Submitted:
22 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Revised FIGO (2018) and TNM (2021) cervical cancer staging.
| TNM CATEGORYa | FIGO STAGEa | CRITERIA | Imaging findings |
| T (TUMOR)b | T CRITERIA | Tumour delineation by imaging | |
| TX | Primary tumour cannot be assessed | Non-diagnostic imaging examination US: Acoustic limitations in depicting the tumour due to e.g. abnormal position of uterus, severe pelvic infection with pyometra/actinomycosis/tuberculosis MRI: Severe imaging artefacts due to e.g. patient movement or hip implants |
|
| T0 | No evidence of primary tumour | No visible tumour depicted by imaging US: Regular contours of exocervix and endocervical canal, homogeneous echogenicity of cervical stroma, regular arrangement of cervical vessels and pericervical fascia. MRI: The four cervical zones are depicted on T2W images with high signal intensity endocervical canal, intermediate signal intensity plicae palmatae, low signal intensity fibrous stroma, and intermediate signal intensity outer stromal ring. |
|
| T1 | Carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded) | Tumour confined to the cervix (not necessarily visible in T1a); assessment of maximum tumour diameter is critical to substage T1b1-T1b3. The measurement of lateral +/- cranial tumour-free distance is optional | |
| T1ac | IA | Carcinoma with maximum depth ≤5 mm | Limited resolution for US or MRI to detect T1a cancers (invasive cancer diagnosed by microscopy) |
| T1a1 | IA1 | Stromal invasion ≤3 mm (diagnosed in biopsy) | |
| T1a2 | IA2 | Stromal invasion >3 mm and ≤5 mm (diagnosed in biopsy) | |
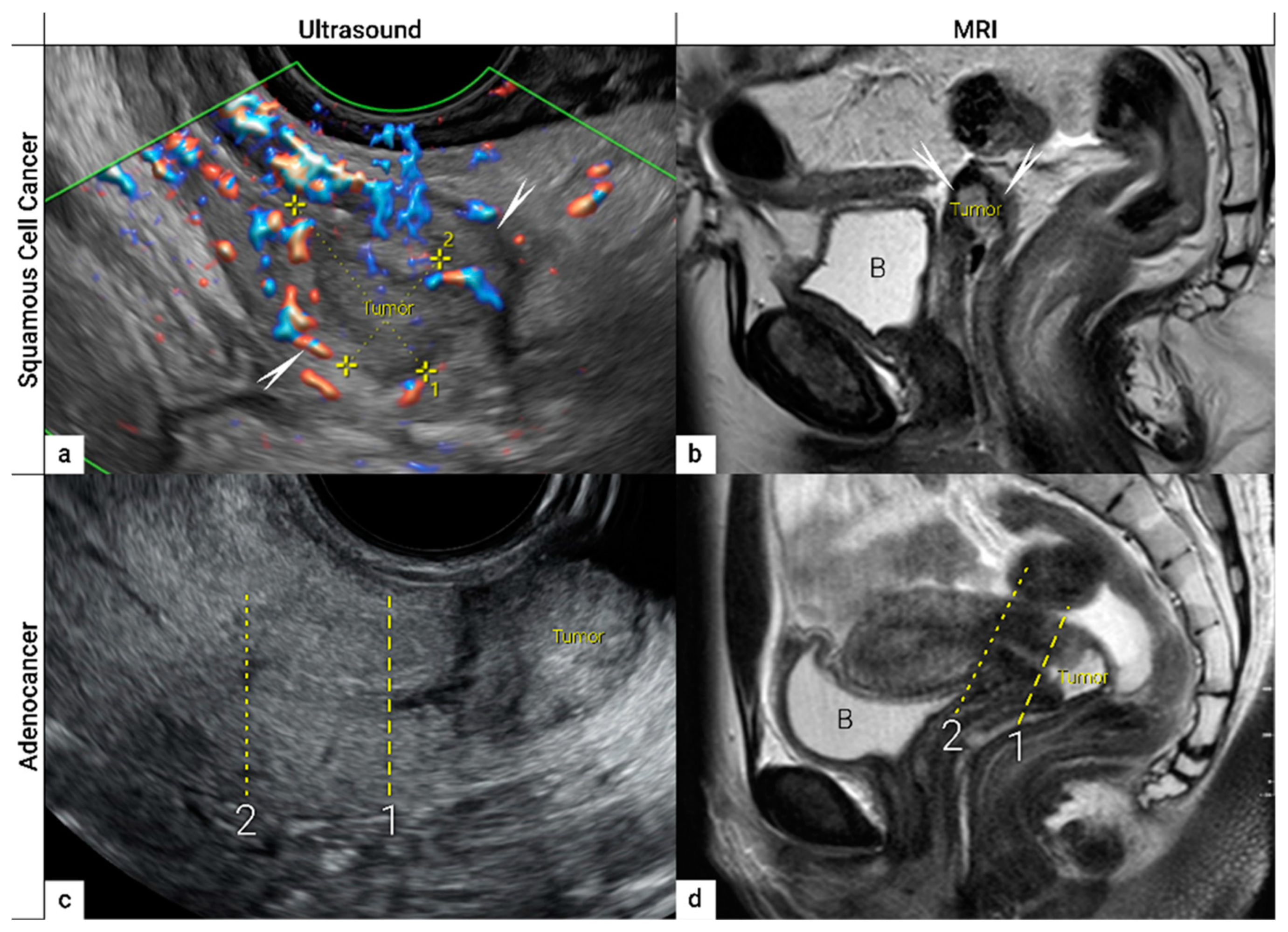
