1. Introduction
Over half of the individuals with Parkinson’s disease (PD) develop freezing of gait (FOG) [
1], one of the most debilitating features of PD and one of the major reasons for falls and reduced quality of life [
2,
3]. FOG is defined as a brief, episodic absence, or marked reduction of forward progression of the feet despite the intention to walk that typically occurs during step initiation and turns [
4]. The transition between the quiet stance and step initiation requires anticipatory postural adjustments (APA) [
5]. APAs are abnormal in individuals with PD and FOG (freezers) and associated with FOG severity [
6,
7]. Freezers have delayed step initiation associated with repetitive APA [
8,
9], as if they were unable to inhibit their postural preparation and release the stepping programme [
4]. It has been hypothesized that freezers may have the inability to inhibit their postural state and initiate stepping [
4,
7,
9,
10]. This deficit in step initiation has been proposed to result from a lack of central inhibition [
11].
We have investigated the involvement of the spinal cord during postural preparation to initiate a step in young and older adults [
12]. Presynaptic inhibition (PSI) is one of the most important spinal cord mechanisms responsible for modulating sensory feedback at the spinal level for walking [
13] and postural preparation [
7,
12,
14,
15,
16]. Increases in the PSI levels may decrease Ia afferents inputs onto motoneurons during posture and gait, through activation of GABAergic primary afferent depolarization interneurons [
15] that are controlled from supraspinal motor tracts [
17]. In animal models, the precision of skilled movements (reach trajectory and velocity of the forepaw to the food) depends on sensory feedback and its refinement by GABAergic interneurons, as the higher presynaptic control is required during the precision of skilled movement [
18].
Previous studies have demonstrated higher PSI levels of the ankle muscles during situations requiring high motor stability (standing on a foam mat), which is consistent with a high proprioceptive feedback demand in healthy individuals [
19]. We have hypothesized that older compared to young adults require a higher presynaptic control of the soleus muscle to compensate for the decreased supraspinal modulation on impaired APAs for step initiation. We found higher levels of PSI of the soleus muscle during impaired APA for older than young adults [
12]. Higher PSI inhibition levels were associated with decreased APA amplitude. Like older adults, freezers can be thought to compensate for the lack of central inhibition during APA for step initiation using a higher presynaptic control.
We have recently demonstrated that during impaired APAs, freezers have a loss of PSI of the soleus muscle compared to non-freezers and age-matched healthy controls, whereas the other groups have PSI during APAs [
7]. The loss of PSI of the soleus muscle during step initiation was associated with small APA amplitudes and FOG severity in freezers, suggesting that the lack of central inhibition of locomotor regions would be reflected in the loss of PSI of the soleus muscle during impaired APAs for step initiation in freezers.
Freezers compared to non-freezers have a dysfunction in the locomotor network that involves mesencephalic locomotor region (MLR), supplementary motor area (SMA), subthalamic nucleus, and cerebellar locomotor region [
20]. Freezers compared to non-freezers showed greater functional connectivity between SMA and MLR and between the SMA and cerebellar locomotor region, which indicate a failed attempt by the brain to compensate for the lack of automatic control of gait by the basal ganglia [
20]. The abnormal functional connectivity between MLR and SMA was associated with FOG severity [
20].
MLR [
21] and SMA [
22,
23] have neurons involved in the APA regulation. MLR [
24] and SMA [
25] send projections to reticulospinal neurons, which are known to regulate APA in animal model (cat) [
26]. Reticulospinal neurons also mediate PSI during fictive locomotion in an animal model (cat) [
17]. Thus, the loss of PSI of the soleus muscle during step initiation found only in freezers would be associated with abnormal MLR and SMA activity during step initiation.
Therefore, this study aimed to identify which locomotor brain region (MLR, SMA, subthalamic nucleus, and cerebellar locomotor region) during an functional magnetic resonance image (fMRI) protocol of the APA task for step initiation would explain the loss of PSI of the soleus muscle during the APA task for step initiation. We used a leg-lifting task in an event-related fMRI protocol that was recently validated by our group as having characteristics similar to step initiation [
27,
28,
29,
30,
31]. We have included these locomotor brain regions as they presented the beta change of the blood oxygenation level-dependent (BOLD) signal during the fMRI protocol of the APA task for step initiation. The beta change is a proxy of change in brain activity during the task, as we have previously published [
27,
28,
29,
30,
31].
Freezers have decreased BOLD signal within the MLR but not within SMA during a fMRI protocol that simulates walking, which has been correlated with the FOG severity [
32]. Thus, we hypothesized that MLR activity during an fMRI protocol of the APA task for step initiation would explain the loss of PSI of the soleus muscle during APA for step initiation in freezers.
2. Materials and Methods
2.1. Ethical approval
The study was approved by the University of Sao Paulo Ethical Committee (School of Physical Education and Sport – ref. 2011/12), registered at the National Clinical Trial (RBR-83VB6B), and was performed in agreement with the Declaration of Helsinki. All individuals provided written informed consent.
2.2. Participants
Freezers diagnosed according to the UK Brain Bank criteria [
33] were recruited from the Movement Disorders Clinic in the School of Medicine at the University of São Paulo. Inclusion criteria were: (1) stable dopaminergic therapy for at least two months before and during the experimental period; (2) presenting FOG during ON-medication state (scored >1 on the New FOG Questionnaire [
34] and identified by a movement disorders specialist by videos of objective tests, such as turning clockwise and counter-clockwise); (3) Hoehn & Yahr stages 3 – 4; (4) 49-85 years of age; (5) able to walk safely without walking aids; (6) no physical exercise practice in the three months preceding study commencement; (7) Mini-Mental State Examination > 23 [
35]; (8) absence of other neurological disorders, significant arthritis, musculoskeletal or vestibular disorders; (9) absence of severe tremor, claustrophobia, and metal in the body; and (10) high quality of brain volumes acquired during the fMRI (head motion <1 mm) [
36].
2.3. Study procedures
For this study, we used clinical, behavioral, PSI of the soleus muscle for step initiation, and fMRI data from our previous study only for freezers, as only they presented loss of PSI of the soleus muscle during step initiation and performed the fMRI protocol for step initiation [
7,
30]. Freezers were assessed in the ON-medication state within 1.5 to 2 hours of taking their morning dose of dopaminergic medication.
2.4. Outcome assessments
2.4.1. Clinical assessments
Clinical assessments included motor severity measured using the Unified PD Rating Scale motor subsection (UPDRS-III) [
37], disease duration (years since diagnosis), levodopa-equivalent daily dosage scores calculated according to standardized methods [
38], subjective FOG assessed by the New FOG Questionnaire scores [
34], and cognitive inhibition assessed using the Stroop Color-Word Test – Victoria version [
39].
2.4.2. Behavioral assessments
FOG severity using the FOG-ratio during a 2-minute turning task, as previously published [
40].
APA amplitude and duration for step initiation, as previously published [
7,
12]. Briefly, we used the force platform (AMTI ORG-7) to measure APA. The onset of the APA during step initiation was defined as the time between the abrupt increase of the mediolateral force amplitude (i.e. 2 standard deviations above the mean baseline force) and the onset of the step. The duration of APA was calculated as the time between the onset of APA and the onset of the step. Step onset was identified by the marker on the right malleolus (2 standard deviations above the mean of the baseline foot displacement in the anteroposterior direction). Mediolateral force amplitude during the step task was normalized by the distance between the malleoli of the individual (N/cm).
Electromyography (EMG) and co-contraction ratio. Self-adhesive surface disc EMG electrodes (1 cm in diameter) placed on the soleus and tibialis anterior muscles were used to record the EMG signals. The skin was shaved when necessary and cleaned with a solution of alcohol to reduce the impedance at the skin–electrode interface. The reference electrode was placed on the skin over the patella. The EMG signals were amplified (×1000) and bandpass filtered (10–1000 Hz) and storage on the computer of the Nicolet Viking Quest portable EMG apparatus (CareFusion, San Diego, CA, USA]. We analyzed the rectified and averaged EMG recordings during the APA task that were measured over a 100 ms epoch that preceded tibial nerve stimulation (test H-reflex) or the common peroneal nerve stimulation (conditioned H-reflex), as previously described [
7,
12]. A co-contraction ratio was also calculated to express the rectified and averaged EMG amplitude for tibial anterior muscle relative to the rectified and averaged EMG for soleus muscle.
