentadinium laticinctum | 13 | 1 | 2 | 2 | 1 | 4 | 1 | |||||
| 2. | Batiacasphaera sphaerica | 68 | 3 | 3 | 4 | 5 | 3 | 1 | 20 | 22 | 3 | 2 | |
| 3. | Spiniferites ramosus s.l. | 81 | 162 | 135 | 120 | 117 | 5 | 138 | 2 | 153 | 65 | 109 | 128 |
| 4. | Batiacasphaera sp. A | 79 | 12 | 87 | 43 | 41 | 44 | 32 | 16 | 51 | 1 | ||
| 5. | Operculodinium centrocarpum | 27 | 51 | 5 | 59 | 96 | 5 | 37 | 9 | 18 | 3 | 25 | 29 |
| 6. | Melitasphaeridium ?pseudorecurvatum | 3 | |||||||||||
| 7. | Homotryblium tenuispinosum * | * | * | * | |||||||||
| 8. | Melitasphaeridium choanophorum | 4 | 15 | 3 | 13 | 4 | 6 | 3 | 12 | ||||
| 9. | Pyxidinopsis psilata | 7 | 3 | 1 | 1 | 2 | 1 | 12 | |||||
| 10. | Systematophora placacantha | 6 | 41 | 16 | 10 | 12 | 5 | 2 | 1 | 7 | 3 | ||
| 11. | Lingulodinium machaerophorum | 9 | 5 | 11 | 14 | 2 | 15 | 12 | 3 | 5 | |||
| 12. | Labyrinthodinium truncatum | 5 | 10 | 12 | 7 | 2 | 2 | 2 | |||||
| 13. | Reticulatosphaera actinocoronata | 1 | 3 | 5 | 1 | 6 | 18 | 6 | 2 | ||||
| 14. | Dapsilidinium sp. | 2 | |||||||||||
| 15. | Operculodinium sp. C | 2 | |||||||||||
| 16. | Spiniferites pseudofurcatus | 1 | 2 | 1 | 3 | 5 | |||||||
| 17. | Hystrichostrogylon sp. | 1 | |||||||||||
| 18. | Reticulatosphaera ? sp. | 1 | |||||||||||
| 19. | Spiniferites sp. A | 3 | 4 | 4 | 2 | 2 | 3 | 3 | 6 | ||||
| 20. | Lejeunecysta sp. | 1 | 1 | 2 | |||||||||
| 21. | Hystrichosphaeropsis obscura | 1 | 4 | ||||||||||
| 22. | Pyxidinopsis ? sp. | 1 | 1 | ||||||||||
| 23. | Cordosphaeridium cf. minimum | 2 | 1 | 8 | 1 | ||||||||
| 24. | Melitasphaeridium pseudorecurvatum | 1 | 1 | 1 | 1 | 1 | 4 | 1 | |||||
| 25. | Nematosphaeropsis labyrinthus | 6 | 1 | 15 | 1 | 119 | |||||||
| 26. | Cerodinium sp.* | * | * | ||||||||||
| 27. | Homotryblium plectilum * | * | * | * | |||||||||
| 28. | Impagidinium sp. | 1 | 1 | 6 | 5 | 1 | |||||||
| 29. | Hystrichokolpoma rigaudiae | 2 | 1 | 1 | 2 | ||||||||
| 30. | Cordosphaeridium minimum | 1 | 1 | 1 | 3 | ||||||||
| 31. | Operculodinium sp. B | 4 | 2 | ||||||||||
| 32. | Wetzeliella sp.* | * | * | ||||||||||
| 33. | Charlesdowniea sp.* | * | |||||||||||
| 34. | Operculodinium sp. A | 1 | 2 | 1 | |||||||||
| Age | Lower Sarmatian | ||||||||||||||||||||||
| Lithostratigraphy | Machów Formation | ||||||||||||||||||||||
| Syndesma Beds | Krakowiec Clays | ||||||||||||||||||||||
| Sample | 29 | 31 | 33 | 35 | 37 | 39 | 41 | 43 | 47 | 49 | 51 | 53 | 55 | ||||||||||
| 1. | Pentadinium laticinctum | 3 | 1 | 2 | 13 | 1 | |||||||||||||||||
| 2. | Batiacasphaera sphaerica | 9 | 1 | ||||||||||||||||||||
| 3. | Spiniferites ramosus s.l. | 12 | 242 | 135 | 3 | 25 | 24 | 52 | 141 | 12 | 31 | 50 | |||||||||||
| 4. | Batiacasphaera sp. A | 1 | 127 | ||||||||||||||||||||
| 5. | Operculodinium centrocarpum | 8 | 10 | 2 | 22 | 3 | |||||||||||||||||
| 6. | Melitasphaeridium ?pseudorecurvatum | ||||||||||||||||||||||
| 7. | Homotryblium tenuispinosum * | ||||||||||||||||||||||
| 8. | Melitasphaeridium choanophorum | 2 | |||||||||||||||||||||
| 9. | Pyxidinopsis psilata | ||||||||||||||||||||||
| 10. | Systematophora placacantha | 4 | 24 | 3 | 71 | 72 | 3 | 78 | 31 | ||||||||||||||
| 11. | Lingulodinium machaerophorum | 13 | 15 | 27 | 13 | ||||||||||||||||||
| 12. | Labyrinthodinium truncatum | 1 | |||||||||||||||||||||
| 13. | Reticulatosphaera actinocoronata | 1 | 25 | ||||||||||||||||||||
| 14. | Dapsilidinium sp. | 2 | 1 | ||||||||||||||||||||
| 15. | Operculodinium sp. C | ||||||||||||||||||||||
| 16. | Spiniferites pseudofurcatus | 1 | |||||||||||||||||||||
| 17. | Hystrichostrogylon sp. | ||||||||||||||||||||||
