1. Introduction
The global generation rate of municipal solid waste (MSW) has been increasing steadily and thus calls for tangible and sustainable management measures. From ~0.6 billion tonnes per year in the 1960s, the rate escalated to around 2 billion tonnes per year in the 2010s with projections indicating a further surge to ~3.5 billion tonnes per year by the 2050s [
1]. Over 60% of MSW is ultimately disposed of in terrestrial solid waste disposal sites (SWDS), including sanitary landfills and unregulated dumpsites. Currently, there are approximately 300,000-500,000 existing SWDS worldwide, receiving >1.2 billion tonnes of MSW annually and hundreds of billion tonnes of MSW cumulatively over time [
2]. Land disposal is expected to remain the predominant management method for MSW in most countries in the next few decades [
2].
MSW biodegradation in SWDS is the most studied process to date. Organic constituents of MSW biodegrade in SWDS, leading to emissions of greenhouse gas (GHG), mainly consisting of methane (CH
4) and carbon dioxide (CO
2). CH
4 exhibits a high global warming potential (GWP), which is 28 times stronger than CO
2 over a 100-year time horizon and 80 times stronger over a 20-year time horizon [
3]. SWDS have been recognized as the third largest anthropogenic source of global CH
4 emission, which accounts for ~20% of all the anthropogenic CH
4 emissions between 2000-2017 with a total carbon (C) flux, including CO
2 and CH
4, on the order of ~100 Tg C/year [
4]. Some huge sites emit CH
4 at rates on the order of 1000 Gg/year, which are even comparable to those of “ultra-emitters” in the oil and gas industry [
5]. SWDS also possess considerable potential for emission reduction, representing one of the largest anthropogenic CH
4 sources that can be controlled at relatively low costs through technological interventions. Up to ~40% reduction of CH
4 emissions (~25 Tg/year) can be reduced via retrofitting SWDS [
6]. Besides the global warming effect, these gas emissions are pivotal in the global biogeochemical cycle of C, which has been studied extensively [
7,
8]. The magnitude of C flux related to global SWDS is even comparable to those of some important natural processes, for example, volcano emissions (100~400 Tg C/year) [
9] and accumulation in tidal wetlands (~54 Tg C/year) [
10].
Global SWDS are not only huge C reservoirs and emission sources, but also cumulate several tens of other elements with tremendous yet poorly quantified masses. They are hypothesized to contain a more diverse array of elements and compounds within the same footprints than any other natural or anthropogenic sites. The wide variety of waste constituents and elements, highly variable and heterogeneous environments, and confined spaces of SWDS give them unique biogeochemical conditions that differ from other places on Earth [
11]. However, the transformation and transport processes of elements other than C and a handful of regulated pollutants have been sporadically studied [
12,
13] and poorly summarized. Given the immense cumulative masses of disposed elements, understanding their transformation and transport processes is critical for effective site management and mitigation of environmental and health risks.
To further widen the scope of solid waste-related research, we summarize the existing knowledge on C and other major elements (N, P, S, Cl, metals, etc.) in SWDS and organize the content into the following four sections: 1) disposal rates; 2) transformation and transport processes; 3) impacts on environmental quality and public health; and 4) potential resource recovery and site remediation measures.
2. Global disposal of MSW and constituting elements
It is important yet challenging to quantify the masses of disposed MSW and constituting elements globally. We first review reported national MSW generations and physical compositions, which are highly variable. Then, we quantitatively summarize the elemental compositions of different waste constituents. The information in this section lays the foundation for the assessments of disposed elements in global sites, which are highly variable and have not been systematically calculated.
2.1. Disposal of MSW
Global and national MSW generation and disposal data have been gathered and compared systematically since the 1990s. MSW generation is highly dependent on the income level. High-income countries with 16% of the global population produce 34% of the world’s MSW, while low-income countries with 9% of the global population generate only 5% of the world’s MSW. Lower-middle and upper-middle-income countries, representing 43% and 32% of the world’s population, contribute 29% and 32% to global MSW generation, respectively [
14]. The continuous acceleration in global MSW generation is mainly driven by economic growth and increasing living standards in developing countries. By 2050, MSW generation is expected to triple in low-income countries and double in lower-middle-income countries, which are primarily concentrated in regions like the Middle East and North Africa, Sub-Saharan Africa, South Asia, and East Asia [
2].
It is critical to distinguish the cumulative masses of MSW disposed of in dumpsites and landfills. Dumpsites typically have little containment system and lack management, which are more common in low-income and middle-income countries. On the contrary, landfills refer to engineered disposal sites that are prevalent in high-income countries. A rough estimation is that, among the land-disposed waste in 2018, 660 million tonnes were disposed of in dumpsites (~45%) and 800 million tons (~55%) in landfills [
1,
14]. Over the next few decades, a higher landfill-to-dumpsite ratio is expected due to the gradual transition from unregulated dumping to sanitary landfilling in developing countries. Nevertheless, it is crucial to maintain continued attention on dumpsites considering the cumulative masses of disposed waste and impacts of unregulated disposal on waste management, environmental and health impacts, circular economy opportunities, and climate resilience [
14].
2.2. Physical and elemental compositions of MSW.
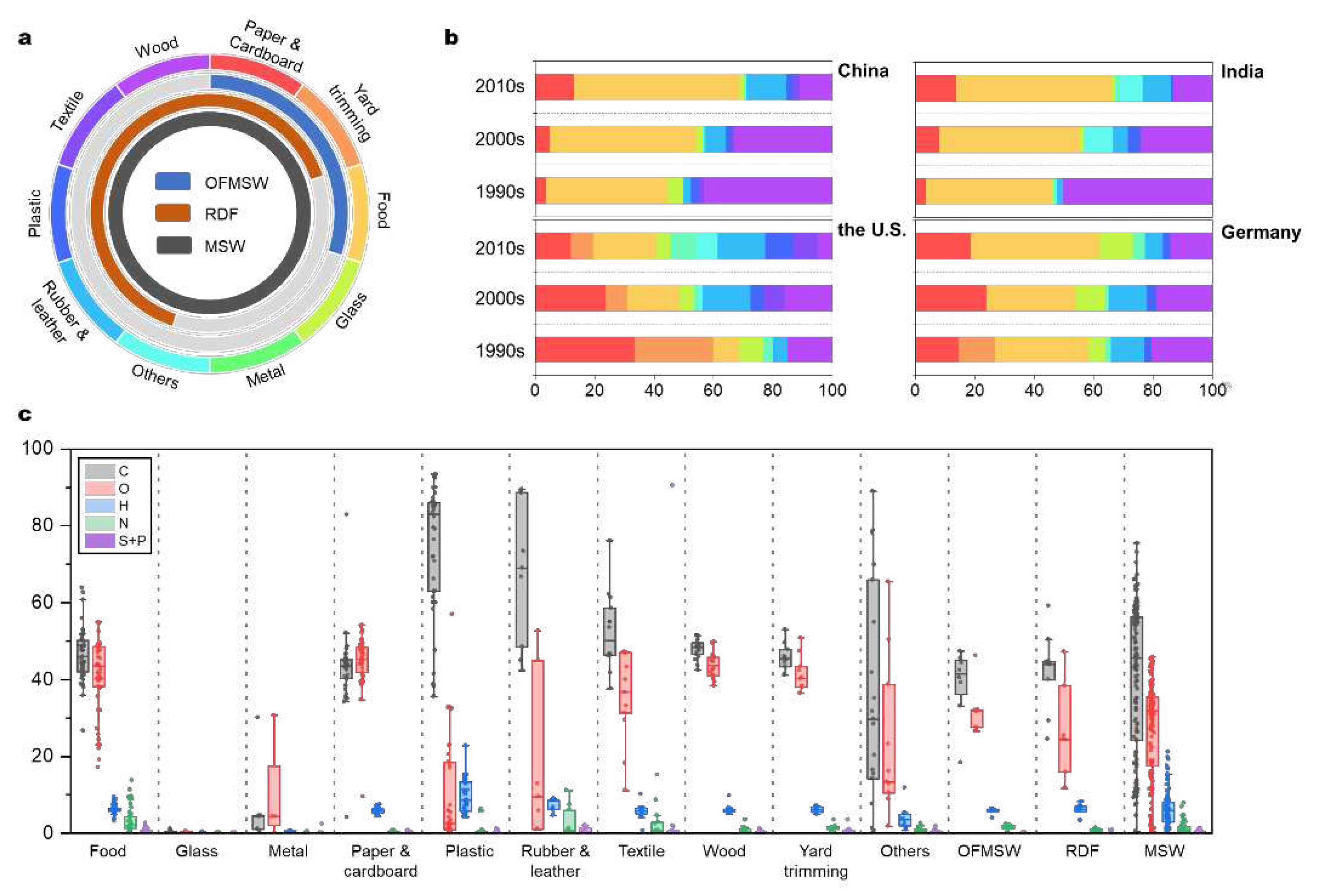
Despite abundant studies reporting MSW physical compositions worldwide, there is currently a lack of a categorization standard, and only the United States (U.S.) and some European Union (E.U.) countries report their country- and/or state-level waste compositions regularly. MSW is most often characterized based on the macroscopic properties, such as by the appearance and origin of waste. Most MSW studies utilized this approach and reported the percentages of each constituent [
15,
16]. In this review, we categorize the physical composition of MSW into ten broadly recognized constituents [
14], which are food, glass, metal, paper and cardboard, plastic, rubber and leather, textile, wood, yard trimming, and others (
Figure 1a). Waste composition varies significantly across different geographic scales, which is influenced by generation time, location, lifestyle, waste management policy, and infrastructure. For example, with rising income levels, the waste compositions in the two largest developing countries, China and India, have evolved significantly from the 1990s to the 2010s [
17,
18]. Over the past three decades, there have been up to 10% increases in the percentages of paper, cardboard, and plastics. During the same period, China and India exhibited higher percentages of organic waste compared to the U.S. and Germany, but lower plastics, metal, wood, rubber, and leather (
Figure 1b). Waste management policy and infrastructure also changed the percentages of specific constituents, for example, the percentages of paper & cardboard in the U.S. and Germany decreased due to substitutions by plastics [
19,
20]. Food waste accounts for ~44% of the global raw MSW by mass and constitutes the largest waste constituent, which impacts significantly on resource wastage and environmental quality [
21,
22].
The physical makeup of MSW is fundamental to understanding the elements within SWDS. The elemental compositions of different waste constituents are essential for computations involving transformation, transport, storage, recovery, and remediation of disposed elements [
23]. This is also referred to as the ultimate analysis of waste in biochemical and thermal treatment [
24]. In fact, the available studies reporting elemental compositions of bulk waste or waste constituents are mostly in the fields of composting [
25], anaerobic digestion [
26], and incineration [
27]. To date, elemental composition has been rarely considered for waste disposal yet, the reasons are twofold: 1) high variability and heterogeneity of waste prevent accurate estimations of elements; 2) current assessments and modeling of SWDS rely on macroscopic and generalized data, e.g., physical parameters (volume, mass) and index values (biochemical oxygen demand (BOD), chemical oxygen demand (COD)). In addition to improving the estimations of GHG emissions from SWDS that have drawn tremendous attention [
6], quantification of MSW elemental composition also enables the calculations of inputs and storages of elements in SWDS, which involve even greater masses than the GHG emissions but are seldomly considered. Incorporating MSW elemental composition also promises better assessments of the transformation and transport processes of elements and more effective management, recovery, and remediation strategies [
28].
