1. Introduction
Follicular cysts, a prevalent ovarian disorder, contribute to infertility in mammals. These cysts emerge when a follicle fails to undergo ovulation at its expected time due to various factors, such as genetics, imbalances in sex hormones and nutrition, aging, gene dysregulation, and stress. This leads to the cyst’s enlargement and accumulation of fluid [
1,
2]. Among these factors, an imbalanced distribution of sex hormones resulting from disruption in the hypothalamic-pituitary-ovarian (HPO) axis is widely acknowledged as the trigger for cyst formation [
3]. However, beyond endocrine disturbances, alterations in intraovarian genes and proteins exert their influence on ovarian development and folliculogenesis, potentially contributing to the formation of cysts.
Cystic ovaries display distinctive gene and protein expression profiles in contrast to dominant or other follicles within a healthy ovary [
3,
4]. The shifts in gene expression within ovarian cells remain intricately connected to the etiology of cystic ovarian conditions [
5,
6]. In cattle, granulosa cells (GCs) obtained from persistent follicles show specific gene expression patterns [
6]. These alterations in gene expression profiles within ovarian cells concurrently impact follicle diameter [
7,
8,
9]. In cattle, ovaries harboring follicles exceeding a diameter of 25 mm in diameter are classified as cystic [
10]. The precise causes and mechanisms underlying cyst formation are not yet fully elucidated.
GCs and follicle diameter are intricately linked, as GCs play a crucial role in orchestrating the growth and maturation of ovarian follicle, subsequently influencing their dimensions. By secreting an array of growth factors and signaling molecules, GCs facilitate the growth and differentiation of the oocyte, while also generating substantial levels of estrogen, a fundamental element in fostering follicular development. Anomalies in GC proliferation can precipitate the formation of cysts. Dysfunction or impairment of GCs can engender infertility, irregular menstrual cycles, and an array of other reproductive disorders. In cattle afflicted with cystic ovaries, the normal trajectory of folliculogenesis remains incomplete with abnormal GCs, culminating in the emergence of persistent cystic follicles [
11,
12].
Ion channels affect several cellular processes encompassing cell proliferation, apoptosis, senescence, migration, and volume regulation. The intricate dance of water and ions through ion channels located at the cell membrane plays a crucial role in regulating ovarian cellular activities, including the complex processes of folliculogenesis [
13]. The interplay between large molecules and ions, secreted by oocytes or GCs, precipitates an osmotic pressure gradient that draws water into the antrum [
14]. In the formation of the ovarian follicular antrum, cell volume regulation emerges as a cornerstone mechanism. Follicles undergo expansion until osmolality equilibrium is achieved both inside and outside the cells. This swelling, in turn, triggers the activation of Cl
- or K
+ channels. Notably, K
+ and Cl
− channels have emerged as pivotal agents orchestrating regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) processes [
15].
In contrast to Cl
- channels, there is a scarcity of reports regarding the involvement of K
+ channels in the formation of ovarian cysts. A few K
+ channels, K
ATP, K
Ca (IK, SK, BK), Kv4.2, and two-pore domain potassium (K
2P) channels are detected in the ovary [
16,
17]. Among K
+ channels, it is the K
2P channels that distinctly orchestrate osmotic water movement through their adept control of the resting membrane potential and facilitation of K
+ efflux in various cells, including mouse zygotes [
18]. In the mammalian ovary and female germ cells, the TWIK-related acid-sensitive K
+ channels (TASKs; TASK-1, TASK-3, and TASK-5), belong to the K
2P channel family, are expressed [
17,
19,
20]. Moreover, another member of the K
2P channel family, TASK-2, is linked to apoptosis through volume regulation [
21]. In breast cancer cells, downregulation of TASK-3 leads to heightened cell migration and invasion [
22], while also inducing cellular senescence and inhibiting growth [
23]. The modulation of TASK channels is expected to exert an influence on the formation of ovarian cysts by affecting cell volume and growth.
This study was conducted to investigate the potential influence of TASK channels on the formation of cystic follicles in bovine ovaries. Ion concentration and TASK channel expression level were compared in follicular fluid (FF) and GCs obtained from small-sized (5 to 10 mm in diameter) and large-sized (> 25 mm) follicles (SF and LF) in Korean cattle.