2.4.3. Test and conditioned H-reflexes
The soleus H-reflex was induced by stimulating the posterior tibial nerve in the left leg via a monopolar stimulating electrode (1 ms rectangular pulse) over the popliteal fossa using a constant-current stimulator [Nicolet Viking Quest portable EMG apparatus, CareFusion, San Diego, CA, USA]. The anode was placed proximally to the patella. H-reflexes were recorded using two self-adhesive surface disc surface EMG electrodes (1 cm in diameter) placed on the soleus muscle, below the insertion of the gastrocnemius muscles, with interelectrode distance of 3–4 cm. Reflex responses were measured as the peak-to-peak amplitude of the soleus H-reflex. H-reflexes were evoked with 10-s intervals. Stimulus intensities were increased in steps of 0.05 mA, starting below the soleus H-reflex threshold and increasing up to supramaximal intensity to measure the M
max. The sensitivity of the soleus H-reflex to inhibitory and facilitatory effects depends critically on its size [
41]. Then, the soleus H-reflex was evoked at an intensity corresponding to 20–25% of maximal motor response, resulting in a soleus H-reflex on the ascending portion of its recruitment curve, and in most individuals a small motor response [
42].
PSI of the soleus H-reflex was evoked by the conditioning stimulation (1 ms rectangular pulses) of the common peroneal nerve through bipolar surface electrodes (0.5 cm in diameter) placed 1–3 cm distal to the neck of the fibula in the left leg [
13,
43,
44,
45]. Motor responses were recorded using two self-adhesive surface disc electrodes (1 cm in diameter) placed on the tibialis anterior muscle. An interval of 100 ms was used between the conditioned H-reflex and the H-reflex test. At a conditioning-test interval of 100 ms, stimulation of the common peroneal nerve evokes an inhibition that is attributed to PSI [
13,
43,
46,
47]. A stimulation intensity of 1.1 × motor threshold is submaximal for activation of all inhibitory interneurons, allowing both facilitatory and inhibitory effects be observed [
16,
41,
43,
48]. Several different subsets of interneurons transmit PSI to Ia terminals projecting to several motoneuron pools (see more details in [
49]. We checked that the stimulation evoked a motor response in the tibialis anterior muscle without a motor response in the peroneal muscles. Also, care was taken to ensure that the conditioning stimulus was applied at a position where the threshold for a motor response (motor threshold) in the tibialis muscle was lower than the motor threshold in the peroneal muscle, preventing vigorous contractions of the tibialis anterior muscle, which could be monitored throughout the experiments. Additionally, the size of the test H-reflex was maintained constant throughout the experiments, thus reducing the bias in the amount of inhibition (PSI), as it depends on the size of the test H-reflex. Because the soleus H-reflex is often depressed in the quiet stance [
13,
14,
50], and mainly in the early part of the stance phase of walking [
13], the test stimulus intensity during the APA was adjusted so that the reference (unconditioned) H-reflex attained the same size as in the quiet stance (our control condition), as previously published ref [
7,
12]. We matched the size of the H-reflex because it is more susceptible to inhibition/facilitation depending on the size of the test H-reflex relative to maximal motor response (Crone
et al. 1990).
2.4.4. Assessment of PSI of the soleus muscle during APA for step initiation
To trigger H-reflexes (test and conditioned) during APAs for step initiation, we used the force platform (AMTI ORG-7) to detect the abrupt increase of mediolateral force amplitude. When the APA amplitude exceeded 10-20% of the mean of the mediolateral force (corresponding to 2 standard deviations above the mean of the baseline force) an electrical stimulus was automatically triggered. The baseline force threshold was calculated through LabVIEW software, as previously pubslished [
7,
12].
2.4.5. Beta of the BOLD signal change of locomotor regions during step initiation
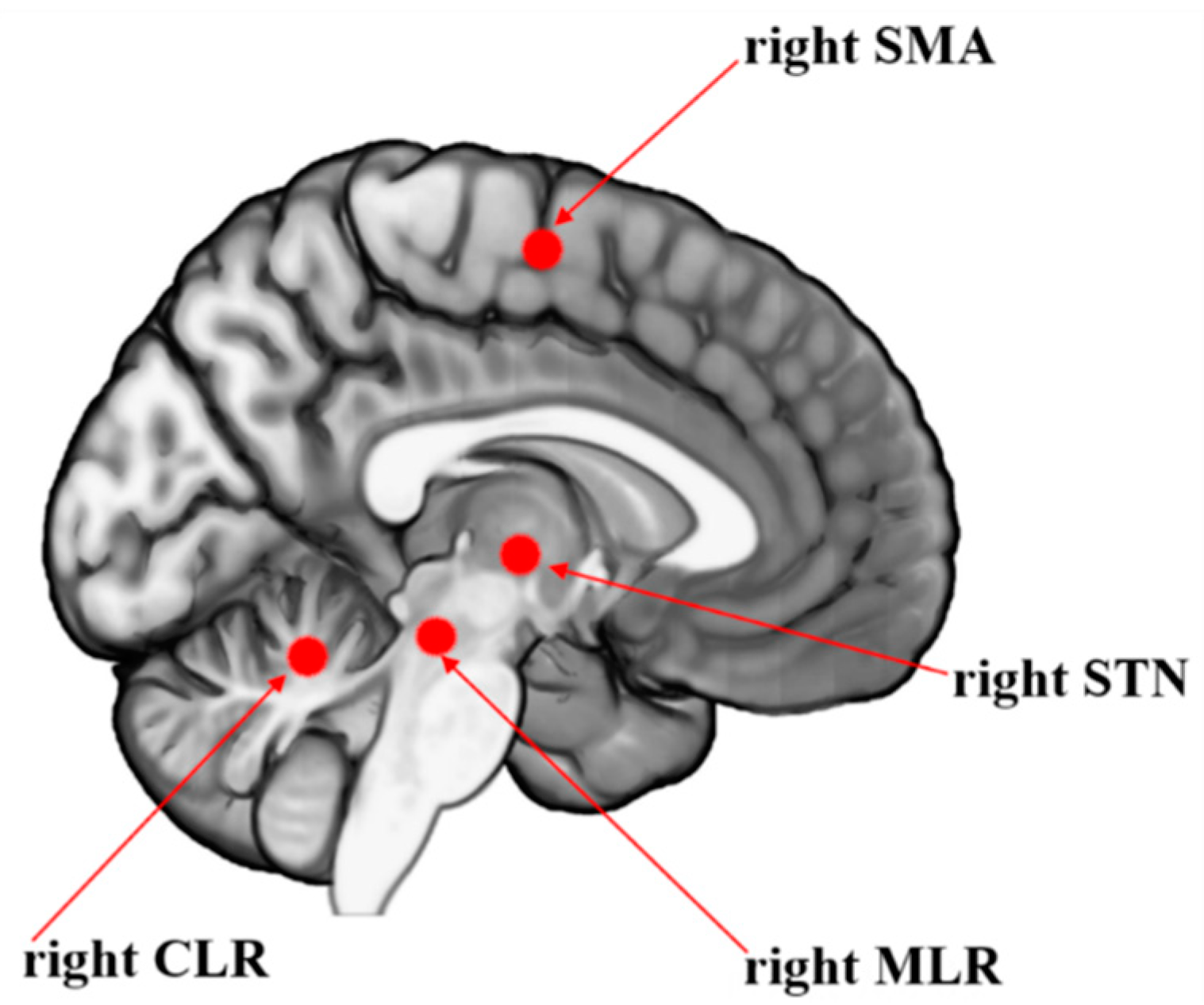
The beta of the BOLD signal change of locomotor regions of interest (SMA, subthalamic nucleus, MLR, cerebellar locomotor region), known to initiate and pace gait, were measured during an event-related fMRI protocol of step initiation [
27,
28,
29,
30]. These locomotor brain regions presented the beta change of the BOLD signal during the fMRI protocol of the APA task for step initiation. The beta change is a proxy of change in brain activity during the task [
27,
28,
29,
30,
31]. We evoked the conditioned and H-reflex test in the left leg in all participants, the same leg required during the initiation of APA inside the scanner, as freezers tend to show predominant involvement of right-sided brain circuitry [
11,
51]. Images were obtained using a 3.0 T MR system (Achieva, Philips Medical Imaging, The Netherlands), 32-channel head coil (80 mT/m gradient maximum amplitude). BOLD-sensitive images were acquired using T2*-weighted gradient echo-planar imaging (EPI): TR, 2.000 ms; TE, 30 ms; 40 slices; 3.0-mm slice thickness; 0.3-mm interslice gap; 3.0- mm
3 isotropic voxels; 240 volumes (acquisition time, 8 minutes). Anatomical T1-weighted 3-D images were used for reference and image registration (T1-FFE; TR, 7 s; TE, 3.2 s; 180 slices; Flip angle, 8°; 1 mm
3 isotropic voxels). fMRI data were processed using FSL software version 6.0 (
www.fmrib.ox.ac.uk/fsl/) [
52]. The volumes were preprocessed with an algorithm designed to reduce head movement (MCFLIRT), spatial smoothing (FWHM, 5 mm), [
53] and images were affine registration to the standard space of MNI-152 (12 DoF) [
54,
55]. As previously recommended, the level of head motion was less than 1 mm to avoid erroneous inference on neuronal function [
36]. MCFLIRT is a motion correction tool based on FLIRT (FMRIB's Linear Image Registration Tool), which is a fully automated robust, and accurate tool for linear (affine) intra- and inter-modal brain image registration used in FEAT (FMRI Expert Analysis Tool) from FSL. The advantages of MCFLIRT are accurate to 0.1mm in tests, end-slice corruption is minimized, plots of motion parameters and RMS movement can be produced (FEAT reports the motion parameters and can use them as regressors), and it works by serial registrations of each EPI in a time-series to the halfway timepoint image. Further, the calculated motion parameters by the MCFLIRT tool during the pre-procedure were added in the statistic model as confounders/covariates, using the standard option, which considered only 6 parameters of motion correction (3 for translation and 3 for rotation) during the analysis, in addition to the task-related regressors [
54,
56]. The event of interest was the time from the onset of the first stimulus until 1 s after leg lifting onset, as detailed previously [
28]. A linear model was implemented to estimate the BOLD signal in the event of interest compared to resting periods. The beta of BOLD signal change of regions of interest was extracted using the featquery tool processing routine from FSL. The peak coordinates of the regions of interest of the right hemisphere during step initiation were SMA: x = 3, y = -13, z = 61, radius = 8 mm [
32], subthalamic nucleus: x = -11, y = -14, z = -3, radius = 8 mm [
20], MLR: x = 6, y = -30, z = -19, radius = 6 mm [
20], cerebellar locomotor region: x = 7, y = -52, z = -16, radius = 6 mm [
20], which are illustrated in
Figure 1.
