Submitted:
22 January 2024
Posted:
22 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Prognostic Biomarkers for Treating Gliomas
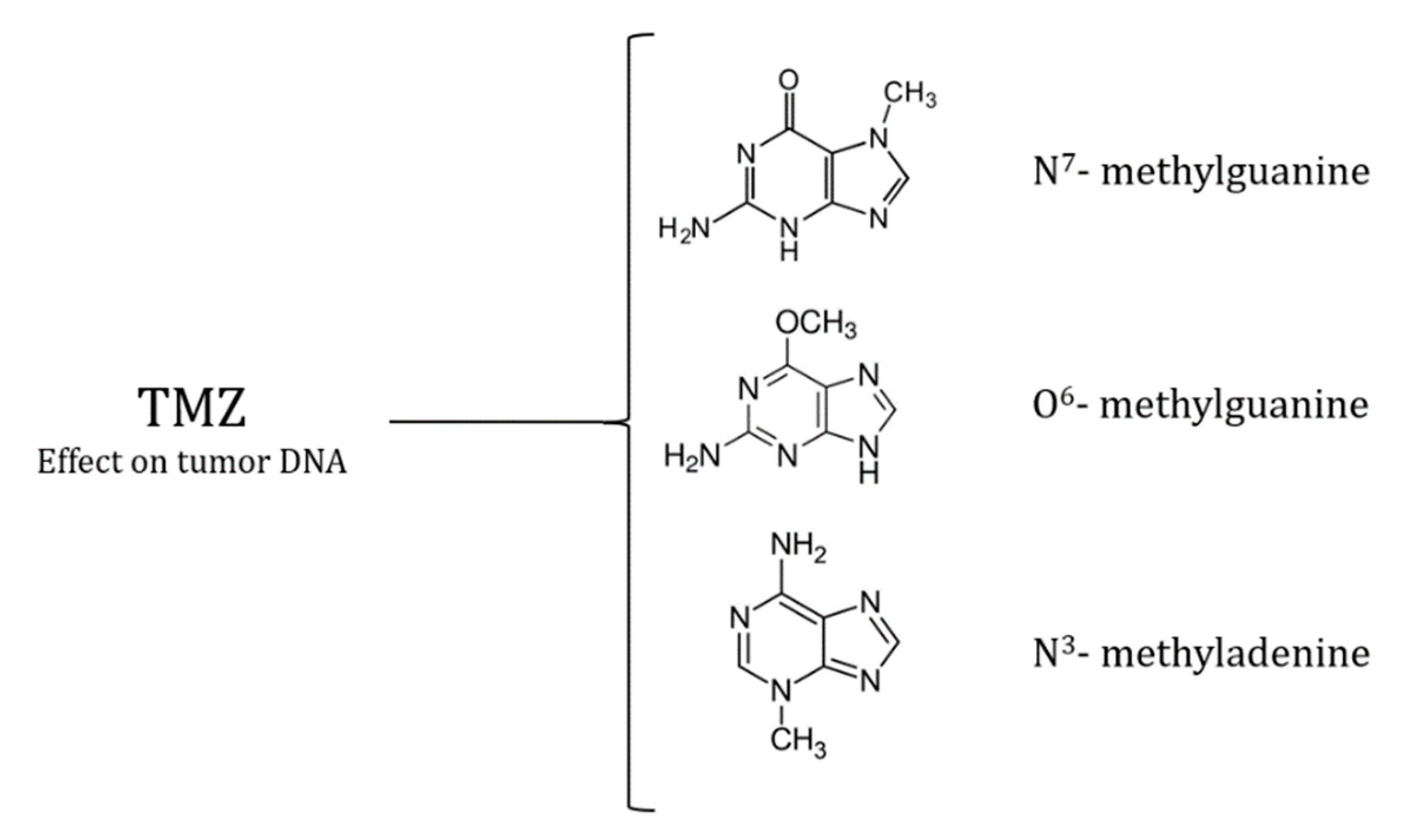
Temozolomide
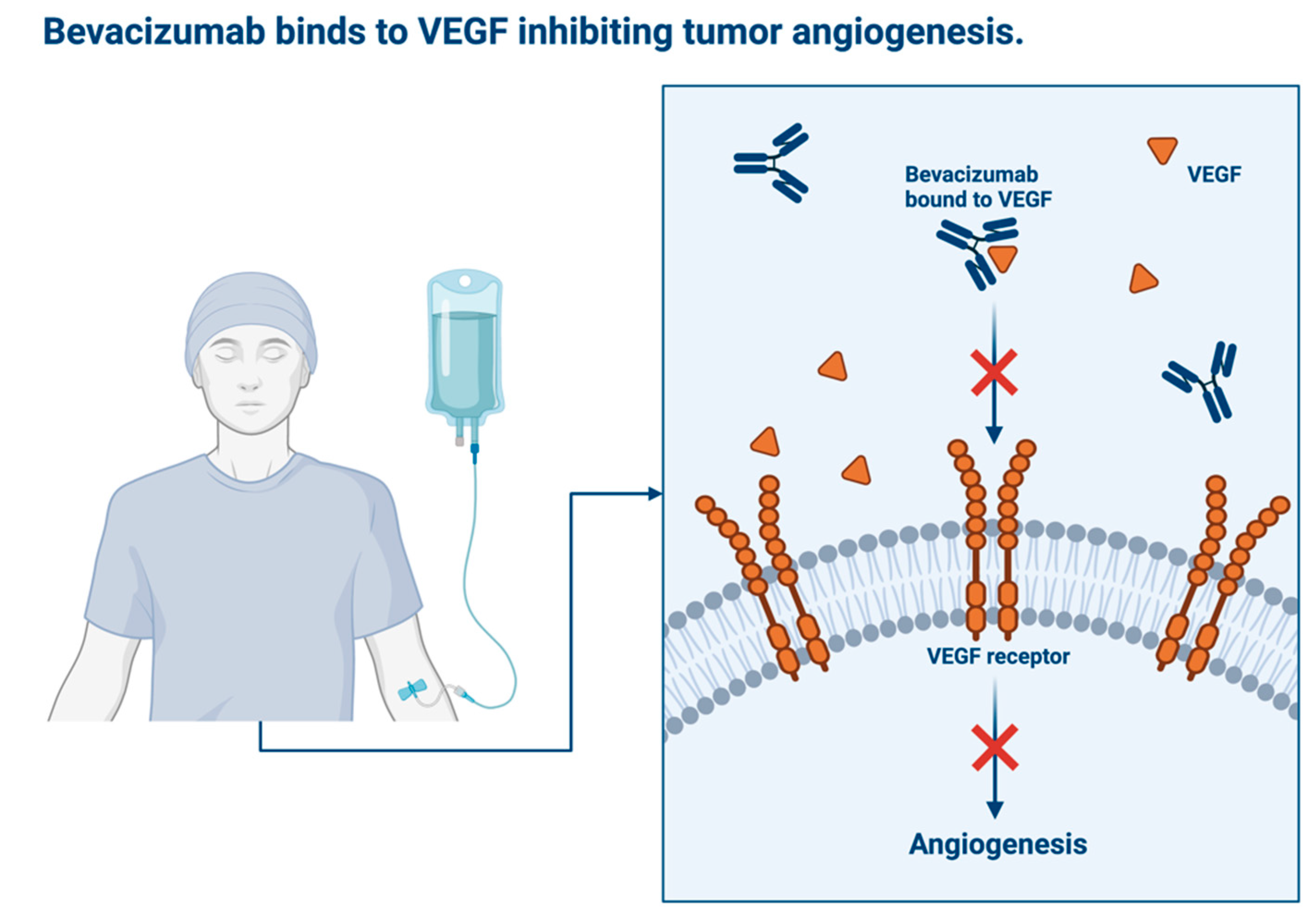
Avastin
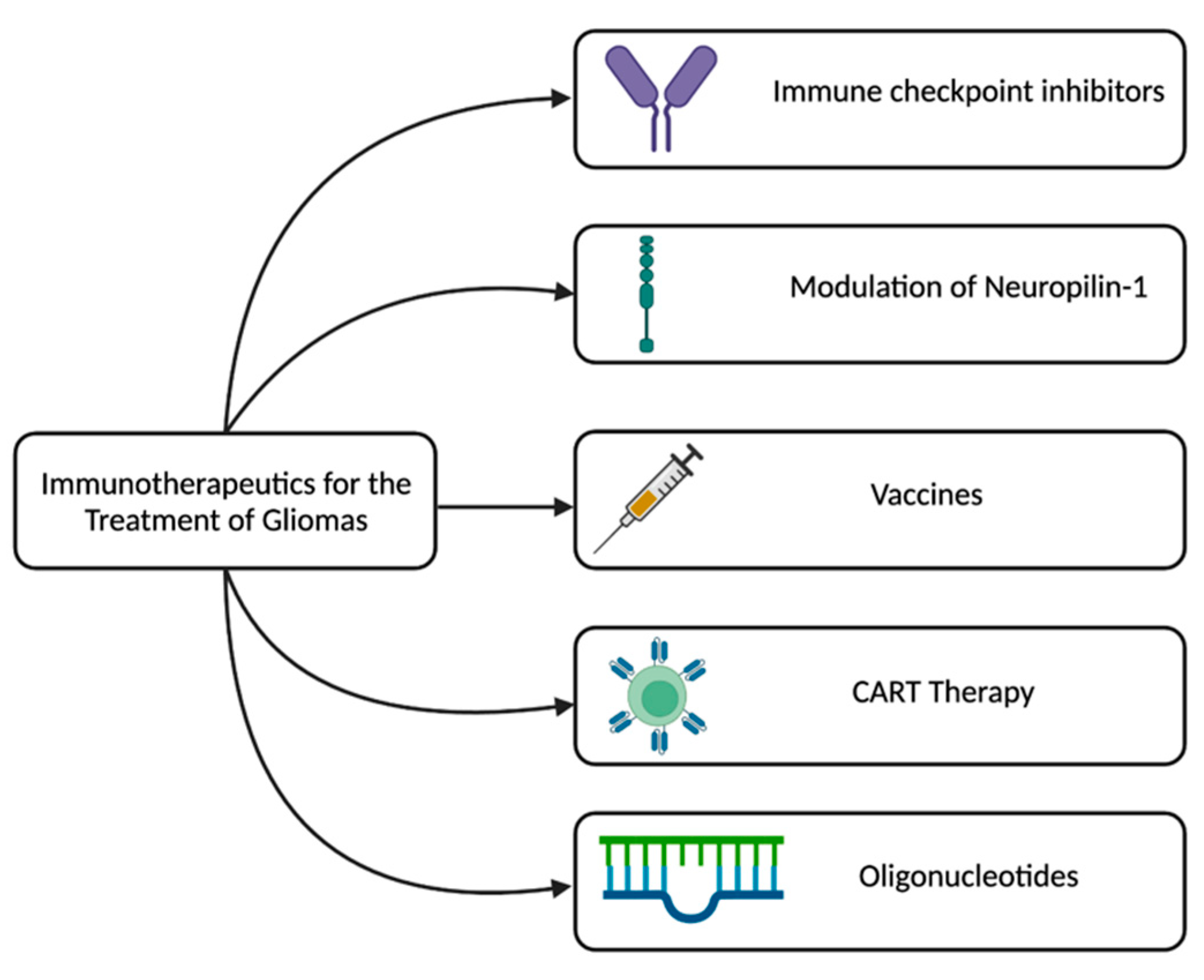
Emerging Immune Modulators
Ongoing Clinical Trials
Conclusions
References
- Zhang Y, Xie J, Han G, et al. [Detection and clinical significance of myeloid-derived suppressor cells in peripheral blood of patients with rectal carcinoma]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:798–802.
- Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20:iv1–iv86.
- Xu S, Tang L, Li X, et al. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020;476:1–12. [CrossRef]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. [CrossRef]
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231–1251. [CrossRef]
- Velázquez Vega JE, Brat DJ, Ryken TC, et al. The role of neuropathology in the management of newly diagnosed glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2020;150:143–164.
- Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139:603–608.
- Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. [CrossRef]
- Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508.
- Watanabe T, Nakamura M, Kros JM, et al. Phenotype versus genotype correlation in oligodendrogliomas and low-grade diffuse astrocytomas. Acta Neuropathol. 2002;103:267–275. [CrossRef]
- Chai R, Li G, Liu Y, et al. Predictive value of MGMT promoter methylation on the survival of TMZ treated IDH-mutant glioblastoma. Cancer Biol Med. 2021;18:272–282. [CrossRef]
- Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–186. [CrossRef]
- Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374:1344–1355. [CrossRef]
- van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021;22:813–823.