3. Discussion
Ovaries harboring follicles larger than 25 mm in diameter are categorized as cystic in cattle [
10]. The follicles utilized in this study, possessing diameters surpassing 25 mm, were characterized as follicular cysts based on histological features and hormone levels (E
2/P
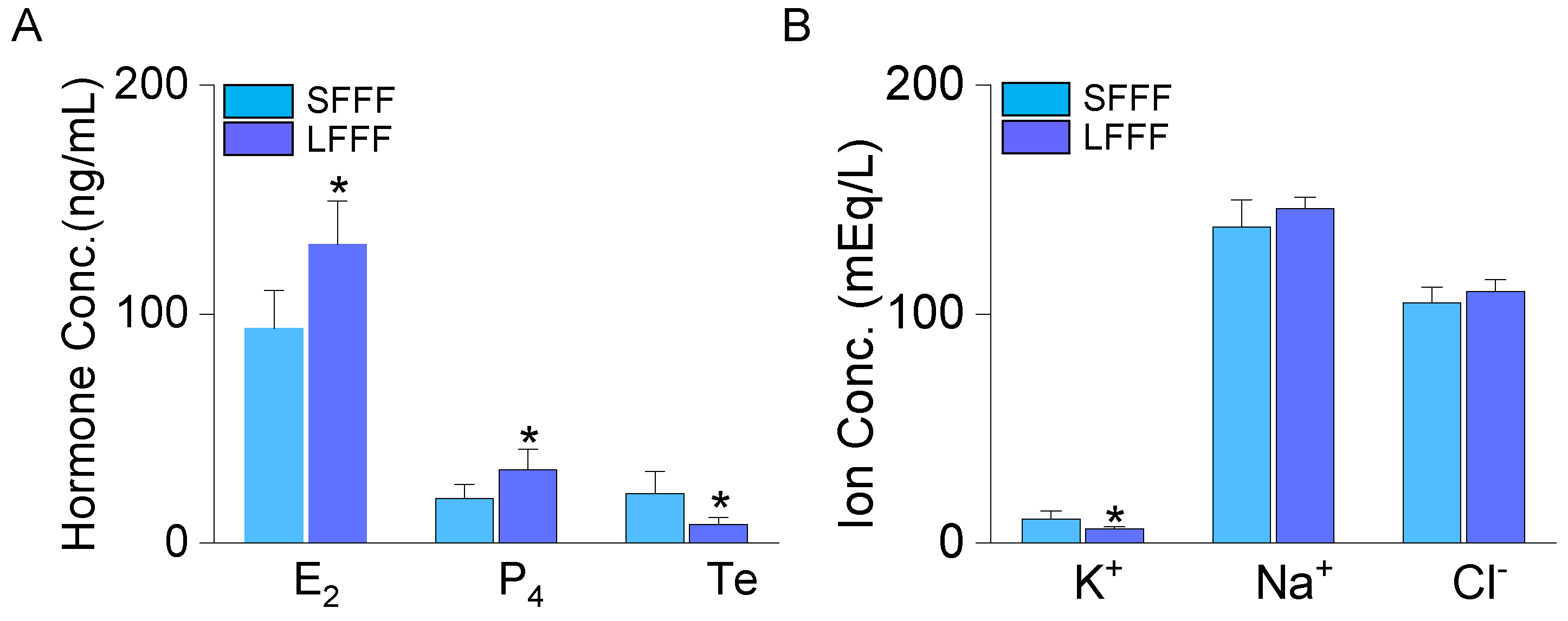
4 ratio > 1 and low testosterone). A follicular cyst, typically a fluid-filled sac found on the ovarian surface, arises when a follicle grows without rupturing or releasing its oocyte due to hormonal signal dysfunction. This study focused on gene expression changes rather than hormonal variations, which have been established in numerous studies as contributors to follicular cyst development.
In the LFFF, the lower K
+ concentration ([K
+]) compared to SFFF enabled us to discern alterations in the expression of K
+ channels. Consistent with our findings, the [K
+] in FF declined as follicle size grew, despite the compared follicle sizes being slightly different [
24]. Unlike K
+, our findings indicated no notable difference in Na
+ and Cl
- concentrations between SF and LF. If the decrease in [K
+] was due to dilution from fluid accumulation, we would have expected a concurrent reduction in in Na
+ and Cl
- concentrations. The isolated decrease in [K
+] in LFFF is believed to result from the dysregulation of K
+ channels or transporters. Regulating [K
+] inside and outside the cell via K
+ channels and transporters in the cell membrane is crucial for preserving the resting membrane potential and various cellular functions [
25]. In general, when K
+ carrier proteins function properly, the extracellular [K
+] ([K
+]
e) remains tightly controlled within a specific range (typically 3.5-5.0 mmol/L). Changes in [K
+] affect cellular processes, such as cell growth, apoptosis, and maintenance of cell volume [
26,
27]. A relatively high [K
+] is sustained in FF.
An interesting finding emerged while investigating the significance of elevated [K
+] levels in FF [
28]. A past study demonstrated that the [K
+] in
in vivo FF closely resembled that in body fluid. However, FF obtained from ovaries at slaughterhouse exhibited a notable increase in [K
+] compared to that in body fluid [
28]. The underlying cause of this [K
+] disparity between the two groups remains unresolved, possibly resulting from blockade of K