| IB | Carcinoma with deepest stromal invasion>5 mm, limited to the cervix uteri with size measured by maximum tumour diameter. | US: Highly vascularized hypoechogenic (squamous-cell carcinoma) or iso- / hyperechogenic lesion (adenocarcinoma) with intact hyperechogenic pericervical fascia and positive sliding sign between tumour and bladder/rectum. MRI: Tumour has intermediate to high signal on T2W images. Dynamic CE-T1W images depicts tumour as hyperintense in the arterial phase and iso- or hypointense in the venous phase. Tumours characteristically exhibit restricted diffusion on DWI (hyperintensity on high b-value images and low intensity on the ADC maps). Tumor does not disrupt the hypointense peripheral stromal ring (best seen on T2W images). |
|
| T1b1d | IB1d | Carcinoma with >5 mm depth of stromal invasion and ≤2 cm in greatest dimension. | |
| IB2 | Carcinoma >2 cm and ≤4cm in greatest dimension. | ||
| IB3 | Carcinoma >4 cm in greatest dimension. | ||
| T2 | II | Carcinoma invades beyond the uterus but does not extend to the lower one-third of the vagina or to the pelvic wall | Tumour extends beyond the cervix. Infiltration of upper two-third of vagina or pericervical fascia by US or MRI. Confident diagnosis of parametrial invasion is made in the presence of the spiculated tumor-parametrial interface, soft tissue mass in parametrium, encasement of periuterine vessels, and ureter. |
| T2a | IIA | Involvement limited to the upper two-thirds of the vagina without parametrial invasion | US: Highly vascularized tumour infiltrates upper two third of hypoechogenic vaginal wall, maximum diameter of primary tumor is critical to substageT2a1/T2a2. MRI: Involvement of the upper two-third of the vagina is seen as segmental loss of the T2-hypointensity of the vaginal wall. |
| T2a1 | IIA1 | Carcinoma ≤4 cm in greatest dimension | |
