1. Introduction
Viral pathogenesis is a process of virus infection and multiplication, resulting in disease manifestation in an infected host. The viral pathogenesis is categorized into two types attributed to dissimilar mechanisms that cause disorders in the host. 1. The virus-induced cytopathogenesis occurs primarily due to virus multiplication in infected cells (1, 2). Viruses could induce either cell death, which leads to severe tissue injuries, or cell transformation for the formation of neoplasia. 2. On contrary with cytopathogenesis, immunopathogenesis is mainly mediated by aberrant immune responses to viruses in the host. Virus-induced immunopathogenesis could be further divided into two very distinct immune mechanisms. The vaccine-induced immunopathogenesis emerged in vaccinated persons immediately in a subsequent epidemic to encounter a natural virus infection (3, 4). Another distinctive immunopathogenesis caused by virus infections, designated as ‘cytokine storm’, revealed an overall increase in innate cell number, a concomitant reduction in T cell number and an early elevation in cytokine levels with high mortality of disease outcomes (5, 6).
Vaccination has long been recognised as a most effective measure to mitigate severity of epidemic as well as pandemic outbreaks of infectious diseases since Dr. Edward Jenner discovered a cowpox inoculation in 1797 (7). Following a smallpox eradication programme from World Health Organization (WHO), smallpox was officially declared eradicated globally in a human population in 1980 (8). And now, 25 vaccine introductions were reported by WHO in 2021, which was not including coronavirus disease 2019 (COVID-19) vaccine introductions. Efficacious vaccinations are not only able to elicit preventive immune responses against infections of pathogenic viruses but to alleviate pathogenic symptoms in vaccinated entities, inclusive of ‘cytokine storming pathogenesis’. If a vaccine-induced immunity does eliminate infection as well as multiplication of infectious viruses, virus-induced cytopathogenesis and ‘cytokine storming’ will be unlikely to occur in any of vaccinated entities. Moreover, the spread of contagious viruses in a human population would be halted by a herd immunity generated by effective vaccination coverage. What we couldn’t foresee very well in vaccination against pathogenic viruses is the vaccine-induced pathogenesis, which occurred in vaccinated entities following a subsequent encounter with natural virus infections.
The devastating consequence of 1918 pandemic influenza, which killed more people than those die in the World War 1, could be partly ascribed to ‘cytokine storming pathogenesis’ (6). This disastrous outcome has been interpreted well with the lack of an effective global health measure to mitigate the pandemic crisis, for example, deficit of effective global vaccination coverage. In 2009, a pandemic outbreak of a novel swine influenza continued to breakthrough defence borders in the global health system (9). The failure of the global health system was demonstrated unequivocally once again with another pandemic outbreak of COVID-19 in 2019 (10). Governments as well as pharmaceutical manufacturers were exploiting a shotgun strategy to rush into an emergent production of all kinds of vaccination materials and anti-viral agents with least restriction of safety concerns. The loss of life and economic crisis caused by pandemic outbreaks are recognised as an evidently factual consequence without disturbing voice or any of arguments from all aspects and relevant fields globally. In fact, we have lost a long-standing wining race against pathogenic viruses this time. We have been taken to a limit to cope with such a pandemic catastrophe with all potential measures and available materials without any further concern on safety issues since effective vaccination coverage was thought to outweigh the risk of a substandard safety for vaccination itself.
Here, we aim to discuss vaccine-associated pathogenesis concerning vaccination materials and strategies from the past to the recent events. These crucial subjects as in the following sections will be represented with a recent progress in a scientific way in detail as well as with clarity. We elucidate the developmental hurdles of each vaccination material as well as their proposed advantages with current concepts in the fields. Finally, we will discuss an obvious dilemma that those proposed advantages have confronted in real clinical trials to indicate a future prospect to circumvent the obstacle caused by vaccine-induced pathogenesis.
2. Live Attenuated Vaccines
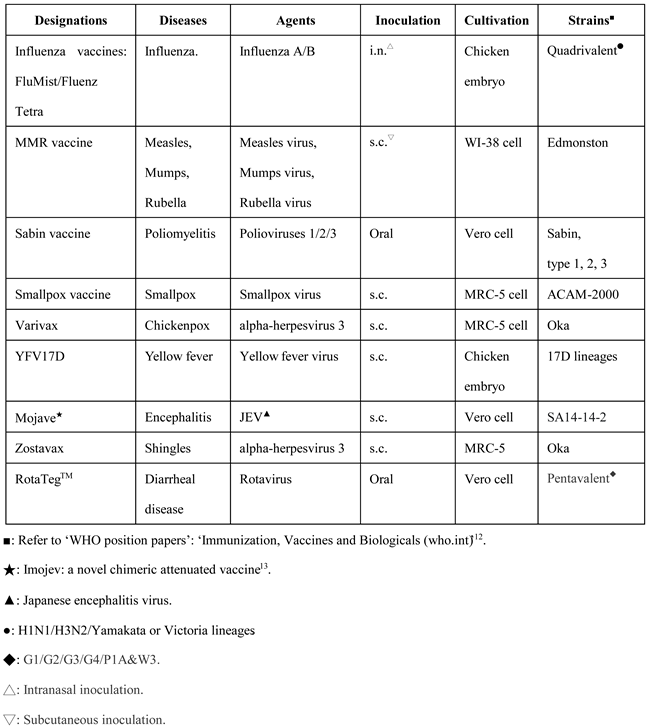
The first successful and widely used vaccine is a cowpox inoculation discovered by ‘Dr. Edward Jenner’ in 1796. Since then, there have been more live attenuated vaccines exploited for global vaccination coverage. At the moment, more than 9 types of live attenuated vaccines available worldwide. A list of live attenuated vaccines in current use as examples are presented in
Table 1 (11). These are successful attenuated ones to protect against all sorts of viral diseases, which were made by traditional measures or novel recombinant technologies.
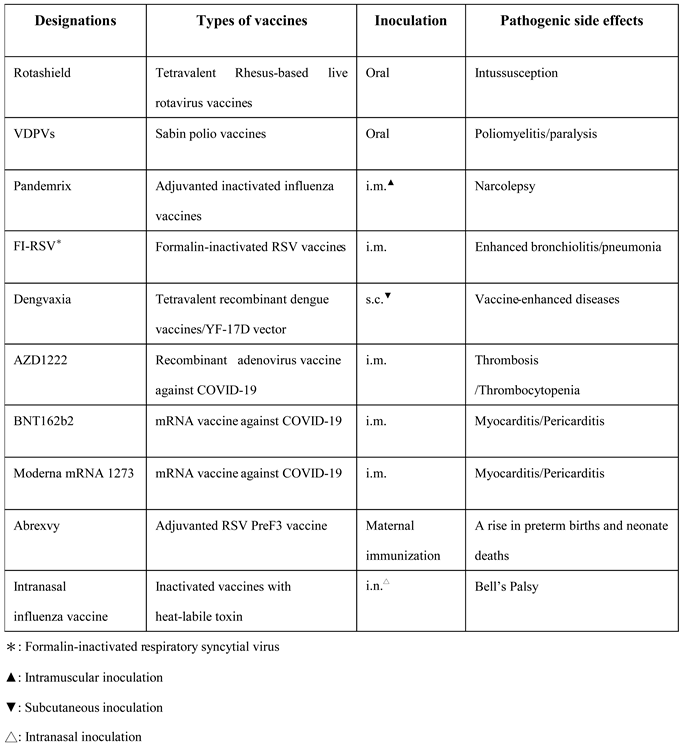
Nevertheless, live attenuated vaccines have caused several unpleasant vaccination-related incidents recently. In 1998, a clinical studies of a tetravalent rhesus-based live attenuated rotavirus vaccine, which was designated as ‘Rotashield’, induced an unpleasant pathogenesis ‘intussusception’ in United States of America (14). The Rotashield was withdrawn immediately from the market following the observation of an obvious pathogenic consequence. In 21st century, the continued reports of adverse effects have shed a dark shadow in the research field for the development of live attenuated vaccines. Multiple emergences of global circulating vaccine-derived polioviruses (VDPVs) released a warning signal to the global health system, resulting in the replacement of current vaccine strain with an inactivated option or in the development of safer attenuated ones (15, 16) (
Table 2). The lack of concern for pathogenesis has casted a dark shadow over the development of live attenuated vaccination. We urgently require a scientific approach to design an innocuous vaccination coverage for live attenuated options excluding a vaccine-induced pathogenesis.
