Submitted:
19 January 2024
Posted:
22 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
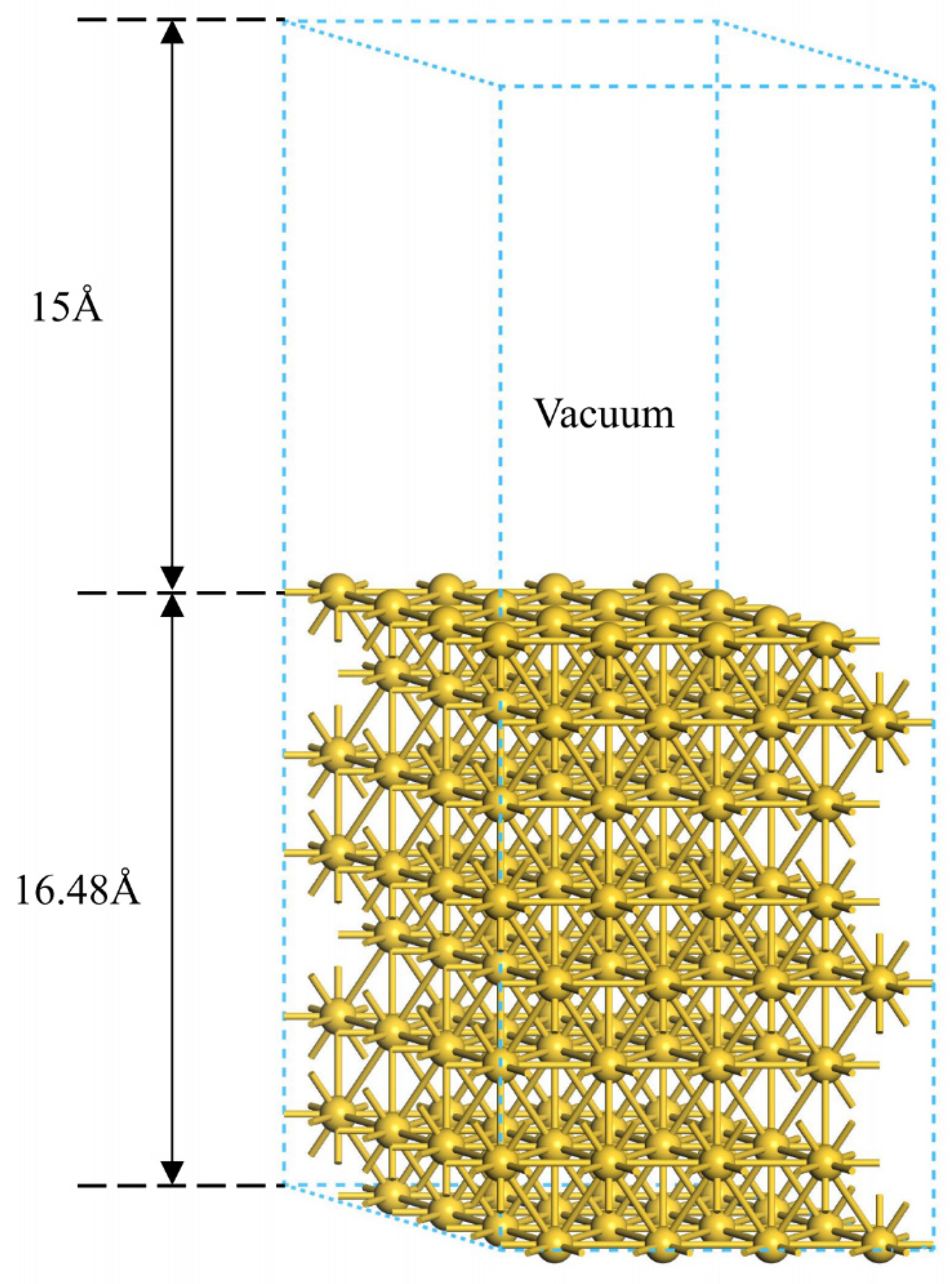
2.1. Model and Parameters
2.2. Adsorption Energy Calculation