| 18. | Reticulatosphaera ? sp. | ||||||||||||||||||||||
| 19. | Spiniferites sp. A | 6 | 1 | ||||||||||||||||||||
| 20. | Lejeunecysta sp. | 3 | 1 | ||||||||||||||||||||
| 21. | Hystrichosphaeropsis obscura | ||||||||||||||||||||||
| 22. | Pyxidinopsis ? sp. | ||||||||||||||||||||||
| 23. | Cordosphaeridium cf. minimum | 1 | 1 | 5 | 12 | 9 | |||||||||||||||||
| 24. | Melitasphaeridium pseudorecurvatum | 1 | |||||||||||||||||||||
| 25. | Nematosphaeropsis labyrinthus | ||||||||||||||||||||||
| 26. | Cerodinium sp.* | ||||||||||||||||||||||
| 27. | Homotryblium plectilum * | ||||||||||||||||||||||
| 28. | Impagidinium sp. | 1 | |||||||||||||||||||||
| 29. | Hystrichokolpoma rigaudiae | ||||||||||||||||||||||
| 30. | Cordosphaeridium minimum | 2 | 3 | 6 | |||||||||||||||||||
| 31. | Operculodinium sp. B | ||||||||||||||||||||||
| 32. | Wetzeliella sp.* | ||||||||||||||||||||||
| 33. | Charlesdowniea sp.* | ||||||||||||||||||||||
| 34. | Operculodinium sp. A | ||||||||||||||||||||||
| Age | Upper Badenian | ||||||||||||||||||||||
| Lithostratigraphy | Machów Formation | ||||||||||||||||||||||
| Pecten Beds | Syndesmya Beds | ||||||||||||||||||||||
| Sample | 8 | 9 | 11 | 13 | 15 | 17 | 19 | 21 | 23 | 25 | 27 | 28 | |||||||||||
| DINOFLAGELLATE CYSTS | |||||||||||||||||||||||
| 35. | Areosphaeridium sp.* | * | |||||||||||||||||||||
| 36. | Glaphyrocysta semitecta* | * | |||||||||||||||||||||
| 37. | Areosphaeridium diktyoplokum* | * | * | * | |||||||||||||||||||
| 38. | Deflandrea phosphoritica* | * | * | ||||||||||||||||||||
| 39. | Lingulodinium sp. A | 1 | |||||||||||||||||||||
| 40. | Cleistosphaeridium sp. | 3 | |||||||||||||||||||||
| 41. | Areoligera sp.* | * | * | ||||||||||||||||||||
| 42. | Polysphaeridium subtile | 19 | 1 | 5 | 5 | ||||||||||||||||||
| 43. | Cordosphaeridium sp.* | * | * | ||||||||||||||||||||
| 44. | Batiacasphaera micropapillata | 6 | |||||||||||||||||||||
| 45. | Polysphaeridium zoharyi | 1 | 5 | ||||||||||||||||||||
| 56. | Batiacasphaera hirsuta | 13 | 95 | 10 | 39 | ||||||||||||||||||
| 47. | Membranophoridium aspinatum * | * | |||||||||||||||||||||
| 48. | Impagidinium pallidum | 1 | 3 | 2 | |||||||||||||||||||
| 49. | Areosphaeridium michoudii * | * | |||||||||||||||||||||
| 50. | Operculodinium ? sp. | 1 | 5 | 2 | |||||||||||||||||||
| 51. | Palaeohystrichophora ? sp.* | * | |||||||||||||||||||||
| 52. | Cleistosphaeridium sp. A* | * | |||||||||||||||||||||
| 53. | Operculodinium sp. D | 3 | |||||||||||||||||||||
| 54. | Operculodinium sp. E | 1 | |||||||||||||||||||||
| 55. | Operculodinium sp. F | 7 | |||||||||||||||||||||
| 56. | Hystrichokolpoma sp. | 3 | |||||||||||||||||||||
| 57. | Achomosphaera sp. | 21 | |||||||||||||||||||||
| 58. | Pentadinium sp. A | ||||||||||||||||||||||
| 59. | Selenopemphix brevispinosa? | ||||||||||||||||||||||
| 60. | Hystrichosphaeropsis sp. | ||||||||||||||||||||||
| 61. | Selenopemphix nephroides | ||||||||||||||||||||||
| 62. | Systematophora ?ancyrea | ||||||||||||||||||||||
| 63. | Operculodinium? sp. | ||||||||||||||||||||||
| 64. | Surculosphaeridium longifurcatum* | ||||||||||||||||||||||
| 65. | Phthanoperidinium comatum* | ||||||||||||||||||||||
| 66. | Isabelidinium sp.* | ||||||||||||||||||||||
| TOTAL COUNTS | 305 | 298 | 321 | 266 | 313 | 60 | 289 | 54 | 319 | 185 | 314 | 276 | |||||||||||
| Age | Lower Sarmatian | |||||||||||||
| Lithostratigraphy | Machów Formation | |||||||||||||
| Syndesma Beds | Krakowiec Clays | |||||||||||||
| Sample | 29 | 31 | 33 | 35 | 37 | 39 | 41 | 43 | 47 | 49 | 51 | 53 | 55 | |
| 35. | Areosphaeridium sp.* | |||||||||||||
