1. Introduction
Cervical cancer involves the development of irregular cells within the cervix lining. Cervical cancer stands as one of the foremost causes of cancer-related mortality among women [
1]. As of 2018, cervical cancer, with approximately 570,000 reported cases and 311,000 recorded deaths worldwide, holds the position as the fourth most frequently diagnosed cancer and the fourth leading cause of cancer-related mortality in women [
2]. Nevertheless, around 85% of global cervical cancer deaths occur in underdeveloped or developing countries, where the death rate is 18 times higher in low-income and middle-income nations compared to wealthier countries [
3]. NACT, which is becoming more popular as a prospective therapeutic option, has received a lot of attention lately. The distant and local recurrences rates were demonstrated to decrease in NACT arm, while PFS and OS survival exhibited significant increases.
GLOBOCAN predicts that there were 311,365 deaths from cervical cancer and 569,874 new cases globally in 2018 [
4]. A total of 8,450,000 woman aged 15 years or older in Romania are at risk of developing cervical cancer. As per the latest estimates, approximately 3,380 women receive a diagnosis of cervical cancer each year, and 1,805 of the pass away due to the condition [
5]. The gold standard for treatment of locally advanced cervical cancer has traditionally been the simultaneous use of chemotherapy, radiation, and brachytherapy, with cisplatin playing a central role. CCRT considerably improves survival outcomes when compared to radiation alone, according to meta-analyses of randomized studies. However, therapeutic failure is still possibly, especially when there is a chronic pelvic illness and locoregional recurrences.
By giving chemotherapy before the main therapy, which is either radiation or extensive surgery, NACT provides a unique method for treating locally advanced cervical cancer. By lowering the tumor's size, improving its radiosensitivity, and perhaps curing micro metastatic disease, this strategy tries to address multiple important elements of the illness [
6]. By incorporating NACT, medical experts aim to enhance OS and disease-free survival rates for patients with stage III cervical cancer.
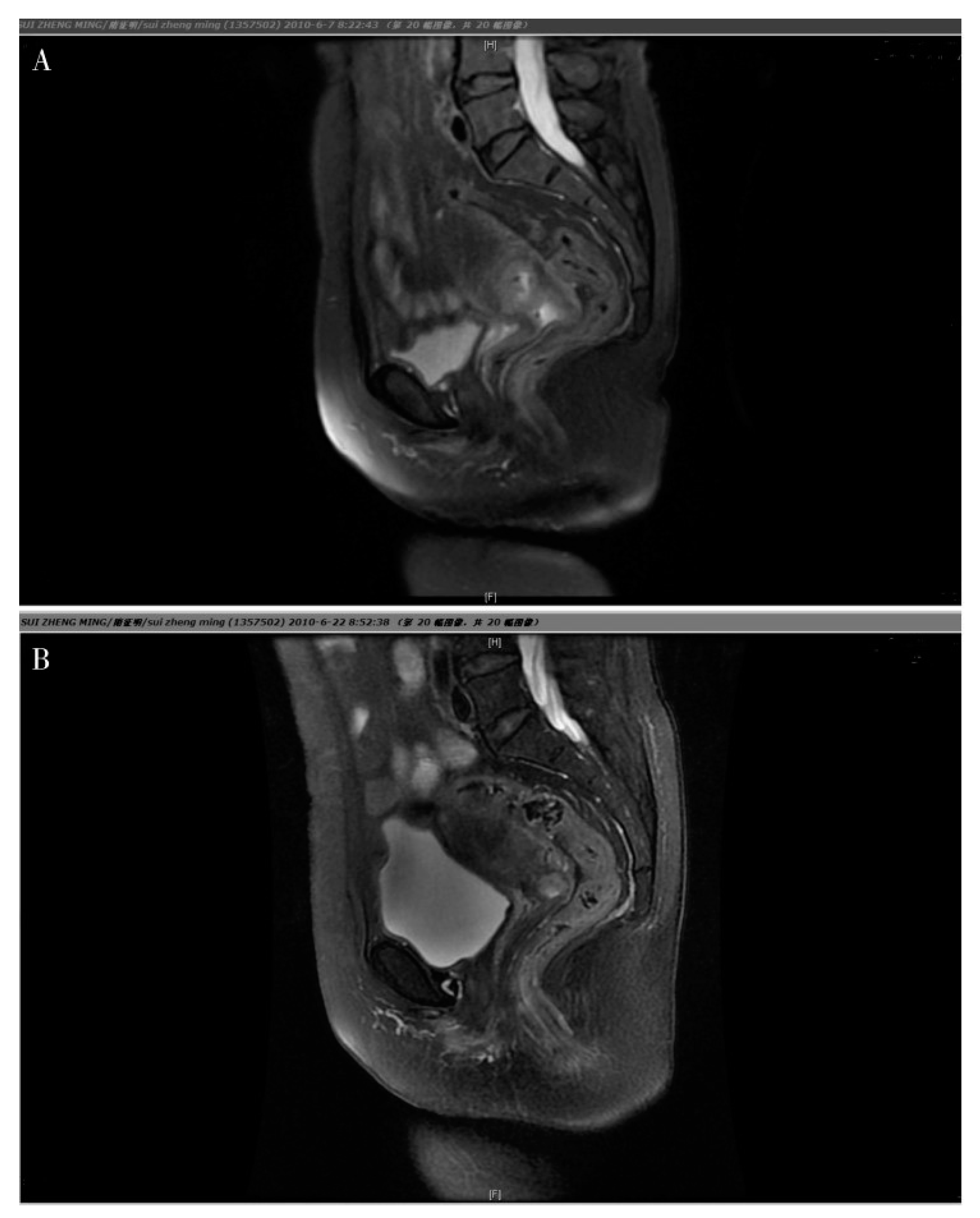
Figure 1.