2.5. Statistical Analyses
Shapiro-Wilk and Levene’s tests were used to test the normality and homogeneity, respectively. We used logarithm transformation for the beta of the BOLD signal change and PSI of the soleus muscle data and we achieved normality. Then, one-tailed Pearson correlation coefficients were calculated among the variables as follow: clinical (UPDRS-III score, disease duration, medication dosage, clinical FOG score, and cognitive inhibition), behavioral (objective FOG severity, APA amplitude, APA duration, rectified and averaged EMG amplitude for tibial anterior and soleus muscle, and co-contraction ratio), PSI of the soleus muscle during step initiation, and the beta of the BOLD signal change of locomotor regions during step initiation (MLR, SMA, subthalamic nucleus, and cerebellar locomotor region). Then, we performed a linear multiple regression (PROC REG of SAS) using the stepwise method for PSI of the soleus muscle during step initiation as the dependent variable. To explain the variance of the dependent variable in the regression model, we used the clinical, behavioral, and beta of the BOLD signal change of regions of interest as independent variables. We also included age and height as independent variables, as these variables are known to be related to the H-reflex [
57,
58]. To avoid collinearity, we included the independent variables in the linear multiple regression analysis if they presented a
P-value ≤0.05 and a correlation lower than 0.7 between them [
59].
Results were presented as mean and standard deviation (SD). Statistical procedures were performed using SAS 9.2® (Institute Inc., Cary, NC, USA), and the significance level was set at P≤0.05.
3. Results
3.1. Participants
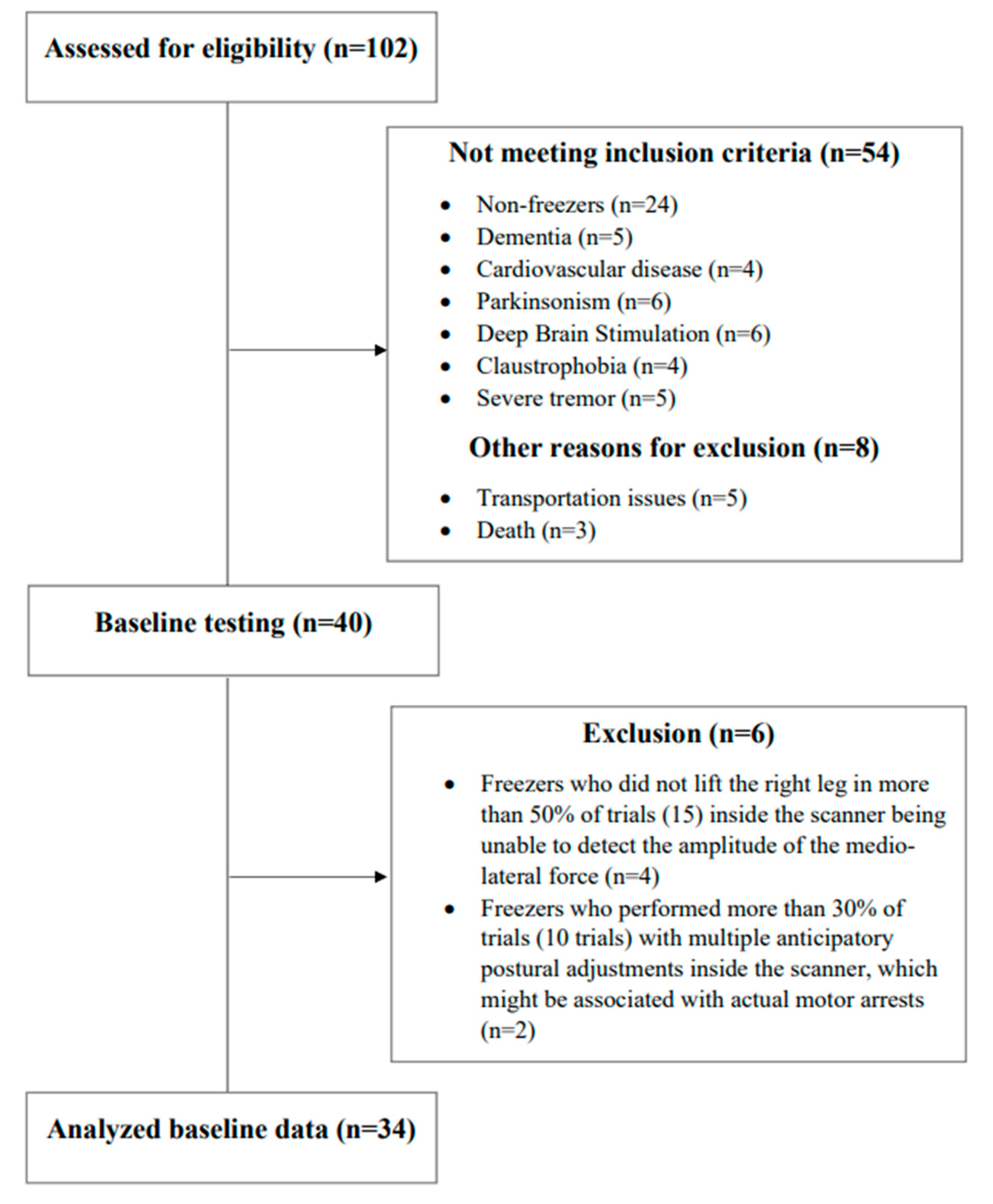
Forty freezers involved in our previous study [
30] performed baseline testing (clinical, behavioral, PSI of the soleus muscle for step initiation, and an fMRI protocol for step initiation), with six of them being excluded from the analysis (four participants were unable to lift the right leg (no detected APA in the left leg) in more than 50% of trials inside the scanner, and two participants performed more than 30% of trials with multiple APAs inside the scanner) as detailed in
Figure 2. The demographic, clinical, and behavioral variables, as well the values of PSI of the soleus muscle for step initiation and the beta of the BOLD signal change of regions of interest are presented in
Table 1.