+ transport and altered protein expression in ovaries obtained from slaughterhouse. The ovaries employed in this study were sourced from slaughterhouses and displayed a relatively elevated [K
+] in comparison to body fluid. Further studies are essential to discern the underlying reasons for the augmented [K
+] levels in ovarian FF obtained from the slaughterhouse.
Dysfunctional K
+ channels in the reproductive system hinder the HPO axis, leading to reduced fertility [
29]. Ovarian [K
+] and K
+ channels are pivotal in E
2 and P
4 synthesis and secretion [
16,
30], influencing follicular development. GCs release hormones, with a substantial portion of GC-produced estrogen entering the FF. Estrogen receptors alpha and beta (ERα and ERβ) function as hormone-responsive transcription factors, directly regulate the expression of their target genes. TASK-3 was one of the proteins regulated by E
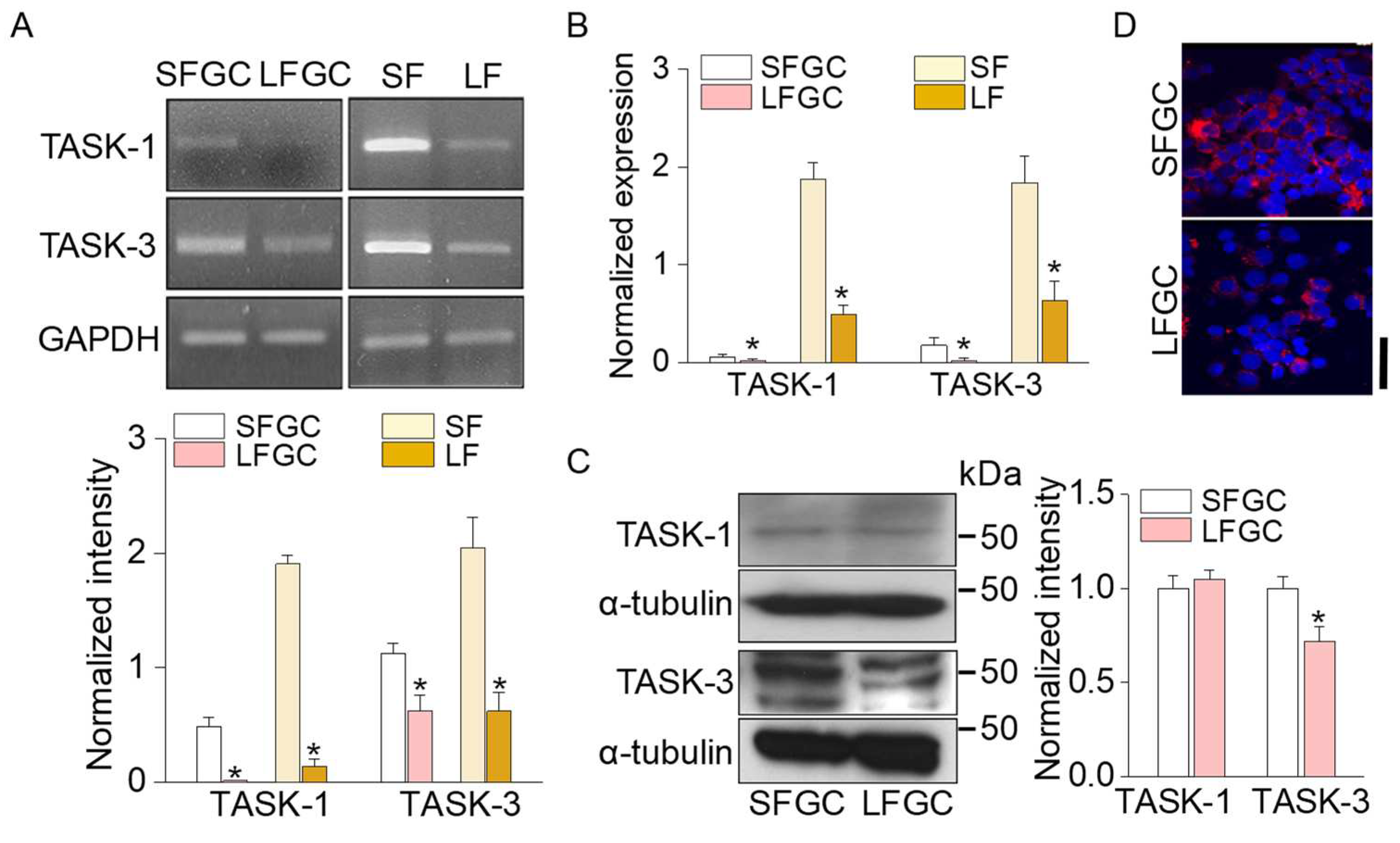
2. Utilizing the transcription element search system (TESS) software, it was revealed that TASK-3’s promoter region encompasses three predictive estrogen response elements. E
2 treatment notably upregulated TASK-3 expression in GCs (data not shown). However, in LFGCs, ERα and ERβ expression was comparatively low when contrasted with SFGCs (data not shown). While E
2 increases the expression level of TASK-3
in vitro, the limited ERs expression is projected to curtail its influence. Unraveling the intricate interplay among TASK-3, ERα, and mediating molecules necessitates further investigation. A reduced ER expression in LFGCs might be linked to the decreased expression of TASK-3.
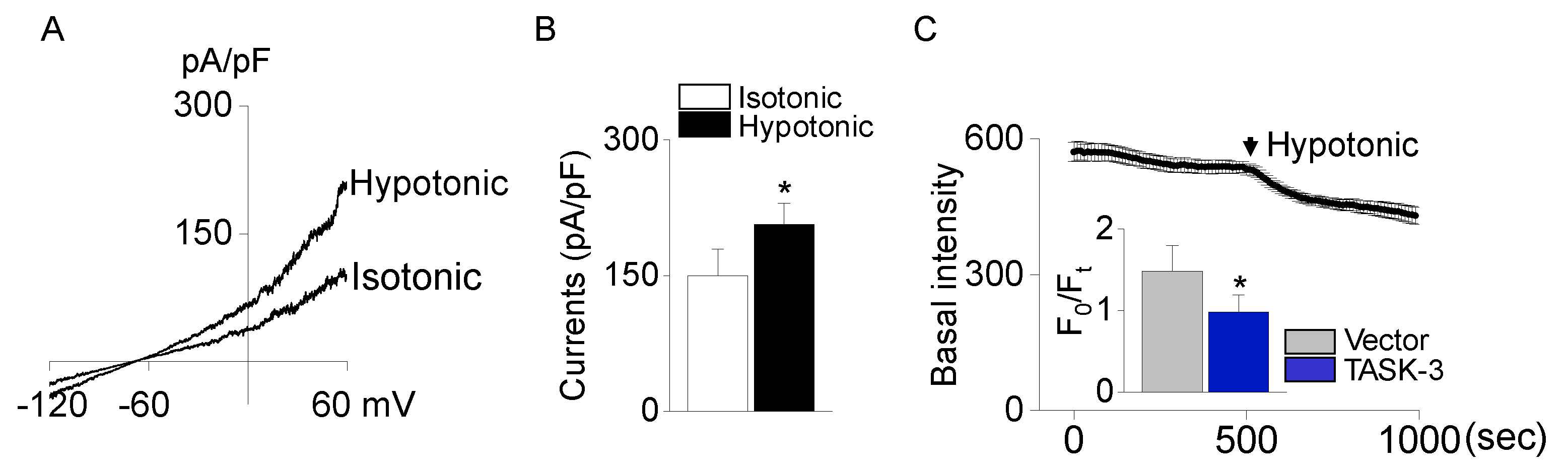
Diminished TASK-3 expression in GCs is likely to lead to the inhibition of K
+ efflux, impaired RVD, depolarized membrane potential, and cellular senescence. TASK-3 channel activation in GC cells drives water expulsion, with osmotic changes due to high molecular compounds in FF enhancing water movement to the FF [
14,
31]. Fluid outflow from GCs can boost FF volume, triggering ion channels in GCs to balance osmotic pressure. AQP4 might collaborate with TASK-3 for water and ion transport in GCs [
32]. However, given the reduced levels of AQP4 in LFGCs [
33], there may be limitations in water and ion transport.