Magnetic Resonance Imaging (MRI) for a cervical carcinoma, objective response of NACT. A. Prior to chemotherapy, the tumor was large and it was difficult to distinguish it from the surrounding tissues. B. following NACT, the cervix returned to it’s natural shape and the tumor reduced considerably.
Figure 1.
Magnetic Resonance Imaging (MRI) for a cervical carcinoma, objective response of NACT. A. Prior to chemotherapy, the tumor was large and it was difficult to distinguish it from the surrounding tissues. B. following NACT, the cervix returned to it’s natural shape and the tumor reduced considerably.
Early trials examining NACT in cervical cancer have shown encouraging findings. 515 cervical cancer patients in a phase III study in Mexico either got regular CCRT or CCRT + adjuvant treatment with gemcitabine and cisplatin [
7]. Since the publication of two pilot studies in the 1980s, cervical cancer patients have been using NACT as a prophylactic therapy before undergoing drastic surgery. Similar results were reported by Sardi and colleagues [
8]. Subsequent trials investigating the role of NACT have bolstered initial findings, affirming feasibility and yielding encouraging outcomes. The overall response rate ranges from 66 to 85%, with a substantial proportion of patients achieving pathological complete responses [
9]. This is true despite significant variations in the treatment plan and schedule. Multiple Phase III clinical trials have been systematically conducted to date, aiming to comprehensively elucidate the actual influence and effect of NACT in individuals diagnosed with locally advanced cervical cancer. In the management of stage III cervical cancer, NACT is the focal point of this research, as a numerous systemic reviews have extensively examinate its impact on treatment. The primary objective is to investigate the therapeutic efficacy and role of NACT, relying on a comprehensive analysis of international medical literature to discern its specific function in this clinical setting.
2. Materials and Methods
To acquire insights into treatments for advanced cervical cancer until the year 2023, an extensive review of pertinent literature, encompassing books and articles, was conducted. The search encompassed esteemed computerized databases such as PubMed, MEDLINE, and Google Scholar. The exploration involved targeted queries incorporating terms such as "neoadjuvant chemotherapy," "stage 3 cervical cancer," "advanced cervical cancer," and "treatment outcomes."
Applying rigorous security, studies of relevance were thoroughly examined, specifically focusing on those providing substantive insights into the application of NACT for managing locally advanced cervical cancer, especially stage 3 cervical cancer. The selection criteria prioritized the inclusion of studies that provided valuable information regarding the efficacy and outcomes associated with neoadjuvant chemotherapy in this clinical context. The methodological approach was systematical undertaken in order to meticulously gather, analyze, and assimilate a comprehensive understanding of the multifaceted effectiveness of NACT, particularly in the context of managing stage 3 cervical cancer.
3. Clinical Evidence and Outcomes
3.1. NACT Followed by Radiotherapy:
The pre- radiotherapy application of NACT has showcased prospective advantages, including the elimination of subclinical distant metastases, reduction in tumor size, rectification of pelvic architecture distortion, and enhancement of radiation delivery [
9]. Numerous randomized clinical trials (RCTs) have systematically explored the significance of NACT preceding definitive radiation. Comparably, the investigation of Chauvergne et al. specifically compared the outcomes of radiotherapy alone with those following NACT, revealing a potentially heightened 3 years DFS in the cohort that underwent chemotherapy followed by radiotherapy, despite a comparable remission rate [
10]. On the contrary, a multicenter study by Herod et al. observed no substantial variations in response rate or OS between NACT and radiotherapy alone for individuals with cervical cancer [
11]. Furthermore, Symonds et al.'s randomized trial exhibited a clinically complete response in patients subjected to NACT followed by radiotherapy [
12]. The collaborative effort undertaken by Meta-analysis Collaboration for NACT in patients with cancer of the cervix, incorporating data from 18 studies, suggests a lack of substantial difference in OS between cohorts subjected to NACT and those receiving radiation alone [
13]. Additionally, examinations involving dose-dense weekly chemotherapy cycles and subsequent radiotherapy in cervical cancer patients demonstrated favorable response rates and manageable adverse events [
14]. The clinical outcomes from diverse studies underscore the imperative for tailored treatment strategies contingent on patient characteristics and tumor staging. Ongoing trials, such as INTERLACE, aim to further scrutinize the relative efficacy of diverse treatment modalities, encompassing standard chemoradiation and induction chemotherapy utilizing paclitaxel [
17]. Furthermore, retrospective studies posit that combining intensity-modulated radiation therapy with intracavitary posterior radiotherapy may represent a secure and recommended approach for individuals with medium- and advanced-stage cervical cancer [
18]. These findings highlight the complexity involved in making treatment decisions for patients with cervical cancer, calling for careful considerations of variety of clinical factors and exploration of diverse therapeutic options.