2.3. Adsorption Experiments
3. Results and Discussion
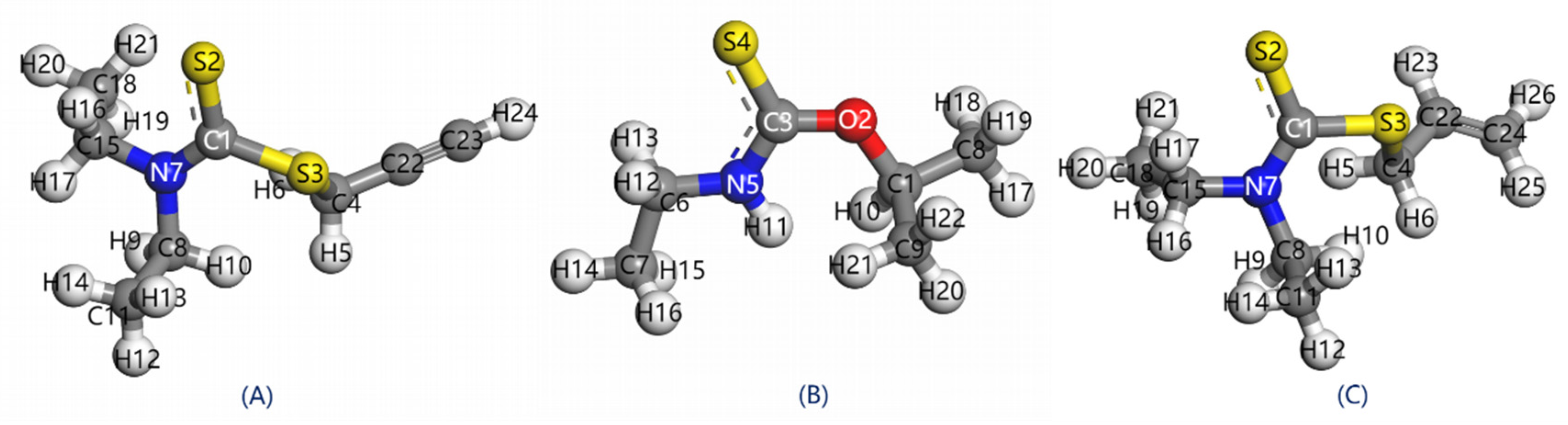
3.1. Structure and Mullinken Population
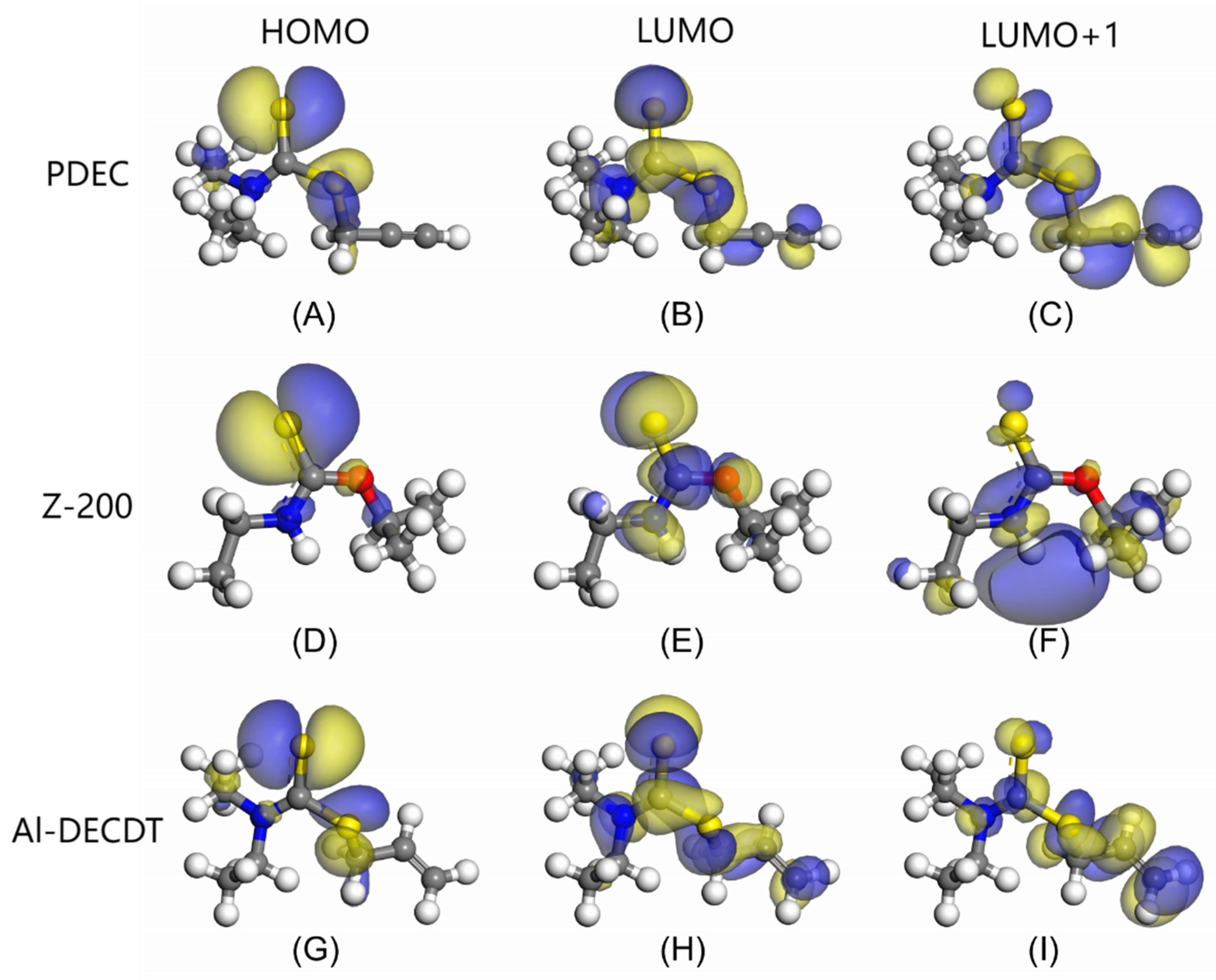
3.2. Frontier Orbital Analysis