To improve the understanding of elemental composition in MSW characterization, we summarize exhaustively the available values reported in the literature (N=397 in 24 countries in
Figure 1c). In addition to the bulk MSW and ten major MSW constituents, some research that reported the elemental compositions of specific groups of constituents are also included, namely organic fraction of MSW (OFMSW) and refuse derived fuel (RDF) (
Figure 1a). Massive and multi-brands productions plus diversified waste management policies and methods introduce great variability to MSW elemental composition, thus even the same MSW constituent based on physical composition show wide ranges of elemental contents (
Figure 1c). Inorganic products (glass and metals) and biobased materials (paper, wood, and yard trimming) show intermediate variabilities in their elemental compositions, while processed organic products (food, plastics, rubber, and textiles) exhibit significant variabilities in the respective elemental compositions. Take food waste as an example, the ranges of C, oxygen (O), and nitrogen (N) contents are 27%-64%, 17%-55%, and 0.2%-14%, respectively. MSW elemental composition also differs with time, country, and waste management strategy, which is due to the same reasons as for MSW physical composition. This results in wide ranges of elemental contents in bulk MSW worldwide, which contains 11%-60% of C, 7%-40% of O, 1%- 10% of hydrogen (H), 0.3%-2.1% of N, and 0.1%-0.6% of sulfur (S) (from 10
th to 90
th percentiles).
3. Transformation and transport processes
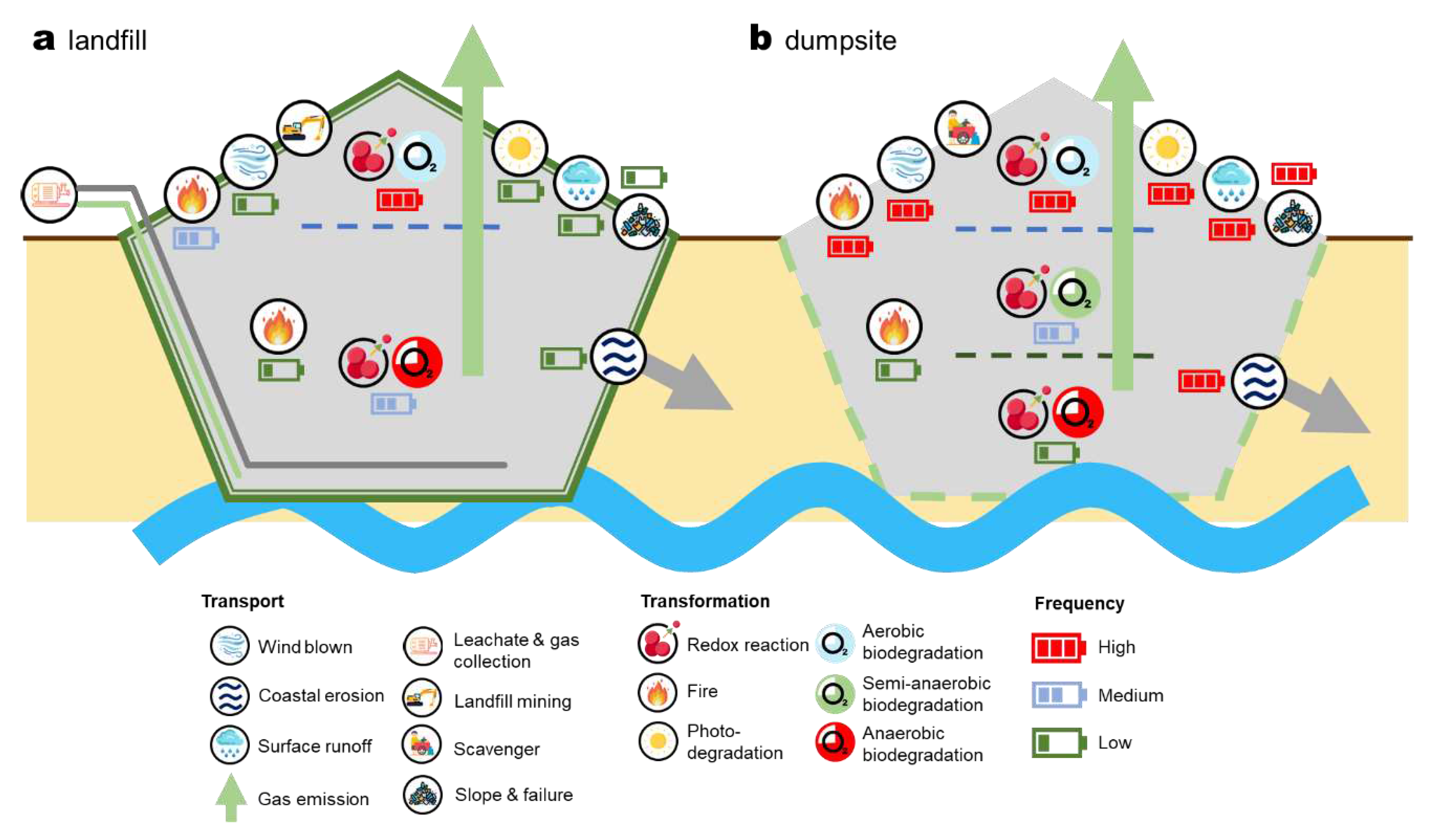
Despite the original intention of permanent isolation and containment, SWDS most often expose MSW to contact with the atmosphere, hydrosphere, and pedosphere. Therefore, SWDS have widely varied environmental conditions, including vertical stress, moisture content, oxygen availability, pH, oxidation-reduction potential, and temperature [
29,
30]. These conditions enable a series of physical, chemical, and microbial reactions occurring in MSW simultaneously or sequentially. Most elements are first transformed to more mobile forms in the aqueous or gaseous phase, which are more easily transported [
31], while some elements are transported directly [
32]. Some transformation processes render the elements less mobile, making them prone to precipitation, complexation, or adsorption [
33]. Original waste and transformed products can be transported within and across site boundaries. Understanding these transformation and transport processes is fundamental to assessing the environmental and health impacts (
Section 4) as well as the resource recovery and site remediation potentials (
Section 5) of SWDS. The following subsections review the major transformation processes, including biochemical degradation [
16] and physicochemical transformation [
34]. We also examine the major transport processes, which are gas collection and emission [
35], leachate collection and leakage [
36], and solid spillage [
37]. The mechanisms, occurrences, magnitudes, and rates of these processes differ greatly in landfills (
Figure 2a) and dumpsites (
Figure 2b), which are exhaustively summarized.
3.1. Biochemical degradation.
Biochemical degradation, or biodegradation in short, is one of the most often studied processes in SWDS since the 1970s [
38], which was already noticed in ancient times [
39]. This process occurs in biodegradable waste rich in C, O, H, N, S, and phosphorus (P) (
Figure 1), which is prevalent in most SWDS globally. The major products of biodegradation include gaseous and aqueous compounds and residual and recalcitrant solids [
40]. Gaseous compounds include CO
2, CH
4, nitrous oxide (N
2O), nitrogen oxide (NO
x), hydrogen sulfide (H
2S), sulfur oxides (SO
x), and other trace organic and inorganic molecules. Aqueous compounds are intermediate and final products of anaerobic degradation, including carbohydrates, amino acids, volatile fatty acids (VFA) [
41], and recalcitrant humic substances [
42]. Additionally, the process involves microbial oxidation and dissolution of heavy metals such as iron (Fe), arsenic (As), chromium (Cr), mercury (Hg), and lead (Pb) [
31]. The mechanisms and intensities of biodegradation vary among dumpsites and landfills. Aerobic biodegradation typically occurs only in the surface layers of landfills with cover systems, while deeper areas predominantly undergo anaerobic biodegradation. In contrast, dumpsites allow extensive contact between MSW and the atmosphere, thus leading to prevalent aerobic biodegradation. For bioreactor and hybrid landfills, which are low in total numbers and distinct from traditional “dry-tomb” landfills, both aerobic and anerobic biodegradation can be manipulated through air and liquid additions [
43].
As a classic biochemical process, biodegradation is commonly characterized by a final degree called biodegradability and a reaction rate that is equivalent to microbial growth and activity rate. Although many laboratory studies measured and reported such values [
15], they are difficult and inaccurate to derive from field measurements besides a few pilot-scale sites with good gas collection and monitoring capabilities [
44,
45]. The major obstacle is that the initial and final conditions of MSW in full-scale sites are almost impossible to obtain, which are necessary for calculating the biodegradability and reaction rate. After the measurements of gas generation, two classic models for data regression are the exponential decay model and Gompertz decay model, both of which proximate Monod’s equation [
46]. Some microbial mechanistic models have been developed, but are more complicated and less practical than the classic models for most sites [
47,
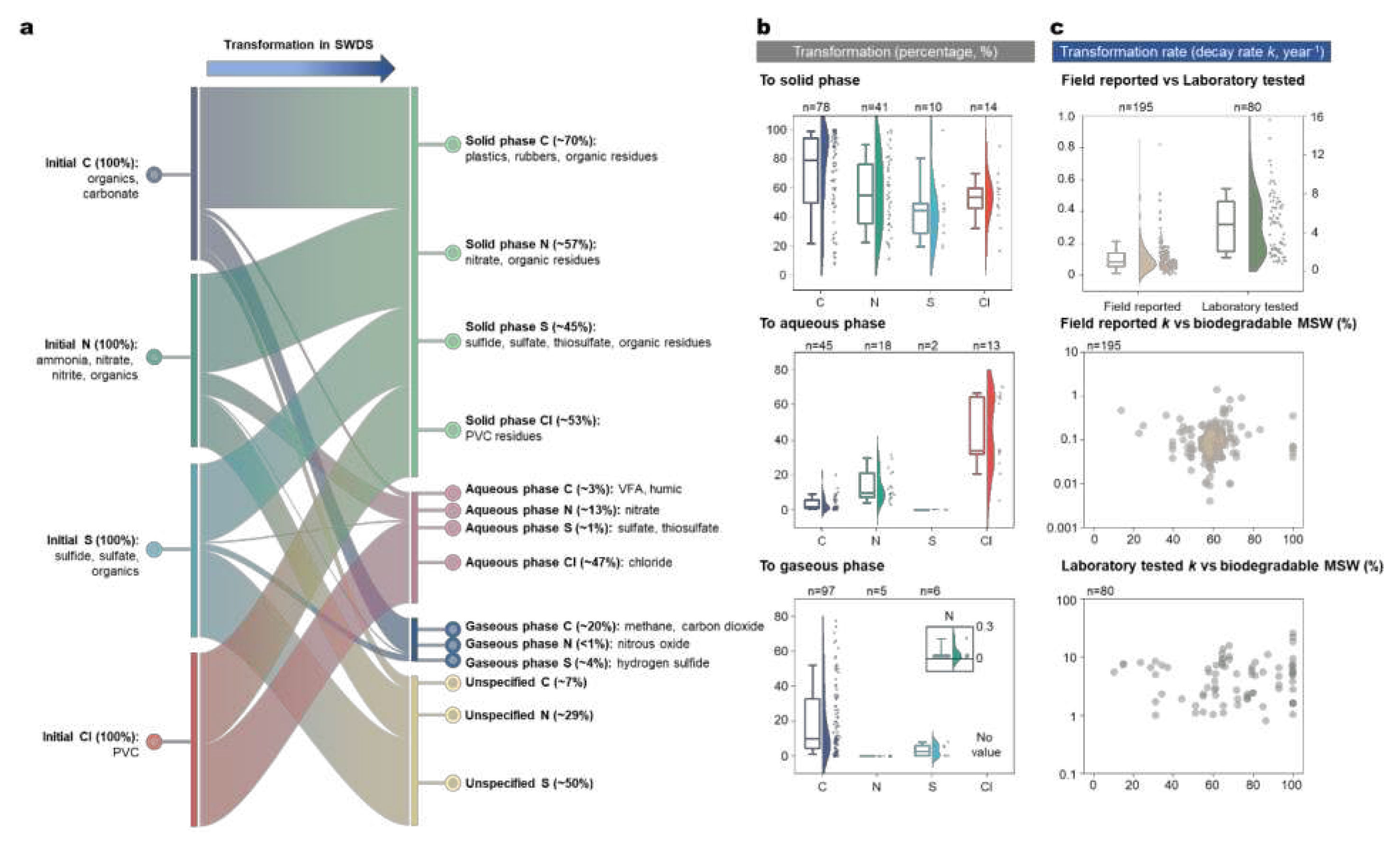
48]. In the following subsections, we provide an overview of the transformation processes of major elements during biodegradation. Considering the scarcity of field measurements, we extensively collect the laboratory tests that reported the forms and contents of major elements before and after biodegradation to calculate the transformation percentages (biodegradability) and rates of each element in gaseous, aqueous, and solid phases (N=129 in
Figure 3b). The average values of the transformation percentages and rates are presented, along with the distributions of major elements in the three phases after biodegradation (
Figure 3a).