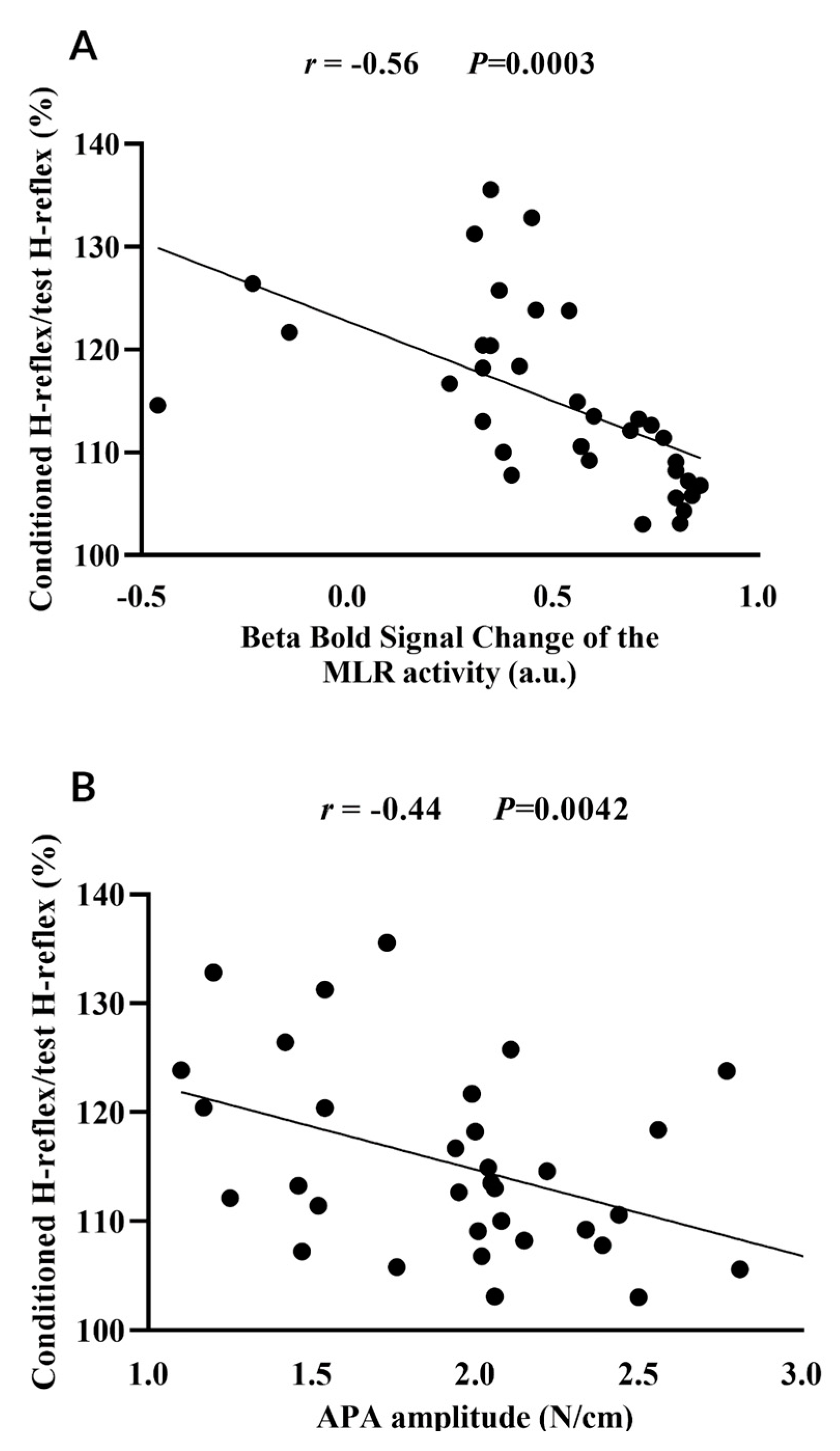
3.2. MLR activity and APA amplitude during step initiation explain the loss of PSI of the soleus muscle for step initiation
MLR activity (beta of BOLD signal change) during step initiation (r = -0.56,
P=0.0003), APA amplitude (r = -0.44,
P=0.0042), FOG-ratio (r = 0.50,
P=0.0011), disease duration (r = 0.49,
P=0.0016), and cognitive inhibition assessed using the Stroop Color-Word Test Stroop (r = 0.31,
P=0.0332) were associated with the loss of PSI of the soleus muscle during step initiation. These variables (except the Stroop Color-Word Test Stroop) entered the linear multiple regression model; however, only the MLR activity during step initiation (R
2=0.32,
P=0.0006) and decreased APA amplitude (R
2=0.13,
P=0.0097) significantly explained together 45% of the loss of PSI of the soleus muscle during step initiation in freezers, as demonstrated in
Table 2.
Figure 3.
Correlation of loss of presynaptic inhibition of the soleus muscle (i.e. ratio of the conditioned H-reflex relative to the test H-reflex) with the beta of blood oxygenation level-dependent (BOLD) signal change of the mesencephalic locomotor region (MLR) (A) and with the anticipatory postural adjustment (APA) amplitude (B) during step initiation. a.u. = arbitrary units.
Figure 3.
Correlation of loss of presynaptic inhibition of the soleus muscle (i.e. ratio of the conditioned H-reflex relative to the test H-reflex) with the beta of blood oxygenation level-dependent (BOLD) signal change of the mesencephalic locomotor region (MLR) (A) and with the anticipatory postural adjustment (APA) amplitude (B) during step initiation. a.u. = arbitrary units.
4. Discussion
This is the first study to show the relationship among MLR activity, APA amplitudes, and loss of PSI of the soleus muscle, all assessed during step initiation in freezers. Although clinical (disease duration) and behavioral (FOG severity during turning) variables were entered in the regression model to explain the loss of PSI of the soleus muscle during step initiation, only MLR activity and APA amplitudes during step initiation explained together 45% of the loss of PSI of the soleus muscle during step initiation in freezers.
4.1. Why do MLR activity and APA amplitudes explain the loss of PSI of the soleus muscle during step initiation in freezers?
MLR activity and APAs amplitude during step initiation may play an important role in the relationships between loss of PSI of the soleus muscle during step initiation and FOG of PD. The lack of central inhibition would be reflected in decreased MLR activity, as MLR receives a strong inhibition from basal ganglia due to the loss of dopaminergic neurons [
60]. MLR is an important locomotor center of the midbrain [
61]. Abnormal MLR inhibition would impair supraspinal motor tracts (e.g., reticulospinal tract) mediating inhibitory interneurons modulating PSI during APA in freezers. Freezers have decreased BOLD signal within the MLR during a fMRI protocol that simulates walking, which has been correlated with the FOG severity [
32]. Freezers also have grey matter atrophy in the MLR [
62]. MLR when stimulated increases postural tone for standing and induces stepping and running in a decerebrate cat [
63,
64]. MLR lesions cause cataplexy, akinesia in rats and immobility attacks reminiscent of the FOG events in PD [
61]. MLR [
21], as well as the reticulospinal tract [
26], has neurons involved in APA regulation. MLR sends projections to reticulospinal neurons [
24] mediating PSI during fictive locomotion in an animal model (cat) [
17]. Reticulospinal neurons receive short-latency orthodromic input from the MLR [
24]. Thus, decreased or absent inputs of MLR may be not exciting the reticulospinal tracts, which are very important for regulating postural muscle tone and locomotion [
65]. The reticulospinal tract modulates the activity of interneurons and motoneurons in spinal segments during posture and locomotion [
66,
67,
68]. Spinal interneurons with GABAergic axo-axonic synapses on primary afferent terminals produce PSI, which regulates the sensorimotor drive during skilled movements in mouse [
18]. In the decerebrate cat, medullary reticular formation-induced generalized motor inhibition [
69] and was associated with PSI of primary afferents [
70]. These inhibitory effects were mediated by inhibitory interneurons [
70,
71,
72]. Spinal GABAergic interneurons mediating PSI are modulated by the reticulospinal tract during locomotion in cats, independent of sensory feedback [
17]. This finding suggests that reticulospinal tract may program different movement pattern modulating presynaptic control to adjust APA during stepping.
The pontomedullary reticular formation in the brainstem is the main source of the reticulospinal tract, playing a well-established role in the control of posture and locomotion [
73]. Reticulospinal tract is crucial for movement control with an important hub for the sensorimotor integration, allowing thus cortical and subcortical structures to appropriately couple voluntary actions with posture and locomotion, like in APA [
74]. APAs are worse in freezers than non-freezers and healthy controls [
7], and APA has been found to be impaired in freezers [
7,
20,
75]. Our previous study demonstrated that decreased APA amplitude during step initiation is associated with FOG severity and loss of PSI during step initiation [
7]. Here, APA amplitude explained 13% of the loss of PSI, which suggests that impaired APAs may be due to abnormal reticulospinal tracts projections on spinal interneurons that modulate PSI during APA for stepping in freezers [
74].