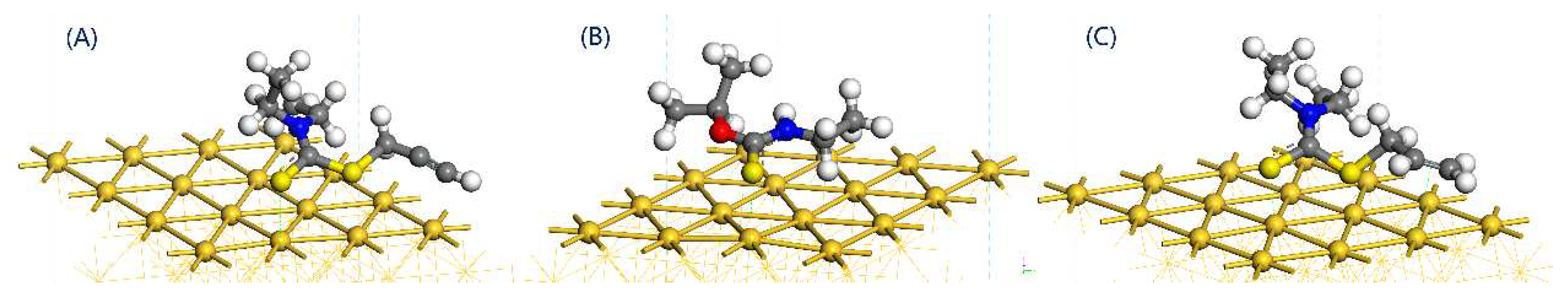
3.3. Adsorption Energy Comparison
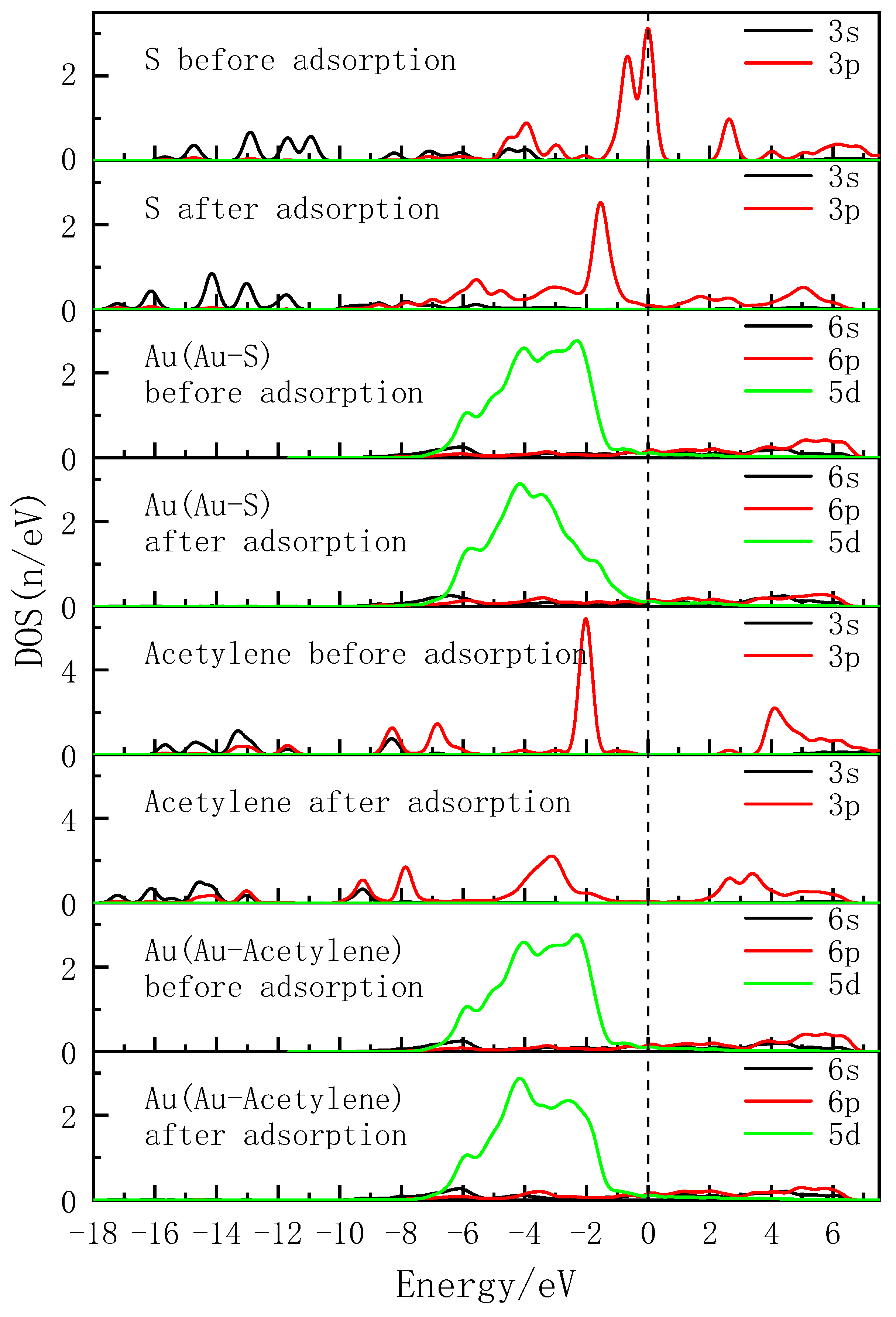
3.4. Density of States Analysis
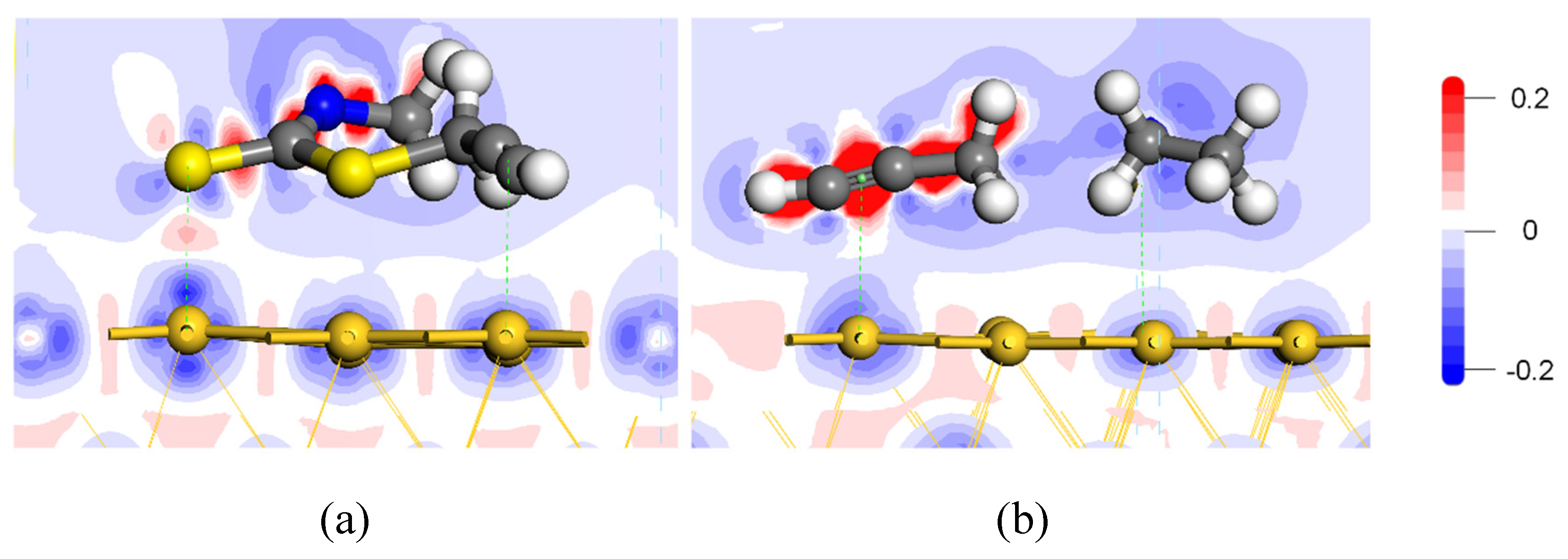
3.5. Electron Density Difference