3.1.1. Biodegradation of carbon
Biodegradable C is converted to biogas containing CH
4 and CO
2, each with 40-60% volumetric concentration. Gaseous C accounts for ~20% of the initially disposed C on average, which ranges from 0.1% to 78% as reported in the laboratory studies due to different waste compositions and biodegradation conditions [
49] (
Figure 3b). Currently, the Intergovernmental Panel on Climate Change’s (IPCC) method uses a first-order decay (FOD) model to estimate gaseous C emission, assuming the reaction rate is proportional to the mass of remaining biodegradable C in the waste [
45]. The resulted rate is called a waste decay rate
k (year
-[
1]). Here, we extensively collect the reported
k values from laboratory tests (N = 80 in 12 countries in
Figure 3c) and field measurements (N = 195 in 18 countries in
Figure 3c). The average value of laboratory-tested
k (5.5 year
-1) is significantly higher than that of field measured
k (0.12 year
-1) (p<0.01), which is due to scale effect, waste heterogeneity, and different temperatures and moisture conditions [
50]. Recently, conventional C emission estimations were found to have high uncertainties and variabilities and largely deviated from the actual values [
51,
52]. Counterintuitively, the field-measured
k and laboratory-tested
k show little correlation with the physical and gravimetric MSW compositions (
Figure 3c). A potential future research direction would be to explore the correlation between
k and MSW elemental composition.
Aqueous C, such as carbohydrates, amino acids, and VFA, is most often measured as BOD, COD, and total organic carbon (TOC) [
53]. The majority of the aqueous C will be finally biodegraded to CO
2, CH
4, and biomass within SWDS or in leachate treatment plants after collection [
54]. The characteristics of leachate humic C, a biodegradation product, have been broadly studied as pollution indicators that influence remediation strategy [
42,
55]. Slow- and non-biodegradable C in the solid phase approximates 70% of the initial C in MSW (
Figure 3b), which consists mainly of recalcitrant polymers (plastics, rubber, composites, and lignin) and transformed residues (humic substances). SWDS are thus significant yet largely unexplored reservoirs of fossil-based C on Earth [
56]. It is difficult, and somewhat arbitrary, to distinguish between slow- and non-biodegradable C, which may not be practical or necessary in a 100-year time horizon [
57]. Despite the recent surge in research on the presence and behaviors of plastics in SWDS [
11,
58], their biodegradation and transport degrees should be minimal. The long-term fates of other recalcitrant polymers remain largely unstudied, which consist of a major portion of the solid C.
3.1.2. Biodegradation of nitrogen
The inputs of N into SWDS primarily exist in the forms of ammonium, nitrate, nitrite, and organic nitrogen [
12]. Only ~1% of the initial N is biodegraded to N
2O, which is frequently emitted from SWDS [
59]. The formation of N
2O requires alternating anaerobic and aerobic conditions, which is common in surface waste. Additionally, the released ammonia (NH
3) may also undergo microbial nitrification in surface waste, leading to N
2O production [
59]. The GWP of N
2O is 273 times that of CO
2 in a 100-year time horizon [
3]. This substantial contribution to global warming has led to studies focusing on the emission patterns and mitigation measures of N
2O [
60]. While the IPCC provides the widely-used FOD emission model of CH
4, there lacks a well-recognized emission model of N
2O despite a few kinetic studies [
61]. There is ~13% of the initial N in the aqueous forms of ammonium, nitrate, and organic nitrogen after biodegradation, which undergoes nitrification and denitrification during leachate treatment and is eventually released as N
2 and NO
x [
53]. The rest of N in the solid phase is in the forms of nitrate and organic residues.
3.1.3. Biotransformation of other elements
The biotransformation processes of O and H are concomitant with C, which are usually estimated using Buswell’s equation once the elemental composition of biodegradable waste is known [
62]. Apart from O and H, the biotransformation processes of S, P, chlorine (Cl), and metal elements have been studied mechanistically without the quantifications of biodegradability and reaction rate. H
2S, known for its distinctive malodor, is a biodegradation product from the reduction of solid and aqueous S [
63]. Once H
2S enters an aerobic zone it is partially microbially oxidized to sulfur oxide (SO
x) [
64]. In addition to H
2S, other reduced S compounds, such as methyl mercaptan (CH
3SH), dimethyl sulfide ((CH
3)
2S), carbon disulfide (CS
2), and dimethyl disulfide ((CH
3)
2S
2) were occasionally detected [
65]. P is an essential nutrient for biodegradation and its transformation has been extensively studied [
66]. The removal of P from landfill leachate is another topic of increasing interest [
67,
68]. Inorganic Cl is primarily found in the forms of salts, such as potassium chloride and sodium chloride that are commonly found in food waste. Some researches indicate that polyvinyl chloride (PVC) is slightly biodegradable, and the Cl in PVC can be transformed to Cl
- through biodegradation and physiochemical erosion [
69]. Approximately 47% of the initial Cl is found in aqueous phase (
Figure 3b).
Generally, over 99% of the initial metals remain in solid phase [
70], while the rest are biotransformed into aqueous phase as side reactions of organic waste biodegradation [
71]. Trace masses of heavy metals may be volatilized as byproducts of microbial activities, although the mechanisms have not been completely understood and the masses remain unclear [
72,
73] (
Figure 4d). The volatilization processes provide unique yet trace-amount pathways for heavy metals to migrate in the gaseous phase, which are analogous to those occur in peatlands and organic-rich sediments [
74]. In fact, SWDS can be an overlooked but important source of volatile heavy metals, the contributions of which to air pollution are not fully understood or quantified.
3.2. Physicochemical transformation
A variety of physicochemical processes occur in SWDS, including compression and fragmentation, dissolution and precipitation, oxidation and reduction, and volatilization. These processes require different conditions as compared to biochemical processes, hence the locations of occurrence may not overlap with those of biochemical processes. The transformed products are usually more mobile than their original forms. Compression and fragmentation are mechanical processes driven by stresses imposed on waste [
75]. Compression densifies waste and reduces its porosity, moisture content, and hydraulic conductivity. This, in turn, retards waste’s exposure and interaction with water, within which most biochemical and physicochemical processes occur. Fragmentation breaks down large solids into smaller and more mobile particles due to tensile and shear stresses [
76]. Brittle waste constituents include food, paper, wood, plastic, rubber, and textile, which are fragmented into the corresponding smaller particles. The smaller food, paper, wood, and even polymer particles facilitate biodegradation due to their higher specific surface areas as compared to the original waste [
77]. The generation of microplastics and microfibers in SWDS received great attention recently [
78,
79], since they are highly mobile and act as vectors for adsorbing and transporting other elements, compounds, and pathogens [
80,
81]. In general, purely physical transformations do not alter waste elemental composition. Instead, they primarily impact the sizes, shapes, and spatial arrangements of waste particles, thereby indirectly influencing the transformation and transport processes of elements.
Leaching or dissolution is the major process to convert inorganic and non-biodegradable solids to aqueous compounds. This process has been extensively studied, particularly for salts and heavy metals in leachate [
53,
82]. In a broad sense, leaching also refers to the mobilizations of recalcitrant organic carbons, such as non-aqueous phase liquids [
83], pharmaceuticals and personal care products (PPCPs) [
84], perfluoroalkyl and poly-fluoroalkyl substances [
85], and humic substances [
53]. On the other hand, soluble carbonates, sulfates, phosphates, and other oxyanions may precipitate with heavy metals in waste layers and leachate collection systems to deteriorate landfill function [
86]. Similarly, minerals may precipitate and form crusts to clog the underlying soil of dumpsites [
87,
88]. Oxidation and reduction occur to multivalent metal ions depending on leachate properties, which alter the valence states and mobilities of heavy metals [
53]. This can result in the relocation and stratification of certain elements in MSW, e.g., Fe, As, Cr, etc. [
89]. Uncontrolled fires [
90] and hydrothermal reactions [
91] also oxidize waste elements. Uncontrolled fires contribute to the emissions of various gaseous pollutants (carbon monoxide, NO
x, SO
x, and particulate matters) and greenhouse gases (CH
4 and CO
2), posing significant threats to ambient air quality [
90,
92]. The concentrations of total N, COD, and heavy metals in the leachate were also elevated shortly after the fires in SWDS [
93]. High-temperature landfills, which are often caused by internal smoldering of flammable waste and aluminum-water reactions, facilitate pyrolysis, ash hydration, and accelerated carbonation of waste [
94].
3.3. Gas collection and emission.
Gas migration in SWDS can be either active or passive. The active collection uses vacuum suction to mobilize gas in the accessible or drainable pores within waste, while other pores are too small to access pneumatically or perched by leachate [
95]. The average efficiency of the gas collection systems in landfills, which is the ratio between gas collection volume and gas generation volume, ranges between 40-80% [
96,
97]. A small number of landfills have been aerated by pumping air under positive pneumatic gradients, primarily aiming to accelerate aerobic biodegradation, reduce water content, and reduce GHG emissions [
98,
99,
100].