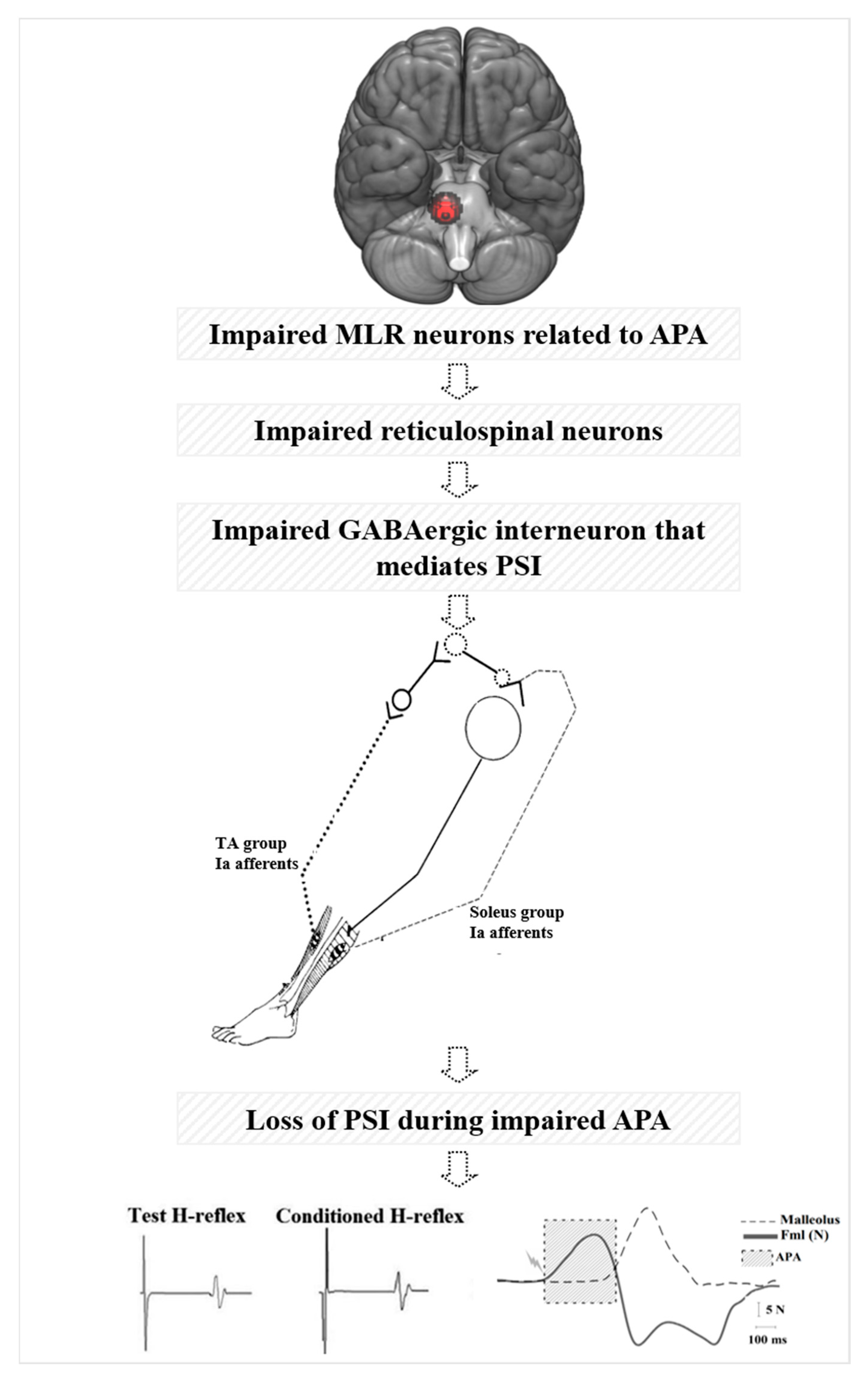
As illustrated in our hypothetical model in
Figure 4, glutamatergic projections from MLR are known to activate both inhibitory and excitatory pathways of the reticulospinal tract from the pontomedullary reticular formation during postural control, gait, and locomotion in cat [
65,
76]. These glutamatergic projections may not be activating both inhibitory and excitatory pathways of the reticulospinal tracts due to MLR atrophy in freezers [
11,
62] or decreased MLR activity during walking [
32]. Inhibitory reticulospinal tract is known to inhibit interneurons and motoneurons via inhibitory interneurons in the spinal cord in cats [
65,
69,
77]. Thus, reticulospinal tract neurons may not be inhibiting GABAergic interneurons that mediate PSI of soleus Ia afferent terminals. As a result, the conditioned H-reflex is not inhibited, leading to loss of PSI during step initiation in freezers that are associated with impaired APA amplitude and FOG severity. We hypothesize that loss of central inhibition (abnormal MLR activity) may be reflected in the loss of spinal inhibition (PSI) for stepping in freezers.
Interestingly, the SMA, a cortical region that contributes to generating self-initiated and multi-segmental voluntary movements [
78], did not enter into the regression model to explain the loss of PSI during step initiation in freezers. SMA sends motor commands to the reticulospinal tract [
25], which coordinates APAs with step initiation [
28]. The reticulospinal tract projects to the spinal motoneuron pool [
76,
79], sending drives to excitatory and inhibitory interneurons mediating PSI during voluntary movements [
17,
32,
80,
81,
82,
83]. SMA has cortico-reticular projections to MLR [
65,
76,
84]. Unlike MLR, SMA is hyperactive in freezers [
85]. Freezers have increased SMA activity during APAs compared to non-freezers [
28]. Freezers have increased functional connectivity between SMA and MLR compared to non-freezers [
86]. The increased functional connectivity between SMA and MLR [
86] would increase the excitability of the cortico-reticular projection arising from SMA on MLR activity. Although the increased connectivity of SMA on the MLR would increase the glutamatergic projections from MLR that are known to activate both inhibitory and excitatory pathways of the reticulospinal tract from the pontomedullary reticular formation during the posture, gait and locomotion in cat [
84]. MLR receives strong projections from the basal ganglia [
60]. Overactivity of the output nuclei of the basal ganglia in freezers may lead to excessive paroxysmal inhibition of the already disordered MLR [
60]. It is possible that inhibition of the basal ganglia on MLR is stronger than the increased excitability of SMA on MLR. Inhibition on MLR via basal ganglia has a negative and stronger influence on spinal inhibitory mechanisms related to postural preparation, such as PSI, before triggering FOG [
10] instead of hyperactivity of SMA on MLR. This is an issue open to future investigation.
4.2. Future directions for treatment strategies
We described some therapies that would improve MLR activity and PSI levels in freezers of PD, such as pedunculopontine nucleus-deep brain stimulation (an invasive therapy), spinal cord stimulation (a semi-invasive therapy), gene therapy, and rehabilitation therapies.
Our findings show that MLR may explain the loss of PSI during APA in freezers. MLR has been implicated in the FOG pathophysiology [
60]. Cellular loss within the MLR is associated with disease progression [
87,
88], which may explain why gait dynamic stability is affected by PD and not responsive to levodopa [
89]. We hypothesize that impaired MLR neurons likely lead to less activity of last-order interneuron via reticulospinal tract [
17], and consequently the loss of PSI in freezers. pedunculopontine nucleus-deep brain stimulation seems to be an effective treatment option for severe PD, although its results in the literature are not conclusive [
90]. Pedunculopontine nucleus-deep brain stimulation seems to mediate effects on the descending reticulospinal control, as bilateral pedunculopontine nucleus-deep brain stimulation alone or plus subthalamic nucleus improved the spinal cord excitability (soleus H-reflex) of six individuals with advanced PD up to normal values of healthy controls [
91]. Future studies should investigate the effects of pedunculopontine nucleus-deep brain stimulation on PSI levels in freezers.
Epidural spinal cord stimulation, a semi-invasive method, has been investigated as a treatment option for gait disorders in PD [
92,
93]. Although spinal cord stimulation has improved gait, APA duration [
94], and FOG episodes [
94,
95], evidence is still inconclusive as these findings were recorded in a small number of individuals [
92,
93]. It has been suggested that this therapy may activate multiple structures along the somatosensory pathway and desynchronizes the pathological cortico-striatal oscillations responsible for the manifestation of PD symptoms [
96,
97]. In addition, proprioceptive signals run primarily through some of the largest myelinated axons comprising the dorsal columns of the spinal cord during electrical stimulation [
96,
97]. In addition to stimulating specific somatosensory pathways, this therapy may also recruit brainstem arousal systems [
96,
97], such as MLR that sends motor commands to the spinal cord to initiate locomotion, via reticulospinal pathways [
98]. This therapy may restore PSI levels in freezers. Future studies should test this hypothesis.
Loss of PSI in freezers suggests that GABAergic interneurons are not activated to inhibit the H-reflex. GABAergic interneurons form axo–axonic contacts with the central terminals of sensory afferents, exerting presynaptic inhibitory control over sensorimotor transmission [
18]. Increased PSI [
16] is a result of decreased Ia afferent inputs onto motoneurons through activation of GABA-ergic primary afferent depolarization interneurons [
15]. GABA is abundant in the spinal cord, it is presented by interneurons only and not by the projection neurons [
99]. GABAergic interneurons are localized largely in the superficial laminae, while some are in deeper laminae of the dorsal and ventral horn [
100]. Subthalamic AAV-GAD (adeno-associated - glutamic acid decarboxylase) injection improved motor signs in hemiparkinsonian macaques [
101] and in individuals with PD with Hoehn and Yahr stage 3 or greater [
102]. The inhibitory neurotransmitter GABA is synthesized by GAD, which is predominant in the ventral horn of the spinal cord [
103]. GAD is the key enzyme involved in the synthesis of the inhibitory neurotransmitter GABA from excitatory glutamate [
104]. The AAV-GAD approach would be important in the spinal cord to restore PSI and improve FOG. Delivery of the gene encoding GAD could increase local GABA production within the spinal cord, restoring the function of GABAergic interneurons that mediate PSI.
We have developed the exercise rehabilitation called resistance training with instability for PD, in which non-freezers [
105] and freezers PD [
30] exercise with load/weight on unstable devices (a BOSU ball placed on the bases of support of individual), increasing the sensorimotor integration. In non-freezers, we observed that 12 weeks of resistance training with instability were effective in increasing PSI levels at rest up to normal values of healthy controls [
45]. In freezers, we observed increased MLR activity and improved APA amplitude after the 12 weeks of resistance training with instability [
30]. Our next step is to verify whether this intervention can restore the PSI levels in freezers, as resistance training with instability is a sensorimotor intervention supposedly activating descending pathways that modulate the PSI [
30].