Without any doubt, live attenuated vaccination could lead to exacerbated responses causing a devastating state of disorder. Moreover, the risk of reverting back into a wild-type or evolving as virulent strains should not be neglected in the vaccine development to select avirulent ones for a vaccination purpose, for example, VDPVs. But the innocuous selection from live attenuated options has become limited and elusive following unfortunate incidents of several vaccination programmes.
3. Inactivated Vaccines
Unlike live attenuated vaccine, the inactivated one reserves its immunogenicity without retaining its replicative capacity either in vitro or in vivo (17). In modern era, several chemical inactivation methods have been introduced to deprive pathogenic microorganisms of infectivity or toxicity derived from their virulent components (18). In fact, the earliest for the use of an inactivated vaccine could be traced back to historical records in ancient China, an invented approach to do the first mucosal immunisation, designated as ‘variolation’ (19).
From 1950’s to 1960’s, Dr. Jonas Salk and Dr. Albert Sabin have developed separate vaccines against poliomyelitis caused by polioviruses (20). Both of them raced for the one to be the main stream of national immunisation programme, later as a standard polio vaccine for global coverage. In 1955, an inactivated polio vaccine developed by Dr. Jonas Salk was successful in a large scale of clinical trial in United States. Unfortunately, there was an infamous contamination event to cause accidental infections in an immunisation campaign, resulting in the termination of the use of Salk vaccine. Following the success of Salk’s inactivated vaccine, Dr. Albert Sabin successfully developed an oral live attenuated one to prevail globally due to its easy-to-use and economical concerns in 1966. Therefore, the advantages of Sabin’s live attenuated oral vaccine soon replaced the Salk one as the most prevalent to prevent global poliomyelitis for decades. Until recently, the Salk vaccine has returned strongly since the global emergence of circulating VDPVs (15, 16). The combination vaccines have included an inactivated polio vaccine for children vaccination all over the world (21, 22).
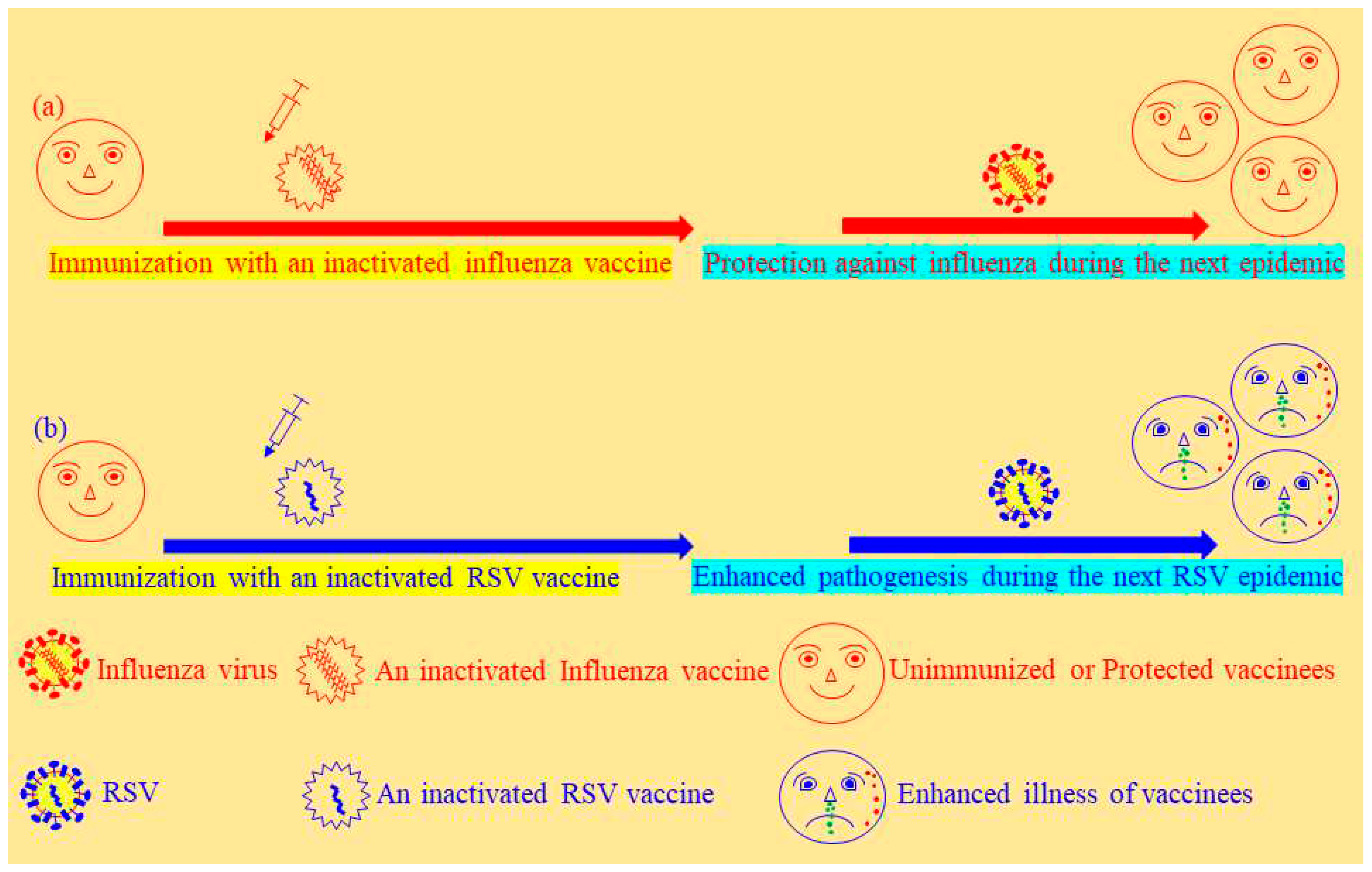
The majority of influenza vaccines in current use are actually made by chemical inactivation of quadrivalent strains of influenza viruses (23-26). Since 1973, the WHO global influenza surveillance and response system has conducted the selection of optimal influenza virus strains as candidate vaccines to confront seasonal epidemic threats annually. There are two key technologies used in the development of suitable candidate strains for vaccination, such as a classical reassortment and reverse genetics. Inactivated influenza vaccines have been very successful in the control of either an epidemic or a pandemic threat of influenza (27, 28) (
Figure 1a). Without exception, serious adverse effects of inactivated influenza vaccines continued to be reported from seasonal or pandemic vaccination programmes, such as Guillain–Barré syndrome and narcolepsy (29, 30). Furthermore, an infamous clinical trial of vaccination causing enhanced illness in young children revealed the limitation of inactivated vaccines to prevent the infection of respiratory syncytial virus (RSV) (3) to which the devastating consequence was represented with another virus alike in calves as well (31) (
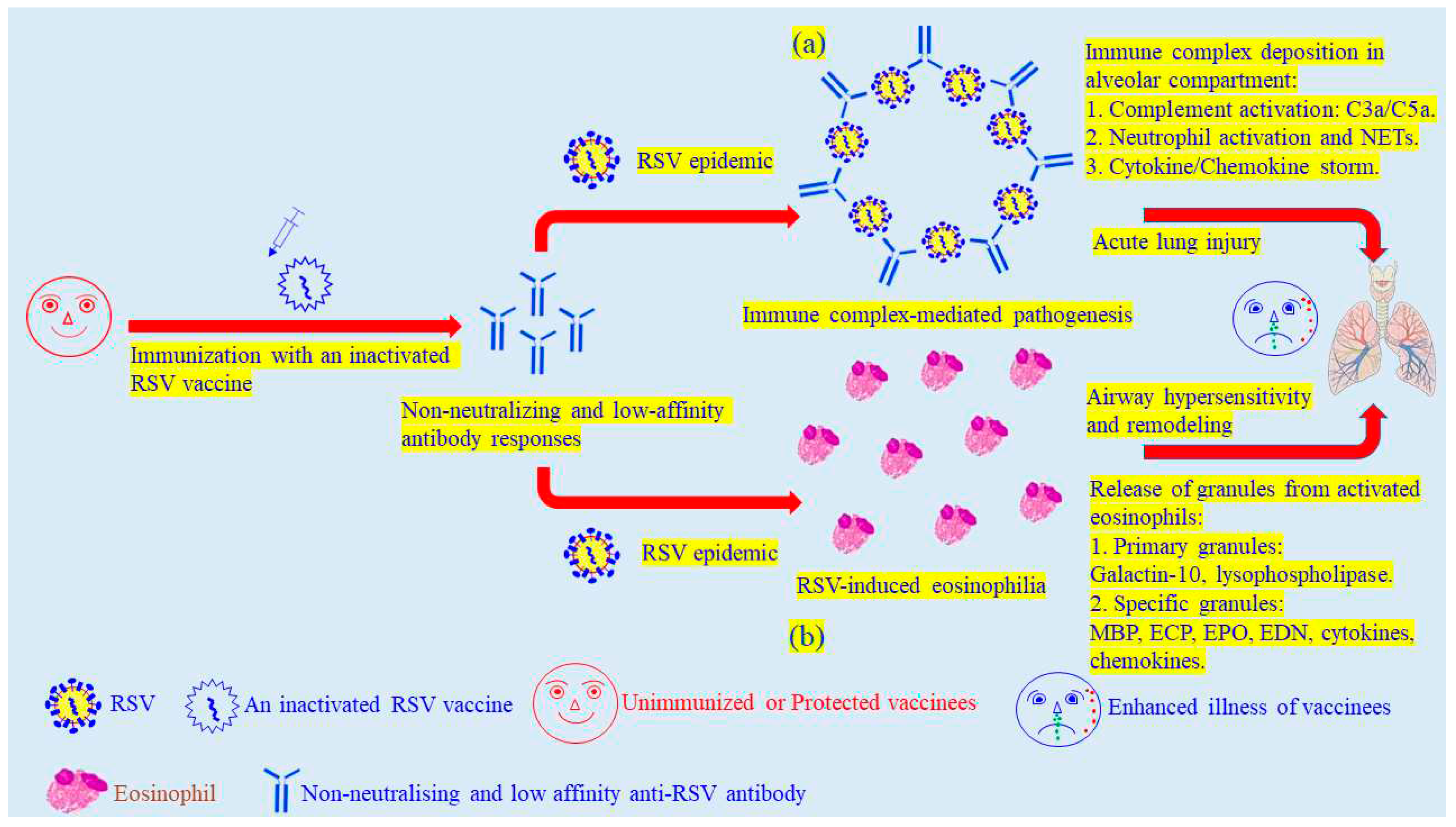
Figure 1b). The detailed mechanisms concerning the pathogenesis induced by an inactivated RSV vaccine are not well-clarified and currently debatable in the fields of immunopathology (See the section of conclusions and
Figure 2) (
Table 2). In certain circumstances, an inactivated vaccine has left too much to be desired to meet a standard requirement of more effective and safer vaccination in livestock, for instance, vaccination against bovine ephemeral fever virus and foot-and-mouth disease viruses (32-34) besides bovine respiratory syncytial virus (BRSV).
Even with magnificent safety records in vaccination against all sorts of viruses, inactivated vaccines provoked unpleasant side effects and enhanced disorders after encountering the same viruses during the next epidemic.
4. Recombinant Vector-Delivered Vaccines