The uncollected gas migrates through shallow waste and cover soil and gets partially oxidized from CH
4 to CO
2, which is beneficial to reduce the overall GWP of gas emission, similar to flaring [
101]. Both aerobic [
102] and anaerobic CH
4 oxidation [
103] have been identified in SWDS, highlighting high spatial heterogeneity and microbial diversity within small footprints. The IPCC’s default fraction of CH
4 oxidation is 10%, which has been challenged by many recent in-situ measurements [
104]. Current estimations of collection and surficial oxidation percentages of CH
4 range between 22-55% [
105], with the rest emitted to the atmosphere. Odor release is a common problem in SWDS caused mainly by H
2S and other S- and N-containing gaseous compounds, which have been monitored in many sites over long periods [
106]. However, their fluxes are even more difficult to directly quantify than GHG. A common practice to estimate H
2S emission is based on the relatively stable volumetric ratio of H
2S to CH
4, which is 36 ppm for H
2S and 50% for CH
4 during biodegradation [
106]. The improved control of H
2S and other odor-causing compounds requires more field monitoring data and a deeper understanding of their generation mechanisms [
63]. Major factors influencing gas emission include climate conditions, waste composition, and SWDS management [
107].
3.4. Leachate collection and leakage
Soluble and suspended compounds can be transported within and out of SWDS via leachate migration, a process that has been extensively reviewed [
53,
55,
108]. The transport processes associated with leachate include surface runoff, leachate drainage, and leachate leakage. Surface runoff is typically less contaminated compared to drained or leaked leachate. Leachate drainage sends leachate to treatment plants via leachate collection systems, which may be clogged due to mineral precipitation (
Section 3.2). Landfill containment systems are prone to failures in the long term, leading to leachate leakages and environmental impacts [
109]. Unregulated dumpsites, typically lacking containment systems and proper management, are much more prone to leakage [
110]. For coastal and riverine SWDS, leakage can also be induced by waste erosion [
111], which can be exacerbated by the impacts of climate change and potential sea level rise. Furthermore, extreme weather events, such as hurricanes, tsunamis, storms, and wildfires, increase the risks of SWDS structures being damaged, which are designed to sequester waste elements permanently [
112].
3.5. Solid spillage
Solid spillage encompasses surface wind transport and waste landslides. Waste in landfills is covered daily, but during daytime operation, small and light particles can be suspended and transported by wind. Waste in unregulated dumpsites, being constantly exposed, are specifically susceptible to being windblown. While the total mass of waste transported in this manner tends to be low, the potential transport distance can reach ~1000 km. An example is the transport of microplastics from SWDS to conservation areas in the U.S. [
32]. Waste landslides, although rare, can mobilize large masses of waste due to excess disposal, storms, or both [
113]. Coastal and riverine SWDS are susceptible to such waste landslides and erosions triggered by tides and waves [
111,
112].
4. Environmental and health impacts
As summarized above, SWDS are not permanent reservoirs of all the disposed waste, instead considerable masses of elements are transformed and transported with SWDS acting as sources. We review systematically the concentrations of major compounds and elements detected in leachate and landfill gas, which serve as the primary mediums for the transport of elements from SWDS. The emitted or leaked compounds have both environmental and health impacts. Monitoring and managing such impacts are major functions and responsibilities of sanitary landfills, while unregulated dumpsites are incapable of doing so and the risks can only be roughly understood.
4.1. Environmental quality impacts.
The releases of gases, leachate, and solids from SWDS pose major threats to the environment, which have been extensively studied for landfills [
87,117] and dumpsites [
110], respectively. The pollutants are often grouped into soluble salts, soluble heavy metals, insoluble particles, biodegradable organics, and trace organics [
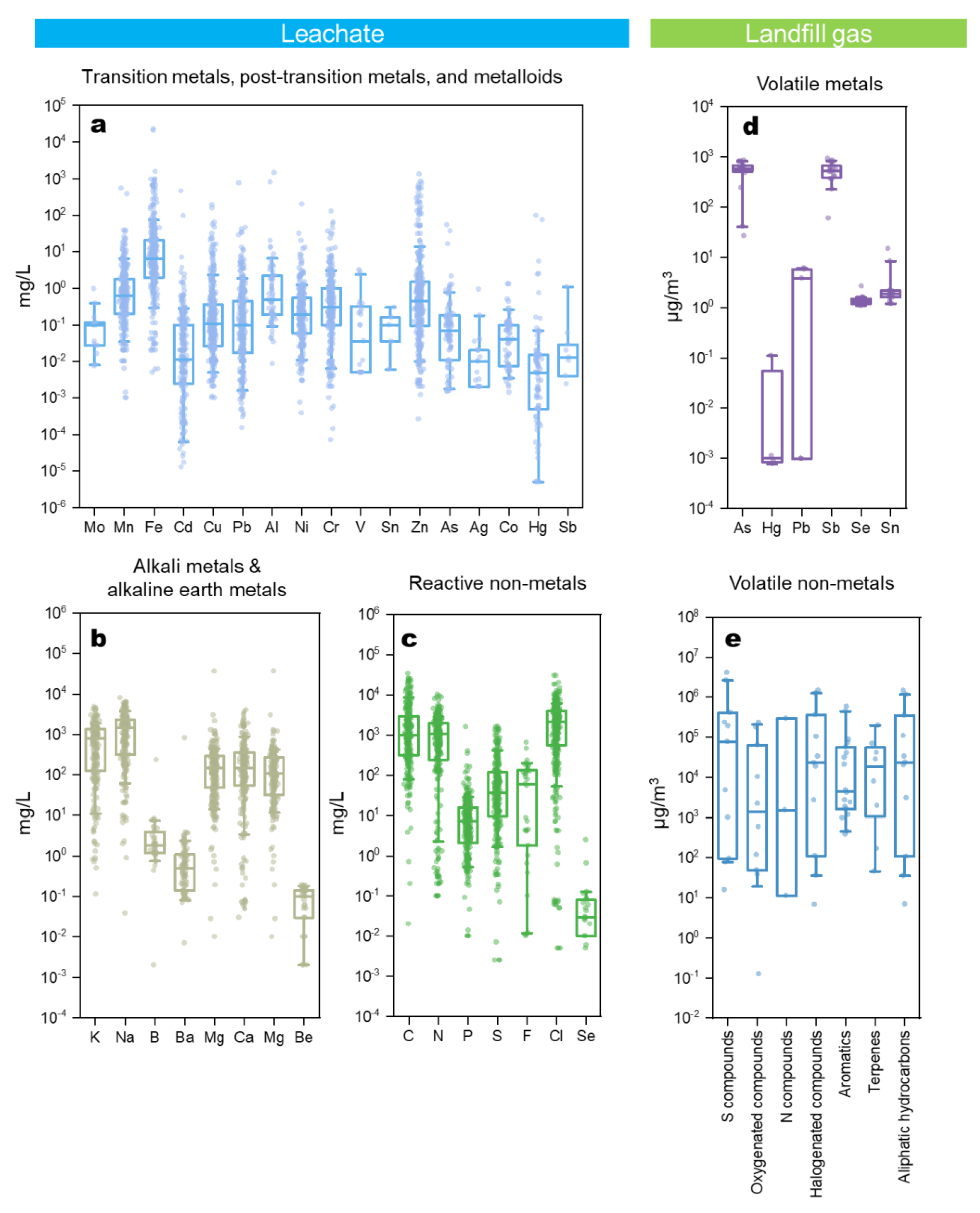
53]. Their impacts are typically categorized based on the environmental mediums, namely air, water, and soil. The pollutants in gaseous and aqueous phases will migrate, either naturally or driven by site operation, to the environment, thereby rendering SWDS intermediate sources. The types of compounds and elements involved in these transport processes can be extensive and vary considerably across different SWDS. Here, we provide an in-depth summary of the concentrations of major compounds and elements found in leachate (N=1,256) and landfill gas (N=90 in
Figure 4). In leachate, most measurements focused on metals (
Figure 4a and 4b) and complex organic compounds (
Figure 4c) [114], which influence ambient water and soil qualities. In landfill gas, pollutants like H
2S and volatile organic compounds (VOC) drew great concerns. The presence of these pollutants in the surrounding environmental mediums of SWDS further indicates their roles as intermediate sources [114]. One example is that PPCPs were detected in the groundwater and surface water surrounding SWDS, which has recently gained research attention but is still understudied [
84,118]. On the other hand, persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs), hexachlorocyclohexane (HCH), and perfluorooctane sulfonic acid (PFOS), may remain stable and immobile in SWDS for decades [119]. Beyond the impacts on the natural environment, a small number of studies focused on the health impacts of disposed waste on animals [120].
Theoretically, complex reactions occurring in SWDS facilitate both detoxification and toxicity amplification of various compounds and elements. It is important to recognize the multiple processes SWDS sustain at the infrastructure-nature interface in addition to acting as reservoirs and intermediate sources. In reality, such studies are scarce given the difficulties in simulating disposal site conditions in the laboratory and the even greater challenge of field sampling and characterization. For example, a few studies found that some halogenated organic compounds can be biodegraded to less toxic products in SWDS, which is also called attenuation [121,122]. Unfortunately, the attenuation process can be extremely slow for POPs [123]. On the other hand, solid and soluble elements can also be transformed into more toxic and bioavailable forms in SWDS. Elements such as Cl and fluorine can be incorporated into VOCs, e.g., carbonyl sulfide and halogenated hydrocarbons, thereby threatening air quality (
Figure 4e) [116]. In the U.S., the VOCs emitted from SWDS account for ~10% of the national VOC emissions [124]. Recent studies even suggested that landfill gas can be a source of anthropogenic gaseous antimony (Sb) and As released in the forms of methylated organics [73] (
Figure 4d). The presence of other volatile metals in landfill gas, including Hg, Pb, selenium (Se), tin (Sn), and tellurium (Te), were also reported [72]. However, it should be noted that the records of volatile metals in landfill gas are overall limited. Further research is needed to improve the mechanistic understanding and quantification of detoxification and toxicity amplification processes in SWDS, which would pave the way for modelling and predicting them.