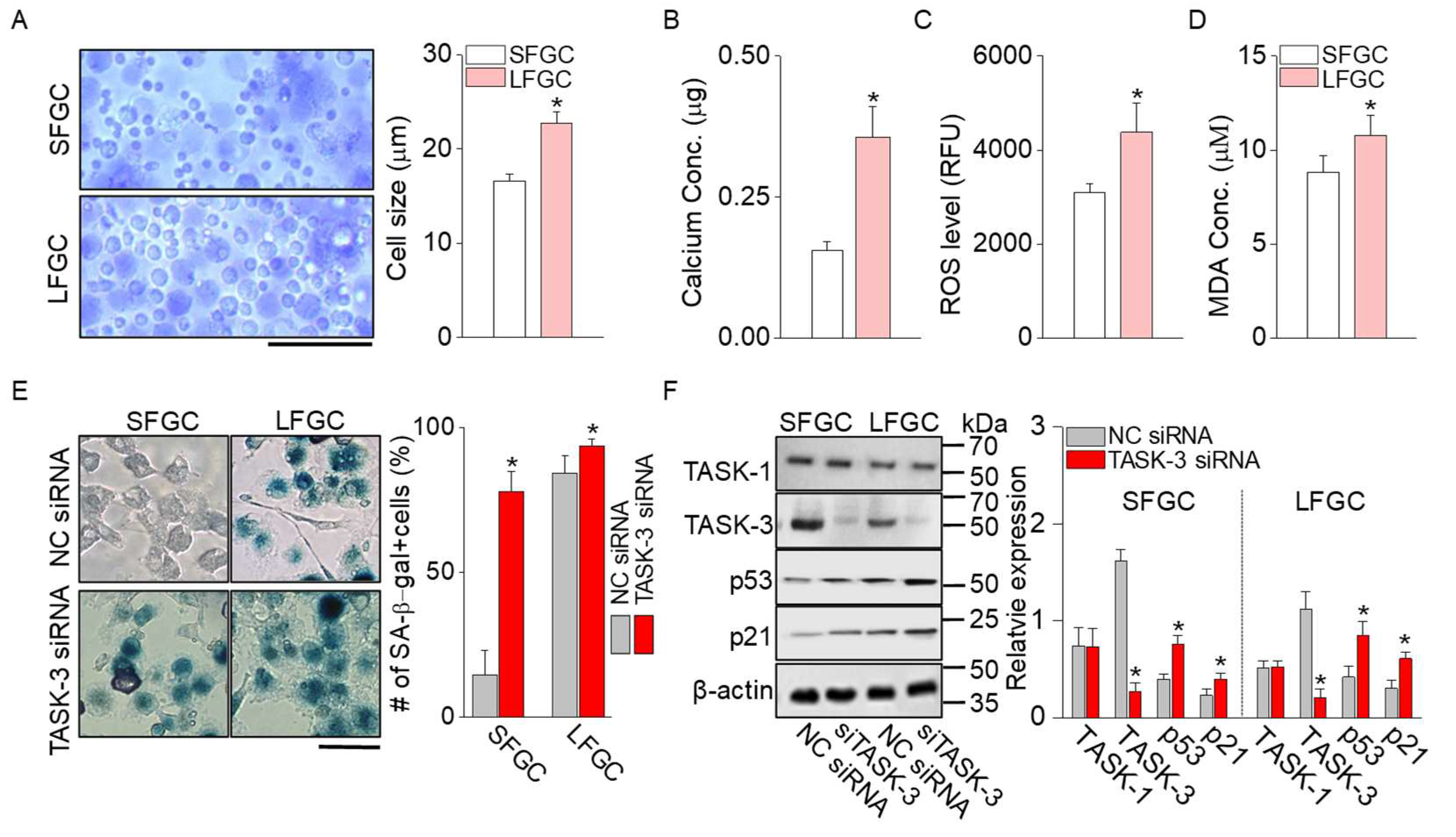
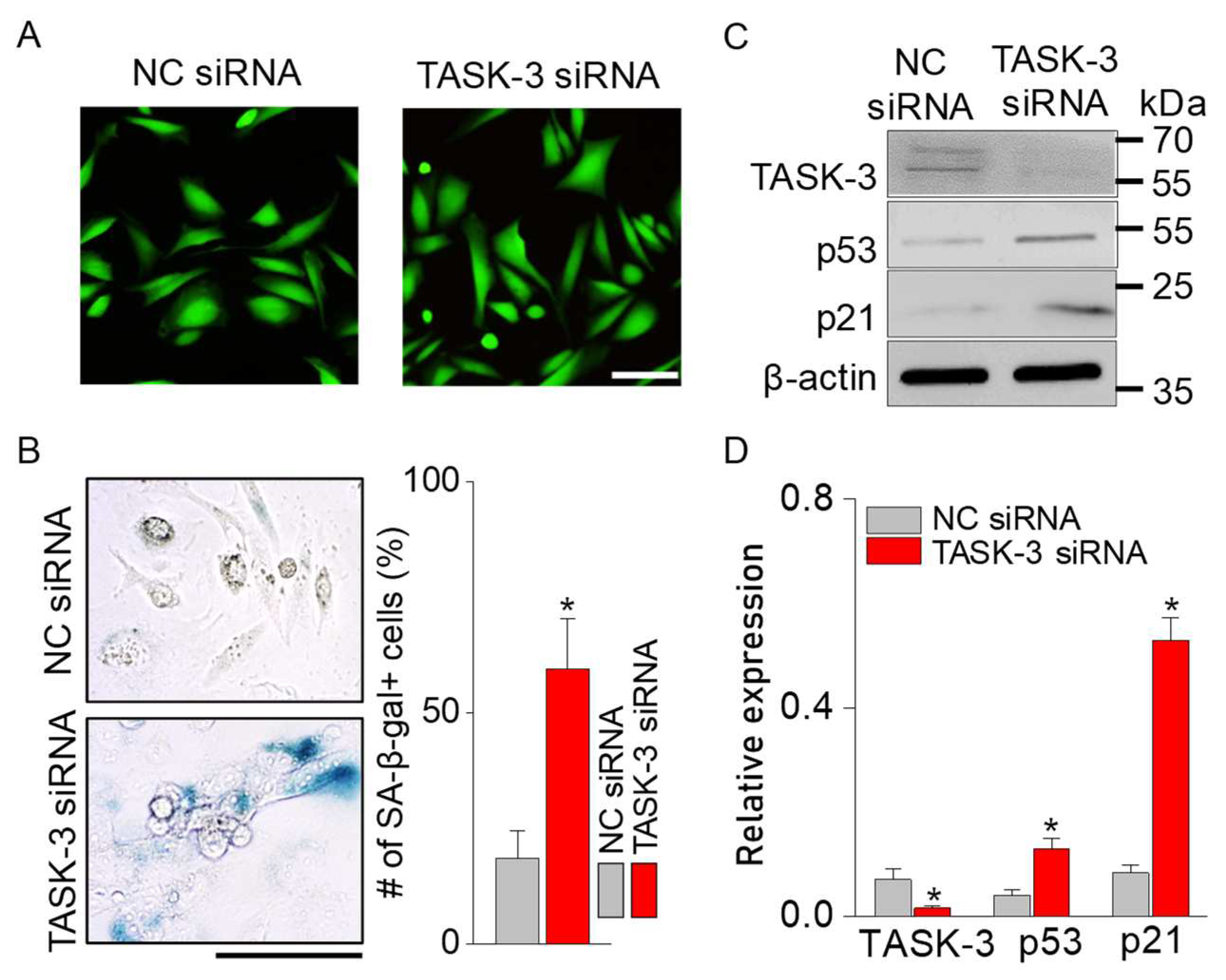
Cellular senescence is a stable and ultimate growth arrest in which cells cannot proliferate despite optimal growth conditions and mitogenic stimuli [
34]. The mechanism of ovarian senescence is shared between cattle and humans. An essential component of ovarian senescence involves the disruption of antioxidant signaling, specifically affecting early-stage oocytes and GCs [
35]. Human GCs exhibit a down-regulation of antioxidative genes [
35], which are closely linked to the decay of ovarian functionality. The TASK-3 channel functions as one of the mitochondrial channels that participate in protecting cells from damage [
36]. Senescent cells exhibit distinct traits such as cellular enlargement, increased SA-β-gal activity, upregulated cell cycle regulators (p53 and p21), and an amplified senescence-associated secretory phenotype [
34,
37]. LFGCs that expressed lower levels of TASK-3 demonstrated an increased cell volume and elevated SA-β-gal activity, along with higher expressions of p53 and p21, when compared to SFGCs. This suggests that reduced TASK-3 expression could play a role in triggering senescence in GCs. The markers of senescence observed in LFGCs were similarly confirmed in cells where TASK-3 was silenced. Furthermore, LFGCs exhibited elevated levels of ROS and Ca