3.2. NACT Followed by Radical Surgery:
A notable spectrum of objective response rates, spanning from 69.4% to 90.2%, coupled with diverse range of pathological optimal response rates, ranging between 21.3% and 48.3%, has been observed in the context of cervical cancer treatment. Additionally, the 5 years DFS rates exhibited variability, falling within a bracket of 55.4% to 71%, while the corresponding 5 year overall survival rates demonstrated a discernible range of 58.9% to 81%. Particularly noteworthy is the consideration of platinum based NACT as a viable alternative to conventional radiotherapy of Concurrent Chemoradiation (CCRT) in cases of cervical cancer, especially those of squamous cell histology. The subsequent recommendation involves the integration of platinum-based NACT followed by the implementation of radical hysterectomy, thereby broadening the scope of therapeutic options and contributing to nuanced approach in the management of cervical cancer. [
19].
In comparison to individuals who underwent primary radical hysterectomy, the combination of NACT followed by radical hysterectomy demonstrated superior PFS with Hazard Ratio (HR) of 0.75 (95% Confidence Interval [CI]: 0.61-0.93, p=0.008) and OS with HR of 0.77 (95% CI: 0.62-0.96, p=0.02). These improvements were consistently observed across various parameters, including total cisplatin (CDDP) dose, chemotherapy cycle length, and tumor stage. These findings contribute valuable insights into the potential benefits of incorporating NACT in the therapeutic approach of cervical cancer management [
19]. Following the strategic administration of NACT, a comprehensive set of positive alterations was observed in various key parameters associated with cervical cancer. Notably, there was a substantial reduction in tumor size, stromal invasion depth, parametrial infiltration, lymph and/ or vascular space involvement, and the occurrence of nodal metastases. These discernible changes collectively signify a promising trend towards a more favorable disease profile. Importantly, the potential implications of these reductions extend beyond mere clinical observations, as they hold the promise of mitigating the need for adjuvant radiotherapy in the subsequent stages of the treatment protocol [
20].
Individuals subjected to NACT afterwards surgery, exhibited several factors linked to a favorable prognosis, notably including an age greater than 35, reduced tumor dimensions, lower stage progression, absence of nodal metastasis, histology type of squamous cell carcinoma, objective clinical response and optimal pathological response [
15,
25]. In a comprehensive study conducted by Colombo et al., an analysis was undertaken involving a cohort of 100 patients diagnosed with advanced cervical cancer who underwent radical hysterectomy with a treatment regimen involving CDDP, vincristine, and bleomycin. Within this cohort, the investigation revealed significant findings, notably emphasizing the critical role of achieving an ideal pathological response. This response is defined by the complete removal of cervical cancer without involvement of negative nodes, coupled with preservation of residual disease limited less than 3 mm stromal invasion. Importantly the study identified this ideal pathological response as a distinct and noteworthy predictor of survival outcomes [
15,
10]. These insights contribute valuable information to the ongoing discourse on treatment strategies for advanced cervical cancer, providing a foundation for more nuanced and personalized approach based on prognostic indicators such as pathological response. The SNAP01 trial demonstrated that a favorable pathological response serves as a significant and independent predictor of survival. The conclusion was drawn from a comparative analysis within the study, which investigated the effectiveness of paclitaxel (PTX) plus ifosfamide (IFO) plus cisplatin (CDDP) regiment, also named TIP regimen), in contrast to the ifosfamide and cisplatin regimen (IP regimen) [
22]. The findings underscore the pivotal role of achieving a positive pathological response in influencing survival outcomes, thereby contributing valuable insights to the broader understanding of effective treatment in context of cervical cancer.
Following surgical intervention, all patients received adjuvant radiation therapy. Benedetti Panici et al. conducted a comparative study involving 441 patients diagnosed with FIGO stage IB2-III cervical cancer. The study aimed to evaluate the efficacy of neoadjuvant platinum-based chemotherapy, administrated at a recommended dosage exceeding 240mg/m2 for 6-8 weeks, followed by radical surgery in comparison to conventional radiotherapy. Both OS and DSF (58.9 vs. 44.5%; p = 0.007 and 0.02 respectively) results from the trial demonstrated the superiority of the treatment group [
24].
Patients who got NACT and then had extensive surgery in the first study had considerably greater four-year overall survival rates (63%) than those who just received radiation (37%). The NACT group saw an improvement in disease-free survival (65% vs. 28%). Similar to the first experiment, the second trial demonstrated a notable contrast in overall survival rates (65% vs 48%) following a 7-year follow-up between the NACT group that underwent major surgery and the group receiving radiotherapy alone [
15].
In a more extensive research study, the application of NACT followed by radical surgery yielded superior OS and DFS outcomes for patients diagnosed with stage IB2-III cervical cancer compared to conventional radiation [
27]. NACT was discovered to reduce the relative risk of death. Prognostic markers for the disease were lymph node positivity and tumor size. The administration of radiation, however, drew criticism since some patients didn't get the full amount recommended or had treatment delays. According to the research, elevated extents of pWee1 and -H2AX were strongly associated with a reduced complete pathological response rate. The observation suggests that these biomarkers may serve as indicators of chemoresistance in cervical cancer, providing valuable insights into potential mechanisms influencing treatment response. Also, it shows the potential significance of G2/M checkpoint activation in protecting cancer cells from chemotherapy's side effects and indicates that targeting G2/M checkpoint kinases like Wee1 may be a feasible strategy to overcome chemoresistance.
