Submitted:
19 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AAIL@MCM-48 composite
2.3. Characterization
2.4. Adsorption Isotherms
3. Results and Discussion
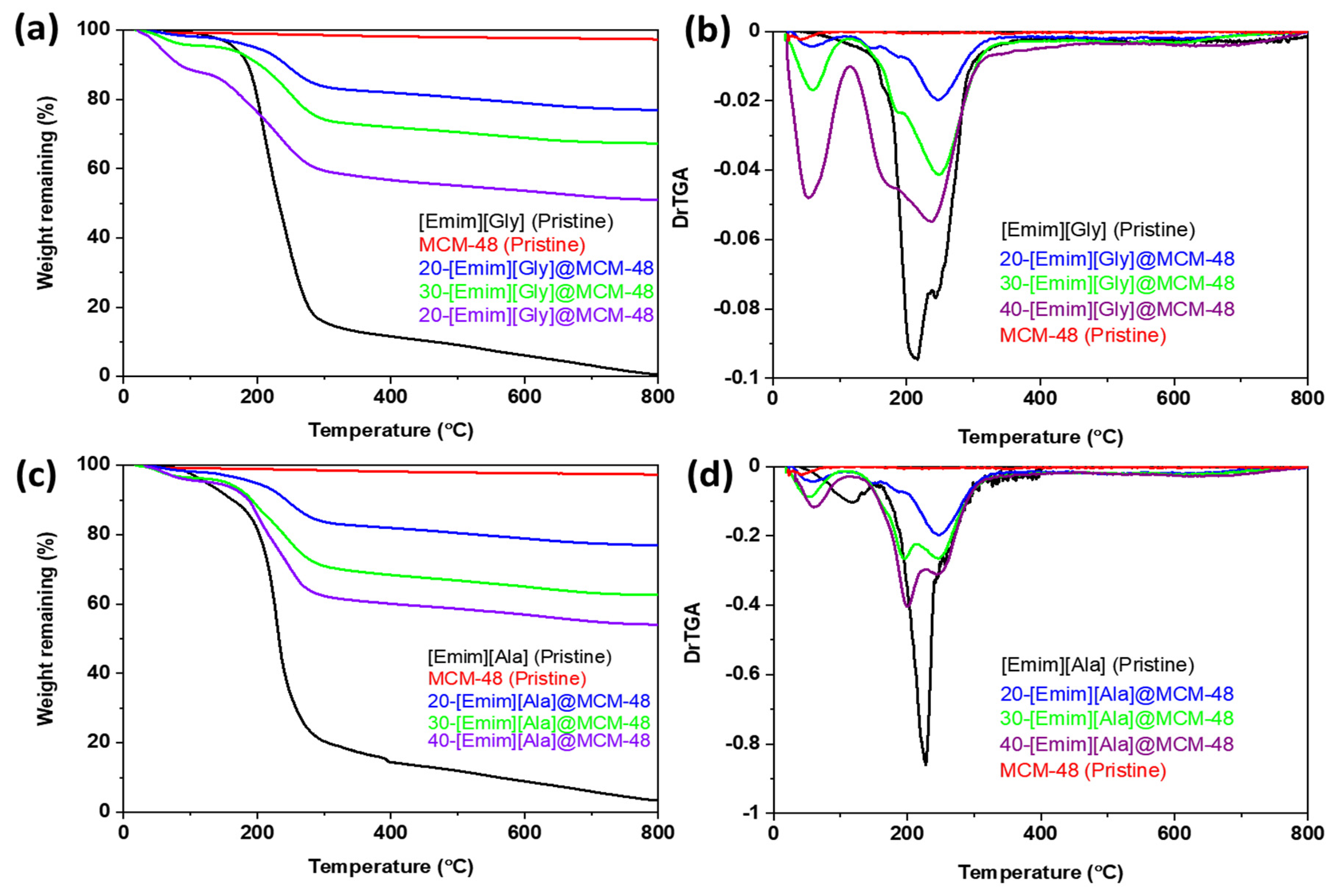
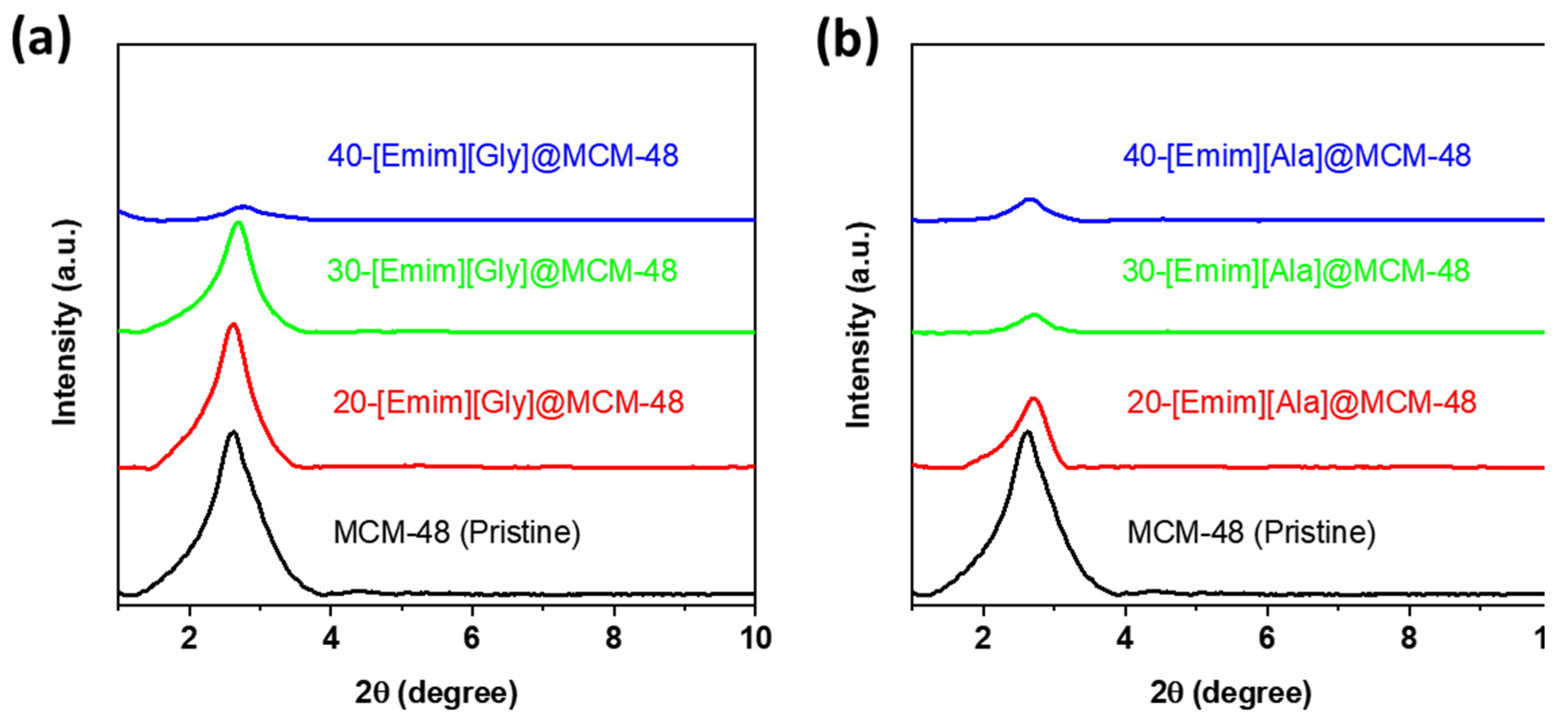
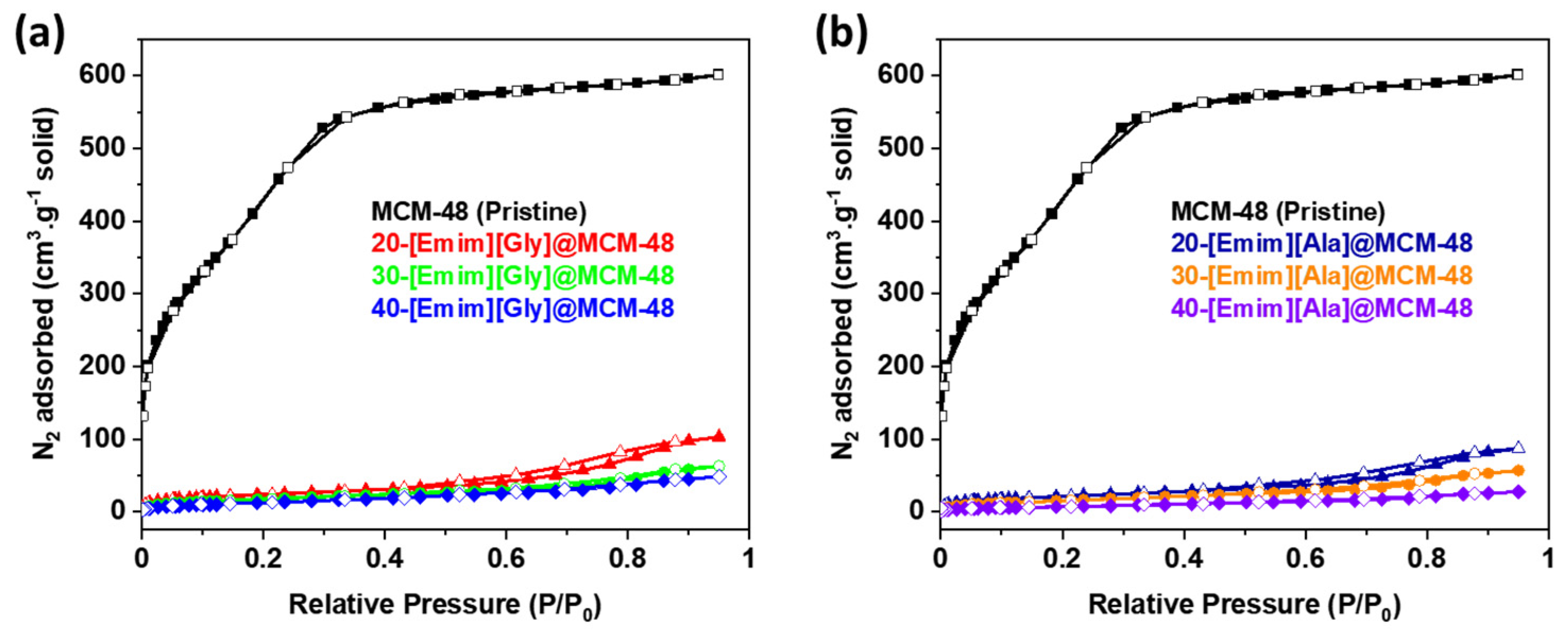
3.1. Characterizations of the AAILs-impregnated Sorbents
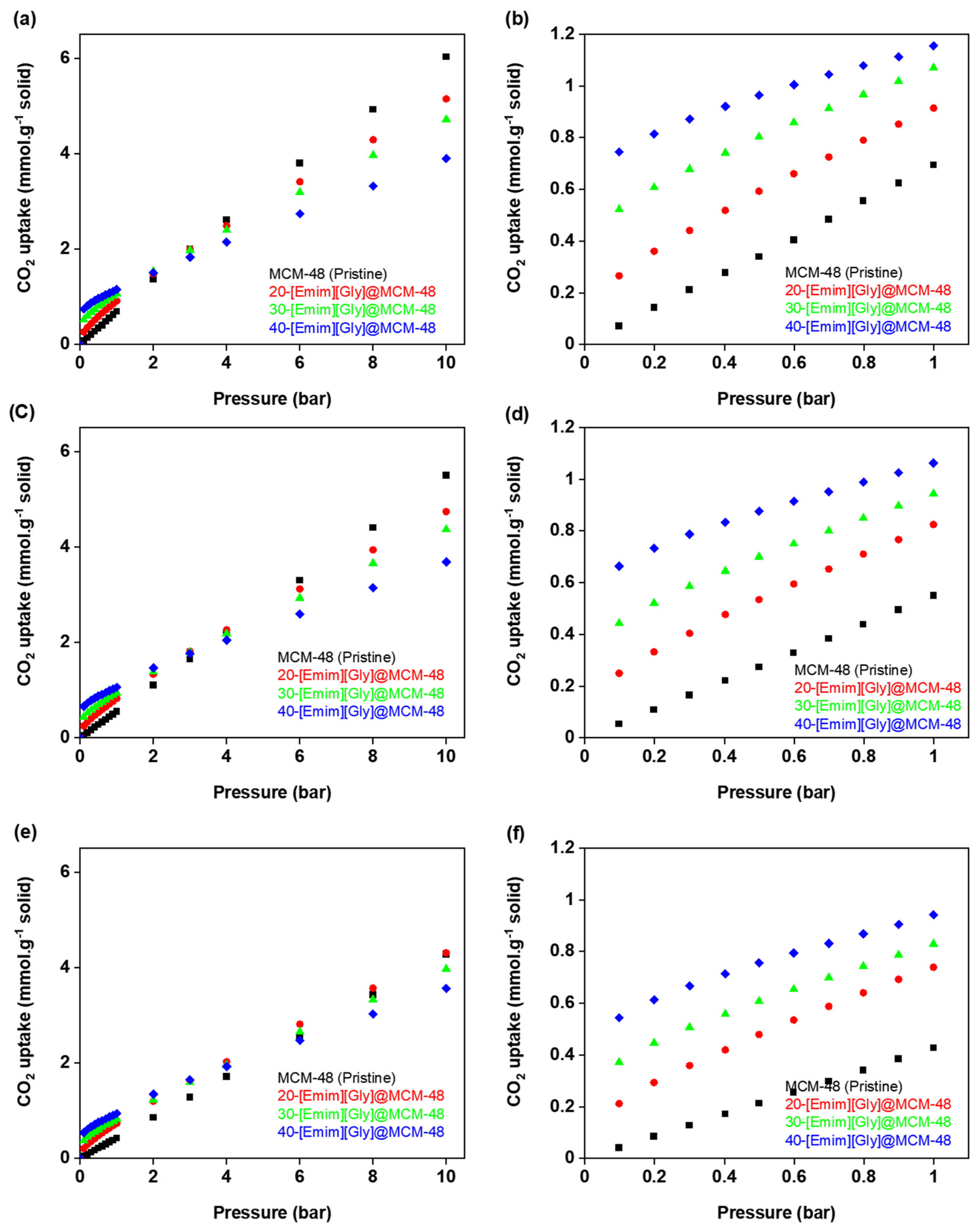
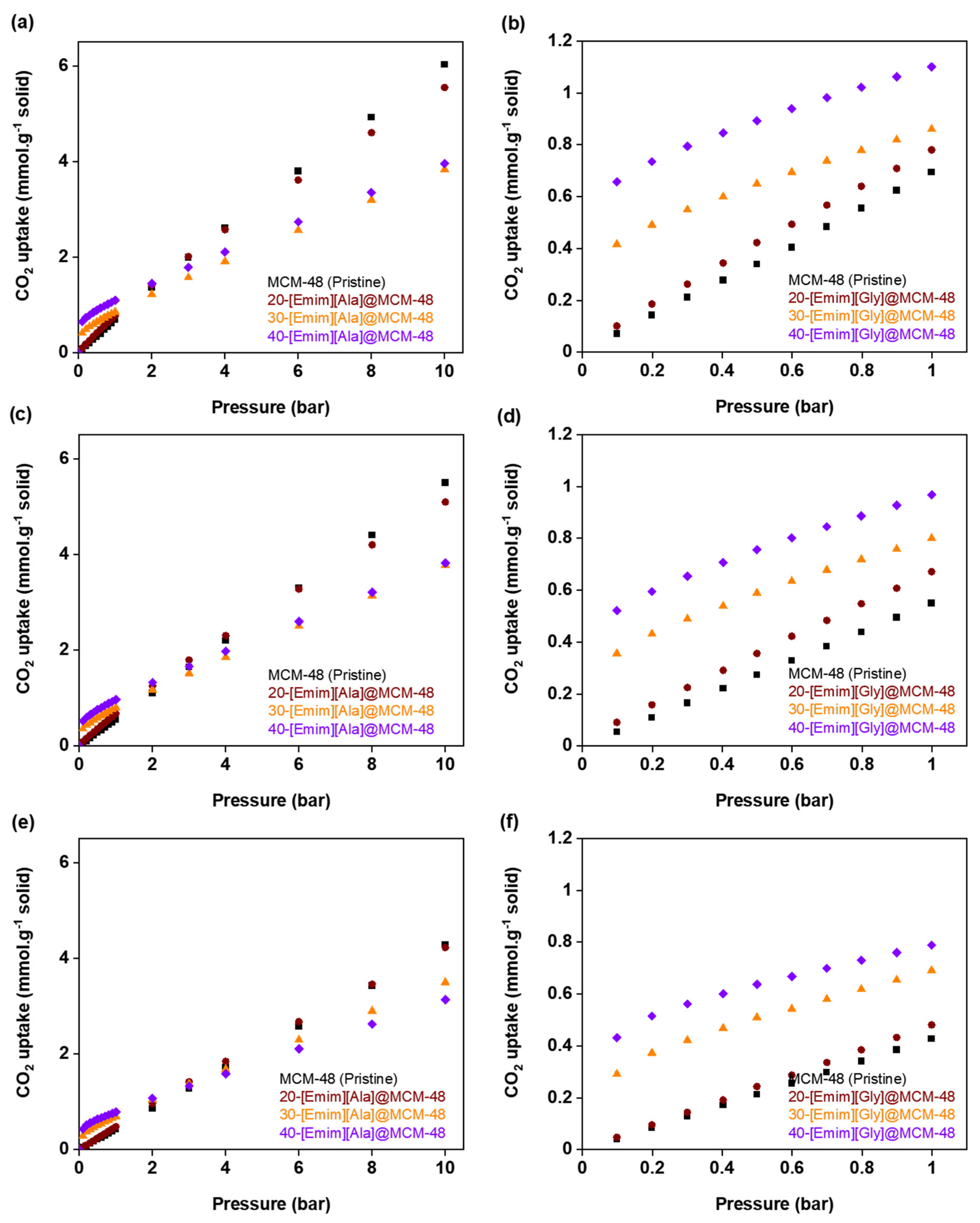
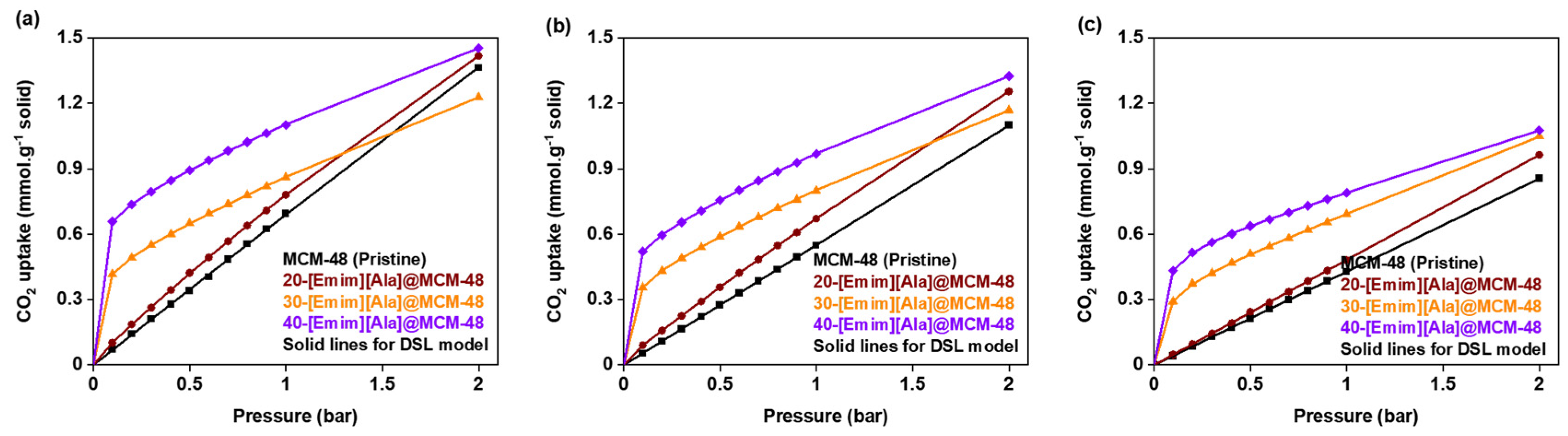
3.2. CO2 Adsorption Isotherms
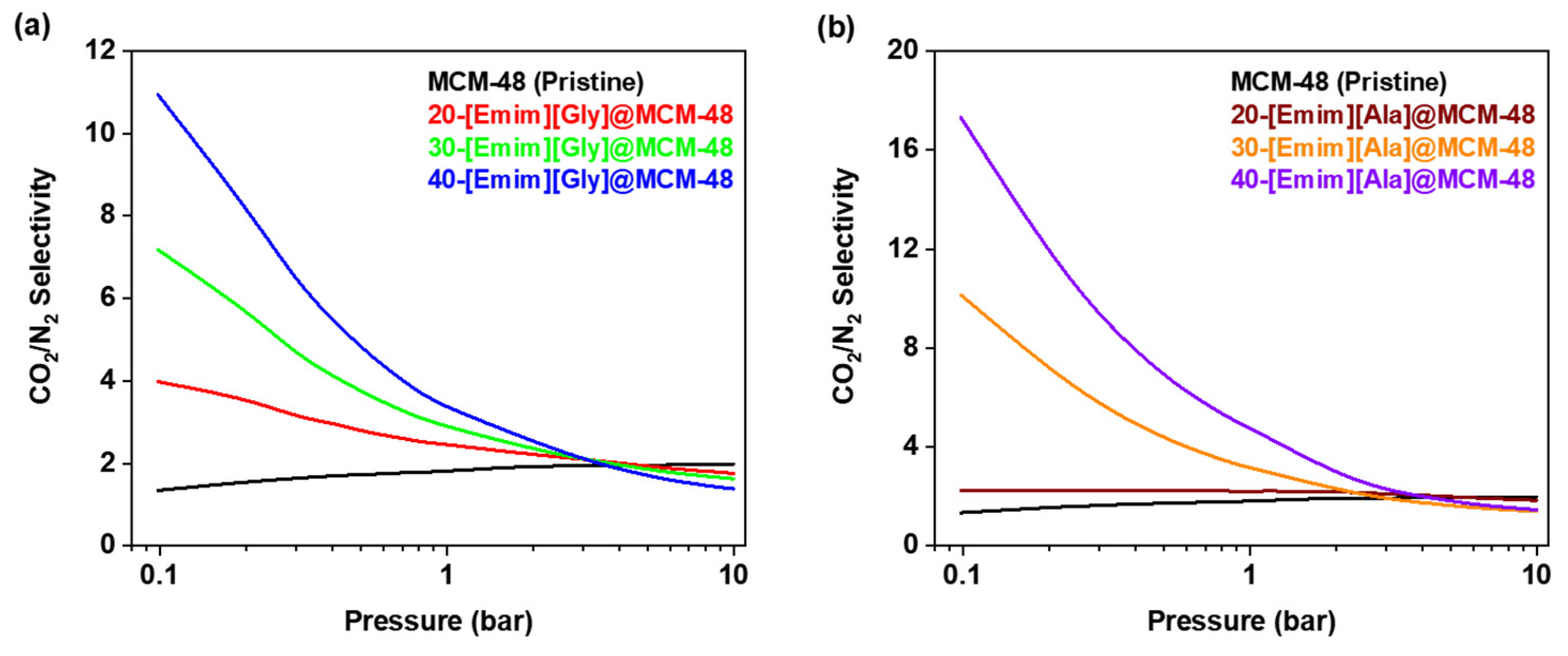
3.3. Selectivity for CO2/N2
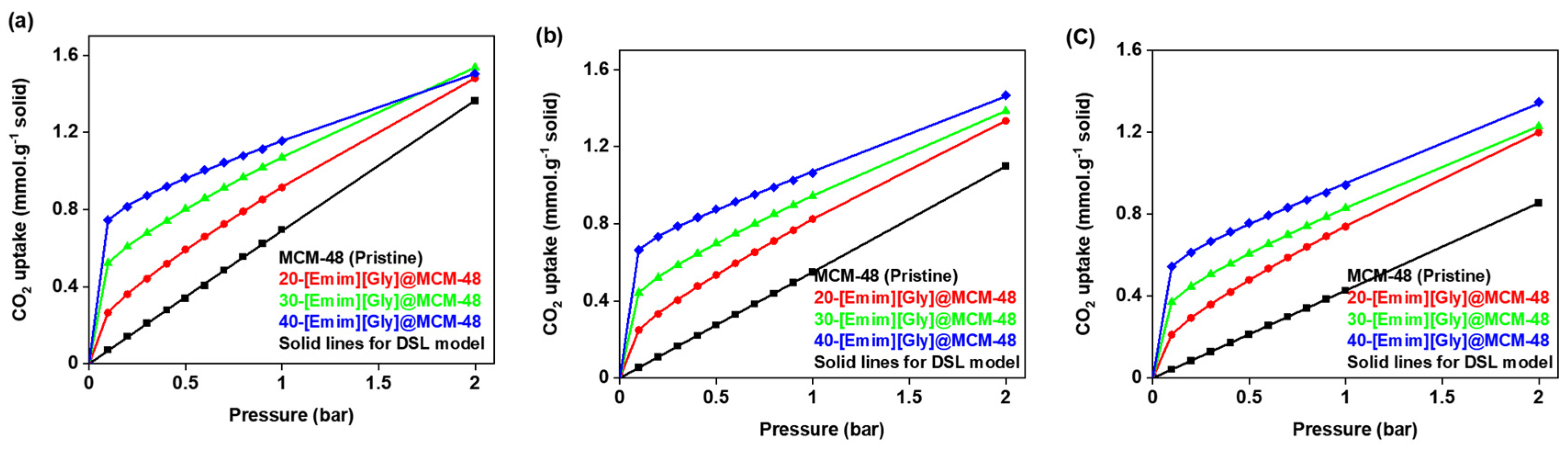
3.4. Equilibrium Isotherm Modeling
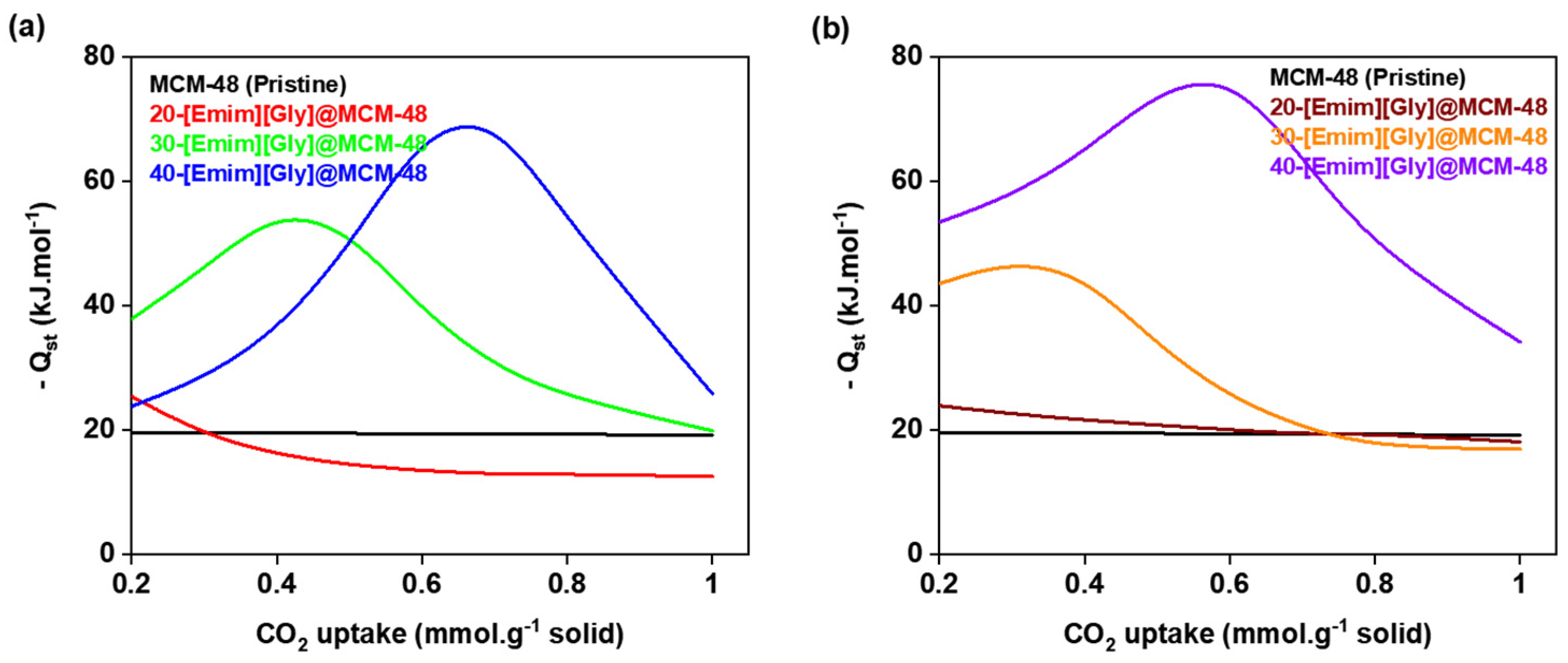
3.5. Isosteric heat of adsorption (Qst)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, S.; Ida, J.; Guliants, V. V.; Lin, J.Y.S. Tailoring