2+, both of which have been linked to cellular senescence [
38,
39]. These factors contribute to a harmful microenvironment for the cells.
This study has several limitations. Firstly, the ovaries used for cellular isolation and gene analysis were obtained from a slaughterhouse. Consequently, we couldn’t determine the biological, genetic, and environmental factors of the donors that could potentially influence ovarian cyst development and TASK-3 expression levels. Additionally, while we observed differences in TASK-3 mRNA and protein levels between SF and LF, only TASK-1 mRNA varied between them. Generally, protein levels remain consistent across tissues [
40], and although normally determined by mRNA levels, this isn’t always the case [
41]. Given the changes at both the mRNA and protein levels, TASK-3 seemed more strongly linked to ovarian cysts than TASK-1, leading us to focus on it. Nonetheless, the role of TASK-1 deserves exploration in future studies, especially since mRNA and protein expression correlation can be low due to factors like post-transcriptional regulation [
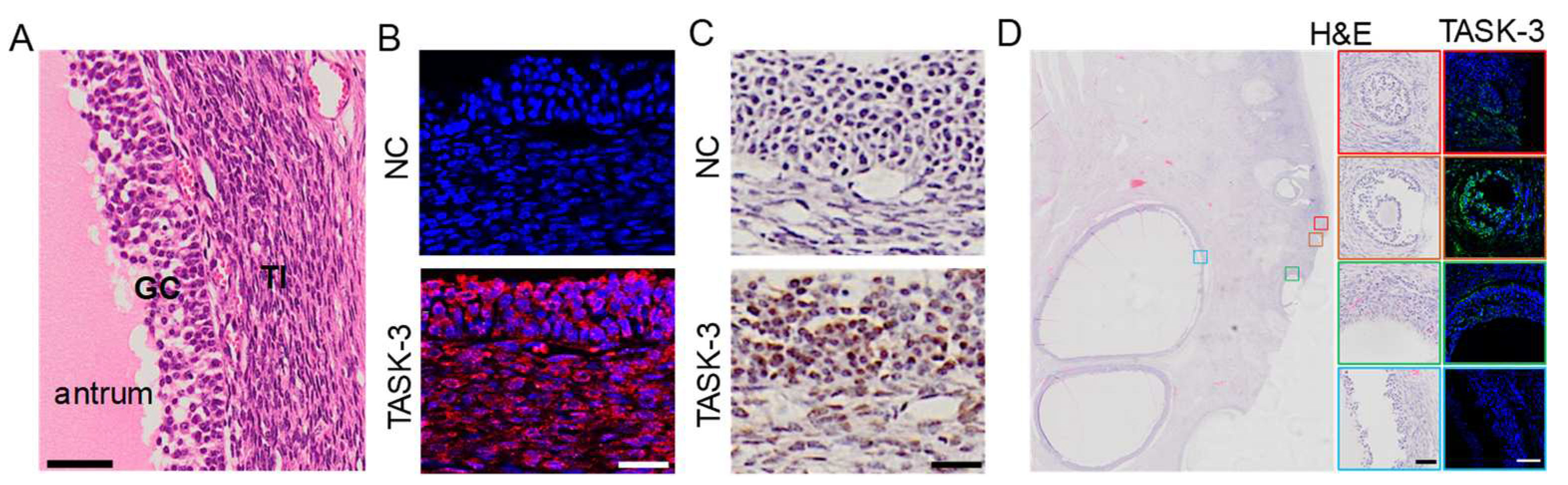
40]. Moreover, immunostaining did not provide a precise representation of TASK-3 expression levels in LF and SF, primarily because of the challenges in fixing the entire cystic follicles.