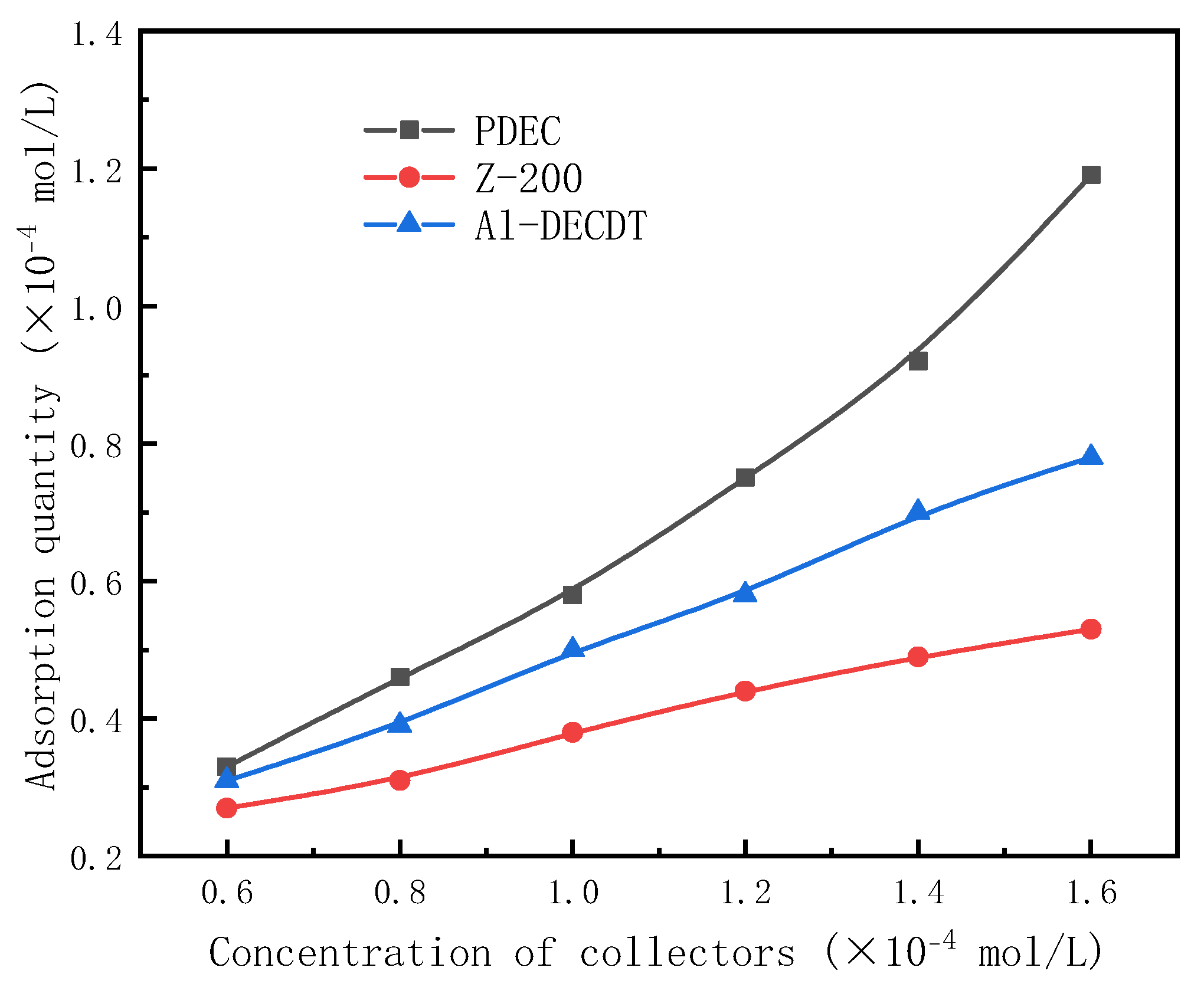
3.6. Adsorption Experiments
4. Conclusions
- (1)
- In PDEC, the carbon-sulfur group form covalent bonds characterized by shorter bond lengths, the reduced carbon-sulfur double bonds and lower Mulliken population of S atom lead to enhanced electron localization. This attribute grants PDEC superior selectivity during its adsorption on mineral surfaces.