Pore Properties of MCM-48 Silica for Selective Adsorption of CO2. J. Phys. Chem. B 2005, 109, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid Amine Sorbents for CO2 Capture by Chemical Adsorption: A Review. Petroleum 2017, 3, 37–50. [Google Scholar]
- Yue, M.B.; Chun, Y.; Cao, Y.; Dong, X.; Zhu, J.H. CO2 Capture by As-Prepared SBA-15 with an Occluded Organic Template. Adv. Funct. Mater. 2006, 16, 1717–1722. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T.; Chinn, D.; Munson, C.L. Amine-Grafted MCM-48 and Silica Xerogel as Superior Sorbents for Acidic Gas Removal from Natural Gas. Ind. Eng. Chem. Res. 2003, 42, 2427–2433. [Google Scholar] [CrossRef]
- Wei, J.; Liao, L.; Xiao, Y.; Zhang, P.; Shi, Y. Capture of Carbon Dioxide by Amine-Impregnated as-Synthesized MCM-41. J. Environ. Sci. 2010, 22, 1558–1563. [Google Scholar] [CrossRef]
- Chen, H.; Liang, Z.; Yang, X.; Zhang, Z.; Zhang, Z. Experimental Investigation of CO2 Capture Capacity: Exploring Mesoporous Silica SBA-15 Material Impregnated with Monoethanolamine and Diethanolamine. Energy and Fuels 2016, 30, 9554–9562. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy and Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of Hybrid Amine-Functionalized MCM-41 Sorbents for CO2 capture. Chem. Eng. J. 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Santos, S.C.G.; Pedrosa, A.M.G.; Souza, M.J.B.; Cecilia, J.A.; Rodríguez-Castellón, E. Carbon Dioxide Adsorption on Micro-Mesoporous Composite Materials of ZSM-12/MCM-48 Type: The Role of the Contents of Zeolite and Functionalized Amine. Mater. Res. Bull. 2015, 70, 663–672. [Google Scholar] [CrossRef]