3.3. NACT Followed by Brachytherapy:
Utilizing NACT, often in conjunction with brachytherapy, presents promising outcomes throughout the handling of FIGO stage III cervical cancer. Research indicates that neoadjuvant chemoradiotherapy (CRT), followed by brachytherapy and radical hysterectomy (RH), proves effective in controlling stage IB2 and IIA cervical cancer, resulting in favorable disease control outcomes [
30]. Furthermore, the amalgamation of neoadjuvant chemotherapy with brachytherapy demonstrates efficacy in managing local lesions, offering a valuable therapeutic approach [
31]. Concurrently, in the contemporary landscape of oncological practice, the well-established standard for effectively managing the complexities of locally advanced cervical cancer prominently involves the comprehensive integration of chemo-radiation with platin based agent, cisplatin, also strategically complemented by the intricate application of brachytherapy. This underscores the significance of integrated approaches in clinical management [
32]. Furthermore, a comprehensive analysis evaluating therapeutic responses between chemo-radiation and the subsequent application of NACT followed by surgery suggests the potential superiority of the latter in specific contexts [
33]. This underscores the dynamic landscape of treatment modalities for stage 3 cervical cancer, with a dedicated emphasis on optimizing outcomes through comprehensive therapeutic strategies.
The application process of brachytherapy involves a meticulous insertion procedure wherein a specialized applicator is carefully placed within the vaginal cavity, accompanied by the insertion of a plastic tub into the uterus. The procedure necessitates the dilation of cervical canal to facilitate the precise position of the instruments. Due to the intricacy of this insertion process and the importance of ensuring patient comfort, such a procedure can be conducted with the administration of either spinal or general anesthesia, providing healthcare practitioners with flexibility in tailoring the approach to individual patient needs and preferences.
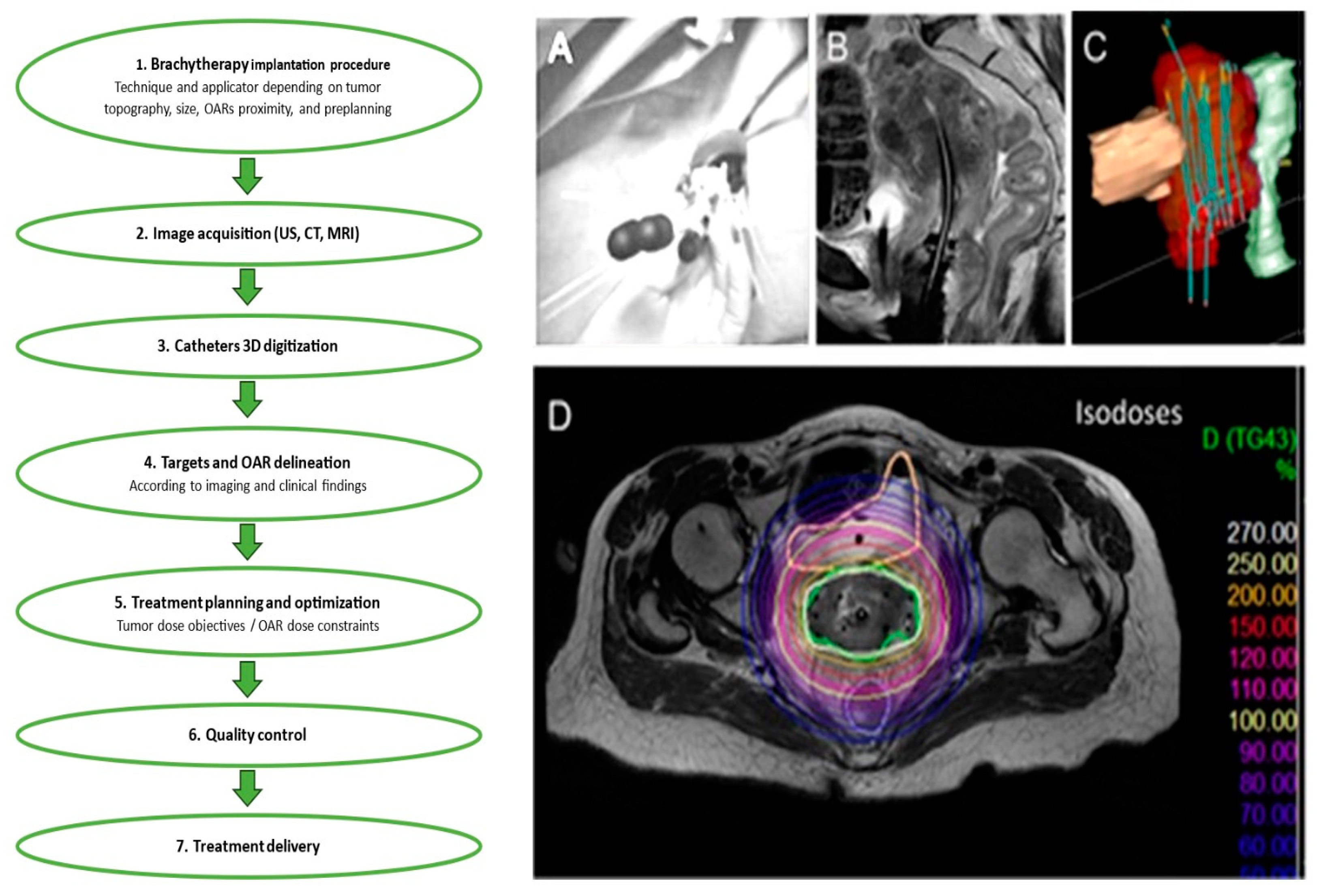
Figure 2.