4. Materials and Methods
4.1. Sample Preparation
Bovine ovaries were obtained from a slaughterhouse and delivered to the laboratory within 2 h, preserved in phosphate-buffered saline (PBS) at 35~39°C containing 100 IU/ml of penicillin and 0.1 mg/ml streptomycin. Ovarian follicles were categorized based on their size: small (5 to 10 mm in diameter) and large (> 25 mm in diameter). Ovaries that had follicles exceeding 25 mm in diameter without a corpus luteum present in either the right or left ovary were identified as follicular cystic ovaries. The large follicles were isolated from follicular cystic ovaries. Follicles were prepared by cutting the perifollicular region with a razor blade. Experimental methods used in this study were partially modified from the procedures performed in the previous studies [
42,
43]. The 80 ovaries with large-sized follicles (LF) and 120 ovaries with only small-sized follicles (SF) were used in this study. Follicular fluid (FF) and granulosa cells (GCs) obtained from small-sized follicles (5 to 10 mm) of 5~10 ovaries were pooled and used for the experiments as a sample.
4.2. Isolation of Follicular Fluid and Granulosa Cells
The isolation of FF and GCs was performed as previously described [
33]. Briefly, FF was carefully aspirated in follicles with a 10 mL syringe fitted with an 18- or 23-gauge needle. The fluid was centrifuged at 1,750 ×g for 10 min, and the supernatant and resultant pellets were used as FF and GCs, respectively. GCs were stained with trypan blue solution (0.4%, Thermo Fisher Scientific, Rockford, IL, USA) to analyze their number and size. GCs suspended in PBS were incubated with an equal volume of trypan blue reagent for 10 min at room temperature. The cells were washed twice with PBS and then observed under a BX-51 microscope (Olympus, Tokyo, Japan).
4.3. Measurement of 17β-Estradiol (E2), Progesterone (P4), and Testosterone
Hormone concentration was measured as detailed in references [
42,
43]. E
2, P
4, and testosterone levels in the FF were ascertained using the Dissociation Enhanced Lanthanide Fluorescence Immunoassay (DELFIA) system (PerkinElmer Life and Analytical Sciences, Wallac Oy, Turku, Finland). Following the manufacturer’s guidelines, each strip was first washed with DELFIA Platewash. Subsequently, 25 µL of either E
2 standards or FF samples were dispensed into the strip wells. This was followed by the addition of 100 µL of the diluted E
2 antiserum solution to each well, and the arrangement was then incubated for 30 min at room temperature with a slow shake. After this incubation, a solution of diluted E
2 (50:1) in an amount of 100 µL was added to each well. The plate was then incubated for 2 h at room temperature with a slow shaking. After this incubation, each strip underwent six washes before an enhancement solution of 200 µL was introduced into every well. A subsequent gentle shake of the plate for 5 min was carried out. The fluorescence levels were recorded using a time-resolved fluorometer (Wallac 1420 VICTOR2
TMD, PerkinElmer) within 1 h of this final shaking procedure. Both standards and FF samples were assessed in duplicate. While evaluating P
4 and testosterone, the same protocol for E
2 was applied, with the exception of a dilution stage that was conducted before the antiserum addition.
4.4. Measurement of Ion Concentrations
Potassium (K+), sodium (Na+), and chloride (Cl-) ion concentrations in the FF were determined with an automatic analyzer (DRI-CHEM 3500i, FUJIFILM, Tokyo, Japan) in line with the manufacturer’s guidelines. For quantitative analysis of the electrolytes in the FF, the potentiometric difference was assessed between two electrodes; one electrode was in contact with a reference liquid holding a known electrolyte concentration, while the other interfaced with the samples.
4.5. Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR) and Real-Time PCR
RT-PCR procedures were performed as previously described [
42,
43] utilizing specific primer pairs, detailed in
Table 1. Bovine follicles and GCs were the source of the total RNA, which was isolated using the Trizol reagent (Invitrogen, CA, USA) following the manufacturer’s guidelines. The resultant total RNA (3 μg) underwent first-strand cDNA synthesis using oligo dT (SuperScript First-Strand Synthesis System, Invitrogen), which then served as a template for PCR amplification employing
Taq polymerase (Takara Bio Inc, Otsu, Shiga, Japan). The synthesized first-strand cDNA’s concentration was determined with a spectrophotometer (NanoDrop® ND-1000, NanoDrop Technologies, Wilmington, DE, USA) and was subsequently employed as the PCR amplification template. The amplification process consisted of an initial denaturation at 94°C for 5 min, followed by 28 or 30 cycles at 94°C for 20 sec, 55°C for 20 sec, 72°C for 20 sec, and a 10 min extension at 72°C. Amplified products were run on 1.5% agarose gels with ethidium bromide. Resultant bands were extracted and underwent direct sequencing using an ABI PRISM® 3100-Avant Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
For real-time PCR, a combination of the Topreal
TM qPCR 2X PreMIX kit (Enzynomics, Daejeon, South Korea), sapphire microplate 96-well, (Greiner bio-one, Kremsmünster, Austria), and the Light Cycler
® 480 II System (Roche, Rotkreuz, Switzerland) was utilized. The PCR parameters included a denaturing cycle (95 °C for 10 min), 45 cycles of PCR (95 °C for 7 s, 56 °C for 7 s, and 72 °C for 10 s), a melting cycle (95 °C for 0 s and 65 °C for 60 s). Relative mRNA levels were determined using the 2
-ΔΔCT method [
44]. In both RT-PCR and real-time PCR analyses, target gene expression was standardized against the glyceraldehyde-3-phosphate dehydrogenase (
GAPDH) level.