- Wang, X.; Akhmedov, N.G.; Duan, Y.; Luebke, D.; Li, B. Immobilization of Amino Acid Ionic Liquids into Nanoporous Microspheres as Robust Sorbents for CO2 Capture. J. Mater. Chem. A 2013, 1, 2978–2982. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Enhancement of Post-Combustion CO2 Capture Capacity by Incorporation of Task-Specific Ionic Liquid into ZIF-8. Microporous Mesoporous Mater. 2021, 111580. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Incorporation of Amino Acid-Functionalized Ionic Liquids into Highly Porous MOF-177 to Improve the Post-Combustion CO2 Capture Capacity. Molecules 2023, 28, 7185. [Google Scholar] [CrossRef]
- Schumacher, K.; Ravikovitch, P.I.; Du Chesne, A.; Neimark, A. V.; Unger, K.K. Characterization of MCM-48 Materials. Langmuir 2000, 16, 4648–4654. [Google Scholar] [CrossRef]
- Xu, J.; Luan, Z.; He, H.; Zhou, W.; Kevan, L. A Reliable Synthesis of Cubic Mesoporous MCM-48 Molecular Sieve. Chem. Mater. 1998, 10, 3690–3698. [Google Scholar] [CrossRef]
- Thi Le, M.U.; Lee, S.Y.; Park, S.J. Preparation and Characterization of PEI-Loaded MCM-41 for CO2 Capture. Int. J. Hydrogen Energy 2014, 39, 12340–12346. [Google Scholar] [CrossRef]
- Boote, B.; Subramanian, H.; Ranjit, K.T. Rapid and Facile Synthesis of Siliceous MCM-48 Mesoporous Materials. Chem. Commun. 2007, 4543–4545. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Ren, J.; Wu, L.; Li, B.G. Preparation and CO2 Sorption/Desorption of N -(3-Aminopropyl)Aminoethyl Tributylphosphonium Amino Acid Salt Ionic Liquids Supported into Porous Silica Particles. Ind. Eng. Chem. Res. 2012, 51, 7901–7909. [Google Scholar] [CrossRef]