| 36. | Glaphyrocysta semitecta* | |||||||||||||
| 37. | Areosphaeridium diktyoplokum* | |||||||||||||
| 38. | Deflandrea phosphoritica* | |||||||||||||
| 39. | Lingulodinium sp. A | |||||||||||||
| 40. | Cleistosphaeridium sp. | |||||||||||||
| 41. | Areoligera sp.* | |||||||||||||
| 42. | Polysphaeridium subtile | 16 | 39 | |||||||||||
| 43. | Cordosphaeridium sp.* | |||||||||||||
| 44. | Batiacasphaera micropapillata | |||||||||||||
| 45. | Polysphaeridium zoharyi | 3 | ||||||||||||
| 56. | Batiacasphaera hirsuta | 1 | ||||||||||||
| 47. | Membranophoridium aspinatum * | |||||||||||||
| 48. | Impagidinium pallidum | |||||||||||||
| 49. | Areosphaeridium michoudii * | |||||||||||||
| 50. | Operculodinium ? sp. | |||||||||||||
| 51. | Palaeohystrichophora ? sp.* | |||||||||||||
| 52. | Cleistosphaeridium sp. A* | |||||||||||||
| 53. | Operculodinium sp. D | |||||||||||||
| 54. | Operculodinium sp. E | |||||||||||||
| 55. | Operculodinium sp. F | |||||||||||||
| 56. | Hystrichokolpoma sp. | 1 | 2 | |||||||||||
| 57. | Achomosphaera sp. | |||||||||||||
| 58. | Pentadinium sp. A | 8 | 1 | |||||||||||
| 59. | Selenopemphix brevispinosa? | 2 | 2 | 3 | 1 | |||||||||
| 60. | Hystrichosphaeropsis sp. | 1 | 1 | |||||||||||
| 61. | Selenopemphix nephroides | 42 | 3 | 3 | 1 | 4 | 2 | |||||||
| 62. | Systematophora ?ancyrea | 65 | 36 | |||||||||||
| 63. | Operculodinium? sp. | 18 | 21 | 28 | 4 | 10 | ||||||||
| 64. | Surculosphaeridium longifurcatum* | * | ||||||||||||
| 65. | Phthanoperidinium comatum* | * | ||||||||||||
| 66. | Isabelidinium sp.* | * | ||||||||||||
| TOTAL COUNTS | 0 | 0 | 36 | 313 | 236 | 35 | 159 | 84 | 155 | 274 | 161 | 131 | 103 | |
3.4. Other aquatic palynomorphs
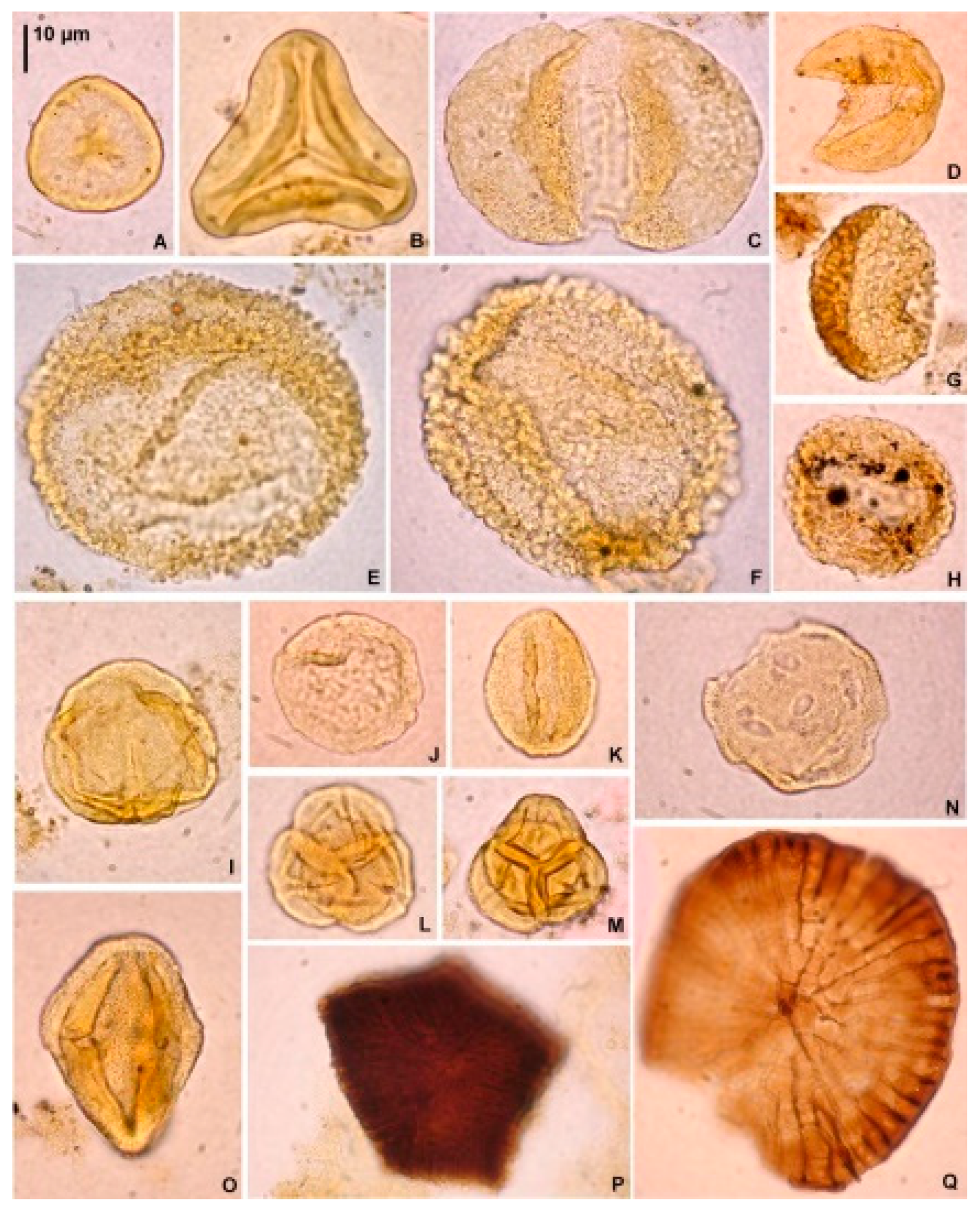
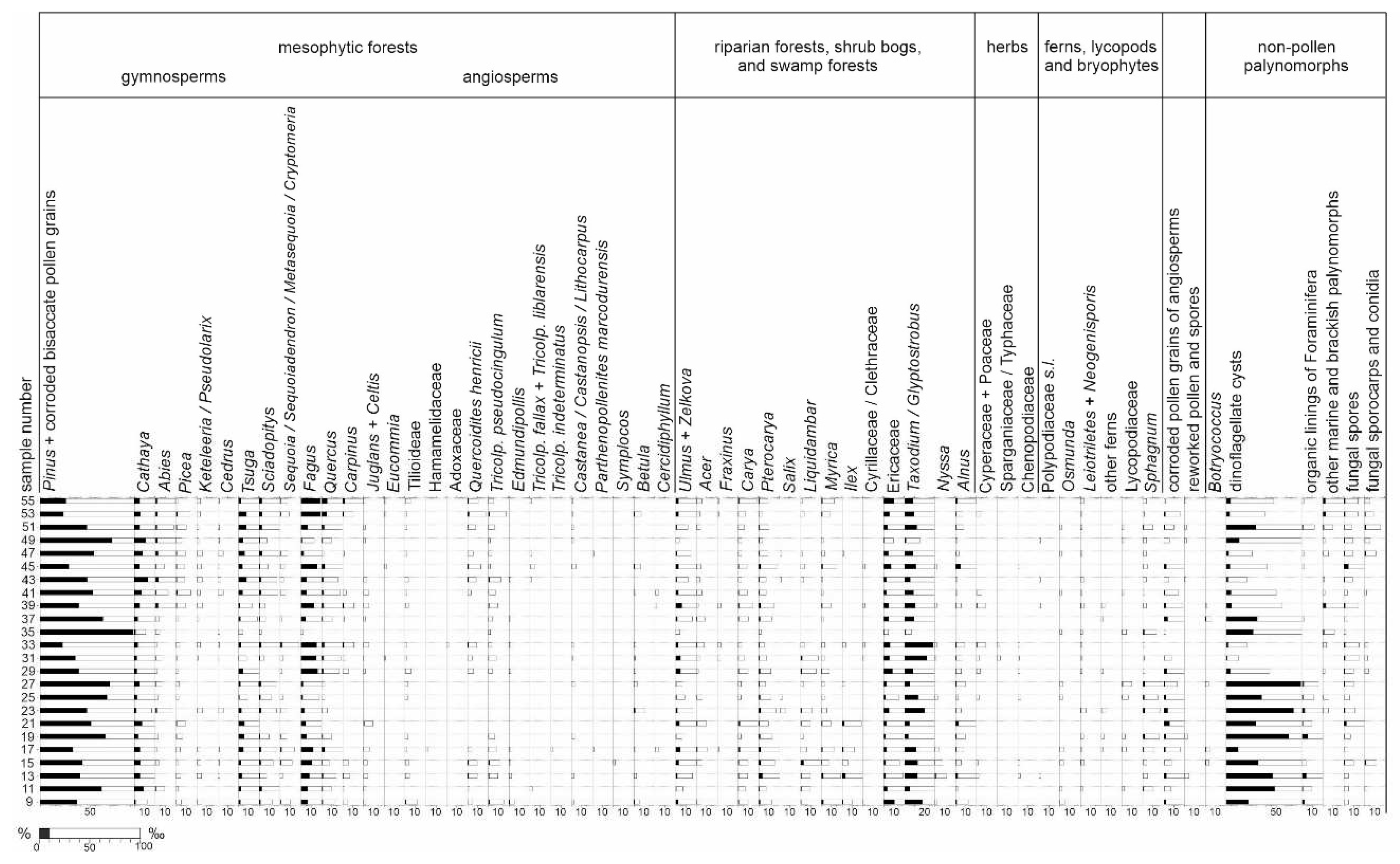
3.5. Spore-pollen analysis
| FOSSIL TAXON | BOTANICAL AFFINITY | ELEMENT |
|---|---|---|
| Spores of plants: | ||
| Baculatisporites sp. | Osmundaceae: Osmunda | P/A |
| Camarozonosporites sp. | Lycopodiaceae: Lycopodiella sect. Campylostachys | P |
| Distverrusporis sp. | Sphagnaceae: Sphagnum | P/A |
| Laevigatosporites sp. | Polypodiaceae, Davalliaceae, and other ferns | P/A |
| Leiotriletes sp. | Lygodiaceae and other ferns | P |
| Neogenisporis sp. | Gleicheniaceae, Cyatheaceae | P1 |
| Retitriletes sp. | Lycopodiaceae: Lycopodium | A |
| Stereisporites sp. | Sphagnaceae: Sphagnum | P/A |
| Verrucatosporites sp. | Davalliaceae, Polypodiaceae, and other ferns | P/A |
| Pollen grains of gymnosperms: | ||
| Abiespollenites sp. | Pinaceae: Abies | A |
| Cathayapollis sp. | Pinaceae: Cathaya | A1 |
| Cedripites sp. | Pinaceae: Cedrus | A1 |
| Inaperturopollenites concedipites (Wodehouse) Krutzsch | Cupressaceae: Taxodium, Glyptostrobus | P2/A1 |
| Inaperturopollenites verrupapillatus Trevisan | Cupressaceae: Taxodium, Glyptostrobus | P2/A1 |
| Keteleeriapollenites sp. | Pinaceae: Keteleeria, Pseudolarix | A1 |
| Piceapollis praemarianus Krutzsch | Pinaceae: Picea | A |
| Piceapollis sp. | Pinaceae: Picea | A |
| Pinuspollenites labdacus (Potonié) Raatz | Pinaceae: Pinus sylvestris type | A |
| Pinuspollenites sp. | Pinaceae: Pinus | A |
| Sciadopityspollenites verticillatiformis (Zauer) Krutzsch | Sciadopityaceae: Sciadopitys | A1 |
| Sciadopityspollenites sp. | Sciadopityaceae: Sciadopitys | A1 |
| Sequoiapollenites sp. | Cupressaceae: Sequoia, Sequoiadendron, Metasequoia | A1 |
| Zonalapollenites gracilis Krutzsch | Pinaceae: Tsuga | A |
| Zonalapollenites verrucatus Krutzsch | Pinaceae: Tsuga | A |
| Zonalapollenites sp. | Pinaceae: Tsuga | A |
| Pollen grains of angiosperms: | ||