- van den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–350.
- Mohile NA, Messersmith H, Gatson NT, et al. Therapy for Diffuse Astrocytic and Oligodendroglial Tumors in Adults: ASCO-SNO Guideline. J Clin Oncol. 2022;40:403–426. [CrossRef]
- Brandes AA, Nicolardi L, Tosoni A, et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253–260. [CrossRef]
- Majzner RG, Theruvath JL, Nellan A, et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res. 2019;25:2560–2574. [CrossRef]
- Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. [CrossRef]
- Rahaman SO, Harbor PC, Chernova O, et al. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. [CrossRef]
- Iwamaru A, Szymanski S, Iwado E, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–2444. [CrossRef]
- Guo X, Zhang Y, Jiao H, et al. The prognostic significance of PD-L1 expression in patients with glioblastoma: A meta-analysis. Front Oncol. 2022;12:925560. [CrossRef]
- Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
- Barciszewska A-M, Gurda D, Głodowicz P, et al. A New Epigenetic Mechanism of Temozolomide Action in Glioma Cells. PLoS One. 2015;10:e0136669. [CrossRef]
- Hombach-Klonisch S, Mehrpour M, Shojaei S, et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther. 2018;184:13–41. [CrossRef]
- Spiro T, Liu L, Gerson S. New cytotoxic agents for the treatment of metastatic malignant melanoma: temozolomide and related alkylating agents in combination with guanine analogues to abrogate drug resistance. Forum (Genova). 2000;10:274–285.
- Jacinto FV, Esteller M. MGMT hypermethylation: a prognostic foe, a predictive friend. DNA Repair (Amst). 2007;6:1155–1160. [CrossRef]
- Kanzawa T, Bedwell J, Kondo Y, et al. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg. 2003;99:1047–1052. [CrossRef]
- Bobola MS, Silber JR, Ellenbogen RG, et al. O6-methylguanine-DNA methyltransferase, O6-benzylguanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res. 2005;11:2747–2755.
- Qian L, Zheng J, Wang K, et al. Cationic core-shell nanoparticles with carmustine contained within O6-benzylguanine shell for glioma therapy. Biomaterials. 2013;34:8968–8978. [CrossRef]
- Stephen ZR, Gebhart RN, Jeon M, et al. pH-Sensitive O6-Benzylguanosine Polymer Modified Magnetic Nanoparticles for Treatment of Glioblastomas. Bioconjug Chem. 2017;28:194–202.
- Liang S, Xu H, Ye B-C. Membrane-Decorated Exosomes for Combination Drug Delivery and Improved Glioma Therapy. Langmuir. 2022;38:299–308. [CrossRef]
- Chu W, Houston ZH, Fletcher NL, et al. Development and Validation of a Targeted Treatment for Brain Tumors Using a Multi-Drug Loaded, Relapse-Resistant Polymeric Theranostic. Biomacromolecules. 2023;24:2674–2690. [CrossRef]
- Marquet G, Dameron O, Saikali S, et al. Grading glioma tumors using OWL-DL and NCI Thesaurus. AMIA Annu Symp Proc. 2007;2007:508–512.
- Ghaffari-Rafi A, Ghaffari-Rafi S, Leon-Rojas J. Role of Temozolomide Regimen on Survival Outcomes in Molecularly Stratified WHO Grade II Gliomas: A Systematic Review. Asian J Neurosurg. 2021;16:14–23. [CrossRef]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [CrossRef]
- Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521–1532. [CrossRef]
- Wahl M, Phillips JJ, Molinaro AM, et al. Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 2017;19:242–251. [CrossRef]
- Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. [CrossRef]
- Villani V, Merola R, Vidiri A, et al. Temozolomide low-dose chemotherapy in newly diagnosed low-grade gliomas: activity, safety, and long-term follow-up. Tumori. 2017;103:255–260. [CrossRef]
- Gao Y, Weenink B, van den Bent MJ, et al. Expression-based intrinsic glioma subtypes are prognostic in low-grade gliomas of the EORTC22033-26033 clinical trial. Eur J Cancer. 2018;94:168–178. [CrossRef]
- Fernandes C, Costa A, Osório L, et al. Current Standards of Care in Glioblastoma Therapy. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): Codon Publications; 2017 [cited 2023 Jun 25]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK469987/.
- Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38.