- (2)
- Analysis of the LUMO+1 orbit indicates that the electrons in acetylene group of PDEC are delocalized, significantly contributing to the molecule’s frontier orbital energy. The C atom in this group (fw+=0.102) demonstrate a robust electron-accepting capacity, underscoring the crucial role of acetylene group in facilitating bonding interactions with mineral surfaces, particularly in accepting electrons from mineral metal atoms to form stable back-donation bonds.
- (3)
- Both the S atom in the carbon-sulfur group and the acetylene group of PDEC establish stable adsorption structures with the Au(1 1 1) surface, adopting a single coordination mode. The adsorption energy as follows: PDEC > Al-DECDT > Z-200. Partial density of states analysis shows that PDEC’s S 3p orbit hybridize with Au 5d orbit, forming robust coordination bonds. Conversely, the C 2p orbit in the acetylene group engage in weaker back-donation bonding with Au 5d orbit. This is further corroborated by the electron density difference and post-adsorption Mulliken population, which confirm the electron donation by PDEC’s S atom to the Au atom, while the acetylene group predominantly accepts electrons, with positive coordination bonds being the primary interaction in the adsorption process.
- (4)
- In adsorption experiments, the adsorption quantity of collectors adsorbed onto gold powder surfaces was quantified using a UV-Visible Spectrophotometer. The adsorption quantity was observed to increase proportionally with the initial concentration, following the order: PDEC > Al-DECDT > Z-200. These experimental outcomes align with the DFT adsorption energy results.
References
- Wu, J.; Ahn, J.; & Lee, J. Gold deportment and leaching study from a pressure oxidation residue of chalcopyrite concentrate. Hydrometallurgy.2021,201, 105583. [CrossRef]
- Yang, H. Y.; Wang, S. H.;Song, X. L., & Pan, H. D. Gold occurrence of Jiaojia gold mine in Shandong province. Transactions of Nonferrous Metals Society of China. 2011,21(9), 2072-2077. [CrossRef]
- Arif, J.; & Baker, T. Gold paragenesis and chemistry at Batu Hijau, Indoneisa: implications for gold-rich porphyry copper deposits. Mineralium Deposita. 2004 39, 523-535. [CrossRef]
- Asamoah, R. K. Specific refractory gold flotation and bio-oxidation products: Research overview. Minerals. 2021, 11(1), 93. [CrossRef]
- Tagirov, B. R.; Filimonova, O. N.;Trigub, A. L.; Vikentyev, I. V.; Kovalchuk, E. V.; Nickolsky, M. S.; Chareev, D. A. The state of gold in phases of the Cu-Fe-S system: In situ X-ray absorption spectroscopy study. Geoscience Frontiers. 2023, 14(3), 101533. [CrossRef]
- El-Sayed, S.; El-Shatoury, E. H.; Abdel-Khalek, N. A.; Abdel-Motelib, A.; Abdel-Khalek, M. A. Influence of Bacillus cereus-gold interaction on bio-flotation of gold in the presence of potassium butyl xanthate. Biointerface Research in Applied Chemistry. 2021, 11(5), 13005-18. [CrossRef]
- Wang, S.; Zhang, L.; Lu, D.; Fu, Y. Identification of abnormal conditions for gold flotation process based on multivariate information fusion and double-channel convolutional neural network. The Canadian Journal of Chemical Engineering. 2023. [CrossRef]
- Akop, C. Developing a bulk circuit suitable for chalcopyrite-pyrite ores with elevated pyrite content in copper-gold ore treatment. 2014.