4.2. Public and occupational health impacts
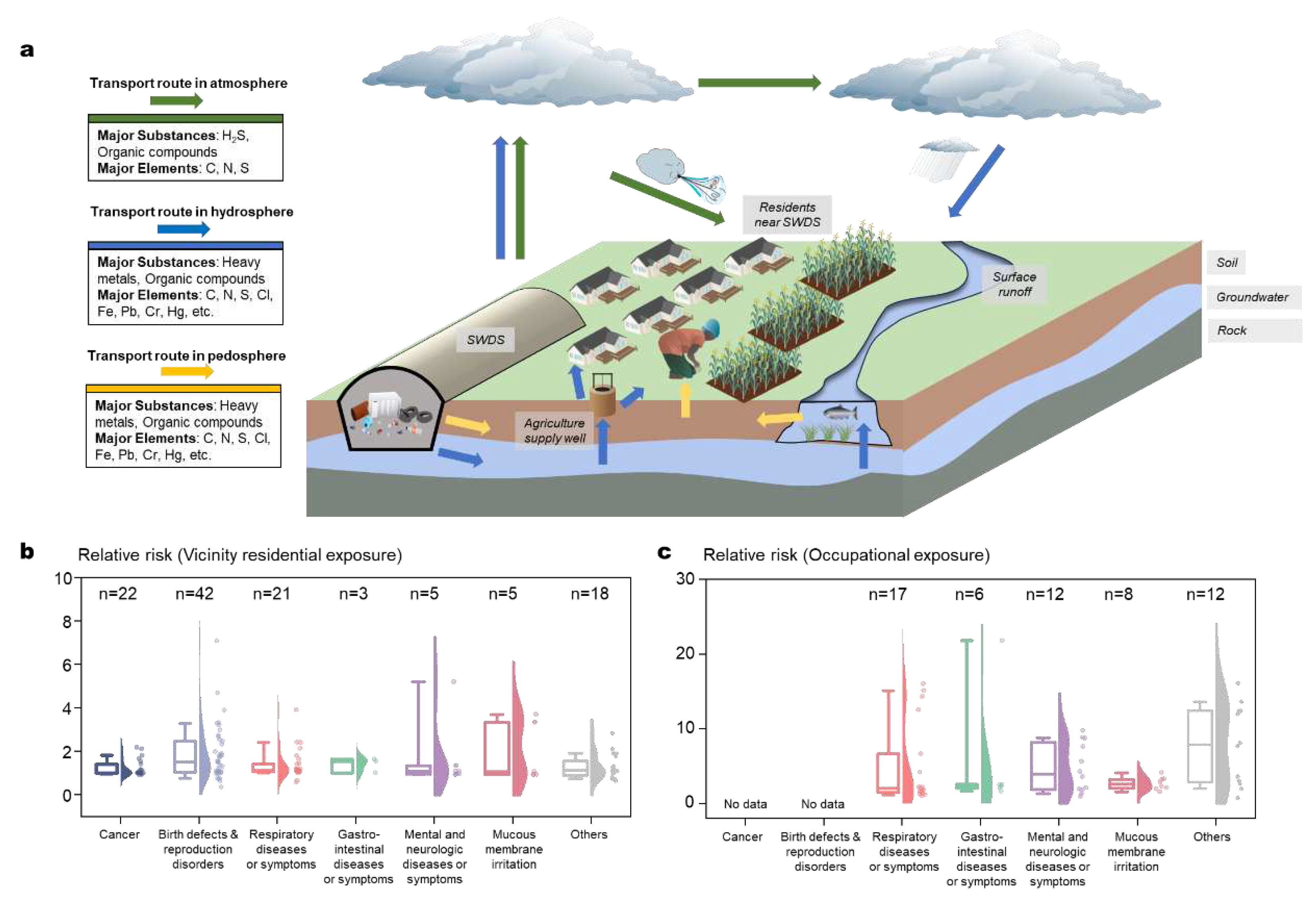
It is widely acknowledged that SWDS pose risks to human health, primarily due to the release and transport of pollutants under varied environmental and urban conditions [125]. Pollutants in SWDS are released through gas emission, leachate leakage, and solid spillage (
Section 3). Thereafter, they participate in the element cycles of the atmosphere, hydrosphere, and pedosphere, which raise the probabilities of human inhalation, ingestion, and dermal absorption (
Figure 5a). We review extensively the negative health impacts of SWDS, including cancers, birth defects and reproduction disorders, respiratory diseases or symptoms, gastro-intestinal diseases or symptoms, mental and neurologic diseases or symptoms, and mucous membrane irritations [126]. The relative health risks are calculated by dividing the SWDS-related risks by the risks of the baseline populations, which are further categorized into those for individuals living near SWDS (N=116 in 11 countries in
Figure 5b) and for disposal site workers (N=55 in 3 countries in
Figure 5c). 75% of the reported relative health risks associated with residential and occupational exposure were greater than 1. Birth defects were frequently reported as a significant health risk [127,128,129], with relative risks ranging from 0.35 to 7.08. The relative risks for occupational exposure are significantly higher than those for vicinity residential exposure. For instance, the relative risks of respiratory diseases average 5.1 for occupational exposure, which is significantly higher than the average of 3.5 for residential exposure (p=0.02). The health of disposal site workers is a critical concern as they are exposed to high concentrations of pollutants for extended periods [130,131].
Despite clear evidence of health impacts induced by pollutants containing organic C, N, S, halogens, metals, and other elements, it is premature and difficult to establish the causal relationships between SWDS and the observed diseases or symptoms [126]. Many available studies suffer from poor exposure measurements and experimental designs, leading to low comparability and inconclusive evidence [126,132]. Well-acknowledged source-pathway-receptor models have not been established for most of the SWDS under investigation. It cannot be generalized which sites and elements and to what degrees the SWDS are threatening public health [133].
5. Resource recovery and site remediation
Considering that ~70% of C, ~57% of N, ~45% of S, ~53% of Cl (
Figure 3a), and more than 99% of metals remain in SWDS after disposal as long-term reservoirs, it is logical and increasingly necessary to design and implement resource recovery and site remediation to promote sustainability and element neutrality. These engineered measures aim to transform SWDS from conventional waste reservoirs and intermediate pollution sources to emerging resource stockpiles [134] and construction land [135].
5.1. Resource recovery
Various types of resources can be recovered from SWDS via gas collection [136], leachate treatment [137], and solid waste mining [138,139]. Long-term transformation and transport processes of elements (
Section 3) are either beneficial or detrimental to resource recovery, which depends on the target resources and approaches. Biogas collection and energy generation rely on biodegradation of organic C, and anaerobic digestion of leachate requires the presence of aqueous C, N, and P generated from biodegradation and leaching of waste. Hence, biodegradation in SWDS should be facilitated if the primary goal is to harvest biogenic energy. For sanitary landfills in developed countries, up to 90 % of the generated gas can be collected, amounting to ~20% of all the disposed C along with comparable masses of O and H and trace masses of N and S. Dumpsites are typically incapable of continuous and effective gas collection. The United Nations supported some Clean Development Mechanism projects for dumpsites in developing countries in Africa, South America, and Asia to help establishing gas recovery and electricity generation facilities, which bring environmental, economic, and social benefits [140]. Leachate collection and anaerobic digestion are evolving and have been systematically reviewed [141,142]. The main products are CH
4, CO
2, and sometimes biobased compounds like VFAs [141]. The recovered CH
4 from both gas collection and leachate treatment can be used for electricity generation (waste-to-energy, WtE) or industrial synthesis (waste-to-material, WtM).
In contrast, waste mining prefers constituents with untransformed elements, mainly targeting electrical and electronic wastes, metals, and polymers including plastics, rubber, and composites. Untransformed compounds and elements are easier to separate and usually have higher purities. Metals, being the most valuable constituent of recovered waste, constantly contribute to over 60% of the revenues of waste mining. Some studies reported that the purities of metals in SWDS are even higher than those in natural ores, which justifies waste mining [143]. The compositions of mined waste vary significantly among OECD and non-OECD countries, which heavily influence the site-specific decisions of waste mining. High-value constituents, such as metals, plastics, rubber and leather, and glass and ceramic, account for higher weight percentages in OECD countries compared to non-OECD countries (N=348 in 32 countries in
Figure 6b). For instance, the average content of metals in mined waste in OECD countries is around 2.9%, nearly double that of non-OECD countries (1.5%). On the contrary, low-value organic waste, including food, paper, wood and yard waste, and textiles, are found to have higher weight percentages in non-OECD countries than in OECD countries (
Figure 6b). Therefore, waste mining needs to be considered cautiously due to potentially low recovery values in non-OECD countries. In addition to WtM recovery, another approach of utilizing mined waste is WtE. After preliminary screening, the combustible fraction of mined waste typically comprises ~40% [144]. Globally, over 50 sites have been explored for mining potential, but less than 10 sites have been partially or fully mined to date [138]. It is an infinitesimal fraction of all the existing SWDS, highlighting the current inhibitory regulatory and economic conditions. However, this situation may change in the future due to various types of international agreements and national initiatives. Notably, the “Waste to Zero” initiative has just been proposed at the United Nations Climate Change Conference in 2023 (COP28), aiming to decarbonize the global waste management sector.
5.2. Site remediation
The motivation for site remediation is the stabilization of waste constituents and the completion of element transformation and transport processes. Otherwise, the sites are considered to be unstable and risky for redevelopment [145]. Several mainstream site remediation techniques include air sparging and leachate pumping [146], barrier and cover systems [147], and deep dynamic compaction [148] (
Figure 6a). Waste mining and removal can be considered as an appropriate remediation technique as well if site remediation is part of the mining objective. A few successful cases have been reported, mostly in developed countries with sufficient budgets and strong demands for urban and suburban construction land [149,150,151,152]. After successful remediations, the sites were ready for redevelopment into shopping malls, parking lots, and industrial parks [153,154]. One remediated site in the United Kingdom has even been turned into a residential area [155].
Overall, the perspective for site remediation is promising, but the associated science and technology call for further development. Scientifically, there is no consensus on which compounds and forms of elements are considered stable and safe after site remediation, although the general understanding is that most elements should be in non-biodegradable and non-leachable forms, such as existing in recalcitrant polymers, insoluble minerals, and humic substances [156]. It remains debatable whether the same environmental criteria for natural soil and sediment should be used to check the contents of elements in stabilized waste, for example, S, P, Cl, and heavy metals. The E.U. has established environmental intervention values, although these values do not distinguish between remediated natural soil and stabilized waste [157]. From an engineering perspective, the remediation techniques are premature and lack abundant case studies and standardization [158]. From policy makers’ perspective, the triple bottom line including environmental and health benefits, economic motivations, and public awareness and acceptance should be considered [159].
6. Summary and future perspectives
Land disposal remains the primary method for managing the surging solid waste globally. SWDS, though intended as final reservoirs for material sequestration, have brought significant environmental and socioeconomic concerns and contributed greatly to climate change due to the various transformation and transport processes of elements. However, these processes are often studied in isolation and seldomly examined in an element-specific context (except for carbon), which impedes the development of effective waste management strategies and a holistic understanding of waste-centric element cycles. In this review, we exhaustively summarize existing literature and establish a database that encapsulates the global-scale records of MSW disposal and the elemental compositions of its constituents. We outline the five dominant transformation and transport processes of elements in SWDS, biochemical degradation, physicochemical transformation, gas migration, leachate migration, and solid spillage. For key elements including C, N, S, Cl, and several metals, we systematically compile the magnitudes and rates of these processes from both laboratory tests and field measurements and estimate element distributions in gaseous, aqueous, and solid phases. Additionally, we examine and quantify the potential environmental impacts and health risks associated with element transformation and transport. Furthermore, we explore the existing knowledge and techniques for resource recovery and site remediation of SWDS and identify current gaps in both scientific understanding and engineering practices. Based on the comprehensive review, we suggest the following future works.
Paradigm shift and standardization of MSW characterization. While many studies reported the properties of MSW [
28], inconsistencies are often observed in their methodologies for waste classification, which hinder the comparability and applicability. Future efforts should aim to establish and promote a uniform MSW characterization standard, including guidelines for sorting, categorization, characterization, and testing of key properties. Moreover, a regular reporting scheme for MSW statistics and properties is necessary. Similar to various GHG emission inventories, we recommend establishing site-scale MSW information records. A comprehensive understanding of the initial statuses and evolutions of MSW properties and comprised elements is crucial for the effective management of waste constituents and elements.