A variety of bacterial or viral vectors have been exploited as vaccine-delivery systems to express protective antigens derived from diverse pathogenic viruses. There are many recombinant vector-delivered vaccines developed against various pathogenic viruses, like human adenovirus type 5 and 26 (Ad5, Ad26), chimpanzee adenovirus (ChAd) and yellow fever 17D (35-39). Successful examples for the development of recombinant vector vaccines are Ervebo (rVSVΔG-ZEBOV-GP), ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines. Ebola viruses continued to cause severe endemic viral diseases representing a serious challenge to public health systems across the continent of Africa (40). A recombinant vesicular stomatitis virus-Zaire Ebola vaccine designated as Ervebo was approved for the current use to prevent the disease caused by Zaire ebolavirus (41). The efficacy of this vaccine was supported by a randomized cluster (ring) vaccination research during the 2014–2016 outbreak in Guinea. 3,775 people in close contact with diagnosed cases of Ebola virus disease (contacts) and their close contacts (contacts of contacts) immediately received the injection of the vaccine to be free from Ebola virus disease 10 or more days following vaccination. The exploitation of low prevalent or chimpanzee adenoviruses as delivery recombinant vectors was very successful to protect against COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (37, 38).
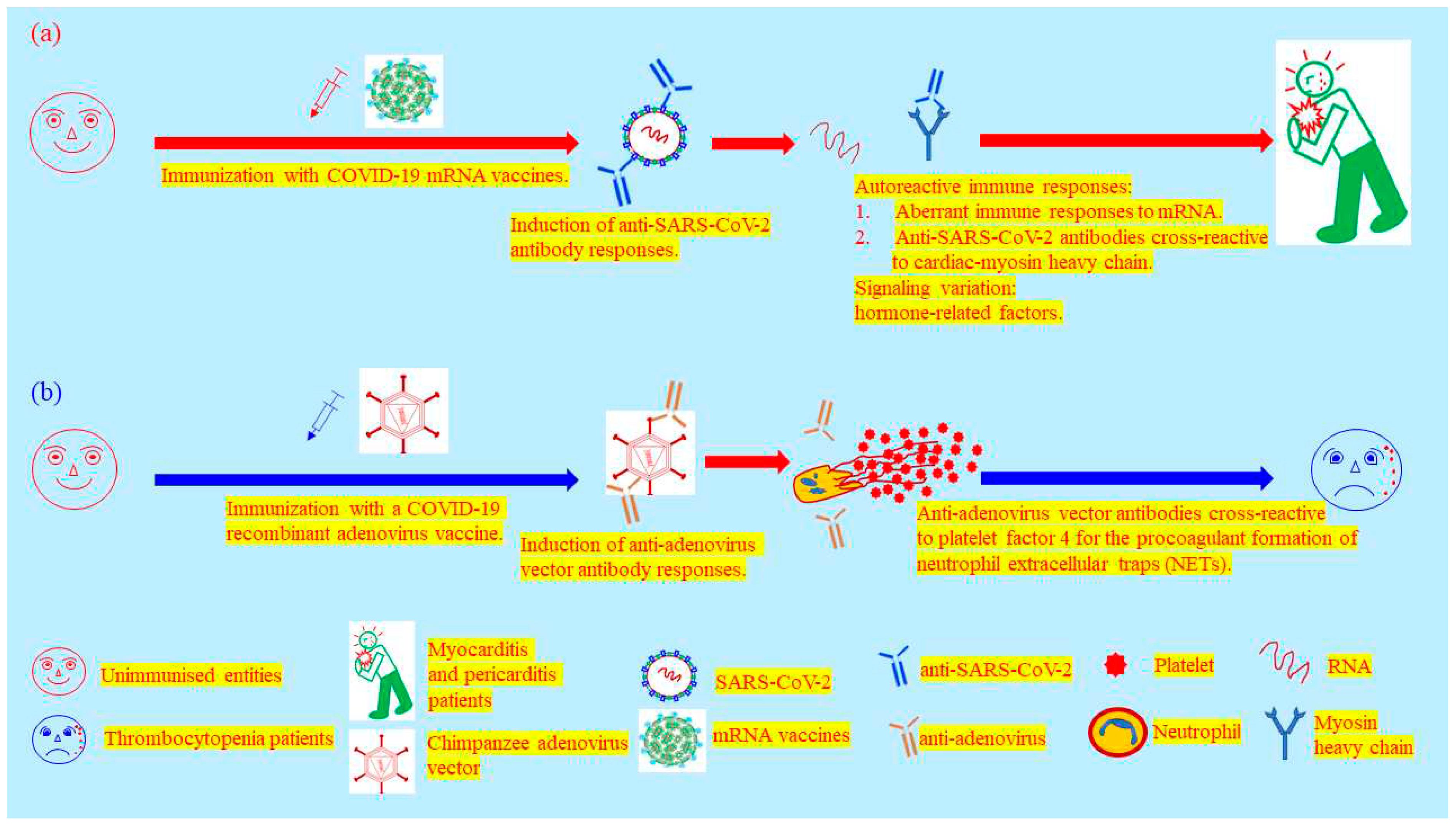
Alarmingly, recombinant vector-delivered vaccines have also failed in several clinical trials against various viral infections (35, 36, 39). Dengvaxia has recently been approved for use only in individuals 9 through 16 years of age with laboratory-confirmed previous dengue infection and living in endemic areas since this recombinant vector-delivered tetravalent vaccine sensitized seronegatives to vaccine-enhanced disease without regard of age (39). Once more, the protective coverage of a recombinant vector-delivered vaccine was restricted by endemic dengue viruses in a real clinical trial, seriously leading to lessen a herd immunity generated by vaccination. For the current status of COVID-19, recombinant adenoviruses have contributed a great deal of effort to halt the pandemic crisis (37, 38). Nevertheless, there was no exceptional status for this novel technology inducing a severe adverse effect. The ChAdOx1 nCoV-19 (AZD1222) induced anti-adenovirus vector antibodies cross-reactive to platelet factor 4 (PF4) to result in the development of immune thrombotic thrombocytopenia in which circulating PF4-reactive antibodies activate platelets as well as neutrophils for the procoagulant formation of neutrophil-extracellular traps (42) (
Figure 3b and
Table 2).
Pathogenic viruses have actually possessed unforeseen measures to divert vaccine-induced immune responses so as to thrive in a human population. The development of recombinant vector-delivered vaccines was stymied by the absence of ‘precision approach’ to tackle complicated pathogenic effects caused by live infectious viruses in vaccinated entities, which would be hard to circumvent even with modern sophisticated recombinant technologies (39, 43-45). In the lack of stringent safety standards, recombinant technologies possess a possibility to bring out the unexpected outcome of severe pathogenesis. In particular for a policy maker amid a serious pandemic crisis, they have no choice indeed but spare the triumphant victory for the microbial world.
5. Genetic Vaccination
Distinguished from recombinant subunit vaccines and recombinant vector-delivered vaccines, genetic vaccines consist of varied types of nucleic acids to express protective antigens from viruses, including plasmid DNA or mRNA molecules (46). Genetic vaccination could present MHC class I-restricted antigens to CD8 T lymphocytes via both endogenous and alternative routes of exogenous processing pathways. The same as live attenuated vaccines as well as recombinant vector-delivered vaccines, the genetic vaccination is able to induce effective cellular responses, in particular for virus-specific CD8 T lymphocyte responses (47). DNA vaccines have been exploited in current use for domesticated animals and wildlife for a while. Safety issues used to be the concern for the development of DNA vaccination owing to the possibility for extrachromosomal elements to integrate into genomes as well as for the production of anti-DNA antibodies. Furthermore, DNA vaccines were considered to be less effective at inducing humoral immune responses than other vaccination approaches (48, 49). The recent success in vaccination against COVID-19 pandemic has eliminated a dark shadow regarding the poor immunogenicity of a DNA plasmid vaccine, which encodes SARS-CoV-2 S protein and IgE signal peptide delivered intradermally by the needle-free PharmaJet Tropis device (50), reaching nearly 67% protective efficacy. Due to its less frequent use, adverse effects of this novel technology could be concealed in the corner without unveiling by few clinical trials.