- Bas, A. D.; Larachi, F. The effect of flotation collectors on the electrochemical dissolution of gold during cyanidation. Minerals Engineering. 2019,130, 48-56. [CrossRef]
- Matveeva, T. N.; Gromova, N. K.; Lantsova, L. B. Experimental Proof of Applicability of Cyclic and Aliphatic Dithiocarbamate Collectors in Gold-Bearing Sulphide Recovery from Complex Ore. Journal of Mining Science. 2021, 57, 123-130. [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Advances in Colloid and Interface Science. 2017, 246, 181-195. [CrossRef]
- Oluwabunmi, K. E.; Adeleke, A. A.; Adetunji, A. R.; Jeje, S. O.; Abioye, A. A.; Adesina, O. A.; Ibitoye, F. P. 2 k Factorial Experiments on Factors that Influence the Recovery of Gold during the Upgrade of Ilesha-Itagunmodi Gold Ore through Froth Flotation. Journal of Minerals and Materials Characterization and Engineering, 2014. [CrossRef]
- Tan, L., Lin, Q., Liu, P., & Fu, L. Studies on the flotation separation of a new thionocarbamate--ZL 4020. Mining and Metallurgical Engineering(China)(China), 1996, 16(3), 26-29.
- Beattie, D. A., Kempson, I. M., Fan, L. J., & Skinner, W. M. Synchrotron XPS studies of collector adsorption and co-adsorption on gold and gold: silver alloy surfaces. International Journal of Mineral Processing, 2009, 92(3-4), 162-168. [CrossRef]
- Ha, C. S.; Nagappan, S. Hydrophobic and Superhydrophobic Organic-Inorganic Nano-Hybrids. CRC Press. 2018. [CrossRef]
- Nosáľová, L.; Maliničová, L.; Kisková, J.; Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Cultivable microbiota associated with gold ore from the rozália gold mine, hodruša-hámre, Slovakia. Geomicrobiology Journal. 2021 38(5), 415-425. [CrossRef]
- Burdonov, A.; Vchislo, N.; Barakhtenko, V.; & Sahabutdinova, T. Synthesized collectors flotation activity study based on fluorine containing and acetylene alcohols. Sustainable Development of Mountain Territories. 2023, 15(3), 707-719. [CrossRef]
- Yushina, T. I. Justification of applying collectors from the class of unsaturated tertiary alcohols in flotation of gold-bearing sulphide ores.2022. [CrossRef]
- Liu, W.; Miller, J. D.; Sun, W.; Hu, Y. Analysis of the selective flotation of elemental gold from pyrite using diisobutyl monothiophosphate. Minerals. 2022, 12(10), 1310. [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Zhong, H.; Zhang, B.; Liu, G.;Yang, Y. Density functional theory study on electronic structure of tetrahedrite and effect of natural impurities on its flotation property. Minerals Engineering. 2021, 169, 106980. [CrossRef]
- Mkhonto, P. P.; Chauke, H. R.; Ngoepe, P. E. The effect of thiol collectors on nickel-rich (110) pentlandite surface using density functional theory. Proceedings of SAIP2017. 2018, 95-100.
- Yuan, M.; Feng, X.; Yan, T. H.; Chen, J.; Ma, X.; Cunha, P.; Wang, Y. Superparamagnetic iron oxide-enclosed hollow gold nanostructure with tunable surface plasmon resonances to promote near-infrared photothermal conversion. Advanced Composites and Hybrid Materials. 2022, 5(3), 2387-2398. [CrossRef]
- Nenchev, G.; Diaconescu, B.; Hagelberg, F.; Pohl, K. Self-assembly of methanethiol on the reconstructed Au (111) surface. Physical Review B. 2009, 80(8), 081401. [CrossRef]
- He, J.; Sun, W.; Chen, D.; Gao, Z.; Zhang, C. Interface interaction of benzohydroxamic acid with lead ions on oxide mineral surfaces: a coordination mechanism study. Langmuir. 2021, 37(11), 3490-3499. [CrossRef]
- He, Y.; Wang, Z.; Ren, Z.; Zheng, R.; Gao, H.; Chen, Z. The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates. Minerals. 2023, 13(8), 1005. [CrossRef]