Development of element transformation and transport models. Currently, the IPCC has established widely used emission and storage models for carbon, while other studies also proposed numerous advanced GHG emission models [
52]. It is necessary to expand the boundary of interest from the waste-atmosphere interface to the whole site and its vicinity, shifting from calculating the emissions of GHG and specific pollutants to managing stocks of various elements. Constructing time-dependent and spatial-resolved mass balance models for different elements in gaseous, aqueous, and solid phases in SWDS will complement the current models [
45] and provide a more holistic understanding of the global impacts of SWDS. Particularly, models for the transformation and transport processes of N-, S-, and halogen-containing compounds are urgently needed, which should depict coupled physical, chemical, and biological reactions in SWDS.
Management of beneficial elements and mitigation of detrimental elements: Various strategies for pollution reduction, emission mitigation, and resource recovery in SWDS should be explored. Engineered measures, like improving cover system, implementing leachate recirculation, and waste mining, should be optimized to accommodate site- and regional-specific needs. Future research should focus on understanding the lifecycle impacts and costs of different engineered measures and their corresponding effects on waste compounds and elements. Additionally, policy-driven research and assessments are needed to support decision making. The ultimate goal of SWDS management should be to achieve regional and global sustainability and neutralities of elements.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Y.W. and X.F. conceptualized the article, conducted the majority of the analysis, and made all the figures. L.R., D.Z., F.C., A.H., S.S., H.Z., M.F., K.Y., Z.L., G.L., and H.H. contributed to the general design of the manuscript, including text structure and data illustration. C.Z. and S.M. contributed to the preparation and analysis of Supplementary Information. The manuscript was written by Y.W. and X.F. with revisions from all the co-authors.
Acknowledgements
Y.W. would like to acknowledge Nanyang Technological University, Singapore, for providing a research scholarship for this study. Y.W. and X.F. thank the support from Debris of the Anthropocene to Resources (DotA2) Lab at Nanyang Technological University.
Conflicts of Interest
The authors declare no competing interest.
References
- Chen, D. M.-C., Bodirsky, B. L., Krueger, T., Mishra, A. & Popp, A. The world’s growing municipal solid waste: trends and impacts. Environmental Research Letters 15, 074021, (2020). [CrossRef]
- Kaza, S., Shrikanth, S. & Chaudhary, S. More growth, less garbage. (2021).
- IPCC. Summary for policymakers Synthesis report of the IPCC sixth assessment report (AR6), (2023).
- Saunois, M. et al. The global methane budget 2000–2017. Earth system science data 12, 1561-1623, (2020).
- Lauvaux, T. et al. Global assessment of oil and gas methane ultra-emitters. Science 375, 557-561, (2022). [CrossRef]
- Hoy, Z. X., Woon, K. S., Chin, W. C., Van Fan, Y. & Yoo, S. J. Curbing global solid waste emissions toward net-zero warming futures. Science 382, 797-800, (2023). [CrossRef]
- Bogner, J., Abdelrafie Ahmed, M., Diaz, C., Faaij, A., Gao, Q., Hashimoto, S., Mareckova, K., Pipatti, R., Zhang, T. in Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (ed B. Metz, Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A.) (Cambridge University Press, 2007).
- Bogner, J., Spokas, K., Burton, E., Sweeney, R. & Corona, V. Landfills as atmospheric methane sources and sinks. Chemosphere 31, 4119-4130, (1995). [CrossRef]
- Gerlach, T. Volcanic versus anthropogenic carbon dioxide. Eos, Transactions American Geophysical Union 92, 201-202, (2011). [CrossRef]
- Wang, F. et al. Global blue carbon accumulation in tidal wetlands increases with climate change. National Science Review 8, nwaa296, (2021). [CrossRef]
- Fei, X. et al. The distribution, behavior, and release of macro-and micro-size plastic wastes in solid waste disposal sites. Critical Reviews in Environmental Science and Technology, 1-24, (2022).
- Berge, N., Reinhart, D. & Townsend, T. The fate of nitrogen in bioreactor landfills. Critical Reviews in Environmental Science and Technology 35, 365-399, (2005).
- Nanda, S. & Berruti, F. Municipal solid waste management and landfilling technologies: a review. Environmental Chemistry Letters 19, 1433-1456, (2021). [CrossRef]
- Kaza, S., Yao, L., Bhada-Tata, P. & Van Woerden, F. What a waste 2.0: a global snapshot of solid waste management to 2050. Report No. 1464813299, (The World Bank, 2018).
- Fei, X. & Zekkos, D. Coupled experimental assessment of physico-biochemical characteristics of municipal solid waste undergoing enhanced biodegradation. Géotechnique 68, 1031-1043, (2018). [CrossRef]
- Eleazer, W. E., Odle, W. S., Wang, Y. S. & Barlaz, M. A. Biodegradability of municipal solid waste components in laboratory-scale landfills. Environ. Sci. Technol. 31, 911-917, (1997). [CrossRef]
- Sharma, K. D. & Jain, S. Overview of municipal solid waste generation, composition, and management in India. Journal of Environmental Engineering 145, 04018143, (2019). [CrossRef]
- Guo, W. et al. Solid waste management in China: Policy and driving factors in 2004–2019. Resources, Conservation and Recycling 173, 105727, (2021). [CrossRef]
- Weitz, K. A., Thorneloe, S. A., Nishtala, S. R., Yarkosky, S. & Zannes, M. The impact of municipal solid waste management on greenhouse gas emissions in the United States. Journal of the Air & Waste Management Association 52, 1000-1011, (2002). [CrossRef]
- Nelles, M., Gruenes, J. & Morscheck, G. Waste management in Germany–development to a sustainable circular economy? Procedia Environmental Sciences 35, 6-14, (2016).
- Zhu, J. et al. Cradle-to-grave emissions from food loss and waste represent half of total greenhouse gas emissions from food systems. Nature Food 4, 247-256, (2023). [CrossRef]
- Crippa, M. et al. Food systems are responsible for a third of global anthropogenic GHG emissions. Nature Food 2, 198-209, (2021). [CrossRef]
- Ma, S., Zhou, C., Chi, C., Liu, Y. & Yang, G. Estimating Physical Composition of Municipal Solid Waste in China by Applying Artificial Neural Network Method. Environmental Science & Technology 54, 9609-9617, (2020). [CrossRef]
- Baawain, M., Al-Mamun, A., Omidvarborna, H. & Al-Amri, W. Ultimate composition analysis of municipal solid waste in Muscat. Journal of cleaner production 148, 355-362, (2017). [CrossRef]
- Iqbal, M. K., Shafiq, T., Hussain, A. & Ahmed, K. Effect of enrichment on chemical properties of MSW compost. Bioresource technology 101, 5969-5977, (2010). [CrossRef]
- Chickering, G. W., Krause, M. J., Inglett, K. S. & Townsend, T. G. Municipal Solid Waste Biodegradability and Methane Potential: Influence of Fiber Content and Elemental Composition. Environmental Engineering Science 39, 15-28, (2022). [CrossRef]
- Pirotta, F., Ferreira, E. C. & Bernardo, C. Energy recovery and impact on land use of Maltese municipal solid waste incineration. Energy 49, 1-11, (2013). [CrossRef]
- Götze, R., Boldrin, A., Scheutz, C. & Astrup, T. F. Physico-chemical characterisation of material fractions in household waste: Overview of data in literature. Waste Management 49, 3-14, (2016). [CrossRef]
- Fei, X., Zekkos, D. & Raskin, L. Archaeal community structure in leachate and solid waste is correlated to methane generation and volume reduction during biodegradation of municipal solid waste. Waste Management 36, 184-190, (2015). [CrossRef]
- Townsend, T. G. et al. Sustainable practices for landfill design and operation. (Springer, 2015).
- Xiaoli, C., Shimaoka, T., Xianyan, C., Qiang, G. & Youcai, Z. Characteristics and mobility of heavy metals in an MSW landfill: Implications in risk assessment and reclamation. Journal of Hazardous Materials 144, 485-491, (2007). [CrossRef]
- Brahney, J., Hallerud, M., Heim, E., Hahnenberger, M. & Sukumaran, S. Plastic rain in protected areas of the United States. Science 368, 1257-1260, (2020). [CrossRef]
- Burlakovs, J. et al. Mobility of metals and valorization of sorted fine fraction of waste after landfill excavation. Waste and biomass valorization 7, 593-602, (2016). [CrossRef]
- Arora, B., Mohanty, B. P., McGuire, J. T. & Cozzarelli, I. M. Temporal dynamics of biogeochemical processes at the Norman Landfill site. Water Resources Research 49, 6909-6926, (2013). [CrossRef]
- Bogner, J. & Matthews, E. Global methane emissions from landfills: New methodology and annual estimates 1980-1996. Glob. Biogeochem. Cycle 17, (2003). [CrossRef]
- Christensen, T. H. et al. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 16, 659-718, (2001). [CrossRef]
- GIZ, Leeds, U. o., Eawag-Sandec & Wasteaware. User Manual: Waste Flow Diagram (WFD): A rapid assessment tool for mapping waste flows and quantifying plastic leakage. Version 1.0. (2020).
- Stone, R. & Gupta, R. K. Aerobic and Anaerobic Landfill Stabilization Process. Journal of the Sanitary Engineering Division 96, 1399-1414, (1970). [CrossRef]
- He, P. J. Anaerobic digestion: An intriguing long history in China. Waste Management 30, 549-550, (2010). [CrossRef]
- Erses, A. S., Onay, T. T. & Yenigun, O. Comparison of aerobic and anaerobic degradation of municipal solid waste in bioreactor landfills. Bioresource technology 99, 5418-5426, (2008). [CrossRef]
- Nguyen, P., Kuruparan, P. & Visvanathan, C. Anaerobic digestion of municipal solid waste as a treatment prior to landfill. Bioresource Technology 98, 380-387, (2007). [CrossRef]
- Kang, K.-H., Shin, H. S. & Park, H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water research 36, 4023-4032, (2002). [CrossRef]
- Reinhart, D. R., McCreanor, P. T. & Townsend, T. The bioreactor landfill: Its status and future. Waste Management & Research 20, 172-186, (2002). [CrossRef]
- Oonk, H. et al. Enhanced biodegradation at the Landgraaf bioreactor test-cell. Waste management 33, 2048-2060, (2013). [CrossRef]
- IPCC. Guidelines for national greenhouse gas inventories. Prepared by the National Greenhouse Gas Inventories Programme. Eggleston HS, Buendia L, Miwa K, Ngara T, Tanabe K, editors. Published: IGES, Japan, (2006).
- Fei, X., Zekkos, D. & Raskin, L. Quantification of parameters influencing methane generation due to biodegradation of municipal solid waste in landfills and laboratory experiments. Waste management 55, 276-287, (2016). [CrossRef]
- El-Fadel, M., Findikakis, A. & Leckie, J. Numerical modelling of generation and transport of gas and heat in landfills I. Model formulation. Waste management & research 14, 483-504, (1996). [CrossRef]
- Gawande, N. A., Reinhart, D. R. & Yeh, G.-T. Modeling microbiological and chemical processes in municipal solid waste bioreactor, part I: Development of a three-phase numerical model BIOKEMOD-3P. Waste management 30, 202-210, (2010).