| Aceripollenites sp. | Sapindaceae: Acer | A1 |
| Alnipollenites verus Potonié | Betulaceae: Alnus | P2/A |
| Caprifoliipites sp. | Adoxaceae: Sambucus, Viburnum | P/A1 |
| Carpinipites carpinoides (Pflug) Nagy | Betulaceae: Carpinus | P2/A1 |
| Caryapollenites simplex (Potonié) Raatz | Juglandaceae: Carya | A1 |
| Celtipollenites sp. | Ulmaceae: Celtis | P/A1 |
| Cercidiphyllites minimireticulatus (Trevisan) Ziembińska-Tworzydło | Cercidiphyllaceae: Cercidiphyllum | A1 |
| Chenopodipollis sp. | Amaranthaceae (incl. Chenopodiaceae) | P/A |
| Cupuliferoipollenites oviformis (Potonié) Potonié | Fagaceae: Castanea, Castanopsis, Lithocarpus | P2/A1 |
| Cupuliferoipollenites pusillus (Potonié) Potonié | Fagaceae: Castanea, Castanopsis, Lithocarpus | P2/A1 |
| Cyperaceaepollis neogenicus Krutzsch | Cyperaceae | P/A |
| Cyrillaceaepollenites megaexactus (Potonié) Potonié | Cyrillaceae, Clethraceae | P |
| Edmundipollis sp. | Araliaceae, Cornaceae, Mastixiaceae | P/A |
| Ericipites baculatus Nagy | Ericaceae | A |
| Ericipites ericius (Potonié) Potonié | Ericaceae | A |
| Ericipites sp. | Ericaceae | A |
| Eucommiapollis minor Menke | Eucommiaceae: Eucommia | A1 |
| Faguspollenites cf. verus Raatz | Fagaceae: Fagus | A |
| Faguspollenites sp. | Fagaceae: Fagus | A |
| Fraxinipollis oblatus Słodkowska | Oleaceae: Fraxinus | P/A |
| Graminidites sp. | Poaceae: Pooideae | P/A |
| Ilexpollenites iliacus (Potonié) Thiergart | Aquifoliaceae: Ilex | P/A1 |
| Ilexpollenites margaritatus (Potonié) Thiergart | Aquifoliaceae: Ilex | P2 |
| Intratriporopollenites sp. | Malvaceae: Tilioideae | P/A1 |
| Juglanspollenites sp. | Juglandaceae: Juglans | A1/P2 |
| Myricipites sp. | Myricaceae: Myrica | P2/A1 |
| Nyssapollenites sp. | Nyssaceae: Nyssa | P2/A1 |
| Parthenopollenites marcodurensis (Pflug & Thomson) Traverse | Vitaceae | P/A1 |
| Periporopollenites orientaliformis (Nagy) Kohlman-Adamska & Ziembińska-Tworzydło | Altingiaceae: Liquidambar | A1 |
| Periporopollenites stigmosus (Potonié) Thomson & Pflug | Altingiaceae: Liquidambar | A1 |
| Polyatriopollenites stellatus (Potonié) Pflug | Juglandaceae: Pterocarya | A1 |
| Quercoidites henricii (Potonié) Potonié, Thomson et Thiergart | Fagaceae: Quercus | P2/A1 |
| Quercopollenites rubroides Kohlman-Adamska & Ziembińska-Tworzydło | Fagaceae: Quercus | A1 |
| Quercopollenites sp. | Fagaceae: Quercus | A1 |
| Salixipollenites capreaformis Planderová | Salicaceae: Salix | A |
| Salixipollenites sp. | Salicaceae: Salix | A |
| Sparganiaceaepollenites sp. | Sparganiaceae, Typhaceae | P/A |
| Symplocoipollenites vestibulum (Potonié) Potonié | Symplocaceae: Symplocos | P |
| Tricolporopollenites fallax (Potonié) Krutzsch | Fabaceae | P/A |
| Tricolporopollenites indeterminatus (Romanowicz) Ziembińska-Tworzydło | Hamamelidaceae | P2 |
| Tricolporopollenites liblarensis (Thomson) Hochuli | Fabaceae | P/A |
| Tricolporopollenites pseudocingulum (Potonié) Thomson & Pflug | Fagaceae?, Styracaceae? | P/A1 |
| Trivestibulopollenites betuloides Pflug | Betulaceae: Betula | A |
| Ulmipollenites undulosus Wolff | Ulmaceae: Ulmus | A2 |
| Ulmipollenites sp. | Ulmaceae: Ulmus | A2 |
| Zelkovaepollenites sp. | Ulmaceae: Zelkova | A1 |
4. Discussion
4.1. Aquatic palaeoenvironment
4.2. Plant communities and terrestrial palaeoenvironment
4.3. Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laskarev, V. Sur les equivalentes du Sarmatien supérieur en Serbie. In: Recueil de traveaux ofert a M. Jovan Cvijic par ses amis et collaborateurs; Vujević, P., Ed.; Državna Štamparija, Beograd, 1924; pp. 73-85.