The earliest publication regarding the use of mRNA vaccine is derived from an attempt to generate CD8 T lymphocyte responses (51). Since COVID-19 pandemic, mRNA vaccines have desperately been employed in several clinical trials preventing COVID-19 pandemic (52, 53). These mRNA vaccines have advantages in the generation of both cellular and humoral immune responses. The mRNA vaccines provided a little better efficacy to protect against COVID-19 than others in clinical trials, permitted urgently with a substandard safety requirement. But without privileged status, mRNA vaccines are the same as others in use to induce unwanted pathogenic effects in vaccinees, such as pericarditis and myocarditis (54) (
Table 2). A higher risk for causing pericarditis and myocarditis was confirmed in those who received mRNA COVID-19 vaccinations compared with unvaccinated individuals in the absence of SARS-CoV-2 infection. There are three proposed mechanisms mediating mRNA vaccine-induced myocarditis. 1. The exploitation of mRNA vaccines could potentially elicit aberrant immune responses to RNA molecule itself albeit modified nucleoside building blocks deployed in the design of mRNA vaccines. 2. The induction of stronger anti-spike protein antibody responses could be potentially cross-reactive to cardiac myosin heavy chain. 3.The signaling variations mediated by hormone-related factors may explain the sexual differences of risk ratio in pathophysiology (55) (
Figure 3a). The pathogenesis caused by genetic vaccines is far from expectation of many vaccinologists, which were considered as uppermost options on the shelf, stockpiled to be a final defence line of public health.
6. Adjuvanted Vaccination and Subunit Vaccines
Adjuvants are either natural or synthetic substances to enhance immunogenicity of all sorts of antigens for vaccination. Subunit vaccines are classified into several types with regard to their biochemical components as well as chemical structures (56, 57). Poor immunogenicity of microbial components generally requires added adjuvants to improve their immunogenicity. Adjuvanted vaccinations have been successful to improve protective efficacy for influenza virus, hepatitis B virus (HBV), human papilloma virus (HPV) and Varicella-Zoster virus (VZV). Even with a nanoparticle vaccine that generates an effective immune response without exogenous adjuvanticity (58), it has doubtless to be integrated with an exogenous adjuvant in vaccination regimen to increase its protective efficacy.
The aluminum hydroxide adjuvant is the first to be recommended for human use to vaccinate against microbial diseases. It has been renovated and modernized to be integrated with versatile added-in materials for formulation with a variety of antigens against diverse pathogenic microorganisms. Now, there are more renovated options licensed available for varied vaccine components to prevent all sorts of epidemic or pandemic infectious diseases (56-59). As a successful example, a nationwide immunisation campaign against hepatitis B has been implemented since July 1984 in Taiwan. The HBV vaccine was a recombinant hepatitis B surface antigen (HBsAg) formulated with aluminum-containing adjuvant. This vaccination campaign actually reduced the incidence of HBV-related diseases in children (57). In 2009, another global vaccination campaign was implemented to halt the novel influenza pandemic. Notwithstanding several successful adjuvanted vaccines available against diverse microorganisms, one of recently developed options unexpectedly caused uneasy vaccine-induced side effects, such as narcolepsy (29, 30). In 1969, the failure of an infamous vaccine trial against RSV was actually elicited by an adjuvanted vaccination with an inactivated RSV antigen (3). Recent phase 3 clinical trials with RSV prefusion protein vaccines, RSVPreF3 (Arexvy) and RSVpreF (Abrysvo), have been successful to protect the elderly aged 60 or more against RSV infections following intramuscular vaccination (60, 61). Despite the successful trials against RSV infections in elderly with an up-to-date technology, a maternal vaccination trial with a prefusion protein was halted over a rise in preterm births and neonatal deaths (61, 62) (
Table 2). The RSVPreF3 (Arexvy) causing a rise in preterm births is an adjuvanted vaccine while another RSVPreF (Abrysvo) containing no adjuvant was claimed to be protective without pathogenic outcomes. It would be improper to conclude that an adjuvant was the main cause for this vaccination-induced incident without approval of substantial and convincing scientific data (62). Nonetheless, the adjuvant could not be disregarded as a potential aetiological factor in these trials.