- Xu, B.; Wu, J.; Dong, Z.; Tao, J.; Qian, L.; Yang, Y. Flotation performance, structure–activity relationship and adsorption mechanism of a newly-synthesized collector for copper sulfide minerals in Gacun polymetallic ore. Appl. Surf. Sci. 2021, 551, 149420. [CrossRef]
- Cao, S.; Cao, Y.; Liao, Y.; Ma, Z. Depression mechanism of strontium ions in bastnaesite flotation with salicylhydroxamic acid as collector. Minerals 2018, 8, 66. [CrossRef]
- Corso, M.; Fernández, L.; Schiller, F.; Ortega, J. E. Au (111)-based nanotemplates by Gd alloying. ACS nano. 2010, 4(3), 1603-1611. [CrossRef]
- Li, M.; Sun, B.; Ao, Z.; An, T.; Wang, G. Atomic-scale identification of influencing factors of sodium dendrite growth on different current collectors. J. Mater. Chem. A 2020, 8, 10199–10205. [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological avenues in mineral processing: Fundamentals, applications and advances in bioleaching and bio-beneficiation. Mineral Processing and Extractive Metallurgy Review. 2023, 44(1), 22-51. [CrossRef]
- Terzi, M.; Kursun, I.; Cinar, M.; Ozdemir, O. Digital image processing (DIP) application on the evaluation of ironrich heavy mineral concentrates produced from river sand using a sequential mineral processing approach. Physicochemical Problems of Mineral Processing. 2021, 57. [CrossRef]
- Yang, K.; Lu, X. C.; Liu, X. D.; Hou, Q. F. Characterization technique ii of mineral material based on probe gas adsorption isotherm: nano-pore structure of porous material. Bulletin of mineralogy petrology and geochemistry. 2006, 25(4), 362-368. [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. J. Phys. Rev. Lett. 2009, 102, 073005. [CrossRef]
- Zierhut, A., Leopold, K., Harwardt, L., Worsfold, P., & Schuster, M. Activated gold surfaces for the direct preconcentration of mercury species from natural waters. Journal of Analytical Atomic Spectrometry, 2009, 24(6), 767-774. [CrossRef]
| Collectors | Bond length (Å) | Mullinken population of bond | Mullinken population of atom charge | ||||
|---|---|---|---|---|---|---|---|
| PDEC | C1-S2 | C1-S2 | S2 | C1 | N7 | S3 | C23 |
| 1.669 | 0.90 | -0.14 | -0.17 | -0.27 | 0.23 | -0.38 | |
| Z-200 | C3-S4 | C3-S4 | S4 | C3 | N5 | O2 | |
| 1.651 | 0.94 | -0.19 | 0.22 | -0.55 | -0.41 | ||
| Al-DECDT | C1-S2 | C1-S2 | S2 | C1 | N7 | S3 | C24 |
| 1.664 | 0.90 | -0.15 | -0.17 | -0.26 | 0.18 | -0.62 | |
| Collectors | Frontline orbital energy (eV) | ||
|---|---|---|---|
| HOMO | LUMO | LUMO+1 | |
| PDEC | -4.708 | -2.077 | -0.608 |
| Z-200 | -4.515 | -0.812 | 0.510 |
| Al-DECDT | -4.456 | -1.942 | -0.895 |
| Collectors | Atoms | fw+ | fw- |
|---|---|---|---|
| PDEC | S2 | 0.280 | 0.421 |
| C1 | 0.079 | 0.017 | |
| N7 | 0.039 | 0.026 | |
| S3 | 0.158 | 0.154 | |
| C23 | 0.102 | 0.081 | |
| Z-200 | S4 | 0.365 | 0.572 |
| C3 | 0.154 | 0.078 | |
| N5 | 0.052 | 0.028 | |
| O2 | 0.064 | 0.013 | |
| Al-DECDT | S2 | 0.266 | 0.414 |
| C1 | 0.068 | 0.019 | |
| N7 | 0.040 | 0.026 | |
| S3 | 0.138 | 0.161 | |
| C24 | 0.065 | 0.034 |
| Collectors | Adsorption energy (KJ/ mol) | S-Au bond length (Å) |
|---|---|---|
| PDEC | -71.46259119 | 2.580 |
| Z-200 | -58.05373004 | 2.521 |
| Al-DECDT | -59.43253585 | 2.557 |
| Atomic label | Adsorption status | s | p | d | Charge/e |
|---|---|---|---|---|---|
| S | Before adsorption | 1.83 | 4.31 | 0.00 | -0.14 |
| After adsorption | 1.83 | 4.17 | 0.00 | 0.00 | |
| Au(Au-S) | Before adsorption | 0.91 | 0.53 | 9.65 | -0.09 |
| After adsorption | 0.85 | 0.72 | 9.62 | -0.18 | |
| C | Before adsorption | 1.15 | 3.23 | 0.00 | -0.38 |
| After adsorption | 1.17 | 3.19 | 0.00 | -0.36 | |
| Au(Au-C) | Before adsorption | 0.91 | 0.53 | 9.65 | -0.09 |
| After adsorption | 0.83 | 0.46 | 9.63 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