- Harzhauser, M., Piller, W.E. Benchmark data of a changing sea — palaeogeography, palaeobiogeography and events in the Central Paratethys during the Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 253, 8-31. [CrossRef]
- Śliwiński, M., Bąbel, M., Nejbert, K., Olszewska-Nejbert, D., Gąsiewicz, A., Schreiber, B.C., Be-Nowitz, J.A., Layer, P. Badenian–Sarmatian chronostratigraphy in the Polish Carpathian Foredeep. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 326–328, 12–29. [CrossRef]
- Palcu, D.V., Kouwenhoven, T.J., Krijgsman, W. Reply to “Comment on the Badenian-Sarmatian extinction event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys (Palcu et al., 2015)” by Silye and Filipescu (2016). Glob. Planet. Change 2016, 145, 141–142.
- Silye, L., Filipescu, S. Comment on “The Badenian-Sarmatian Extinction Event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys domain” (Palcu et al., 2015). Glob. Planet. Change 2016, 145, 17–19.
- Peryt, T.M., Kasprzyk, A. Carbonate-evaporite sedimentary transitions in the Badenian (middle Miocene) basin of southern Poland. Sediment. Geol. 1992, 76, 257–271. [CrossRef]
- Peryt, D., Gedl, P., Peryt, T.M. Marine transgression(s) to evaporite basin: The case of middle Miocene (Badenian) gypsum in the Central Paratethys, SE Poland. Jour. Palaeogeogr. 2020, 9, 16. [CrossRef]
- Peryt, D., Garecka, M., Peryt, T.M. Foraminiferal and calcareous nannoplankton biostratigraphy of the upper Badenian–lower Sarmatian strata in the SE Polish Carpathian Foredeep. Geol. Quart. 2021, 65, 18. [CrossRef]
- Łuczkowska, E. Foraminiferal zones in the Miocene, south of the Holy Cross Mts. Bull. Acad. Pol. Sci., Sér. Sci. Géol. Géogr. 1963, 11, 29-34.
- Łuczkowska, E. The micropaleontological stratigraphy of the Miocene in the region of Tarnobrzeg-Chmielnik (in Polish with English summary). Pr. Geol. 1964, 20, 1-72.
- Łuczkowska, E. A new zone with Praeorbulina indigena (Foraminiferida, Globigerinidae) in the Upper Badenian (Tortonian s.s.) of Central Paratethys. Roczn. Polsk. Tow. Geol. 1971, 40, 445–448.
- Odrzywolska-Bieńkowa, E. Micropaleontological stratigraphy of the Miocene of central part of the Carpathian Foredeep (in Polish with English summary). Prz. Geol. 1975, 23, 597–603.
- Czepiec, I. Biostratigraphy and paleoenvironment of Sarmatian marginal zone of Poland (in Polish with English summary). Kwart. AGH, Geologia 1996, 22, 309–338.
- Kirchner, Z. Miocene stratigraphy of the Central Carpathian Foreland based on microfaunal studies (in Polish with English summary). Acta Geol. Pol. 1956, 6, 421-450.
- Cicha, I., Rögl, F., Rupp, Ch., Čtyroká, J. (eds.). Oligocene-Miocene foraminifera of the Central Paratethys. Abh. Senckenberg. Naturforsch. Ges. 1998, 549, 1-325.
- Filipescu, S. Anomalinoides dividens bioevent at the Badenian/Sarmatian boundary – a response to paleogeographic and paleoenvironmental changes. Studia Univ. Babeş-Bolyai, Geologia 2004, 49 (2), 21–26.
- Dumitriu, S.D., Loghin, S., Dubicka, Z., Melinte-Dobrinescu, M.C., Paruch-Kulczycka, J., Ionesi, V. Foraminiferal, ostracod, and calcareous nannofossil biostratigraphy of the latest Badenian – Sarmatian interval (Middle Miocene, Paratethys) from Poland, Romania and the Republic of Moldova. Geol. Carpath. 2017, 68, 419–444.
- Ney, R., Burzewski, W., Bachleda, T., Górecki, W., Jakóbczak, K., Słupczyński, K. Outline of paleogeography and evolution of lithology and facies of Neogene layers on the Carpathian Foredeep (in Polish with English summary). Prace Geol. 1974, 82, 3-65.
- Dziadzio, P., Maksym, A., Olszewska, B. Miocene deposition in the eastern part of the Carpathian Foredeep in Poland (in Polish with English summary). Prz. Geol. 2006, 54, 413–420.
- Alexandrowicz, S.W., Garlicki, A., Rutkowski, J. Podstawowe jednostki litostratygraficzne miocenu zapadliska przedkarpackiego (in Polish). Kwart. Geol. 1982, 26, 470-471.
- Peryt, T.M. The beginning, development and termination of the Middle Miocene Badenian salinity crisis in Central Paratethys. Sediment. Geol. 2006, 188-189. 379-396. [CrossRef]
- de Leeuw, A., Tulbure, M., Kuiper, K.F., Melinte-Dobrinescu, M.C., Stoica, M., Krijgsman, W. New 40Ar/39Ar, magnetostratigraphic and biostratigraphic constraints on the termination of the Badenian Salinity Crisis: Indications for tectonic improvement of basin interconnectivity in Southern Europe. Glob. Planet. Change 2018, 169, 1-15. [CrossRef]
- Pawłowska, K., Kubica, B. Karta otworu wiertniczego Babczyn-2 (in Polish; unpublished). Archive CUG, Warszawa, 1960.
- Jasionowski, M., Peryt, T.M. Historia badań. In: Budowa geologiczna Polski, tom I. Stratygrafia, część 3a Kenozoik Paleogen Neogen, Peryt, T.M., Piwocki, M., Eds.; Polish Geological Institute, 2004; pp. 203–212.
- Czapowski, G. Wschodnia część zapadliska. In: Budowa geologiczna Polski, tom I. Stratygrafia, część 3a Kenozoik Paleogen Neogen, Peryt, T.M., Piwocki, M., Eds.; Polish Geological Institute, 2004; pp. 239–245.
- Palcu, D.V., Golovina, L.A., Vernyhorova, Y.V., Popov, S.V., Krijgsman, W. Middle Miocene paleoenvironmental crises in Central Eurasia caused by changes in marine gateway configuration. Glob. Planet. Chang. 2017, 158, 57-71. [CrossRef]
- Odrzywolska-Bieńkowa, E. Porównanie wybranych głębszych profile mikrofaunistycznych rejonu świętokrzyskiego i lubelskiego (in Polish). Sprawozd. Posiedz. Kom. Nauk. PAN, Kraków 1972, 16, 493-494.
- Van der Zwaan, G.J. Paleoecology of late Miocene Mediterranean Foraminifera. Utrecht Micropaleont. Bull. 1982, 25.
- Culver, S. New foraminiferal depth zonation of the northwestern Gulf of Mexico. Palaios 1988, 3, 69–85. [CrossRef]
- Lutze, G.F., Thiel H. Epibenthic foraminifera from elevated microhabitats; Cibicidoides wuellerstorfi and Planulina ariminensis. Jour. Foram. Res. 1989, 19, 153–158. [CrossRef]
- Verhallen, P. Late Pliocene to early Pleistocene Mediterranean mud-dwelling foraminifera; influence of a changing environment on community structure and evolution. Utrecht Micropaleont. Bull. 1991, 40, 1–219.