Infections with different serotypes of dengue viruses have long been recognised as an obvious obstacle for the development of effective vaccines, which could cause a serious viral haemorrhagic fever. The inferior quality of anti-viral antibody responses facilitated the infections of hetero-serotype dengue viruses, designated as antibody-dependent enhancement (ADE) (39). Currently live recombinant dengvaxia vaccine is only available for aged 9 years and older who have evidence of previous dengue infection. The limitation of this licensed vaccine has prompted a variety of groups to develop new vaccination approaches to reach a better herd immunity against dengue diseases. A passive immunisation of therapeutic antibodies and a live attenuated recombinant dengue vaccine based on serotype 2 backbone have actually been extensively explored and developed in phase 3 clinical trials. Besides the exploitation of passive antibody therapy and other recombinant vaccines, alternative options are to design vaccine components avoiding pathogenic domains of viral proteins, for instance, the implementation with fragmented proteins or with synthetic peptides (63, 64). Again, these approaches require innocuous and efficacious adjuvants to enhance their immunogenicity to be effective against dengue infections. But the good option is really hard to obtain from a poverty shelf at the moment. ADE has caused various vaccine-indued diseases halting the development for both therapeutic antibodies and advanced vaccination technologies (39, 65). Without concern over the quality of antibody responses like ‘antibody affinity’, ADE could not be neglected as an obvious hurdle for the development of effective vaccines against dengue infections.
Mucosal vaccination is an old approach that has been renovated to be a hot research topic recently (18-20). A non-invasive immunisation could be an attractive inoculation mode to bypass an invasive injection process. Intranasal and oral immunisations were two most prevalent approaches in development. Cholera toxin B and nontoxic heat-labile enterotoxin B have been developed and tested in both pre-clinical and clinical trials against pathogenic infections (66, 67). Facing the same challenge as other modern methods, the use of nontoxic fragment of bacterial toxins encountered a serious obstacle regarding adverse effects, such as Bell’s Palsy, following mucosal immunisation (68) (
Table 2). The recent development for mucosal immunisation is to employ a nanoscale measure to deliver oral nanoparticle vaccines with special coatings in a controlled-release mode (69-71). Amid a series of enchanting success against infectious viruses with nanoscale vaccinations, a nanoparticle vaccination approach is not always effective against an intractable virus concerned deeply with vaccine-induced pathogenesis, like RSV (57, 58, 72).
7. Veterinary Vaccination
The safety standards for livestock and poultry are not as stringent as those for humans in the development of effective vaccines. Just as a successful story of smallpox vaccination in humans, a famous infectious disease of cattle, like rinderpest, has been eradicated by a worldwide vaccination campaign, which was officially announced by the United Nations Food and Agriculture Organization and the World Organization for Animal Health. Veterinary virus infections not only cause mortality but severely reduce the production yield in livestock and poultry. Some of veterinary viruses could even result in zoonoses affecting a large proportion of world’s human population, such as avian influenza viruses and SARS-CoV.
Besides conventional inactivated vaccines, the introduction of new generations of vaccines for veterinary use is not really far behind those applied in human subjects already, for example, using equine influenza virus vaccine (73), recombinant equine West Nile virus vaccine (Recombitek) (74) and recombinant avian influenza vaccines (75). Several veterinary viruses have presented tough challenges for vaccine development, and now none is available economically and stably for an intractable African swine fever virus (ASFV) despite the effective live attenuated one available with limitation, ASFV-G-ΔI177L (32-34, 76). Conventional approaches are not eligible for surmounting these viral diseases, in particular only few available on a traditional shelf at present. In the same boat with human vaccines, veterinary vaccines have certainly confronted the similar challenge derived from vaccine-induced pathogenesis subsequent to virus infection, for example, infection of feline immunodeficiency virus (FIV) (4, 77, 78).
8. Conclusions
The review article is aiming for discussion of the discrepancy between protection and pathogenesis following vaccination (
Figure 1). The chemically-inactivated influenza vaccines have been very successful at protection against either seasonal influenza epidemics or pandemic outbreaks for decades. The adjuvanted chemically-inactivated influenza vaccine was demonstrated to be effective against influenza infections in elderly and young children without causing vaccine-enhanced pathogenesis (25) (
Figure 1a). In comparison with inactivated influenza vaccines, a notorious catastrophe of vaccine-enhanced pathogenesis caused by an adjuvanted formalin-inactivated RSV vaccine has actually hindered the development of safer and more effective vaccines against RSV-induced bronchiolitis and pneumonia in infants and young children since 1969 (
Figure 1b). Since then, there has been none of effective vaccine available on market to protect new-born and young children for decades. There continue to be a discrepancy as well as a disputation regarding detailed mechanisms between the vaccine-enhanced pathogenesis and protective immune responses against influenza and RSV infections, induced by adjuvanted formalin-inactivated vaccines. Immune complexes were suggested to mediate the vaccine-enhanced illness in vaccinees due to the poor quality of anti-RSV antibody responses (3, 79-81). The non-neutralising and low-affinity antibodies could be easily forming immune complexes during the next encountering RSV infection. The deposition of immune complexes in the alveolar compartments could trigger complement activation cascades to release C3a and C5a, which would further bring out neutrophil infiltration and storming of cytokines and chemokines. These cascade events finally caused severe injuries of lower respiratory tracts in infants and young children (
Figure 2a) (82). Moreover, non-neutralising antibody responses facilitate RSV multiplication to elicit eosinophilia in lung tissues, further leading to airway hypersensitivity and remodeling in vaccinated infants and young children (
Figure 2b) (83). On the other hand, the vaccine-induced eosinophilia generated by Th2 responses used to be the main paradigm to illustrate the cause of this particular vaccine-elicited event (84, 85). Alternatively, another hypothesis respecting specific CD8 T lymphocyte responses is improbable to be excluded from available options on a table, based on scientific observations (86-89). Peculiarly following vaccination, the specific CD8 T lymphocyte is able to reduce viral load and to mediate protection against severe disease at early stages of virus infections (90, 91). In recent clinical efficacy trials, RSVPreF3 and RSVpreF vaccines have been successful at inducing protective responses against RSV diseases in elderly (60, 61). Again, the same vaccine candidate continued to induce unexpected pathogenic events, causing a rise in preterm births and neonate deaths (60, 61, 62). This serious outcome has brought out serious debates and arguments for a maternal vaccination against RSV diseases. The mechanisms and causes behind are currently seen as a vague figure in vaccinology. The mechanisms concerning vaccinated pathogenesis could not be easily figured out in this debatable field for the moment. Many successful trials to prevent various virus infections have indeed turned the recognised paradigm regarding T-helper 1/T-helper 2 (Th1/Th2) subset-mediated protection mechanisms into a controversial issue in ‘Immunology’ (57, 58, 60, 61), which currently favours the hypothesis supporting the role of ‘antibody affinity’ in the protection against RSV diseases (79-81) (
Figure 2). The specialized Th1 responses are not essential for protection against obligately intracellular pathogens following vaccination in these clinical trials, like viruses.