Finally, recent studies have demonstrated the benefit of vibration (100 – 120 Hz) on FOG, suggesting this strategy as a novel therapy for freezers [
106,
107,
108,
109,
110]. Vibration is an external somatosensory cue that involves enhanced proprioceptive processing while the vibration is provided in the feet or wrists [
106,
107,
108,
109]. The signals resulting from vibration ascend the spinal cord, which may reach the cortico-subcortical brain areas (e.g., thalamus and sensorimotor cortices) and interact with the motor system to improve gait [
111]. Interestingly, PSI is responsive to vibration in healthy individuals [
112,
113,
114,
115,
116]. Thus, this therapy can be assumed to have a potential to restore PSI levels and improve APA and MLR activity in freezers.
4.3. Limitations
This study has some limitations. First, all individuals were assessed in the ON medication state, not reflecting their true disease state. Second, although fMRI imposes a restrictive environment, with limitations to assessing usual step initiation in a standing position, fMRI is the gold standard for
in vivo imaging of the brain human to assess cortical and subcortical areas and presents high spatial resolution and optimal signal-to-noise ratio [
117]. We used our fMRI protocol that simulates step initiation [
27,
28,
29,
30,
31], which has been validated to APA outside and inside the scanner [
118]. Third, our participants had no FOG episode, thus we do not know whether the loss of PSI and the MLR activity would change during FOG episodes.
5. Conclusions
Decreased MLR activity during a simulated APA task is related to a higher loss of PSI during APA for step initiation. MLR activity and APA amplitudes during step initiation explained together 45% of the loss of presynaptic inhibition during step initiation in freezers. Deficits in central and spinal inhibitions during APA may be related to FOG pathophysiology.
Author Contributions
Conceptualization, C.S.B., F.B.H., and C.U.; methodology, C.S.B., F.B.H., D.B.C., A.C.L.P., and C.U.; software, C.S.B., C.U.; validation, C.S.B., C.U.; formal analysis, C.S.B., D.B.C., and A.C.L.P.; investigation, C.S.B., C.U.; resources, C.S.B., C.U.; data curation, C.S.B., D.B.C., A.C.L.P., and C.U.; writing—original draft preparation, C.S.B.; writing—review and editing, J.L.O.L., D.B.C., A.C.L.P., M. P. N., E. C. T. M., E.C.T.M., F.H.M., E.R.B., L.A.T., E.A.J., F.B.H., C.U., and C.S.B.; visualization, J.L.O.L., D.B.C., A.C.L.P., M. P. N., E. C. T. M., E.C.T.M., F.H.M., E.R.B., L.A.T., E.A.J., F.B.H., C.U., and C.S.B.; supervision, C.S.B.; project administration, C.S.B., C.U.; funding acquisition, C.S.B., C.U. All authors have read and agreed to the published version of the manuscript.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo under award numbers 2015/13096-1, 2016/13115-9, and 2018/16909-1, the Conselho Nacional de Desenvolvimento Científico e Tecnológico under award numbers 406609/2015-2, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior under award numbers 88887.388143/2019-00 and 03085/2015-0, and Oregon Health & Science University Fellowship for Diversity in Research.
Institutional Review Board Statement
All subjects signed informed consent forms approved by the University of Sao Paulo Ethical Committee (School of Physical Education and Sport – ref. 2011/12), registered at the Clinical Trial Registration: Brazilian Clinical Trials Registry (RBR-83VB6B) and UTN-U1111-1215-9956.
Informed Consent Statement
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Data Availability Statement
Data available upon request.
Acknowledgments
We would like to thank the participants from the Movement Disorders Clinic, School of Medicine, University of Sao Paulo, for their commitment to the study, Eden Marcos Braga de Oliveira who helped with technical support, Martina Mancini for the intellectual support, and FAPESP, CNPq, CAPES, and OFDIR.
Conflicts of Interest
The authors declare no conflicts of interest, except FBH. FBH has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. FBH also consultants with Biogen, Neuropore, Sanofi, Adamus, Abbott, and Takeda. This potential individual conflict has been reviewed and managed by Oregon Health & Science University.
References
- Amboni, M.; Stocchi, F.; Abbruzzese, G.; Morgante, L.; Onofrj, M.; Ruggieri, S.; Tinazzi, M.; Zappia, M.; Attar, M.; Colombo, D.; et al. Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study. Parkinsonism & related disorders. 2015, 21, 644–649. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Movement disorders : official journal of the Movement Disorder Society. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Moore, O.; Peretz, C.; Giladi, N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Movement disorders : official journal of the Movement Disorder Society. 2007, 22, 2192–2195. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: moving forward on a mysterious clinical phenomenon. The Lancet. Neurology. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.L. Balance control during gait initiation: State-of-the-art and research perspectives. World journal of orthopedics. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Schlenstedt, C.; Mancini, M.; Nutt, J.; Hiller, A.P.; Maetzler, W.; Deuschl, G.; Horak, F. Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson's Disease? Front Aging Neurosci. 2018, 10, 36. [Google Scholar] [CrossRef]
- Lira, J.L.O.; Ugrinowitsch, C.; Coelho, D.B.; Teixeira, L.A.; de Lima-Pardini, A.C.; Magalhaes, F.H.; Barbosa, E.R.; Horak, F.B.; Silva-Batista, C. Loss of presynaptic inhibition for step initiation in parkinsonian individuals with freezing of gait. J Physiol. 2020, 598, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Experimental brain research. 2007, 179, 29–42. [Google Scholar] [CrossRef]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Recovery from Multiple APAs Delays Gait Initiation in Parkinson's Disease. Front Hum Neurosci. 2017, 11, 60. [Google Scholar] [CrossRef]
- Lira, J.L.O.; Ugrinowitsch, C.; Coelho, D.B.; Teixeira, L.A.; de Lima-Pardini, A.C.; Magalhaes, F.H.; Barbosa, E.R.; Horak, F.B.; Silva-Batista, C. Reply from Jumes Leopoldino Oliveira Lira, Carlos Ugrinowitsch, Daniel Boari Coelho, Luis Augusto Teixeira, Andrea Cristina de Lima-Pardini, Fernando Henrique Magalhaes, Egberto Reis Barbosa, Fay B. Horak, and Carla Silva-Batista. J Physiol. 2022, 600, 421–422. [Google Scholar] [CrossRef]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Nutt, J.G.; Fair, D.A.; Horak, F.B. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013, 136, 2405–2418. [Google Scholar] [CrossRef] [PubMed]
- Filho, S.S.; Coelho, D.B.; Ugrinowitsch, C.; de Souza, C.R.; Magalhães, F.H.; de Lima-Pardini, A.C.; de Oliveira É, M.B.; Mattos, E.; Teixeira, L.A.; Silva-Batista, C. Age-Related Changes in Presynaptic Inhibition During Gait Initiation. The journals of gerontology. Series A, Biological sciences and medical sciences. 2021, 76, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Capaday, C.; Lavoie, B.A.; Comeau, F. Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Canadian journal of physiology and pharmacology. 1995, 73, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.; Meunier, S.; Pierrot-Deseilligny, E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988, 111, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Rudomin, P.; Schmidt, R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental brain research. 1999, 129, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. The Journal of physiology. 1987, 389, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Frigon, A.; Gossard, J.P. Independent control of presynaptic inhibition by reticulospinal and sensory inputs at rest and during rhythmic activities in the cat. J Neurosci. 2013, 33, 8055–8067. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.J.; Croce, K.R.; Huang, Z.J.; Abbott, L.F.; Jessell, T.M.; Azim, E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature. 2014, 509, 43–48. [Google Scholar] [CrossRef]
- Baudry, S.; Duchateau, J. Age-related influence of vision and proprioception on Ia presynaptic inhibition in soleus muscle during upright stance. The Journal of physiology. 2012, 590, 5541–5554. [Google Scholar] [CrossRef]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Carpenter, S.D.; Fair, D.A.; Nutt, J.G.; Horak, F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014, 9, e100291. [Google Scholar] [CrossRef]
- Sinnamon, H.M.; Jassen, A.K.; Vita, L.A. Brainstem regions with neuronal activity patterns correlated with priming of locomotor stepping in the anesthetized rat. Neuroscience. 2000, 99, 77–91. [Google Scholar] [CrossRef]
- Gurfinkel, V.S.; Lipshits, M.I.; Lestienne, F.G. Anticipatory neck muscle activity associated with rapid arm movements. Neurosci Lett. 1988, 94, 94,104–108. [Google Scholar] [CrossRef]
- Viallet, F.; Massion, J.; Massarino, R.; Khalil, R. Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res. 1992, 88, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Skinner, R.D. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain research. 1987, 411, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, R.; Kojima, S.; Edama, M.; Onishi, H. Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals. Brain Sci. 2020, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Drew, T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004, 92, 2217–2238. [Google Scholar] [CrossRef] [PubMed]