The illustration shows the steps that make up the brachytherapy procedure. This example shows how to: (A) perform an implantation; (B) acquire images; (C) digitize 3-dimensional (3D) catheters; and (D) demarcate targets and organs at risk (OARs). Treatment delivery, quality control, and treatment planning and optimization (D) come next.
Figure 2.
The illustration shows the steps that make up the brachytherapy procedure. This example shows how to: (A) perform an implantation; (B) acquire images; (C) digitize 3-dimensional (3D) catheters; and (D) demarcate targets and organs at risk (OARs). Treatment delivery, quality control, and treatment planning and optimization (D) come next.
Figure 3.
Following a systemic refinement of treatment protocols, there has been a gradual elevation in the Therapeutic Index among patients diagnosed with locoregionally advanced cervix cancer undergoing brachytherapy.
Figure 3.
Following a systemic refinement of treatment protocols, there has been a gradual elevation in the Therapeutic Index among patients diagnosed with locoregionally advanced cervix cancer undergoing brachytherapy.
4. Advances and Considerations in Neoadjuvant Chemotherapy:
Since 1999, the standard approach for managing locoregionally advance cancer of the cervix bas been concurrent chemoradiation, a therapeutic strategy that has demonstrated a substantial enhancement in OS, making a noteworthy 12% improvement when compared to radiotherapy alone [
9]. Nevertheless, despite these improvements, metastatic disease is still responsible for a significant portion of cervical cancer mortality. In order to enhance outcomes in this illness context, it is thus becoming more important to investigate new systemic methods.
Prior to final radiotherapy, NACT has traditionally been thought to be useless or even detrimental. However, current research indicates that variables like cisplatin dose-intensity and cycle durations may affect how effective NACT is. Promising outcomes were shown in a Phase II research that used a weekly treatment of carboplatin and paclitaxel for six weeks prior to definitive chemoradiation [
4]. In a comprehensive retrospective analysis, Zhang et al. conducted a comparative examination of the efficacy and safety between primary surgery and sequential approach of NACT followed by surgical therapy in a cohort of patients diagnosed with locally advanced cervical carcinoma [
34]. This meticulous investigation sought to unravel not only the outcomes in terms of treatment effectiveness but also the paramount aspect of safety associated with these two distinct therapeutic pathways. Through the use of propensity score matching, a total of 639 locally advanced cervical carcinoma patients were separated into NACTS and primary surgery groups. Regarding DFS and overall survival OS, NACTS revealed equivalent results to PS. Notably, NACTS resulted in a drop in intermediate-risk variables, which thus lowered the need for adjuvant radiation. The DFS with NACTS was enhanced in patients with bigger tumor diameters (5 cm) or increased SCC (5 ng/ml). The potential advantages of paclitaxel with platinum neoadjuvant chemotherapy in the treatment of LACC are highlighted by this research, especially in instances with certain high-risk characteristics.
The current INTERLACE experiment, a randomized Phase III investigation that seeks to further evaluate the advantages of NACT in this environment, is being conducted as a result of these positive results [
9]. For locally advanced cervical cancer, Song et al. looked at the effects of switching from low-dose-rate (LDR) to high-dose-rate (HDR) intracavitary brachytherapy (ICBT) during radical concurrent chemoradiotherapy. 76 patients who received treatment between 2008 and 2014 were the subject of a retrospective investigation. Comparable long-term results and toxicity profiles to LDR brachytherapy were seen after switching to HDR-ICBT. Significantly the 5 year PFS rate achieved an impressive 63.7%, while OS rate stood at 69.3% [
35]. The work highlights the viability of HDR-ICBT in the combined treatment method and implies that image-guided brachytherapy and increased systemic therapy may provide opportunities for improvement. An important predictive factor that affects patient survival is nodal involvement.
When compared to surgery alone, the approach of NACT succeeded by surgical intervention demonstrated a significant enhancement in clinical outcomes for patients with locoregional illness. This comprehensive strategy contributed to a notable 23% reduction in the likelihood of mortality [
7]. Compared to patients who had surgery without receiving previous chemotherapy, those who got NACT had decreased frequencies of nodal metastatic disease, vascular involvement, and the invasion of parametrial tissue. Additionally, it was observed that individuals who positively responded to NACT displayed significantly improved clinical outcomes in comparison to their counterparts who did not exhibit a favorable response to the treatment. NACT, succeeded with aggressive surgical therapy, has been substantiated in clinical trials to substantially reduce the risk of mortality by 34% (HR: 0.65; p=0.0004). Furthermore, this combined treatment approach has demonstrated a significant elevation in the 5-year viability, escalating it from 50% to a noteworthy 64% [
36]. Unfortunately, a notable percentage of individuals still required additional radiation subsequent to surgical operation, posing challenges in thoroughly assessing the cost-effectiveness and influence of preoperative treatment on healthcare outcomes.