- Krause, M. J., Chickering, G. W. & Townsend, T. G. Translating landfill methane generation parameters among first-order decay models. Journal of the Air & Waste Management Association 66, 1084-1097, (2016). [CrossRef]
- Amini, H. R., Reinhart, D. R. & Mackie, K. R. Determination of first-order landfill gas modeling parameters and uncertainties. Waste management 32, 305-316, (2012). [CrossRef]
- Maasakkers, J. D. et al. Using satellites to uncover large methane emissions from landfills. Science Advances 8, eabn9683, (2022). [CrossRef]
- Spokas, K., Bogner, J. & Corcoran, M. Modeling landfill CH4 emissions: CALMIM international field validation, using CALMIM to simulate management strategies, current and future climate scenarios. Elem Sci Anth 9, 00050, (2021).
- Kjeldsen, P. et al. Present and long-term composition of MSW landfill leachate: a review. Critical reviews in environmental science and technology 32, 297-336, (2002). [CrossRef]
- Barlaz, M. A. Carbon storage during biodegradation of municipal solid waste components in laboratory-scale landfills. Global biogeochemical cycles 12, 373-380, (1998). [CrossRef]
- Teng, C., Zhou, K., Peng, C. & Chen, W. Characterization and treatment of landfill leachate: A review. Water research 203, 117525, (2021). [CrossRef]
- Stubbins, A., Law, K. L., Muñoz, S. E., Bianchi, T. S. & Zhu, L. Plastics in the Earth system. Science 373, 51-55, (2021). [CrossRef]
- Chamas, A. et al. Degradation rates of plastics in the environment. ACS Sustainable Chemistry & Engineering 8, 3494-3511, (2020). [CrossRef]
- Lau, W. W. Y. et al. Evaluating scenarios toward zero plastic pollution. Science 369, 1455-1461, (2020). [CrossRef]
- Rinne, J. et al. Nitrous oxide emissions from a municipal landfill. Environ. Sci. Technol. 39, 7790-7793, (2005). [CrossRef]
- Zhang, H., Yan, X., Cai, Z. & Zhang, Y. Effect of rainfall on the diurnal variations of CH4, CO2, and N2O fluxes from a municipal solid waste landfill. Science of the Total Environment 442, 73-76, (2013). [CrossRef]
- Wu, C., Shimaoka, T., Nakayama, H. & Komiya, T. Kinetics of nitrous oxide production by denitrification in municipal solid waste. Chemosphere 125, 64-69, (2015). [CrossRef]
- Buswell, A. & Mueller, H. Mechanism of methane fermentation. Industrial & Engineering Chemistry 44, 550-552, (1952).
- Ko, J. H., Xu, Q. & Jang, Y.-C. Emissions and Control of Hydrogen Sulfide at Landfills: A Review. Critical Reviews in Environmental Science and Technology 45, 2043-2083, (2015). [CrossRef]
- Heaney, C. D. et al. Relation between malodor, ambient hydrogen sulfide, and health in a community bordering a landfill. Environmental research 111, 847-852, (2011). [CrossRef]
- Kim, K.-H., Choi, Y. J., Jeon, E. C. & Sunwoo, Y. Characterization of malodorous sulfur compounds in landfill gas. Atmospheric Environment 39, 1103-1112, (2005). [CrossRef]
- Lou, Z. et al. Landfill refuse stabilization process characterized by nutrient change. Environmental engineering science 26, 1655-1660, (2009). [CrossRef]
- Luo, Y., Li, R., Sun, X., Liu, X. & Li, D. The roles of phosphorus species formed in activated biochar from rice husk in the treatment of landfill leachate. Bioresource technology 288, 121533, (2019). [CrossRef]
- Khanzada, Z. T. Phosphorus removal from landfill leachate by microalgae. Biotechnology Reports 25, e00419, (2020). [CrossRef]
- Peng, B.-Y. et al. Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environment International 145, 106106, (2020). [CrossRef]
- Øygard, J. K., Måge, A. & Gjengedal, E. Estimation of the mass-balance of selected metals in four sanitary landfills in Western Norway, with emphasis on the heavy metal content of the deposited waste and the leachate. Water research 38, 2851-2858, (2004). [CrossRef]
- Flyhammar, P. Estimation of heavy metal transformations in municipal solid waste. Science of the Total Environment 198, 123-133, (1997). [CrossRef]
- Feldmann, J. & Hirner, A. V. Occurrence of volatile metal and metalloid species in landfill and sewage gases. International Journal of Environmental Analytical Chemistry 60, 339-359, (1995). [CrossRef]
- de Oliveira, F. D. G., Robey, N. M., Smallwood, T. J., Spreadbury, C. J. & Townsend, T. G. Landfill gas as a source of anthropogenic antimony and arsenic release. Chemosphere 307, 135739, (2022). [CrossRef]
- Haynes, K. M. et al. Gaseous mercury fluxes in peatlands and the potential influence of climate change. Atmospheric Environment 154, 247-259, (2017). [CrossRef]
- Fei, X. & Zekkos, D. Comparison of direct shear and simple shear responses of municipal solid waste in USA. Environmental Geotechnics 5, 158-167, (2017). [CrossRef]
- Zekkos, D. & Fei, X. Constant load and constant volume response of municipal solid waste in simple shear. Waste Management 63, 380-392, (2017). [CrossRef]
- Tokiwa, Y., Calabia, B. P., Ugwu, C. U. & Aiba, S. Biodegradability of plastics. International journal of molecular sciences 10, 3722-3742, (2009).
- Wang, Y., Lu, X. & Fei, X. Property changes of conventional plastic waste mixed with municipal solid waste after 10-year degradation experiments simulating landfill conditions. Journal of Hazardous Materials Letters 2, 100047, (2021). [CrossRef]
- He, P., Chen, L., Shao, L., Zhang, H. & Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics?-Evidence of microplastics in landfill leachate. Water research 159, 38-45, (2019). [CrossRef]
- Lu, X., He, H., Wang, Y., Guo, Y. & Fei, X. Masses and size distributions of mechanically fragmented microplastics from LDPE and EPS under simulated landfill conditions. Journal of Hazardous Materials 445, 130542, (2023). [CrossRef]
- Sun, J. et al. Revisiting microplastics in landfill leachate: unnoticed tiny microplastics and their fate in treatment works. Water Research 190, 116784, (2021). [CrossRef]
- Scott, J., Beydoun, D., Amal, R., Low, G. & Cattle, J. Landfill management, leachate generation, and leach testing of solid wastes in Australia and overseas. Critical Reviews in Environmental Science and Technology 35, 239-332, (2005). [CrossRef]
- Santos, A., Fernandez, J., Guadano, J., Lorenzo, D. & Romero, A. Chlorinated organic compounds in liquid wastes (DNAPL) from lindane production dumped in landfills in Sabinanigo (Spain). Environmental pollution 242, 1616-1624, (2018). [CrossRef]
- Yu, X. et al. Municipal solid waste landfills: an underestimated source of pharmaceutical and personal care products in the water environment. Environ. Sci. Technol. 54, 9757-9768, (2020). [CrossRef]
- Robey, N. M., da Silva, B. F., Annable, M. D., Townsend, T. G. & Bowden, J. A. Concentrating per-and polyfluoroalkyl substances (PFAS) in municipal solid waste landfill leachate using foam separation. Environ. Sci. Technol. 54, 12550-12559, (2020). [CrossRef]
- Fleming, I. R., Rowe, R. K. & Cullimore, D. R. Field observations of clogging in a landfill leachate collection system. Can. Geotech. J. 36, 685-707, (1999).
- Vaverková, M. D. Landfill impacts on the environment. Geosciences 9, 431, (2019). [CrossRef]
- Akinbile, C. O. Environmental impact of landfill on groundwater quality and agricultural soils in Nigeria. Soil and Water Research 7, 18-26, (2012). [CrossRef]
- Halim, C. E. et al. Comparison between Acetic Acid and Landfill Leachates for the Leaching of Pb(II), Cd(II), As(V), and Cr(VI) from Cementitious Wastes. Environmental Science & Technology 38, 3977-3983, (2004). [CrossRef]
- Dabrowska, D., Rykala, W. & Nourani, V. Causes, Types and Consequences of Municipal Waste Landfill Fires—Literature Review. Sustainability 15, 5713, (2023).
- Berge, N. D. et al. Hydrothermal Carbonization of Municipal Waste Streams. Environmental Science & Technology 45, 5696-5703, (2011). [CrossRef]
- Bihałowicz, J. S., Rogula-Kozłowska, W. & Krasuski, A. Contribution of landfill fires to air pollution – An assessment methodology. Waste Management 125, 182-191, (2021). [CrossRef]
- Øygard, J. K., Måge, A., Gjengedal, E. & Svane, T. Effect of an uncontrolled fire and the subsequent fire fight on the chemical composition of landfill leachate. Waste Management 25, 712-718, (2005). [CrossRef]
- Hao, Z. et al. Heat Generation and Accumulation in Municipal Solid Waste Landfills. Environmental Science & Technology 51, 12434-12442, (2017). [CrossRef]
- Barlaz, M. A., Chanton, J. P. & Green, R. B. Controls on landfill gas collection efficiency: instantaneous and lifetime performance. Journal of the Air & Waste Management Association 59, 1399-1404, (2009). [CrossRef]
- Mønster, J., Samuelsson, J., Kjeldsen, P. & Scheutz, C. Quantification of methane emissions from 15 Danish landfills using the mobile tracer dispersion method. Waste Management 35, 177-186, (2015). [CrossRef]
- Spokas, K., Bogner, J., Corcoran, M. & Walker, S. From California dreaming to California data: Challenging historic models for landfill CH4 emissionsCalifornia landfill emissions. Elementa: Science of the Anthropocene 3, (2015).
- Ritzkowski, M. & Stegmann, R. Controlling greenhouse gas emissions through landfill in situ aeration. International Journal of Greenhouse Gas Control 1, 281-288, (2007). [CrossRef]
- Ritzkowski, M. & Stegmann, R. Landfill aeration worldwide: Concepts, indications and findings. Waste Management 32, 1411-1419, (2012). [CrossRef]
- Liu, L. et al. The in situ aeration in an old landfill in China: Multi-wells optimization method and application. Waste Management 76, 614-620, (2018). [CrossRef]
- Spokas, K. A. & Bogner, J. E. Limits and dynamics of methane oxidation in landfill cover soils. Waste Management 31, 823-832, (2011). [CrossRef]
- Gebert, J., Groengroeft, A. & Pfeiffer, E.-M. Relevance of soil physical properties for the microbial oxidation of methane in landfill covers. Soil Biology and Biochemistry 43, 1759-1767, (2011). [CrossRef]
- Parsaeifard, N., Sattler, M., Nasirian, B. & Chen, V. C. Enhancing anaerobic oxidation of methane in municipal solid waste landfill cover soil. Waste management 106, 44-54, (2020). [CrossRef]
- Scheutz, C. et al. Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste management & research 27, 409-455, (2009). [CrossRef]
- Chanton, J. P., Powelson, D. K. & Green, R. B. Methane oxidation in landfill cover soils, is a 10% default value reasonable? Journal of environmental quality 38, 654-663, (2009).