- de Lima-Pardini, A.C.; Coelho, D.B.; Nucci, M.P.; Boffino, C.C.; Batista, A.X.; de Azevedo Neto, R.M.; Silva-Batista, C.; Barbosa, E.R.; Cohen, R.G.; Horak, F.B.; et al. Brain networks associated with anticipatory postural adjustments in Parkinson's disease patients with freezing of gait. Neuroimage Clin. 2020, 28, 102461. [Google Scholar] [CrossRef]
- de Lima-Pardini, A.C.; de Azevedo Neto, R.M.; Coelho, D.B.; Boffino, C.C.; Shergill, S.S.; de Oliveira Souza, C.; Brant, R.; Barbosa, E.R.; Cardoso, E.F.; Teixeira, L.A.; et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Scientific reports. 2017, 7, 43088. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Yano, B.; Martini, D.N.; Horak, F.B.; de Lima-Pardini, A.; Almeida, F.; Santana, V.P.; Lima, D.; Batista, A.X.; Marquesini, R.; Lira, J.; et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Movement disorders : official journal of the Movement Disorder Society. 2021, 36, 152–163. [Google Scholar] [CrossRef]
- Silva-Batista, C.; de Lima-Pardini, A.C.; Nucci, M.P.; Coelho, D.B.; Batista, A.; Piemonte, M.E.P.; Barbosa, E.R.; Teixeira, L.A.; Corcos, D.M.; Amaro, E., Jr.; et al. A Randomized, Controlled Trial of Exercise for Parkinsonian Individuals With Freezing of Gait. Movement disorders : official journal of the Movement Disorder Society. 2020, 35, 1607–1617. [Google Scholar] [CrossRef]
- Moreira-Neto, A.; Ugrinowitsch, C.; Coelho, D.B.; de Lima-Pardini, A.C.; Barbosa, E.R.; Teixeira, L.A.; Amaro, E., Jr.; Horak, F.B.; Mancini, M.; Nucci, M.P.; et al. Freezing of gait, gait initiation, and gait automaticity share a similar neural substrate in Parkinson's disease. Hum Mov Sci. 2022, 86, 103018. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Matar, E.; Ward, P.B.; Bolitho, S.J.; Gilat, M.; Pearson, M.; Naismith, S.L.; Lewis, S.J. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain : a journal of neurology. 2013, 136, 1204–1215. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Rochester, L.; Herman, T.; Vandenberghe, W.; Emil, G.E.; Thomaes, T.; Giladi, N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait & posture. 2009, 30, 459–463. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Seto, E.; Sela, G.; McIlroy, W.E.; Black, S.E.; Staines, W.R.; Bronskill, M.J.; McIntosh, A.R.; Graham, S.J. Quantifying head motion associated with motor tasks used in fMRI. NeuroImage. 2001, 14, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Elton, R. UPDRS Program Members. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M. Calne DB, editors. Recent developments in Parkinson’s disease, Vol. 2. Florham Park, NJ: Macmillan Healthcare Information. 1987, p.153–163, 293–304.
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement disorders official journal of the Movement Disorder Society. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Troyer, A.K.; Leach, L.; Strauss, E. Aging and response inhibition: Normative data for the Victoria Stroop Test. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006, 13, 20–35. [Google Scholar] [CrossRef]
- Mancini, M.; Smulders, K.; Cohen, R.G.; Horak, F.B.; Giladi, N.; Nutt, J.G. The clinical significance of freezing while turning in Parkinson's disease. Neuroscience. 2017, 343, 222–228. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Mazieres, L.; Morin, C.; Nielsen, J.; Pierrot-Deseilligny, E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Experimental brain research. 1990, 81, 35–45. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Jespersen, B.; Nielsen, J. Reciprocal Ia inhibition between ankle flexors and extensors in man. The Journal of physiology. 1987, 389, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Patikas, D.A.; Kotzamanidis, C.; Robertson, C.T.; Koceja, D.M. The effect of the ankle joint angle in the level of soleus Ia afferent presynaptic inhibition. Electromyography and clinical neurophysiology. 2004, 44, 503–511. [Google Scholar] [PubMed]
- Magalhaes, F.H.; Elias, L.A.; da Silva, C.R.; de Lima, F.F.; de Toledo, D.R.; Kohn, A.F. D1 and D2 Inhibitions of the Soleus H-Reflex Are Differentially Modulated during Plantarflexion Force and Position Tasks. PloS one. 2015, 10, e0143862. [Google Scholar] [CrossRef] [PubMed]
- Silva-Batista, C.; Mattos, E.C.; Corcos, D.M.; Wilson, J.M.; Heckman, C.J.; Kanegusuku, H.; Piemonte, M.E.; Tulio de Mello, M.; Forjaz, C.; Roschel, H.; et al. Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson's disease. J Appl Physiol (1985). 2017, 122, 1–10. [Google Scholar] [CrossRef]
- Iles, J.F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. The Journal of physiology. 1996, 491, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Earles, D.; Vardaxis, V.; Koceja, D. Regulation of motor output between young and elderly subjects. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2001, 112, 1273–1279. [Google Scholar] [CrossRef]
- Geertsen, S.S.; Lundbye-Jensen, J.; Nielsen, J.B. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. Journal of applied physiology. 2008, 105, 915–922. [Google Scholar] [CrossRef]
- Knikou, M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008, 171, 1–12. [Google Scholar] [CrossRef]
- Baudry, S.; Lecoeuvre, G.; Duchateau, J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. Journal of applied physiology. 2012, 112, 296–304. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Yuan, Y.; Zhang, L.; Ding, J.; Wang, J.; Zhang, J.; Zhang, K.; Wang, J. Alterations of functional and structural connectivity of freezing of gait in Parkinson's disease. Journal of neurology. 2016, 263, 1583–1592. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Fletcher, P.C.; Henson, R.N.; Worsley, K.J.; Brett, M.; Nichols, T.E. Guidelines for reporting an fMRI study. NeuroImage. 2008, 40, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Human brain mapping. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Buschbacher, R.M. Normal range for H-reflex recording from the calf muscles. American journal of physical medicine & rehabilitation. 1999, 78, S75–S79. [Google Scholar] [CrossRef]
- Morita, H.; Shindo, M.; Yanagawa, S.; Yoshida, T.; Momoi, H.; Yanagisawa, N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Experimental brain research. 1995, 104, 167–170. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Carré, G.C.G.; García Márquez, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; Münkemüller, T.; McClean, C.J.; et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2012, 36, 27–46. [Google Scholar] [CrossRef]
- Lewis, S.J.; Barker, R.A. A pathophysiological model of freezing of gait in Parkinson's disease. Parkinsonism & related disorders. 2009, 15, 333–338. [Google Scholar] [CrossRef]
- Sherman, D.; Fuller, P.M.; Marcus, J.; Yu, J.; Zhang, P.; Chamberlin, N.L.; Saper, C.B.; Lu, J. Anatomical Location of the Mesencephalic Locomotor Region and Its Possible Role in Locomotion, Posture, Cataplexy, and Parkinsonism. Frontiers in neurology. 2015, 6, 140. [Google Scholar] [CrossRef]
- Snijders, A.H.; Leunissen, I.; Bakker, M.; Overeem, S.; Helmich, R.C.; Bloem, B.R.; Toni, I. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain a journal of neurology. 2011, 134, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Shik, M.L.J.B. Control of walking and running by means of electrical stimulation of the midbrain. Biophys 1966, 11, 659–666. [Google Scholar]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiological reviews. 1976, 56, 465–501. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. Journal of neural transmission 2016, 123, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Habaguchi, T.; Ohtinata-Sugimoto, J.; Saitoh, K.; Sakamoto, T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003, 119, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Dubuc, R.; Gossard, J.P. Dynamic sensorimotor interactions in locomotion. Physiological reviews. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.M.; Liu, J.; Hedlund, P.B.; Akay, T.; Pearson, K.G. Descending command systems for the initiation of locomotion in mammals. Brain research reviews. 2008, 57, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Kohyama, J.; Matsuyama, K.; Mori, S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience. 2001, 103, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Engberg, I.; Lundberg, A. Primary afferent depolarization evoked from the brain stem and the cerebellum. Archives italiennes de biologie. 1966, 104, 73–85. [Google Scholar]
- Jankowska, E.; Lund, S.; Lundberg, A.; Pompeiano, O. Inhibitory effects evoked through ventral reticulospinal pathways. Archives italiennes de biologie. 1968, 106, 124–140. [Google Scholar]