- Brawanski KR, Sprung S, Freyschlag CF, et al. Influence of MMR, MGMT Promotor Methylation and Protein Expression on Overall and Progression-Free Survival in Primary Glioblastoma Patients Treated with Temozolomide. Int J Mol Sci. 2023;24:6184. [CrossRef]
- Stupp R, Pavlidis N, Jelic S, et al. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol. 2005;16 Suppl 1:i64-65. [CrossRef]
- Angara K, Borin TF, Arbab AS. Vascular Mimicry: A Novel Neovascularization Mechanism Driving Anti-Angiogenic Therapy (AAT) Resistance in Glioblastoma. Transl Oncol. 2017;10:650–660. [CrossRef]
- Wang H, Guo J, Wang T, et al. Efficacy and safety of bevacizumab in the treatment of adult gliomas: a systematic review and meta-analysis. BMJ Open. 2021;11:e048975. [CrossRef]
- Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. [CrossRef]
- Ellis LM. Bevacizumab. Nat Rev Drug Discov. 2005;Suppl:S8-9.
- Ghiaseddin A, Peters KB. Use of bevacizumab in recurrent glioblastoma. CNS Oncol. 2015;4:157–169. [CrossRef]
- Gerriets V, Kasi A. Bevacizumab. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Jun 25]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK482126/.
- Salmaggi A, Eoli M, Frigerio S, et al. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol. 2003;62:297–303. [CrossRef]
- Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–6625.
- Birner P, Piribauer M, Fischer I, et al. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: evidence for distinct angiogenic subtypes. Brain Pathol. 2003;13:133–143. [CrossRef]
- Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55:91–100. [CrossRef]
- Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs. 2010;2:165–175. [CrossRef]
- Muller YA, Chen Y, Christinger HW, et al. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure. 1998;6:1153–1167. [CrossRef]
- García-Romero N, Palacín-Aliana I, Madurga R, et al. Bevacizumab dose adjustment to improve clinical outcomes of glioblastoma. BMC Med. 2020;18:142. [CrossRef]
- Blumenthal DT, Mendel L, Bokstein F. The optimal regimen of bevacizumab for recurrent glioblastoma: does dose matter? J Neurooncol. 2016;127:493–502.
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. [CrossRef]
- Li M, Kroetz DL. Bevacizumab-induced hypertension: Clinical presentation and molecular understanding. Pharmacol Ther. 2018;182:152–160. [CrossRef]
- Lin X, Daras M, Pentsova E, et al. Bevacizumab in high-grade glioma patients following intraparenchymal hemorrhage. Neurooncol Pract. 2017;4:24–28. [CrossRef]
- Castro BA, Aghi MK. Bevacizumab for glioblastoma: current indications, surgical implications, and future directions. Neurosurg Focus. 2014;37:E9. [CrossRef]
- McBain C, Lawrie TA, Rogozińska E, et al. Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev. 2021;5:CD013579. [CrossRef]
- Taal W, Oosterkamp HM, Walenkamp AME, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. [CrossRef]
- Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377:1954–1963. [CrossRef]
- Vredenburgh JJ, Desjardins A, Reardon DA, et al. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol. 2009;11:80–91. [CrossRef]
- Szklener K, Mazurek M, Wieteska M, et al. New Directions in the Therapy of Glioblastoma. Cancers (Basel). 2022;14:5377. [CrossRef]
- Field KM, Simes J, Nowak AK, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17:1504–1513. [CrossRef]
- Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787.
- Yang S-B, Gao K-D, Jiang T, et al. Bevacizumab combined with chemotherapy for glioblastoma: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8:57337–57344. [CrossRef]
- Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [CrossRef]
- Thomas AA, Ernstoff MS, Fadul CE. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012;18:59–68. [CrossRef]
- Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. [CrossRef]
- Conrad CA. Chemotherapy for metastatic tumors to the central nervous system. Curr Oncol Rep. 2001;3:490–494. [CrossRef]
- Pellerino A, Franchino F, Soffietti R, et al. Overview on current treatment standards in high-grade gliomas. Q J Nucl Med Mol Imaging. 2018;62:225–238. [CrossRef]
- Vauleon E, Avril T, Collet B, et al. Overview of cellular immunotherapy for patients with glioblastoma. Clin Dev Immunol. 2010;2010:689171. [CrossRef]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. [CrossRef]
- Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13:3–13.
- Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001;52:401–410.
- Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991;12:370–374. [CrossRef]
- Ghouzlani A, Kandoussi S, Tall M, et al. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Front Immunol. 2021;12:679425. [CrossRef]
- Yamanaka R. Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med. 2008;14:228–235. [CrossRef]
- Zhao T, Li C, Ge H, et al. Glioblastoma vaccine tumor therapy research progress. Chin Neurosurg J. 2022;8:2. [CrossRef]
- Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–1385. [CrossRef]
- Van Gool S, Maes W, Ardon H, et al. Dendritic cell therapy of high-grade gliomas. Brain Pathol. 2009;19:694–712.
- Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res. 2014;20:5620–5629. [CrossRef]
- Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. [CrossRef]
- Yeo AT, Charest A. Immune Checkpoint Blockade Biology in Mouse Models of Glioblastoma. J Cell Biochem. 2017;118:2516–2527. [CrossRef]
- 90. Kaminska B, Ciechomska IA, Cyranowski S. Autophagy in brain tumor immune evasion and responses to immunotherapy. Autophagy in Immune Response: Impact on Cancer Immunotherapy [Internet]. Elsevier; 2020 [cited 2023 Jun 25]. p. 29–52. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128196090000031.
- Goswami S, Walle T, Cornish AE, et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med. 2020;26:39–46. [CrossRef]
- Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. [CrossRef]
- Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–2167. [CrossRef]
- Brown NF, Ng SM, Brooks C, et al. A phase II open label, randomised study of ipilimumab with temozolomide versus temozolomide alone after surgery and chemoradiotherapy in patients with recently diagnosed glioblastoma: the Ipi-Glio trial protocol. BMC Cancer. 2020;20:198. [CrossRef]
- Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res. 2016;4:124–135. [CrossRef]
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. [CrossRef]
- Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20:674–686. [CrossRef]
- Miyauchi JT, Chen D, Choi M, et al. Ablation of Neuropilin 1 from glioma-associated microglia and macrophages slows tumor progression. Oncotarget. 2016;7:9801–9814. [CrossRef]
- Smith GT, Radin DP, Tsirka SE. From protein-protein interactions to immune modulation: Therapeutic prospects of targeting Neuropilin-1 in high-grade glioma. Front Immunol. 2022;13:958620. [CrossRef]
- Caponegro MD, Moffitt RA, Tsirka SE. Expression of neuropilin-1 is linked to glioma associated microglia and macrophages and correlates with unfavorable prognosis in high grade gliomas. Oncotarget. 2018;9:35655–35665. [CrossRef]
- Luksik AS, Yazigi E, Shah P, et al. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers (Basel). 2023;15:1414. [CrossRef]
- Bagley SJ, Desai AS, Linette GP, et al. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20:1429–1438. [CrossRef]
- Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. [CrossRef]
- Goff SL, Morgan RA, Yang JC, et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J Immunother. 2019;42:126–135. [CrossRef]
- Krichevsky AM, Uhlmann EJ. Oligonucleotide Therapeutics as a New Class of Drugs for Malignant Brain Tumors: Targeting mRNAs, Regulatory RNAs, Mutations, Combinations, and Beyond. Neurotherapeutics. 2019;16:319–347. [CrossRef]
- Yoon S, Rossi JJ. Therapeutic Potential of Small Activating RNAs (saRNAs) in Human Cancers. Curr Pharm Biotechnol. 2018;19:604–610. [CrossRef]
- Kelley ML, Strezoska Ž, He K, et al. Versatility of chemically synthesized guide RNAs for CRISPR-Cas9 genome editing. J Biotechnol. 2016;233:74–83. [CrossRef]
- Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013;15:4–27.
- Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518.
- Kirkpatrick JP, Sampson JH. Recurrent malignant gliomas. Semin Radiat Oncol. 2014;24:289–298.
- Kim MM, Umemura Y, Leung D. Bevacizumab and Glioblastoma: Past, Present, and Future Directions. Cancer J. 2018;24:180–186.
- Thomas AA, Fisher JL, Hampton TH, et al. Immune modulation associated with vascular endothelial growth factor (VEGF) blockade in patients with glioblastoma. Cancer Immunol Immunother. 2017;66:379–389. [CrossRef]
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–486. [CrossRef]
- Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