In 1979, the Guillain–Barré syndrome induced following influenza vaccination did discourage the significance and benefit of vaccination against annually seasonal influenza infections (29). Recently, the mechanism of this rare event has preferred the multiple-factor hypothesis to another one for cross-reactive autoimmunity. In addition, the 2009 swine novel influenza outbreak raised a wave of global vaccination campaign to halt the spreading of this pandemic crisis. The outcome of this campaign presented a safety issue of vaccination with the potential risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (92). Cross-reactive autoimmunity was soon recognised as the major fault of vaccination (93). This recognised pathogenesis mechanism has been disputed with a retraction of published research results as well as with a recent progress in the field of autoimmunity (94-96). In spite of outweighing benefits from vaccination, accumulated harmful ramifications should not be disregarded as ordinary incidental events.
A nationwide HBV vaccination programme benefited the general public health in Taiwan since 1984. Nonetheless, HBsAg would certainly generate not only protective antibody responses but unpleasant immune reactions, for instance, inducing hypersensitivity and unnecessary cellular immunity (97-99). Some unpleasant immune reactions could even result in devastating consequences (99-101). An adjuvant could not be neglected as one of important aetiological factors to significantly affect consequences of vaccine-induced immune responses (30, 80, 92, 99, 102, 103) notwithstanding that many of severe adverse effects require further investigations to circumnavigate a superficial and paradoxical issue, such as vaccination-induced autism. We have long relied on the trial-and-error way to develop new vaccination materials and approaches to prevent various infectious diseases. Could the successful story of HBV vaccine just be a purely fortunate coincidence in the lack of a precision approach to circumvent pathogenic consequences without circumspectly scientific considerations on vaccination designs and strategies (101-104)? We should certainly look into the detailed working mechanisms of the successful story of HBV vaccination, which could lead us to a better path of science with respect to a precision opinion. The invisible enemy of mankind has actually evolved to defeat sophisticated arsenals devised by human intelligence, e.g., RSV, FIV and human immunodeficiency virus (HIV) (3, 35, 36, 61, 72, 77, 78, 105). Have we been really lost in a long winning race against pathogenic species of germ world? How are we possible to refuel our poverty shelf with state-of-the-art and advantageous options so as to keep a safe and rejoicing life in our homeland on this planet? The adjuvants and delivery systems are certainly reshaping the outcome of vaccine-induced immunity, resulting in potential vaccine-related pathogenesis in vaccinated individuals (29, 30, 39, 42, 54, 62, 102). The approach of ‘precision medicine’ could therefore circumvent vaccine-induced aberrant pathogenic effects, exploiting the scientific design not simply on antigen itself but on adjuvants as well as delivery systems to achieve the highest level of ‘innocuous vaccination’. Under the circumstances of poverty shelf, we could not offer to thrive in the absence of a precision option based on evolutionary concepts removing the pathogenic complicacy (39, 80, 81, 90, 102, 104, 106, 107).
Author Contributions
Dr. Chia-Yu Chi were putting a lot of efforts for research works of influenza viruses in Taiwan, R.O.C. Dr. Chih-Chiang Yang has been developing various oral delivery nanoparticle systems for pharmaceutical industries in Taiwan, R.O.C. Dr. Shiou-Chih Hsu has discovered an unexpected adjuvant-induced pathogenesis at ‘GRC, Academia Sinica’ in Taiwan, R.O.C. and been developing T lymphocyte-based vaccination strategies and materials at ‘National Health Research Institute’ in Taiwan, R.O.C. Dr. Chih-Peng Chang was contributing to researches in the pathogenesis of dengue viruses in southern Taiwan, R.O.C.
Funding
This review article received no funding.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed.
Acknowledgments
I am thankful to the funding support as well as research resources offered by ‘Genomics Research Centre, Academia Sinica’ for research on the adjuvanted vaccination against influenza viruses as well as for an inspiration to write this review article, derived from several discussions with Academician Lai (Michael Ming-Chiao Lai) whose relevant contributions are in the research field of coronaviruses at ‘Institute of Molecular Biology, Academia Sinisa’, Taiwan, R.O.C.
Conflicts of Interest
None of conflict interest.
References
- Danthi, P. Viruses and diversity of cell death. Anu Rev Virol 2016, 3, 533–553. [Google Scholar] [CrossRef]
- Butel, J.S. Viral carcinogenesis: relevation and molecular mechanisms and etiology and human disease. Carcinogenesis 2000, 21, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Vennema, H.; De Groot, R.J.; Harbour, D.A.; Dalderup, M.; Gruffydd-Jones, T.; Horzinek, M.C.; Spaan, W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunisation. J Virol 1990, 64, 1407–1409. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N Engl J Med 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Baxby, D. Edward Jenner’s unpublished cowpox inquiry and the royal society: Everard home’s report to Sir Joseph Banks. Medical history 1999, 43, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Alecaut, A.B.; Malberg, D.R.; Charter, R.S.; Lane, J.M. Smallpox in the Republic of Guinea, West Africa. II. Eradication using mobile teams. Am J Trop Med Hyg 1977, 26, 765–774. [Google Scholar] [CrossRef]
- Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; Uyeki, T.M. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009, 360, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.W.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479-480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Who.int/teams. Geneva: WHO Online Resources. Immunisation, Vaccines and Biologicals; 2022. Available online: https://www.who.int/teams/immunisation-vaccines-and-biologicals. (accessed on 7 November 2023).
- Appaiahgari, M.B.; Vrati, S. IMOJEV(®): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines 2010, 9, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.E. Rotavirus vaccines and intussusception risk. Curr Opin Gastroenterol 2005, 21, 20–25. [Google Scholar]
- Who.int/emergencies/disease. Geneva: WHO Online Resources. Circulating vaccine-derived poliovirus type 2 (cVDPV2) Indonesia; 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON430 (accessed on 7 November 2023).