In addition, there is currently no comparison between NACT followed by major surgery and the standard of therapy at this time, chemoradiation. The outcomes of the upcoming EORTC study 55994 are anticipated to provide important light on this contrast [
9]. To reduce the requirement of post-surgical radiation and prevent cumulative adverse effects linked to threesome therapeutic approach (systemic therapy with chemotherapeutics, extensive surgery, radiation treatment), in the age of individualized treatment, it becomes imperative to carefully chose individuals for NACT succeeded by operation. Future research should concentrate on identifying the patients who will gain the most from this strategy without having their quality of life significantly impacted, especially in relation to sexual, gastrointestinal, and urinary dysfunctions.
To preserve reproductivity capability and also preserving the ovary in young females, avoiding radiation would be optimal. These individuals could undergo a concise and intensive treatment plan, later on for a medical assessment and a laparoscopy to determine if lymph nodes were involved. Only patients who had an ideal response and negative nodes would thereafter have open surgery [
9]. This approach would reduce the necessity for postoperative combined chemotherapy and radiation, therefore decrease the elevated morbidity associated with the combination of extensive surgery and radiotherapy.
5. FIGO Stage III Cervical Cancer:
In the exploration of studies focused on FIGO stage IIIC cervical cancer, the available literature sheds light on critical treatment modalities. In a pivotal study by Ye et al., a comprehensive exploration was conducted to compare the outcomes of two prominent treatment modalities for individuals diagnosed with cervical cancer at stage FIGO IB2, IIA, or IIIC. The study meticulously examinate the efficacy of NACT followed by radical surgical procedure in direct comparison with widely established approach involving concomitant chemotherapy and radiotherapy [
37]. Primary endpoint of this investigation delves into these nuances of OS and DFS, providing a comprehensive analysis with 5-year cutoff point after treatment [
38].
In the context of neoadjuvant chemotherapy, Gupta et al.'s research delves into its efficacy when followed by radical surgery [
39]. The research compromises subgroup analyses, unveiling a statistically significant detriment in DFS within the NACT plus operation group, particularly among patients with FIGO stage IIIC [
39]. Additional perspectives are provided by Gadducci et al., highlighting the established approach of CCRT and brachytherapy as the standard of care for cervical cancer at an advanced local stage [
40]. Furthermore, the results from Hsieh et al.’s study suggests that definitive chemoradiotherapy stand out as a viable and effective treatment alternative for cervical cancer at FIGO stageIB2 when juxtaposed with extensive surgical intervention, whether or not NACT is incorporated [
41].
Investigating the effectiveness and safety of NACT for individuals diagnosed with international staging system IB3 and IIA2 cervical cancer, Hu et al.'s research underscores concurrent platinum-based chemoradiation (CCRT) as the endorsed therapeutic regimen, aligning with the guidelines stipulated by the National Comprehensive Cancer Network [
42]. Gupta et al. contribute to the scholarly discourse with a meticulous comparative analysis, scrutinizing the effectiveness and adverse effects profile of NACT followed by extensive surgical intervention in contrast to established cisplatin-based methodologies. Furthermore, a comprehensive research synthesis conducted by Yang et al. posits that the introduction of surgery subsequent to NACT in patients diagnosed with FIGO stage I–II cervical cancer yields comparable outcomes to surgical intervention in isolation, thereby shedding illumination on the efficacy of this particular treatment paradigm.
In considering adverse events, it is imperative to note that the studies presented lack a detailed exposition of specific adverse effects correlated with neoadjuvant chemotherapy. To attain a comprehensive understanding of treatment-related adverse events and their ramifications on the quality of life for patients, a thorough exploration of literature specifically delving into adverse events and post-treatment quality of life assessments is strongly recommended.
6. Conclusion:
Considering that the 4th most frequent cancer by incidence and mortality amongst woman globally is cervical cancer it is clear that the disease continues to pose a serious threat to global healthcare. The actual gold standard treatment for locally advanced cervical cancer is CCRT, however it is still difficult to estimate the prognosis, especially in stage III illness. Recently, attention has been drawn to neoadjuvant chemotherapy (NACT), which has become a possible treatment alternative. When irradiation is added to clinical trials of NACT, the outcomes have been mixed. There may be gains in terms of metastasis reduction and improved radiation delivery, but there are no appreciable survival benefits over radiotherapy alone. A higher percentage of OS and PFS was observed when NACT was combined with radical surgery, it has shown more promising result than surgery alone. The utility of using NACT, the tumor’s size, parametrial infiltration, nodal metastases, and vascular space invasion may be reduced, which may lessen the requirement for adjuvant radiation treatment. In the future it might be essential to enhance management opportunities of locally advance disease of the cervix and lessen its negative effect on patients’ quality of life by finding biomarkers of predicting indications of tailor NACT therapy.