- Gurkan, B.E.; De La Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Rebelo, L.P.N.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Ionic Liquid-Impregnated Metal-Organic Frameworks for CO2/CH4 Separation. ACS Appl. Nano Mater. 2019, 2, 7933–7950. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance. ACS Appl. Mater. Interfaces 2016, 8, 30992–31005. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of Acetate-Based Ionic Liquids into a Zeolitic Imidazolate Framework (ZIF-8) as Efficient Sorbents for Carbon Dioxide Capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
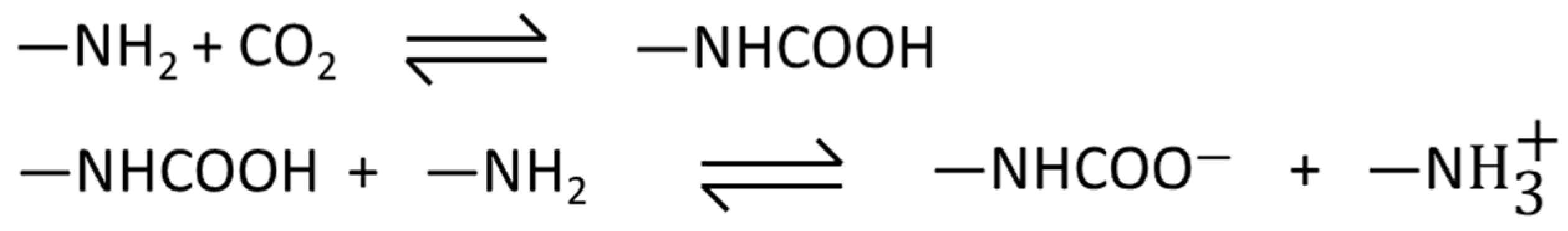
| Name | Abbreviation | Structure |
|---|---|---|
| 1-Ethyl-3-methylimidazolium | [Emim] | 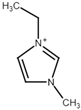 |