- Alleman, M.M.; Jorba, J.; Henderson, E.; Diop, O.M.; Shaukat, S.; Traoré, M.A.; Wiesen, E.; Wassilak, S.G.F.; Cara, C.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks - Worldwide, January 2020-June 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.E. The theory of immunity from contagious diseases. Botanical Gazette 1886, 11, 241–245. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Plotkin, S.L. The development of vaccines: how the past led to the future. Nat Rev Microbiol 2011, 9, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Needham, J. The earliest mentions of inoculation. In Science and civilisation in China, Medicine, 6th ed.; Gwei-djen, L., Sivin, N., Eds.; Cambridge University Press: Cambridge, U.K, 2000; Volume 6, p. 134. [Google Scholar]
- Katz, S.L. From culture to vaccine—Salk and Sabin. N Engl J Med 2004, 351, 1485–1487. [Google Scholar] [CrossRef]
- Skibinski, D.A.G.; Baudner, B.C.; Singh, M.; O'Hagan, D.T. Combination vaccines. J Glob Infect Dis 2011, 3, 63–72. [Google Scholar] [CrossRef]
- Marshall, G.S.; Happe, L.E.; Lunacsek, O.E.; Szymanski, M.D.; Woods, C.R.; Zahn, M.; Russell, A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007, 26, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-R.; Chen, G.-W.; Yang, C.-C.; Yang, W,-Z.; Liu, D.-P.; Lin, J.-H.; Chiu, S.-C.; Chen, H.-Y.; Tsao, K.-C.; Huang, C.-G; et al. Laboratory-based surveillance and molecular epidemiology of influenza virus in Taiwan. J Clin Microbiol 2005, 43, 1651–1661. [CrossRef]
- de Graaf, H.; Faust, S.N. Fluarix quadrivalent vaccine for influenza. Expert Rev Vaccines 2015, 14, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Valdez, A.; Valdez-Zapata, G.; Patel, S.S.; Castelli, F.V.; Garcia, M.G.; Jansen, W.T.; Arora, A.K.; Heijnen, E. MF59-adjuvanted influenza vaccine (FLUAD®) elicits higher immune responses than a non-adjuvanted influenza vaccine (Fluzone®): A randomized, multicentre, Phase III pediatric trial in Mexico. Hum Vaccin Immunother 2018, 14, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-Y.; Wang, S.-M.; Lin, C.-C.; Wang, H.-C.; Wang, J.-R.; Su, I.-J.; Liu, C.-C. Clinical features of children infected with different strains of influenza B in southern Taiwan. The Pediatr Infect Dis J 2008, 27, 640–645. [Google Scholar] [CrossRef]
- Price, A.M.; Flannery, B.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J.; Phillips, C.H.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; et al. Influenza vaccine effectiveness against influenza A(H3N2)-related illness in the United States during the 2021-2022 influenza season. Clin Infect Dis 2023, 76, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- de Whalley, P.C.S.; Pollard, A.J. Pandemic influenza A (H1N1) 2009 vaccination in children: a UK perspective. J Pediatr Child Health 2013, 49, E183–E188. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Victor, M. Influenza vaccination and the Guillain–Barré syndrome. N Engl J Med 1998, 339, 1845–1846. [Google Scholar] [CrossRef]
- Persson, I.; Granath, F.; Askling, J.; Ludvigsson, J.F.; Olsson, T.; Feltelius, N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med 2014, 275, 172–190. [Google Scholar] [CrossRef]
- Gershwin, L.J.; Scheleglet, E.S.; Gunther, R.A.; Anderson, M.L.; Woolums, A.R.; Larochelle, D.R.; Boyle, G.A.; Friebertshauser, K.E.; Singer, R.S. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 1998, 16, 1225–1236. [Google Scholar] [CrossRef]
- Della Porta, A.J.; Snowdon, W.A. An experimental inactivated virus vaccine against bovine ephemeral fever 2. do neutralizing antibodies protect against infection? Vet Microbiol 1979, 4, 197–208. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Chen, S.-H.; Chou, C.-C.; Ting, L.-J.; Itakura, C; Wang, F.-I. Bovine ephemeral fever in Taiwan (2001-2002). J Vet Med Sci 2005, 67, 411–416.
- Belsham, G.J. Towards improvements in foot-and-mouth disease vaccine performance. Acta Vet Scand 2020. 62. 20. [CrossRef]
- The Lancet HIVs Editorial. What future for HIV vaccines? Lancet HIV 2023, 10, e143. [Google Scholar] [CrossRef]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013, 369, 2083–2092. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef] [PubMed]
- Wojda, T.R.; Valenza, P.L.; Cornejo, K.; McGinley, T.; Galwankar, S.C.; Kelkar, D.; Sharpe, R.P.; Papadimos, T.J.; Stawicki, S.P. The Ebola outbreak of 2014-2015: From coordinated multilateral action to effective disease containment, vaccine development, and beyond. J Glob Infect Dis 2015, 7, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Grais, R.F.; Kennedy, S.B.; Mahon, B.E.; Dubey, S.A.; Grant-Klein, R.J.; Liu, K.; Hartzel, J.; Coller, B.-A.; Welebob, C.; Hanson, M,E.; et al. Estimation of the correlates of protection of the rVSVΔG-ZEBOV-GP Zaire ebolavirus vaccine: a post-hoc analysis of data from phase 2/3 clinical trials. Lancet Microbe 2021, 2, e70–e78. [CrossRef]
- Greinacher A., Selleng K., Palankar R., Wesche J., Handtke S., Wolff M., Aurich K., Lalk M., Methling K., Völker U., et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 2256–2268. [CrossRef] [PubMed]
- Huang, K.-J.; Yang, Y.-C.; Lin, Y.-C.; Huang, J.-H.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, C.-C.; Lei, H.-Y. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol 2006, 176, 2825–2832. [Google Scholar] [CrossRef]
- Wang, S.-M.; Chen, I.-C.; Su, L.-Y.; Huang, K.-J.; Lei, H.-Y.; Liu, C.-C. Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin Vac Immunol 2010, 17, 1517–1523. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lei, H.-Y.; Lin, Y.-S.; Liu, H.-S.; Wu, H.-L. Yeh T-M. Dengue virus-induced autoantibodies bind to plasminogen and enhance its activation. J Immunol 2011, 187, 6483–6490. [CrossRef] [PubMed]
- Tang, D.-C.; DeVit, M.; Johnston, S.A. Genetic immunisation is a simple method for eliciting an immune response. Nature 1992, 356, 152–154. [Google Scholar] [CrossRef]
- Hsu, S.C. (Stephen). The Role of CD8 T Cells in the Control of Infectious Disease and Malignancies. In Topley and Wilson’s Microbiology and Microbial Infections: Immunology, 10th ed.; Kaufmann, S.H.E., Steward, M.W., Eds.; Holder Arnold: London, U.K, 2007; Volume 10, pp. 419–434. [Google Scholar]
- Taylor, G.; Brucea, C.; Barbetb, A.F.; Wyld, S.G.; Thomas, L.H. DNA vaccination against respiratory syncytial virus in young calves. Vaccine 2005, 23, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B.; et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis 1998, 178, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020, 383, 2603–2615. [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: a systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Hromic-Jahjefendic, A.; Sezer, A.; Aljabali, A.A.A.; Serrano-Aroca, A.; Tambuwala, M.M.; Uversky, V.N.; Redwan, E.M.; Barh, D.; Lundstrom, K. COVID-19 vaccines and myocarditis: An overview of current evidence. Biomedicines 2023, 11, 1469. [Google Scholar] [CrossRef]
- Shirley, M. 20-valent pneumococcal conjugate vaccine: A review of its use in adults. Drugs 2022, 82, 989–999. [Google Scholar] [CrossRef]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 1997, 336, 1855–1859. [Google Scholar] [CrossRef]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001, 93, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in vaccine adjuvants. Methods Mol Biol 2022, 2412, 145–178. [Google Scholar] [PubMed]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023, 388, 1465–1471. [Google Scholar] [CrossRef]
- Papi, A.; Ison, A.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Maternal RSV vaccine: Further analysis is urged on preterm births. BMJ 2023, 381, 1021. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Yang, M.; Chen, H.-W.; Wang, S.; Chang, C.-P.; Ho, T.-S.; Kao, Y.-S.; Tien, S.-M.; Lin, H.-H.; Chang, P.-C.; et al. A novel chimeric dengue vaccine candidate composed of consensus envelope protein domain III fused to C-terminal-modified NS1 protein. Vaccine 2022, 40, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies against modified NS1 wing domain protect against dengue virus infection. Sci Rep 2017, 7, 6975. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, W.; Nanbo, A.; Maruyama, J.; Marzi, A.; Takada, A. A complement component C1q-mediated mechanism of antibody-dependent enhancement of Ebola virus infection. PLoS Negl Trop Dis 2020, 14, e0008602. [Google Scholar] [CrossRef]
- Clements, J.D.; Hartzog, F.L.; Lyon, F.L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 1988, 6, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Northrup, R.S.; Fauci, A.S. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis 1972, 125, 672–673. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s Palsy in Switzerland. N Engl J Med 2004, 350, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-K.; Yang, C.C.; Wang, W.-C.; Chien, T.-Y.; Wu, C.-C.; Chin, L.-C. inventors; Oral composition, method for manufacturing and use thereof; Medical and Pharmaceutical Industry Technology and Development Centre, assignee. Taiwan patent 1764159 (202045166). 2022 May 11.