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera. Longman, Avon, 1991; pp. 408.
- Murray, J.W. Ecology and Applications of Benthic Foraminifera. Cambridge University Press, Cambridge, 2006; pp. 426.
- Sjoerdsma, P.G., van der Zwaan, G.J. Simulating the effect of changing oceanic flux and oxygen content on the distribution of benthic foraminifers. Marine Micropaleont. 1992, 19, 163–180.
- Gonera, M. Foraminiferida and palaeoenvironment of the Badenian Formations (Middle Miocene) in Upper Silesia (Poland) (in Polish with English summary) (in Polish with English summary). Stud. Natur. 2001, 48, 1–211.
- Hohenegger, J. Estimation of environmental paleogradient values based on presence/absence data: a case study using benthic foraminifera for paleodepth estimation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 217, 115–130. [CrossRef]
- Kouwenhoven, T.J., van der Zwaan, G.J. A reconstruction of late Miocene Mediterranean circulation patterns using benthic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 238, 373–385. [CrossRef]
- Kaminski, M.A. Calibration of the Benthic Foraminiferal Oxygen Index in the Marmara Sea. Geol. Quart. 2012, 56, 757–764. [CrossRef]
- Dumitriu, S.D., Dubicka, Z., Loghin, S., Melinte-Dobrinescu, M.C., Paruch-Kulczycka, J. The evolution of the Carpathian Foredeep Basin during the latest Badenian and Sarmatian (Middle Miocene): inferences from micropalaeontological data. Geol. Quart. 2020, 64, 1004-1022. [CrossRef]
- Van der Zwaan, G.J., Duijnstee, I.A.P., den Dulk, M., Ernst, S.R., Jannink, N.T., Kouwenhoven, T.J. Benthic foraminifers: proxies or problems? A review of paleoecological concepts. Earth-Sci. Rev. 1999, 46, 213–236.
- Van Hinsbergen, D.J.J., Kouwenhoven, T.J., van der Zwaan, G.J. Paleobathymetry in the backstripping procedure: correction for oxygenation effects on depth estimates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 221, 245–265. [CrossRef]
- Holcová, K., Zágoršek, K. Bryozoa, foraminifera and calcareous nannoplankton as environmental proxies of the "bryozoan event" in the Middle Miocene of the Central Paratethys (Czech Republic). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 267, 216-234. [CrossRef]
- Hart, M.B., Fisher, J.K., Smart, C.W., Speers, R., Wall-Palmer, D. Re-colonization of hostile environments by benthic foraminifera: an example from Montserrat, Lesser Antilles Volcanic Arc. Micropaleont. 2022, 69, 1-27. [CrossRef]
- Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Ważyńska, H., Słodkowska, B., Sadowska, A. Atlas of pollen and spores of the Polish Neogene. Vol. 1 – Spores; W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków, 2001; 158 pp.
- Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Ważyńska, H., Sadowska, A. Atlas of pollen and spores of the Polish Neogene. Vol. 2 – Gymnosperms; W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków, 2002; 237 pp.
- Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Słodkowska, B., Ważyńska, H., Sadowska, A. Atlas of pollen and spores of the Polish Neogene. Vol. 3 – Angiosperms (1); W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków, 2009; 233 pp.
- Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Słodkowska, B., Worobiec, E., Durska, E. Atlas of pollen and spores of the Polish Neogene. Vol. 4 – Angiosperms (2); W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków, 2014; 466 pp.
- Nalepka, D., Walanus, A. Data processing in pollen analysis. Acta Palaeobot. 2003, 43, 125–134.
- Hemleben, C., Spindler, M., Anderson, O.R. Modern Planktonic Foraminifera; Springer, New York, 1989; 363 pp.
- Schiebel, R., Bijma, J., Hemleben, C. Population dynamics of the planktic foraminifer Globigerina bulloides from the eastern North Atlantic. Deep-Sea Res. I 1997, 44, 1701–1713. [CrossRef]
- Caron, M. Cretaceous planktic foraminifera. In: Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch-Nielsen, K., Eds.;, Cambridge University Press, 1985; pp. 17–86.
- Schiebel, R., Hemleben, C. Modern planktonic foraminifera. Paläont. Zt. 2005, 79, 135–148.
- Hebbeln, D., Marchant, M., Wefer, G. Seasonal variations of the particle flux in the Peru-Chile Current at 30°S under normal and El Nino conditions. Deep Sea Research Part II: Topical Studies in Oceanography 2000, 47, 2101-2128,.
- Petrizzo, M.R. Palaeoceanographic and palaeoclimatic inferences from Late Cretaceous planktonic foraminiferal assemblages from the Exmouth Plateau (ODP Sites 762 and 763, eastern Indian Ocean). Mar. Micropaleontol. 2002, 45, 117–150. [CrossRef]
- Schiebel, R., Bijma, J., Hemleben, C. Population dynamics of the planktic foraminifer Globigerina bulloides from the eastern North Atlantic. Deep Sea Res. Part I 1997, 44, 1701-1713. [CrossRef]
- Di Stefano, A., Verducci, M., Lirer, F., Ferraro, L., Iaccarino, S.M., Hüsing, S.K., Hilgen, F.J. Paleoenvironmental conditions preceding the Messinian Salinity Crisis in the Central Mediterranean: integrated data from the Upper Miocene Trave section (Italy). Palaeogeogr., Palaeoclimatol., Palaeoecol. 2010, 297, 37–53. [CrossRef]
- Bé, A.W.H., Tolderlund, D.S. Distribution and ecology of living planktonic foraminifera in surface waters of the Atlantic and Indian Oceans. In: The Micropaleontology of Oceans; Funnell, B.M., Riedel, W.R., Eds.; Cambridge University Press, Cambridge, 1971; pp. 105–149.
- Bé, A.W.H. An ecological zoogeographic and taxonomic review of recent planktonic foraminifera. In: The Micropaleontology of Oceans, Ramsay, A.T.S., Ed.; Academic Press, London, 1977; pp. 1–100.
- Thunell R., Reynolds-Sautter, L. Planktonic foraminiferal faunal and stable isotopic indices of upwelling: a sediment trap study in the San Pedro Basin, Southern California Bight. Geol. Soc. Spec. Publ. 1992, 64, 77-91. [CrossRef]
- Schiebel, R., Hemleben, C. Planktic Foraminifers in the Modern Ocean. Springer, Berlin Heidelberg, 2017; pp. 358.