| Glycine | [Gly] | 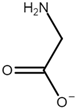 |
| Alanine | [Ala] | 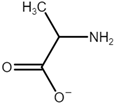 |
| Samples | SBET (m2·g-1) |
SLangmuir (m2·g-1) |
Pore Volume (cm3·g-1) |
|---|---|---|---|
| MCM-48 | 1,638 | 2,700 | 0.93 |
| 20-[Emim][Gly]@ MCM-48 | 87 | 287 | 0.16 |
| 30-[Emim][Gly]@ MCM-48 | 66 | 192 | 0.10 |
| 40-[Emim][Gly]@ MCM-48 | 50 | 155 | 0.07 |
| 20-[Emim][Ala]@ MCM-48 | 79 | 236 | 0.13 |
| 30-[Emim][Ala]@ MCM-48 | 60 | 196 | 0.09 |
| 40-[Emim][Ala]@ MCM-48 | 29 | 117 | 0.04 |
| Model Parameters | 20-[Emim][Gly]@MCM-48 | 30-[Emim][Gly]@MCM-48 | 40-[Emim][Gly]@MCM-48 | ||||||
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 7.277 | 0.239 | 6.204 | 0.520 | 0.450 | 0.390 | 0.780 | 0.691 | 0.572 |
| bA | 0.102 | 37.406 | 0.095 | 83.374 | 67.387 | 52.263 | 95.804 | 101.737 | 81.843 |
| NB | 0.248 | 7.722 | 0.209 | 5.896 | 7.090 | 7.041 | 7.017 | 731.290 | 531.196 |
| bB | 36.92 | 0.083 | 29.783 | 0.105 | 0.076 | 0.068 | 0.058 | 0.001 | 0.001 |
| R2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Model Parameters | 20-[Emim][Ala]@MCM-48 | 30-[Emim][Ala]@MCM-48 | 40-[Emim][Ala]@MCM-48 | ||||||
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 0.026 | 0.023 | 349.867 | 0.433 | 0.374 | 0.357 | 0.679 | 0.531 | 0.525 |
| bA | 53.033 | 10000 | 0.000 | 58.632 | 48.183 | 26.971 | 90.057 | 79.650 | 34.622 |
| NB | 8.784 | 12.152 | 579.453 | 4.647 | 4.692 | 227.845 | 3.908 | 3.753 | 94.954 |
| bB | 0.094 | 0.056 | 0.001 | 0.104 | 0.102 | 0.002 | 0.124 | 0.135 | 0.003 |
| R2 | 1.000 | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