4.6. Western Blot Analysis
Western blotting was conducted based on established protocols [
42,
43]. GCs underwent homogenization in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA, USA) supplemented with 20 mM Tris-HCl (pH 7.5), 150 mM NaCl/1 mM Na
2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na
3VO
4, and 1 μg/ml leupeptin). After incubation at 4°C for 30 min, the samples were centrifuged at 13,000 rpm (16,609 × g, Micro 17TR, Hanil, Korea) for 30 min at 4°C. The protein content of the supernatants was quantified by the Bradford protein assay (Bio-Rad, Hercules, CA, USA). The proteins (50-100 μg/lane) were then subjected to 10% SDS-polyacrylamide gels, followed by transfer to polyvinylidene fluoride (PVDF) membranes (0.45 μm, Millipore, Bedford, MA, USA) in TBS buffer solution containing 25 mM Tris-base, 190 mM glycine, and 20% methanol. Ponceau S staining verified effective transfer, and the destained blots were subsequently blocked. Primary antibodies specific to TASK-1 (1:1000 dilution, Alomone Labs, Jerusalem, Israel), TASK-3 (1:1000 dilution, Sigma, St Louis, MO, USA), p53 (1:200 dilution, Santa Cruz Biotechnology, Inc, Dallas, TX, USA), p21 (1:200 dilution, Santa Cruz Biotechnology), α-tubulin (1:5000 dilution, Sigma), and β-actin (1:5000 dilution, Sigma) were applied and left overnight at 4°C. Afterward, blots were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:3000; Assay Designs, Ann Arbor, MI, USA) at room temperature for 1 h, and protein bands were visualized using the enhanced chemiluminescence (ECL Plus kit; ELPIS, Daejeon, Korea).
4.7. Hematoxylin-Eosin (H&E) Staining
H&E staining of ovaries was carried out based on the methods detailed in previous studies [
42,
43]. After a PBS (0.1 M, pH 7.0) wash, the ovaries were fixed in a 4% (w/v) paraformaldehyde solution, leading to the creation of 4 μm thick paraffin-embedded sections. For histological assessment of follicles, H&E staining was applied. The sections were laid on gelatin-coated slides and allowed to air dry before undergoing deparaffinization. After rinsing with tap water, they were submerged in hematoxylin for 5 min and then validated for thorough staining. A 3-min eosin stain followed. Thereafter, the sections were progressively dehydrated using a graded series of alcohols (70% to 100% ethanol, 3 min each), cleared in xylene, and finally mounted. Images of the stained sections were captured with a BX-51 microscope (Olympus, Tokyo, Japan) equipped with a high-resolution Camedia C-7070 camera (Olympus). For each sample, five sections underwent evaluation.
4.8. Immunostaining
Deparaffinized tissue sections were first washed in PBS, treated with 0.3% H2O2 for 30 min, and then rinsed again in PBS. To reduce non-specific IgG binding, sections were blocked using 1.5% normal goat serum in PBS at room temperature for 30 min. The sections were incubated overnight at 4°C in a humidified chamber with anti-TASK-3 primary antibody (1:200 dilution, Sigma). Following another PBS wash, sections were treated either with cyanine 3 (cy3) anti-rabbit secondary antibody (1:400 dilution, Abcam, Cambridge, UK), Alexa Fluor 488 anti-rabbit secondary antibody (1:400 dilution, Invitrogen), or biotin-conjugated secondary antibody (1:200 dilution, Sigma) diluted in 1.5% normal blocking serum at room temperature for 1 h, followed by three PBS washes. For visualization, immunofluorescence staining was counterstained with 4’,6-diamidino-2-phenylindole (DAPI). For the 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining, sections exposed to biotin-conjugated secondary antibody were further treated with an avidin-biotin-peroxidase complex (ABC Elite kit; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. These samples were then washed with PBS and stained using DAB solution (Sigma) containing 0.03% H2O2 for 3 min. Hematoxylin was used for counterstaining. Images were captured using a confocal laser scanning microscope (Olympus) for fluorescence and a BX-51 microscope (Olympus) for DAB images.
For immunocytochemistry, the isolated GCs were cultured on a cover glass for 24 hours. The cells were permeabilized with 0.2% Triton X-100 for 10 min at room temperature and then washed three times in PBS. Blocking was performed using a buffer containing 2% normal goat serum in 0.1 M PBS for 1 h at room temperature. Cells were then incubated overnight at 4°C with rabbit polyclonal anti-TASK-3 primary antibody (1:100 dilution, Alomone Labs), followed by three additional PBS washes. Cells were next treated with Cy3-conjugated anti-rabbit IgG secondary antibody (1:100 dilution; Invitrogen) for 1 h in the dark, followed by another three washes. Nuclear staining was carried out using DAPI at a concentration of 0.1 μg/mL (Sigma). Finally, cells were wet-mounted using Permount mounting medium (Fisher Chemical, Geel, Belgium) and observed under an Olympus confocal laser scanning microscope.
4.9. Measurement of Free Radical Activity and Calcium and Malondialdehyde (MDA) Concentrations in CGs
The free radical activity, as well as calcium and MDA concentrations, were determined following the methodologies described in a previous study [
45]. To evaluate free radical activity in cell lysates, the Oxiselect
TM In Vitro ROS/RNS assay kit (Cell Biolabs, San Diego, CA, USA) was utilized. The Calcium Detection Assay kit (Abcam) was used for measuring calcium concentration. MDA concentration was gauged using the OxiSelect™ TBARS assay kit (STA-330; Cell Biolabs).