- Darling, K.F., Wade, C.M., Siccha, M., Trommer, G., Schulz, H., Abdolalipour, S., Atsushi Kurasawa, A. Genetic diversity and ecology of the planktonic foraminifers Globigerina bulloides, Turborotalita quinqueloba and Neogloboquadrina pachyderma off the Oman margin during the late SW Monsoon. Mar. Micropaleont. 2017, 137, 64–77. [CrossRef]
- Jorissen, F.J., de Stigter, H.C., Widmark, J.G.V. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleont. 1995, 26, 3-15. [CrossRef]
- Rodrigues, A.R., Gómez Pivel, M.A., Schmitt, P., de Almeida, F.K., Bonetti, C. Infaunal and epifaunal benthic foraminifera species as proxies of organic matter paleofluxes in the Pelotas Basin, south-western Atlantic Ocean. Mar. Micropaleont. 2018, 144, 38-49. [CrossRef]
- Kováč, M., Márton, E., Oszczypko, N., Vojtko, R., Hók, J., Králiková, S., Plašienka, D., Klučiar, T., Hudáčková, N., Oszczypko-Clowes, M. Neogene palaeogeography and basin evolution of the Western Carpathians, Northern Pannonian domain and ad joining areas. Glob. Planet. Chang. 2017, 155, 133–154. [CrossRef]
- Bukowski, K., de Leeuw, A., Gonera, M., Kuiper, K.F., Krzywiec, P. Peryt, D. Badenian tuffite levels within the Carpathian orogenic front (Gdów–Bochnia area, Southern Poland): radio-isotopic dating and stratigraphic position. Geol. Quart. 2010, 54, 449–464.
- Hess, S., Kuhnt, W. Deep-sea benthic foraminiferal recolonization of the 1991 Mt Pinatubo ash layer in the South China Sea. Mar. Micropaleont. 1996, 28, 171-197. [CrossRef]
- Hess, S., Kuhnt, W., Hill, S., Kaminski, M. A., Holbourn, A., de Leon, M. Monitoring the recolonization of the Mt Pinatubo 1991 ash layer by benthic foraminifera. Mar. Micropaleont. 2001, 43, 119-142. [CrossRef]
- Filipescu, S. Anomalinoides dividens bioevent at the Badenian/Sarmatian boundary — a response to paleogeographic and paleoenvironmental changes. Studia Universitatis Babeş-Bolyai, Geologia 2004, 49, 21–26.
- Chaloner, W.G., Muir, M. Spores and floras. In: Coal and coal-bearing strata; Murchison, D.G., Westoll, T.S., Eds.;. Oliver and Boyd, Edinburgh, 1968; pp. 127–146.
- Streel, M., Richelot, C. Wind and water transport and sedimentation of miospores along two rivers subject to major floods and entering the Mediterranean Sea at Calvi (Corsica, France). In: Sedimentation of organic particles; Traverse, A., Ed.; Cambridge University Press, Cambridge, 1994; pp. 59–67.
- Brinkhuis, H. Late Eocene to Early Oligocene dinoflagellate cysts from the Priabonian type-area (northeast Italy): biostratigraphy and palaeoenvironmental interpretation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 107, 121–163.
- Stover, L.E., Brinkhuis, H., Damassa, S.P., de Verteuil, L., Helby, R.J., Monteil, E., Partridge, A.D., Powell, A.J., Riding, J.B., Smelror, M., Williams, G.L. Mesozoic–Tertiary dinoflagellates, acritarchs and prasinophytes. In: Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation, Dallas TX, 1996; Volume 2, pp. 641–750.
- Sluijs, A., Pross, J., Brinkhuis, H. From greenhouse to icehouse; organic-walled dinoflagellate cysts as paleoenvironmental indicators in the Paleogene. Earth-Sci. Rev. 2005, 68, 281–315. [CrossRef]
- Williams, G.L. Dinoflagellate cysts, their classification, biostratigraphy and palaeoecology. In: Oceanic Micropalaeontology, Ramsay, A.T.S., Ed.; Academic Press, London, 1977; pp. 1231–1325.
- Köthe, A. Paleogene dinoflagellates from northwest Germany. Geol. Jb. R. A 1990, 118, 1-111.
- Gedl, P. Middle Miocene dinoflagellate cysts from the Korytnica clays (Góry Świętokrzyskie Mountains, Poland). Ann. Soc. Geol. Pol. 1996, 66, 191–218.
- Schreck, M., Matthiessen, J. Batiacasphaera micropapillata: palaeobiogeographic distribution and palaeoecological implications of a critical Neogene species complex. Micropalaeont. Soc. Spec. Publ. 2013, 5, 301–314.
- Gedl, P. Palynofacies of the Miocene deposits in the Gliwice area (Upper Silesia, Poland). Bull. Pol. Acad. Sci., Earth Sci. 1997, 45, 191–201.
- Rochon, A., de Vernal, A., Turon, J.L., Matthiessen, J., Head, M.J. Distribution of recent dinoflagellate cysts in surface sediments from the North Atlantic Ocean and adjacent seas in relation to sea-surface parameters. Am. Assoc. Strat. Palynol. Found., Contrib. Ser. 1999, 35, 146 pp.
- Schreck, M., Meheust, M., Stein, R., Matthiessen, J. Response of marine palynomorphs to Neogene climate cooling in the Iceland Sea (ODP Hole 907A). Mar. Micropaleont. 2013, 101, 49–67. [CrossRef]
- Gedl, P. Palaeoenvironmental and sedimentological interpretations of the palynofacies analysis of the Miocene deposits from the Jamnica S-119 borehole (Carpathian Foredeep, Poland). Geol. Quart. 1999, 43, 479-492.
- Guy-Ohlson, D. Prasinophycean algae. In: Palynology: Principles and Applications; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation, Dallas, Texas, 1996; Volume 1, pp. 181–189.
- Gedl, P. Dinoflagellate cysts and palynofacies from the upper Badenian (Middle Miocene) of the Roztocze area at Józefów and Żelebsko (Carpathian Foredeep Basin, Poland): palaeoenvironmental implications. Ann. Soc. Geol. Pol. 2016, 86, 273–289.
- Bradford, M.R., Wall, D.A. The distribution of Recent organic-walled dinoflagellate cysts in the Persian Gulf, Gulf of Oman, and northeastern Arabian Sea. Palaeontogr, Abt. B 1984, 192, 16–84.
- Gedl, P., Peryt, D. Dinoflagellate cyst, palynofacies and foraminiferal records of environmental changes related to the Late Badenian (Middle Miocene) transgression at Kudryntsi (western Ukraine). Ann. Soc. Geol. Pol. 2011, 81, 331–349.
- Peryt, D., Gedl, P., Peryt, T.M. Foraminiferal and palynological records of the Late Badenian (Middle Miocene) transgression in Podolia (Shchyrets near Lviv, western Ukraine). Geol. Quart. 2014, 58, 465–484. [CrossRef]
- Gedl, P., Peryt, D., Peryt, T.M. Foraminiferal and palynological organic matter records of the Upper Badenian (Middle Miocene) deposits at Anadoly (marginal part of the Ukrainian Carpathian Foredeep Basin). Geol. Quart. 2016, 60, 517–536. [CrossRef]
- Head, M.J. Neogene occurrences of the marine acritarch genus Nannobarbophora Habib and Knapp, 1982 emend., and the new species N. gedlii. Jour. Paleont. 2003, 77, 382–385. [CrossRef]
- Gedl, P. In situ and recycled dinoflagellate cysts from Middle Miocene deposits at Bęczyn, Carpathian Foredeep, Poland. Studia Geol. Pol. 2005, 124, 371-394.