- Hu, Y.; Litwin, T.; Nagaraja, A.R.; Kwong, B.; Katz, J.; Watson, N.; Irvine, D.J. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core−shell nanoparticles. Nano Lett 2007, 7, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.K.; Yang, C.C.; Lin, T.C.; Chin, L.C.; Ho, P.H. inventors; Medical and Pharmaceutical Industry Technology and Development Centre, assignee. Modified release pharmaceutical composition and method for the treatment of mental disorders. Taiwan patent 1744858 (202037368). 2021 Nov 1.
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simões, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory syncytial virus vaccination during pregnancy and effects in infant. N Engl J Med 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L. ISCOM-matrix-based equine influenza (EIV) vaccine stimulates cell-mediated immunity in the horse. Vet Immunol Immunopathol 2012, 145, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Garch, H.E.I.; Minke, J.M.; Rehder, J.; Richard, S.; Toulemonde, C.E.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V.A. West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet Immunol Immunopathol 2008, 123, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Spackman, E.; Pantin-Jackwood, M. Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird-agricultural interface. Ecohealth 2014, 11, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-ΔI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Trans bound Emerg Dis 2022, 69, e497–e504. [Google Scholar] [CrossRef]
- Siebelink, K.H.; Tijhaar, E.; Huisman, R.C.; Huisman, W.; de Ronde, A.; Darby, I.H.; Francis, M.J. Rimmelzwaan GF, Osterhaus AD. Enhancement of feline immunodeficiency virus infection after immunisation with envelope glycoprotein subunit vaccines. J Virol 1995, 69, 3704–3711. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.M.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J Virol 1997, 71, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.-Y.; Mitzner, W.; et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 2009, 15, 34–41. [Google Scholar] [CrossRef]
- Petty, R.E.; Steward, M.W. The effect of immunological adjuvants on the relative affinity of anti-protein antibodies. Immunology 1977, 32, 49–55. [Google Scholar]
- Chargelegue, D.; Obeid, O.E.; Hsu, S.C.; Shaw, M.D.; Denbury, A.N.; Taylor, G.; Steward, M.W. A peptide mimic of a protective epitope of respiratory syncytial virus selected from a combinatorial library induces virus-neutralizing antibodies and reduces viral load in vivo. J Virol 1998, 72, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A.; Fattahi, F.; Bosmann, M. New insights into molecular mechanisms of immune complex-induced injury in lung. Front Immunol 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F. Eosinophils: structure and functions. Curr Opin Immunol 1994, 6, 85–90. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Clarke, S.L.; Record, F.M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol 1992, 4, 493–500. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Kuiken, T.; Timmerman, H.H.; van Amerongen, G.; Van Den Hoogen, B.G.; Vos, H.W.; Neijens, H.J.; Andeweg, A.C. Osterhaus ADME. Immunisation of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol 2002, 76, 11561–11569. [Google Scholar] [CrossRef]
- Isaacs, D.; Bangham, C.R.; McMicahel, A.J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet 1987, 2, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Braciale, T.J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med 1997, 186, 421–432. [Google Scholar] [CrossRef]
- Zeng, R.-H.; Gong, W.; Fan, C.-F.; Wang, Y.F.; Mei, X.-G. Induction of balanced immunity in BALB/c mice by vaccination with a recombinant fusion protein containing a respiratory syncytial virus G protein fragment and a CTL epitope. Vaccine 2006, 24, 941–947. [Google Scholar] [CrossRef]
- Olson, M.R.; Hartwig, S.M.; Varga, S.M. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol 2008, 181, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Chargelegue, D.; Obeid, O.E.; Steward, M.W. Synergistic effect of immunisation with a peptide cocktail inducing antibody, helper and cytotoxic T-cell responses on protection against respiratory syncytial virus. J Gen Virol 1999, 80, 1401–1405. [Google Scholar] [CrossRef]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef]
- Nohynek, H.; Jokinen, J.; Partinen, M.; Vaarala, O.; Kirjavainen, T.; Sundman, J.; Himanen, S.-L.; Hublin, C.; Julkunen, I.; Olsén, P.; et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 2012, 7, e3353. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán-Arita, A.K.; Kornum, B.R.; Mahlios, J.; Jiang, W.; Lin, L.; Hou, T.; Macaubas, C.; Einen, M.; Plazzi, G.; Crowe, C.; et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med 2013, 5, 216ra176. [Google Scholar] [CrossRef]
- De la Herrán-Arita, A.K.; Kornum, B.R.; Mahlios, J.; J.; Jiang, W.; Lin, L.; Hou, T.; Macaubas, C.; Einen, M.; Plazzi, G.; Crowe, C.; et al. Retraction of the research article: “CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy”. Sci Transl Med 2014, 6, 247rt1.
- Culina, S.; Lalanne, A.I.; Afonso, G.; Cerosaletti, K.; Pinto, S.; Sebastiani, G.; Kuranda, K.; Nigi, L.; Eugster, A.; Østerbye, T.; et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 2018, 3, eaao4013. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fairweather, D. Complexities in the relationship between infection and autoimmunity. Curr Allergy Asthma Rep 2014, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.-Y.; Huang, K.-J.; Shen, C.-L.; Huang, J.L. An antigen-specific hypersensitivity which does not fit into traditional classification of hypersensitivity. J Immunol 1989, 143, 432–438. [Google Scholar] [CrossRef]
- Lei, H.-Y.; Wang, Y.-L.; Lee, S.-C.; Chen, S.H. The effect of pepsin digestion in relation to the pre-S region on hepatitis B surface antigen-induced hypersensitivity. J Immunol 1992, 148, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, R.; Melber, K.; Kuhrober, A.; Janowicz, Z.A. Reimann J. Immunisation with soluble hepatitis B virus surface protein elicits murine H-2 class I-restricted CD8+ cytotoxic T lymphocyte responses in vivo. J Immunol 1994, 152, 1110–1119. [Google Scholar] [CrossRef]
- Kagi, D.; Ledermann, B.; Burki, K.; Zinkernagel, R.M.; Hengartner, H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol 1996, 14, 207–232. [Google Scholar] [CrossRef]
- Kimura, K.; Ando, K.; Tomita, E.; Ohnishi, H.; Ishikawa, T.; Kakumu, S.; Muto, Y.; Moriwaki, H. Elevated intracellular IFN-gamma levels in circulating CD8+ lymphocytes in patients with fulminant hepatitis. J Hepathol 1999, 31, 579–583. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Lin, K.-H.; Tseng, Y.-C.; Cheng, Y.-Y.; Ma, H.-H.; Chen, Y.-C.; Jan, J.-T.; Wu, C.-Y.; Ma, C. The adjuvant-induced vaccinated pathogenesis subsequent to an influenza infection. Immunology 2023; submitted.
- Gherardi, R.K. , Crépeaux G., Authier F.-J. Myalgia and chronic fatigue syndrome following immunisation: macrophagic myofasciitis and animal studies support linkage to aluminum adjuvant persistency and diffusion in the immune system. Autoimmun Rev 2019, 18, 691–705. [Google Scholar] [CrossRef]
- Monath, T.P.; Myers, G.A.; Beck, R.A.; Knauber, M.; Scappaticci, K.; Pullano, T.; Archambault, W.T.; Catalan, J.; Miller, C.; Zhang, Z.-X.; et al. Safety testing for neurovirulence of novel live, attenuated flavivirus vaccines: infant mice provide an accurate surrogate for the test in monkey. Biologicals 2005, 33, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.E.; Bekker, L.-G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120/MF59 in adults. N Engl J Med 2021, 384, 1089–1100. [Google Scholar] [CrossRef]
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Usova, S.V.; Nechaeva, E.A.; Danilenko, E.D.; Pyankov, S.A.; Gudymo, A.S.; Moiseeva, A.A.; Onkhonova, G.S.; et al. Assessment of safety and prophylactic efficacy of the EpiVacCorona peptide vaccine for COVID-19 prevention (Phase III). Vaccines (Basel) 2023, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc R Soc Lond B 1979, 205, 489–511. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).