4.10. Cellular Senescence Assay
Cellular senescence was evaluated using methods previously described [
46] and following the manufacturer’s instruction (BioVision Inc, CA, USA). Briefly, bovine GCs at a density of 2 × 10
4 cells/mL and Chinese hamster ovary (CHO) cells at a density of 5 × 10
4 cells/mL were plated in a 24-well plate and incubated for 24 h at 37°C and 5% CO
2. Subsequent to this incubation, the cells were rinsed twice with PBS and fixed with a fixative solution for 15 min at room temperature. After fixation, the cells were washed thrice with PBS. A staining solution mixture (comprising 470 µL of staining solution, 5 µL of staining supplement, and 25 µL of 20 mg/mL X-gal in DMF) was then added to each well, and the plate was incubated for an additional 48 h at 37°C. Images were captured from five distinct areas per dish using a microscope (Axiovert 40C, Zeiss, Jena, Germany), and cells were counted to determine the average stained cell number. The percentage of SA-β-gal-positive cells in each sample was calculated by taking the ratio of SA-β-gal-positive cells to the total cell count and then multiplying the result by 100.
4.11. Live/dead Cell Staining
Cell viability was assessed using calcein-AM (Thermo Fisher Scientific, Eugene, OR, USA) and propidium iodide (PI, Sigma) based on previously outlined methods [
46]. Viable cells were stained green with calcein-AM, while dead cells with membrane damage were stained red using PI. CHO cells, post-transfection with siRNA at a density of 5 × 10
3 cells/100 μL, were cultured on a glass-bottomed culture dish (SPL, Pocheon, Korea) for 48 h. Post-culturing, cells were rinsed twice with free DMEM and subsequently stained with 2 μM calcein-AM and 3 μg/mL PI for 10 min at 37°C. After staining, cells were washed thrice and visualized using a confocal laser scanning microscope (Olympus) with filter sets tailored for fluorescein isothiocyanate (FITC) and Texas Red.
4.12. Recording of Whole-Cell Current
Whole-cell currents measurement were taken using an Axopatch 200 amplifier (Axon Instruments, Union City, CA, USA). The membrane potential was held at -80 mV, followed by a 1-sec depolarizing voltage pulse, varying between -120 and +60 mV. The patch pipettes exhibited a resistance ranging from 4 to 5 MΩ when filled with the pipette solution. The pipette solution comprised (in mM) 150 KCl, 1 MgCl2, 5 EGTA, and 10 HEPES. The bath solution contained (in mM) 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES. To achieve a pH of 7.3, adjustments were made using either HCl or NaOH (KOH). After the capacitative transients were cancelled, whole-cell currents were measured. The pCLAMP software (Version 8, Axon) was utilized for whole-cell current analysis. Recordings were conducted under ambient conditions.
4.13. Measurement of Cell Volume
Changes in the volume of individual cells were determined by monitoring shifts in the concentration of a trapped fluorescent dye inside the cell, as documented in prior research [
47]. CHO cells, which had been transfected with DNA that encodes for rat TASK-3 (GenBank ID, AF192366) within the pcDNA3.1 vector, were cultured on 25-mm round coverslips. These cells were then treated with calcein-AM at a concentration of 5 μM for 5 min. Before initiating the experiment, they were exposed to an iso-osmotic solution for 30 min. All the experimental observations were made using a confocal laser imaging device (Olympus). The source of light for excitation was set at 488 nm, and emitted light was detected at wavelengths exceeding 515 nm. The results are depicted using the F0/Ft notation. In this context, F and t denotes fluorescence intensity and time, respectively. The value F0 is indicative of the fluorescence intensity when the cell is in an iso-osmotic solution and the time is set to zero. The ratio F0/Ft serves as an indicator of the cell volume.
4.14. Gene Silencing with Small Interfering RNA
Gene silencing assay was conducted according to established protocols [
22]. CHO cells were transfected using either a scrambled siRNA as a negative control (NC, ON-TARGET Non-targeting Pool; Dharmacon, Lafayette, CO, USA) or TASK-3-specific ON-TARGETplus SMARTpool siRNA (Dharmacon). Transfections were performed in serum-free medium using the Magnetofection™ system (Chemcell GmbH, Berlin, Germany), in accordance with the manufacturer’s guidelines. For the transfection mixture, 75 nM of either NC siRNA and rat TASK-3 siRNA was combined with 1.0 μL of PolyMAG (Chemicell GmbH, Berlin, Germany). The mixture was incubated for 20 min at room temperature before being added to 500 μL of serum-free culture medium in individual wells of a 24-well plate. The plate was then placed on a MagnetoFACTOR plate 24 device and incubated for 30 min at room temperature. Following this, the culture medium was replaced with fresh serum-containing medium, and the cells were incubated for an additional 6 h at 37°C in a 95% air and 5% CO
2. Subsequently, the medium was refreshed, and the cells were allowed to grow for an additional two days.
4.15. Data Analysis and Statistics
Images of agarose gels and Western blots were captured using a LAS-4000 luminescent image analyzer (Fujifilm Corp, Tokyo, Japan). Band intensities from gels and blotting membrane were quantified using Sigma Gel image analysis software (version 1.0, Jandel Scientific, CA, USA) or Quantity One software (version 4.6.3), which is compatible with a GS-800 calibrated densitometer (Bio-Rad, CA, USA). Data are presented as mean ± SD. Statistical significance was determined using Student’s t-test, with a p < 0.05 considered significant. All statistical analyses were conducted using OriginPro2020 software (OriginLab Corp., Northampton, MA, USA).
Author Contributions
Conceptualization, D.K. and C.-W.K.; methodology, E.-J.K., M.S.W., J.H.R. and D.K.; software, E.-J.K., M.S.W. and D.K.; validation, E.-J.K. and D.K.; formal analysis, E.-J.K., M.S.W., D.L.C., A.C.C, J.H.R. and D.K.; investigation, E.-J.K., C.-W.K. and D.K.; resources, I.-K.K., data curation, D.K.; writing—original draft preparation, C.W.K. and D.K.; writing—review and editing, D.K.; visualization, E.-J.K. and D.K.; supervision, D.K.L., S.-G.H., J.H. and D.K.; project administration, E.-J.K.; funding acquisition, C.W.K. and D.K. All authors have read and agreed to the published version of the manuscript.