- Jiménez-Moreno, G., Head, M.J., Harzhauser, M. Early and Middle Miocene dinoflagellate cyst stratigraphy of the Central Paratethys, Central Europe. Jour. Micropaleont. 2006, 25, 113–139. [CrossRef]
- Oszast, J. The Miocene vegetation of a sulphur bed at Piaseczno near Tarnobrzeg (Southern Poland) (in Polish with English summary). Acta Palaeobot. 1967, 8, 3–29.
- Sadowska, A. Sarmatian palynoflora from Jamnica near Tarnobrzeg (Carpathian Foredeep) - environmental and climatic implications. Geol. Quart. 1999, 43, 493–498.
- Schlütz, F., Shumilovskikh, L.S. On the relation of Potamomyces armatisporus to the fossil form-type Mediaverrunites and its taxonomical and ecological implications. Fung. Ecol. 2013, 6, 309–315. [CrossRef]
- Worobiec, G., Neumann, F.H., Worobiec, E., Nitz, V., Hartkopf-Fröder, C. New fungal cephalothecoid-like fructifications from central European Neogene deposits. Fung. Biol. 2017, 121, 285–292. [CrossRef]
- Worobiec, G., Worobiec, E., Gedl, P., Kasiński, J.R., Peryt, D., Widera, M. Terrestrial-aquatic wood-inhabiting ascomycete Potamomyces from the Miocene of Poland. Acta Palaeontol. Pol. 2022, 67, 737–744. [CrossRef]
- Hawksworth, D.L., van Geel, B., Wiltshire, P.E. The enigma of the Diporotheca palynomorph. Rev. Palaeobot. Palynol. 2016, 235, 94–98. [CrossRef]
- Worobiec, G., Worobiec, E., Gedl, P., Kowalski, R., Peryt, D., Tietz, O. Fossil history of fungus host-specificity: Association of conidia of fossil Asterosporium asterospermum with macro- and microremains of Fagus. Fungal Biol. 2023, 127, 1312–1320. [CrossRef]
- Worobiec, E., Worobiec, G., Gedl, P. Occurrence of fossil bamboo pollen and a fungal conidium of Tetraploa cf. aristata in Upper Miocene deposits of Józefina (Poland). Rev. Palaeobot. Palynol. 2009, 157, 211–217. [CrossRef]
- Grabowska, I., Słodkowska, B. Katalog profili osadów trzeciorzędowych opracowanych palinologicznie (in Polish). PIG, Warszawa, 1993; pp. 80.
- Ważyńska, H. Introduction. Pr. Państw. Inst. Geol. 1998, 160, 5–8.
- Oszast, J. Pollen analysis of Tortonian clays from Stare Gliwice in Upper Silesia, Poland (in Polish with English summary). Monogr. Botan. 1960, 9, 1–47. [CrossRef]
- Dyjor, S., Sadowska, A. Problem of the Badenian-Sarmatian boundary at Stara Kuźnia region near Kędzierzyn (Silesia) in the light of palinological investigations (in Polish with English summary). Acta Palaeobot. 1984, 24, 27–51.
- Sadowska, A. Former palynological studies of the Tertiary in the western part of Carpathian Foredeep in Poland (in Polish with English summary). Prz. Geol. 1996, 44, 1039–1042.
- Sadowska, A. Miocene palynology in the Gliwice Region (Upper Silesia), Poland. Bull. Pol. Acad. Sci., Earth Sci. 1997, 45, 203-210.
- Durska, E. The Badenian Salinity Crisis in the palynological record: vegetation during the evaporative event (Carpathian Foredeep, southern Poland). Ann. Soc. Geol. Pol. 2017, 87, 213–228. [CrossRef]
- Kita, Z. Palynological analysis of Tortonian deposits from the borehole Kłaj 1 (East of Kraków) (in Polish with English summary). Roczn. Polsk. Tow. Geol. 1963, 33, 517–526.
- Syabryaj, S.V., Stuchlik, L. Palaeofloristic and palaeoclimatic reconstruction on the territories of Ukraine and Poland during the Badenian-Sarmatian. Acta Palaeobot. 2004, 44, 55–68.
- Zachos, J., Pagani, M., Sloan, L., Thomas, E., Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693.
- Syabryaj, S.V., Stuchlik, L. Development of flora and vegetation of the Ukrainian Eastern Carpathians and Polish Western Carpathians in the Neogene. Acta Palaeobot. 1994, 34, 165–194.
- Kvaček Z., Kováč, M., Kovar-Eder, J., Doláková, N., Jechorek, H., Parashiv, V., Kováčová, M., Sliva, L. Miocene evolution of landscape and vegetation in the Central Paratethys. Geol. Carpath. 2006, 57, 295–310.
- Hudáčková, N., Ruman, A., Plašienková, I., Jamrich, M., Halásová, E., Kiss, P., Kováčová, M., Babejová-Kmecová, J., Kováč, M. Badenian/Sarmatian foraminifera shift in the Central Paratethys: two methods comparison – a morphogroup and species approach. In: Proceedings of the Geologica Carpathica 70 Conference, Smolenice Castle, Slovakia, October 9–11, 2019; Broska, I., Kohút, M., Tomašových, A., Eds.; 2019; pp. 144–145.
- Palcu, D.V., Tulbure, M., Bartol, M., Kouwenhoven, T.J., Krijgsman, W. The Badenian-Sarmatian Extinction Event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys domain. Glob. Planet. Change 2015, 133, 346-358. [CrossRef]
- Nováková, P., Rybár, S., Šarinová, K., Nagy, A., Hudáčková, N., Jamrich, M., Teodoridis, V., Kováčová, M. Šujan, M., Vlček, T., Kováč, M. The late Badenian-Sarmatian (Serravallian) environmental transition calibrated by sequence stratigraphy (eastern Danube Basin, Central Paratethys). Geol. Carpath. 2020, 71, 291–313.
- Popov, S.V., Antipov, M.P., Zastrozhnov, A.S., Kurina, E.E., Pinchuk, T.N. Sea-level fluctuations on the northern shelf of the Eastern Paratethys in the Oligocene–Neogene. Stratigr. Geol. Correl. 2010, 18, 200–224. [CrossRef]
- Simon, D., Palcu, D., Meijer, P., Krijgsman, W. The sensitivity of middle Miocene paleoenvironments to changing marine gateways in Central Europe. Geology 2019, 47, 35–38. [CrossRef]
- Peryt, D., Gedl, P., Jurek, K., Więcław, D., Worobiec, E., Worobiec, G., Peryt, T.M. Environmental perturbations around the Badenian/Sarmatian (Middle Miocene) boundary in the Central Paratethys: micropalaeontological and organic geochemical records. Palaeogeogr., Palaeoclimatol., Palaeoecol. 2024. (submitted).
- Brasier, M.D. The ecology and distribution of Recent foraminifera from the reefs and shoals around Barbuda, West Indies. Jour. Foram. Res. 1975, 5, 193–210. [CrossRef]
- Piller, W.E. Harzhauser, M. The myth of the brackish Sarmatian Sea. Terra Nova 2005, 17, 450–455. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