Author Contributions
Conceptualization, O.M.; software O.M.; validation S.M., O.M.; formal analysis, O.M., S.M.; resources, O.M.; data curation, O.M. and S.M.; writing original draft preparation, the entire team; writing-review and editing, O.M. and S.M.; visualization O.M, S.M. and L.L.; supervision, L.L., S.M., project administration, O.M. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
If needed, we will find a way to provide further information.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
NACT—neoadjuvant chemotherapy
CCRT—Cisplatin—based chemotherapy and radiotherapy
MRI— magnetic resonance imagining
RCTs—randomised clinical trials
DFS—disease—free survival
HR—Hazard Ratio
CI—Confidence Interval
RH—Radical Hysterectomy
OS—overall survival
PTX—paclitaxel
IFO—ifosfamide
CDDP—cisplatin
CRT—chemoradiotherapy
OARs—organs at risk
US—ultrasound
CT—computer tomography
LDR—Low—dose—rate
HDR—High—dose—rate
ICBT—intracavitary brachytherapy
FIGO—International Federation of Gynecology and Obstetrics
References
- Mattiuzzi C, Lippi G Cancer statistics: a comparison between World Health Organization (WHO) and Global Burden of Disease (GBD) Eur J Public Health. 2020;30:1026–7. [CrossRef]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [CrossRef]
- Prabhu M, Eckert LO Development of World Health Organization (WHO) recommendations for appropriate clinical trial endpoints for next-generation Human Papillomavirus (HPV) vaccines. Papillomavirus Res. 2016;2:185–9. [CrossRef]
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre, and A. Jemal, “Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA. Cancer J. Clin., vol. 68, no. 6, pp. 394–424, Nov. 2018. [CrossRef]
- L. Stan, “Romania: Romania,” Eur. J. Polit. Res. Polit. Data Yearb., vol. 52, no. 1, pp. 196–207, 2013.
- M. McCormack et al., “A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer,” Br. J. Cancer 2013 10812, vol. 108, no. 12, pp. 2464–2469, May 2013. [CrossRef]
- Dueñas-González et al., “Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix,” J. Clin. Oncol., vol. 29, no. 13, pp. 1678–1685, May 2011. [CrossRef]
- J. E. Sardi et al., “A possible new trend in the management of the carcinoma of the cervix uteri,” Gynecol. Oncol., vol. 25, no. 2, pp. 139–149, 1986. [CrossRef]
- L. Rydzewska, J. Tierney, C. L. Vale, and P. R. Symonds, “Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer,” Cochrane Database Syst. Rev., vol. 2012, no. 12, Dec. 2012. [CrossRef]
- M. Lapresa, G. Parma, R. Portuesi, and N. Colombo, “Neoadjuvant chemotherapy in cervical cancer: an update,” Expert Rev. Anticancer Ther., vol. 15, no. 10, pp. 1171–1181, Oct. 2015. [CrossRef]
- J. Herod et al., “A randomised, prospective, phase III clinical trial of primary bleomycin, ifosfamide and cisplatin (BIP) chemotherapy followed by radiotherapy versus radiotherapy alone in inoperable cancer of the cervix,” Ann. Oncol. Off. J. Eur. Soc. Med. Oncol., vol. 11, no. 9, pp. 1175–1181, 2000. [CrossRef]
- R. P. Symonds et al., “The Scottish and Manchester randomised trial of neo-adjuvant chemotherapy for advanced cervical cancer,” Eur. J. Cancer, vol. 36, no. 8, pp. 994–1001, May 2000. [CrossRef]
- L. Kumar et al., “Neoadjuvant chemotherapy in locally advanced cervical cancer: two randomised studies,” Aust. N. Z. J. Med., vol. 28, no. 3, pp. 387–390, 1998. [CrossRef]
- N. Bhatla, D. Aoki, D. N. Sharma, and R. Sankaranarayanan, “Cancer of the cervix uteri,” Int. J. Gynecol. Obstet., vol. 143, pp. 22–36, Oct. 2018. [CrossRef]
- Gadducci and S. Cosio, “Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer: Review of the Literature and Perspectives of Clinical Research,” Anticancer Res., vol. 40, no. 9, pp. 4819–4828, Sep. 2020. [CrossRef]
- L. Vale, J. F. Tierney, S. E. Davidson, K. J. Drinkwater, and P. Symonds, “Substantial improvement in UK cervical cancer survival with chemoradiotherapy: results of a Royal College of Radiologists’ audit.,” Clin. Oncol. (R. Coll. Radiol)., vol. 22, no. 7, pp. 590–601, Sep. 2010. [CrossRef]
- K. Yu and L. Zhou, “Intensity-Modulated Radiotherapy and Three-Dimensional Conformal Radiotherapy Combined with Intracavitary Posterior Radiotherapy for the Treatment of Medium-Term and Advanced Cervical Cancer: Efficacy, Safety and Prognostic Factors,” Front. Surg., vol. 9, May 2022. [CrossRef]
- T. Mori et al., “Multi-institutional phase II study of neoadjuvant irinotecan and nedaplatin followed by radical hysterectomy and the adjuvant chemotherapy for locally advanced, bulky uterine cervical cancer: A Kansai Clinical Oncology Group study (KCOG-G1201),” J. Obstet. Gynaecol. Res., vol. 45, no. 3, pp. 671–678. [CrossRef]