- Cai, B. et al. Evaluating the impact of odors from the 1955 landfills in China using a bottom-up approach. Journal of Environmental Management 164, 206-214, (2015). [CrossRef]
- Powell, J. T., Townsend, T. G. & Zimmerman, J. B. Estimates of solid waste disposal rates and reduction targets for landfill gas emissions. Nature Climate Change 6, 162-165, (2016). [CrossRef]
- Luo, H., Zeng, Y., Cheng, Y., He, D. & Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Science of the Total Environment 703, 135468, (2020). [CrossRef]
- Rowe, R. K. Long-term performance of contaminant barrier systems. Geotechnique 55, 631-678, (2005). [CrossRef]
- Levis, J. W., Weisbrod, A., Van Hoof, G. & Barlaz, M. A. A review of the airborne and waterborne emissions from uncontrolled solid waste disposal sites. Critical Reviews in Environmental Science and Technology 47, 1003-1041, (2017). [CrossRef]
- Brand, J. H. & Spencer, K. L. Will flooding or erosion of historic landfills result in a significant release of soluble contaminants to the coastal zone? Science of The Total Environment 724, 138150, (2020).
- Fei, X., Fang, M. & Wang, Y. Climate change affects land-disposed waste. Nature Climate Change 11, 1004-1005, (2021). [CrossRef]
- Zhang, S. et al. Quantitative human risk analysis of 2015 Shenzhen dump failure considering influence of urbanization. Journal of Mountain Science 18, 1439-1457, (2021). [CrossRef]
- Ma, S. et al. Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. Journal of Cleaner Production 333, 130234, (2022). [CrossRef]
- Smallwood, T. J., Magnuson, J. K., Thompson, J. T., Lin, A. M. & Townsend, T. G. Insights on volatile metals in landfill gas as determined from advanced treatment media. Journal of Hazardous Materials 462, 132777, (2024). [CrossRef]
- Duan, Z., Scheutz, C. & Kjeldsen, P. Trace gas emissions from municipal solid waste landfills: A review. Waste Management 119, 39-62, (2021). [CrossRef]
- Yaashikaa, P. et al. A review on landfill system for municipal solid wastes: Insight into leachate, gas emissions, environmental and economic analysis. Chemosphere, 136627, (2022). [CrossRef]
- Yu, X. et al. Do high levels of PPCPs in landfill leachates influence the water environment in the vicinity of landfills? A case study of the largest landfill in China. Environment international 135, 105404, (2020). [CrossRef]
- Weber, R., Watson, A., Forter, M. & Oliaei, F. Persistent organic pollutants and landfills-a review of past experiences and future challenges. Waste Management & Research 29, 107-121, (2011). [CrossRef]
- Pfeiffer, M. B., Blackwell, B. F. & DeVault, T. L. Collective effect of landfills and landscape composition on bird–aircraft collisions. Human–Wildlife Interactions 14, 9, (2020).
- Bjerg, P. L., Tuxen, N., Reitzel, L. A., Albrechtsen, H. J. & Kjeldsen, P. Natural attenuation processes in landfill leachate plumes at three Danish sites. Groundwater 49, 688-705, (2011). [CrossRef]
- Scheutz, C., Mosbæk, H. & Kjeldsen, P. Attenuation of methane and volatile organic compounds in landfill soil covers. Journal of Environmental Quality 33, 61-71, (2004).
- Balzer, W., Gaus, M., Gaus, C., Urban, U. & Weber, R. PCDD/F emission from Leblanc Soda factories in Great Britain, France and Germany during the 18th to early 20th century. Organohalogen Compounds, (2008).
- Nair, A. T., Senthilnathan, J. & Nagendra, S. M. S. Emerging perspectives on VOC emissions from landfill sites: Impact on tropospheric chemistry and local air quality. Process Safety and Environmental Protection 121, 143-154, (2019). [CrossRef]
- Tomita, A., Cuadros, D. F., Burns, J. K., Tanser, F. & Slotow, R. Exposure to waste sites and their impact on health: A panel and geospatial analysis of nationally representative data from South Africa, 2008–2015. The Lancet Planetary Health 4, e223-e234, (2020). [CrossRef]
- Mattiello, A. et al. Health effects associated with the disposal of solid waste in landfills and incinerators in populations living in surrounding areas: a systematic review. International journal of public health 58, 725-735, (2013). [CrossRef]
- Dolk, H. et al. Risk of congenital anomalies near hazardous-waste landfill sites in Europe: the EUROHAZCON study. The Lancet 352, 423-427, (1998). [CrossRef]
- Elliott, P. et al. Risk of adverse birth outcomes in populations living near landfill sites. Bmj 323, 363-368, (2001). [CrossRef]
- Vrijheid, M. Health effects of residence near hazardous waste landfill sites: a review of epidemiologic literature. Environmental health perspectives 108, 101-112, (2000). [CrossRef]
- Chalvatzaki, E., Aleksandropoulou, V. & Lazaridis, M. A case study of landfill workers exposure and dose to particulate matter-bound metals. Water, Air, & Soil Pollution 225, 1-19, (2014). [CrossRef]
- Cook, E. & Velis, C. Global review on safer end of engineered life. Engineering X (founded by the Royal Academy of Engineering and the Lloyd’s Register Foundation) (2021).
- Porta, D., Milani, S., Lazzarino, A. I., Perucci, C. A. & Forastiere, F. Systematic review of epidemiological studies on health effects associated with management of solid waste. Environmental health 8, 1-14, (2009). [CrossRef]
- Velis, C. & Mavropoulos, A. Unsound waste management and public health: The neglected link? Waste Management & Research 34, 277-279, (2016).
- Yi, S. Resource recovery potentials by landfill mining and reclamation in South Korea. Journal of environmental management 242, 178-185, (2019). [CrossRef]
- Wen, Y., Zhao, Y., Guan, Z. & Zhang, X. Remodeling of Abandoned Land: A Review of Landscape Regeneration and the Reconstruction of Urban Landfill Sites. Sustainability 15, 10810, (2023). [CrossRef]
- Themelis, N. J. & Ulloa, P. A. Methane generation in landfills. Renewable Energy 32, 1243-1257, (2007). [CrossRef]
- Iskander, S. M., Brazil, B., Novak, J. T. & He, Z. Resource recovery from landfill leachate using bioelectrochemical systems: opportunities, challenges, and perspectives. Bioresource Technology 201, 347-354, (2016). [CrossRef]
- Krook, J., Svensson, N. & Eklund, M. Landfill mining: A critical review of two decades of research. Waste management 32, 513-520, (2012). [CrossRef]
- Zhi, Y., Ma, S., Qin, J., Zhao, Z. & Zhou, C. Assessing the city-level material stocks in landfills and the landfill mining potential of China. Environmental Research 236, 116737, (2023). [CrossRef]
- Couth, R. & Trois, C. Sustainable waste management in Africa through CDM projects. Waste management 32, 2115-2125, (2012). [CrossRef]
- Renou, S., Givaudan, J., Poulain, S., Dirassouyan, F. & Moulin, P. Landfill leachate treatment: Review and opportunity. Journal of hazardous materials 150, 468-493, (2008). [CrossRef]
- Bove, D. et al. A critical review of biological processes and technologies for landfill leachate treatment. Chemical Engineering & Technology 38, 2115-2126, (2015). [CrossRef]
- Gabasiane, T. S., Danha, G., Mamvura, T. A., Mashifana, T. & Dzinomwa, G. Characterization of copper slag for beneficiation of iron and copper. Heliyon 7, e06757, (2021). [CrossRef]
- Jones, P. T. et al. Enhanced Landfill Mining in view of multiple resource recovery: a critical review. Journal of Cleaner Production 55, 45-55, (2013). [CrossRef]
- Hou, D. et al. Sustainable remediation and redevelopment of brownfield sites. Nature Reviews Earth & Environment, 1-16, (2023). [CrossRef]
- Pleasant, S., O'Donnell, A., Powell, J., Jain, P. & Townsend, T. Evaluation of air sparging and vadose zone aeration for remediation of iron and manganese-impacted groundwater at a closed municipal landfill. Science of the total environment 485, 31-40, (2014). [CrossRef]
- Moon, S., Nam, K., Kim, J. Y., Hwan, S. K. & Chung, M. Effectiveness of compacted soil liner as a gas barrier layer in the landfill final cover system. Waste management 28, 1909-1914, (2008). [CrossRef]
- Zekkos, D., Kabalan, M. & Flanagan, M. Lessons learned from case histories of dynamic compaction at municipal solid waste sites. Journal of geotechnical and geoenvironmental engineering 139, 738-751, (2013). [CrossRef]
- Hogland, W. Remediation of an old landsfill site: soil analysis, leachate quality and gas production. Environmental Science and Pollution Research 9, 49-54, (2002).
- Somani, M., Harbottle, M., Datta, M., Ramana, G. V. & Sreekrishnan, T. R. Identification and assessment of appropriate remediation management techniques for the recovery of soil-like material produced in landfill mining. Journal of Environmental Management 348, 119300, (2023). [CrossRef]
- Yellappa, M., Sarkar, O., Reddy, Y. V. R. & Mohan, S. V. Municipal landfill leachate remediation coupling acidogenesis and bioelectrogenesis for biohydrogen and volatile fatty acids production. Process Safety and Environmental Protection 172, 716-726, (2023). [CrossRef]
- Mondal, T., Choudhury, M., Kundu, D., Dutta, D. & Samanta, P. Landfill: An eclectic review on structure, reactions and remediation approach. Waste Management 164, 127-142, (2023). [CrossRef]
- Simis, M., Awang, A. & Arifin, K. From Ex-landfill to Public Park: Impact on Local Community's Quality of Life and Living Environment. Procedia - Social and Behavioral Sciences 222, 763-771, (2016).
- Nai, C. et al. Potentially contamination and health risk to shallow groundwater caused by closed industrial solid waste landfills: Site reclamation evaluation strategies. Journal of Cleaner Production 286, 125402, (2021). [CrossRef]
- Scott, D. I., Longman, M. & Wilson, S. Reclaiming historic landfill sites for residential development: a UK case study. Journal of Environmental Engineering and Science 15, 71-79, (2019). [CrossRef]
- Cossu, R., Raga, R. & Rossetti, D. The PAF model: an integrated approach for landfill sustainability. Waste management 23, 37-44, (2003). [CrossRef]
- Lijzen, J. et al. Technical evaluation of the Intervention Values for Soil/sediment and Groundwater. Human and ecotoxicological risk assessment and derivation of risk limits for soil, aquatic sediment and groundwater. (2001).
- Ye, J., Chen, X., Chen, C. & Bate, B. Emerging sustainable technologies for remediation of soils and groundwater in a municipal solid waste landfill site--A review. Chemosphere 227, 681-702, (2019). [CrossRef]
- Pastre, G. et al. A decision support tool for enhanced landfill mining. Detritus 1, 91-101, (2018).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).