- Takakusaki, K.; Ohta, Y.; Mori, S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Experimental brain research 1989, 74, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.T.; Davidson, A.G.; Buford, J.A. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis). Neuroscience 2009, 163, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Riddle, C.N.; Edgley, S.A.; Baker, S.N. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. The Journal of neuroscience the official journal of the Society for Neuroscience. 2009, 29, 4993–4999. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Nutt, J.G.; Carlson-Kuhta, P.; Stephens, M.; Horak, F.B. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Experimental neurology. 2009, 215, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Kohyama, J.; Matsuyama, K. Medullary reticulospinal tract mediating a generalized motor inhibition in cats: III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience. 2003, 121, 731–746. [Google Scholar] [CrossRef]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Movement disorders official journal of the Movement Disorder Society 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Lundberg, A.; Voorhoeve, P. Effects from the pyramidal tract on spinal reflex arcs. Acta physiologica Scandinavica 1962, 56, 201–219. [Google Scholar] [CrossRef]
- Iles, J.F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. The Journal of physiology 1996, 491, 197–207. [Google Scholar] [CrossRef]
- Meunier, S.; Pierrot-Deseilligny, E.J.E. Cortical control of presynaptic inhibition of Ia afferents in humans. Experimental brain research 1998, 119, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, S.; Spildooren, J.; Heremans, E.; Wenderoth, N.; Swinnen, S.P.; Vandenberghe, W.; Nieuwboer, A. The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cerebral cortex 2014, 24, 3154–3166. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Takahashi, M.; Noguchi, T.; Chiba, R.J.N.; Neuroscience, C. Neurophysiological mechanisms of gait disturbance in advanced Parkinson's disease patients. Neurol. Clin. Neurosci. 2022. [Google Scholar] [CrossRef]
- Lewis, S.J.; Shine, J.M. The Next Step: A Common Neural Mechanism for Freezing of Gait. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry 2016, 22, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Carpenter, S.D.; Fair, D.A.; Nutt, J.G.; Horak, F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PloS ONE 2014, 9, e100291. [Google Scholar] [CrossRef] [PubMed]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson's disease. Brain : a journal of neurology. 2000, 123, 1767–1783. [Google Scholar] [CrossRef]
- Zweig, R.M.; Jankel, W.R.; Hedreen, J.C.; Mayeux, R.; Price, D.L. The pedunculopontine nucleus in Parkinson's disease. Annals of neurology. 1989, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson's Disease. Movement disorders : official journal of the Movement Disorder Society. 2015, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wu, D.; Lin, C.; Cai, H.; Chen, L.; Cai, G.; Ye, Q.; Cai, G. Pedunculopontine Nucleus Deep Brain Stimulation Improves Gait Disorder in Parkinson's Disease: A Systematic Review and Meta-analysis. Neurochemical research. 2020, 45, 709–719. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Palmieri, M.G.; Galati, S.; Stanzione, P.; Peppe, A.; Tropepi, D.; Brusa, L.; Pisani, A.; Moschella, V.; Marciani, M.G.; et al. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson's disease patients. Journal of neural transmission (Vienna, Austria : 1996). 2008, 115, 731–735. [Google Scholar] [CrossRef]
- Streumer, J.; Selvaraj, A.K.; Kurt, E.; Bloem, B.R.; Esselink, R.A.J.; Bartels, R.; Georgiev, D.; Vinke, R.S. Does spinal cord stimulation improve gait in Parkinson's disease: A comprehensive review. Parkinsonism & related disorders. [CrossRef]
- Fonoff, E.T.; de Lima-Pardini, A.C.; Coelho, D.B.; Monaco, B.A.; Machado, B.; Pinto de Souza, C.; Dos Santos Ghilardi, M.G.; Hamani, C. Spinal Cord Stimulation for Freezing of Gait: From Bench to Bedside. Frontiers in neurology. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- de Lima-Pardini, A.C.; Coelho, D.B.; Souza, C.P.; Souza, C.O.; Ghilardi, M.; Garcia, T.; Voos, M.; Milosevic, M.; Hamani, C.; Teixeira, L.A.; et al. Effects of spinal cord stimulation on postural control in Parkinson's disease patients with freezing of gait. eLife. 2018, 7, 2–10. [Google Scholar] [CrossRef]
- Samotus, O.; Parrent, A.; Jog, M. Spinal Cord Stimulation Therapy for Gait Dysfunction in Advanced Parkinson's Disease Patients. Movement disorders : official journal of the Movement Disorder Society. 2018, 33, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Petersson, P.; Siesser, W.B.; Caron, M.G.; Nicolelis, M.A. Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science. 2009, 323, 1578–1582. [Google Scholar] [CrossRef]
- Yadav, A.P.; Nicolelis, M.A.L. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2017, 32, 820–832. [Google Scholar] [CrossRef]
- Fanselow, E.E.; Reid, A.P.; Nicolelis, M.A. Reduction of pentylenetetrazole-induced seizure activity in awake rats by seizure-triggered trigeminal nerve stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000, 20, 8160–8168. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, L.; Wu, M.V.; Ohara, P.T. GABA puts a stop to pain. Current drug targets. CNS and neurological disorders. 2004, 3, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Magoul, R.; Onteniente, B.; Geffard, M.; Calas, A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience. 1987, 20, 1001–1009. [Google Scholar] [CrossRef]
- Emborg, M.E.; Carbon, M.; Holden, J.E.; During, M.J.; Ma, Y.; Tang, C.; Moirano, J.; Fitzsimons, H.; Roitberg, B.Z.; Tuccar, E.; et al. Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007, 27, 501–509. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Mackie, M.; Hughes, D.I.; Maxwell, D.J.; Tillakaratne, N.J.; Todd, A.J. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience 2003, 119, 461–472. [Google Scholar] [CrossRef]
- Luo, J.; Kaplitt, M.G.; Fitzsimons, H.L.; Zuzga, D.S.; Liu, Y.; Oshinsky, M.L.; During, M.J. Subthalamic GAD gene therapy in a Parkinson's disease rat model. Science 2002, 298, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Silva-Batista, C.; Corcos, D.M.; Roschel, H.; Kanegusuku, H.; Gobbi, L.T.; Piemonte, M.E.; Mattos, E.C.; MT, D.E.M.; Forjaz, C.L.; Tricoli, V.; et al. Resistance Training with Instability for Patients with Parkinson's Disease. Med Sci Sports Exerc. 2016, 48, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.; Greenberg, A.; King, E.; McNames, J.; Holmstrom, L.; Horak, F.B.; Mancini, M. Alleviating Freezing of Gait using phase-dependent tactile biofeedback. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2016, 2016, 5841–5844. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Novak, V. Effect of step-synchronized vibration stimulation of soles on gait in Parkinson's disease: a pilot study. Journal of neuroengineering and rehabilitation. 2006, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Smulders, K.; Harker, G.; Stuart, S.; Nutt, J.G. Assessment of the ability of open- and closed-loop cueing to improve turning and freezing in people with Parkinson's disease. Scientific reports. 2018, 8, 12773. [Google Scholar] [CrossRef]
- Phuenpathom, W.; Panyakaew, P.; Vateekul, P.; Surangsrirat, D.; Hiransuthikul, A.; Bhidayasiri, R. Vibratory and plantar pressure stimulation: Steps to improve freezing of gait in Parkinson's disease. Parkinsonism & related disorders 2022, 105, 43–51. [Google Scholar] [CrossRef]
- Klaver, E.C.; van Vugt, J.P.P.; Bloem, B.R.; van Wezel, R.J.A.; Nonnekes, J.; Tjepkema-Cloostermans, M.C. Good vibrations: tactile cueing for freezing of gait in Parkinson's disease. Journal of neurology 2023. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Provitera, V.; Estraneo, A.; Selim, M.M.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Lanzillo, B.; Santoro, L. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain: a journal of neurology. 2008, 131, 1903–1911. [Google Scholar] [CrossRef]
- Souron, R.; Baudry, S.; Millet, G.Y.; Lapole, T. Vibration-induced depression in spinal loop excitability revisited. The Journal of physiology. 2019, 597, 5179–5193. [Google Scholar] [CrossRef]
- Gillies, J.D.; Lance, J.W.; Neilson, P.D.; Tassinari, C.A. Presynaptic inhibition of the monosynaptic reflex by vibration. The Journal of physiology. 1969, 205, 329–339. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Godaux, E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. The Journal of physiology. 1978, 285, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lapole, T.; Deroussen, F.; Pérot, C.; Petitjean, M. Acute effects of Achilles tendon vibration on soleus and tibialis anterior spinal and cortical excitability. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2012, 37, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Lapole, T.; Canon, F.; Pérot, C. Acute postural modulation of the soleus H-reflex after Achilles tendon vibration. Neuroscience letters. 2012, 523, 154–157. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011, 54, 2808–2821. [Google Scholar] [CrossRef]
- de Lima-Pardini, A.C.; de Azevedo Neto, R.M.; Coelho, D.B.; Boffino, C.C.; Shergill, S.S.; de Oliveira Souza, C.; Brant, R.; Barbosa, E.R.; Cardoso, E.F.; Teixeira, L.A.; et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci. Rep. 2017, 7, 43088. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).