- L. Rydzewska, J. Tierney, C. L. Vale, and P. R. Symonds, “Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer,” Cochrane Database Syst. Rev., vol. 2012, no. 12, Dec. 2012. [CrossRef]
- H. Chen, C. Liang, L. Zhang, S. Huang, and X. Wu, “Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study.,” Gynecol. Oncol., vol. 110, no. 3, pp. 308–315, Sep. 2008. [CrossRef]
- Buda et al., “Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study,” J. Clin. Oncol., vol. 23, no. 18, pp. 4137–4145, 2005. [CrossRef]
- J. Kigawa, Y. Minagawa, H. Ishihara, H. Itamochi, Y. Kanamori, and N. Terakawa, “The role of neoadjuvant intraarterial infusion chemotherapy with cisplatin and bleomycin for locally advanced cervical cancer,” Am. J. Clin. Oncol., vol. 19, no. 3, pp. 255–259, Jun. 1996. [CrossRef]
- J. E. Sardi et al., “Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: The final results,” Gynecol. Oncol., vol. 67, no. 1, pp. 61–69, 1997. [CrossRef]
- J. Sardi et al., “Randomized trial with neoadjuvant chemotherapy in stage IIIB squamous carcinoma cervix uteri: an unexpected therapeutic management,” Int. J. Gynecol. Cancer, vol. 6, no. 2, pp. 85–93, Mar. 1996. [CrossRef]
- J. E. Sardi et al., “Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: The final results,” Gynecol. Oncol., vol. 67, no. 1, pp. 61–69, 1997. [CrossRef]
- P. B. Panici et al., “Dose-dense neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer: a phase II study,” Oncology, vol. 89, no. 2, pp. 103–110, Jul. 2015. [CrossRef]
- J. F. Tierney et al., “Neoadjuvant chemotherapy for locally advanced cervix cancer,” Cochrane Database Syst. Rev., no. 4, Jan. 2009. [CrossRef]
- C. Napolitano, F. Imperato, B. Mossa, M. L. Framarino, R. Marziani, and L. Marzetti, “The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib-IIIb): a long-term randomized trial.,” Eur. J. Gynaecol. Oncol., vol. 24, no. 1, pp. 51–59, Jan. 2003, Accessed: Aug. 06, 2023. [Online]. Available online: https://europepmc.org/article/med/12691318.
- P. Benedetti-Panici et al., “Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study,” J. Clin. Oncol., vol. 20, no. 1, pp. 179–188, Jan. 2002. [CrossRef]
- M. Maugeri-Saccà, M. Bartucci, and R. De Maria, “Checkpoint kinase 1 inhibitors for potentiating systemic anticancer therapy,” Cancer Treat. Rev., vol. 39, no. 5, pp. 525–533, Aug. 2013. [CrossRef]
- P. Vici et al., “DNA damage and repair biomarkers in cervical cancer patients treated with neoadjuvant chemotherapy: An exploratory analysis,” PLoS One, vol. 11, no. 3, Mar. 2016. [CrossRef]
- Pearcey, R.; Brundage, M.; Drouin, P.; Jeffrey, J.; Johnston, D.; Lukka, H.; MacLean, G.; Souhami, L.; Stuart, G.; Tu, D. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J. Clin. Oncol. 2002, 20, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 340, 1144–1153.
- Y. Zhang, B. Li, Y. Wang, S. Liu, and H. Wang, “Paclitaxel Plus Platinum Neoadjuvant Chemotherapy Followed by Surgery Versus Primary Surgery in Locally Advanced Cervical Cancer—A Propensity Score Matching Analysis,” Front. Oncol., vol. 10, p. 604308, Dec. 2020. [CrossRef]
- J. Song, N. Alyamani, G. Bhattacharya, T. Le, C. E, and R. Samant, “The Impact of High-Dose-Rate Brachytherapy: Measuring Clinical Outcomes in the Primary Treatment of Cervical Cancer,” Adv. Radiat. Oncol., vol. 5, no. 3, p. 419, May 2020. [CrossRef]
- J. Tierney, “Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis of individual patient data from 21 randomised trials,” Eur. J. Cancer, vol. 39, no. 17, pp. 2470–2486, 2003. [CrossRef]
- Treatment of FIGO 2018 stage IIIC cervical cancer with neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10170857/ (accessed on 1 November 2023).
- Initial treatment for FIGO 2018 stage IIIC cervical cancer: overall survival and disease-free survival outcomes. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10587947/ (accessed on 1 November 2023).
- Neoadjuvant chemotherapy for patients with international staging system IB3 and IIA2 cervical cancer. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-022-10355-3 (accessed on 3 November 2023).
- Neoadjuvant Chemotherapy Followed by Radical Surgery: Subgroup Analyses. Available online: https://ascopubs.org/doi/10.1200/JCO.2017.75.9985 (accessed on 3 November 2023).
- Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer. Available online: https://ar.iiarjournals.org/content/40/9/4819 (accessed on 4 November 2023).
- Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer. Available online: https://www.sciencedirect.com/science/article/pii/S0929664617307325 (accessed on 4 November 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).