| T2a2 | IIA2 | Carcinoma >4 cm in greatest dimension | |
| T2b | IIB | Parametrial tumour invasion but no pelvic side wall extension | US: Tumour infiltrates the hyperechogenic pericervical fascia, negative sliding sign, presence of hypoechogenic tumour projections into hyperechogenic parametria. MRI: Tumour disrupts the hypointense peripheral stroma and extends into the parametrium +/- abutting parametrial vessels on T2W images. |
| T3e | IIIe | Carcinoma involves the lower third of the vagina and/or extends to the pelvic side wall and/or causes hydronephrosis or non-functioning kidney | Tumour infiltration of the lower third of vagina or lateral pelvic side wall. Pelvic side wall infiltration is considered when the tumour causes hydroureter, infiltrates the obturator internus, piriformis, and levator ani muscles, encases the iliac vessels, or invades the pelvic bones on US or MRI. |
| T3a | IIIA | Carcinoma involves the lower third of the vagina, with no extension to the pelvic wall. | US: Highly vascularized, irregular tumour infiltration of the lower third of vagina MRI: Involvement of the lower third of the vagina is suggested by disruption of the normal low-signal-intensity wall on T2- weighted images |
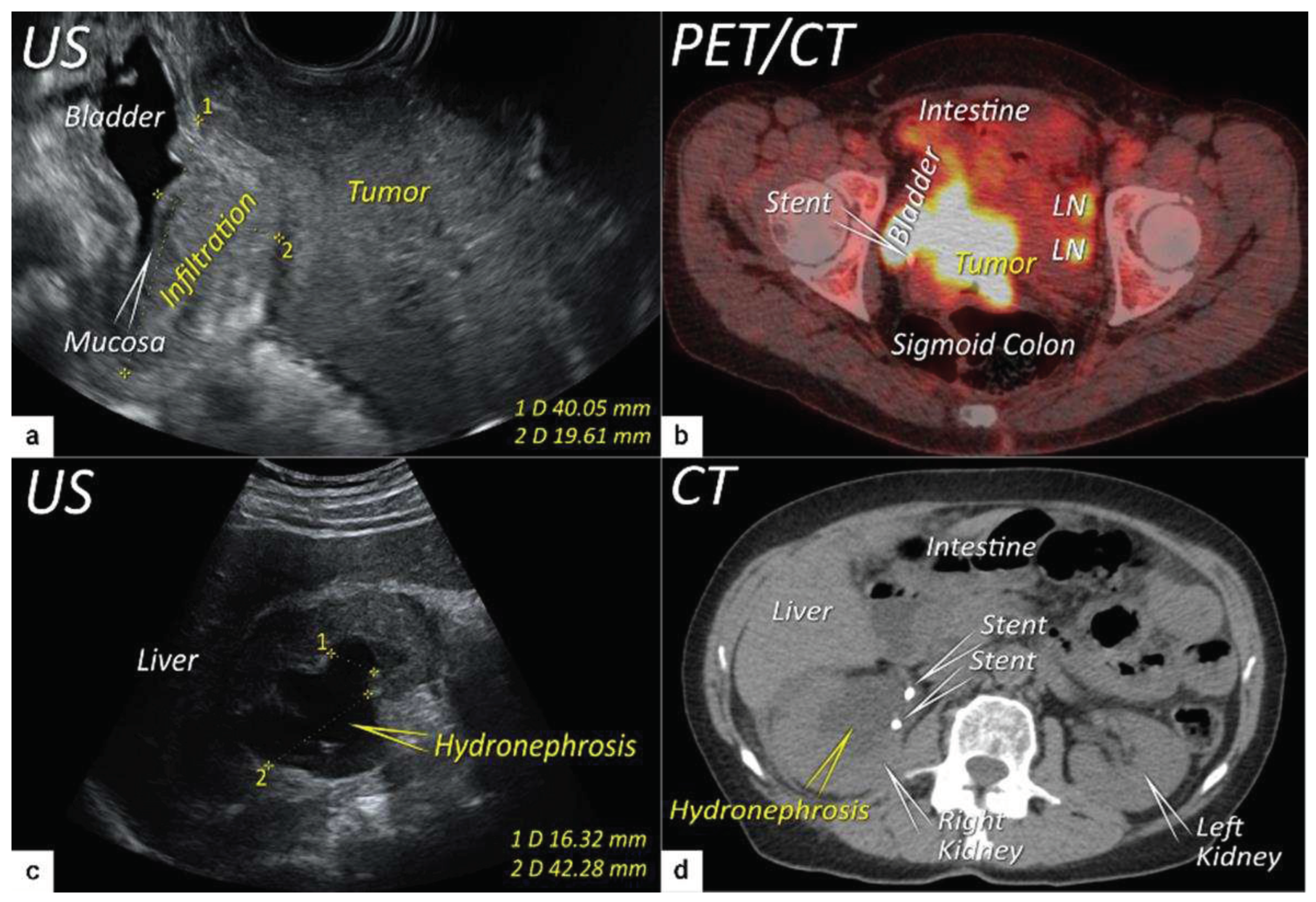
| T3b | IIIB | Tumour extension to the pelvic side wall and/or hydronephrosis or non-functioning kidney (unless known to be due to other cause). | US: Hypoechogenic tumor projections up to pelvic side wall +/- infiltration of iliac vessels, ureters, muscles, presence of hydronephrosis MRI: Hyperintense infiltration up to the pelvic side wall, loss of normal parametrial signal intensity and increased signal intensity in pelvic musculature due to tumour invasion seen on T2W-images. |
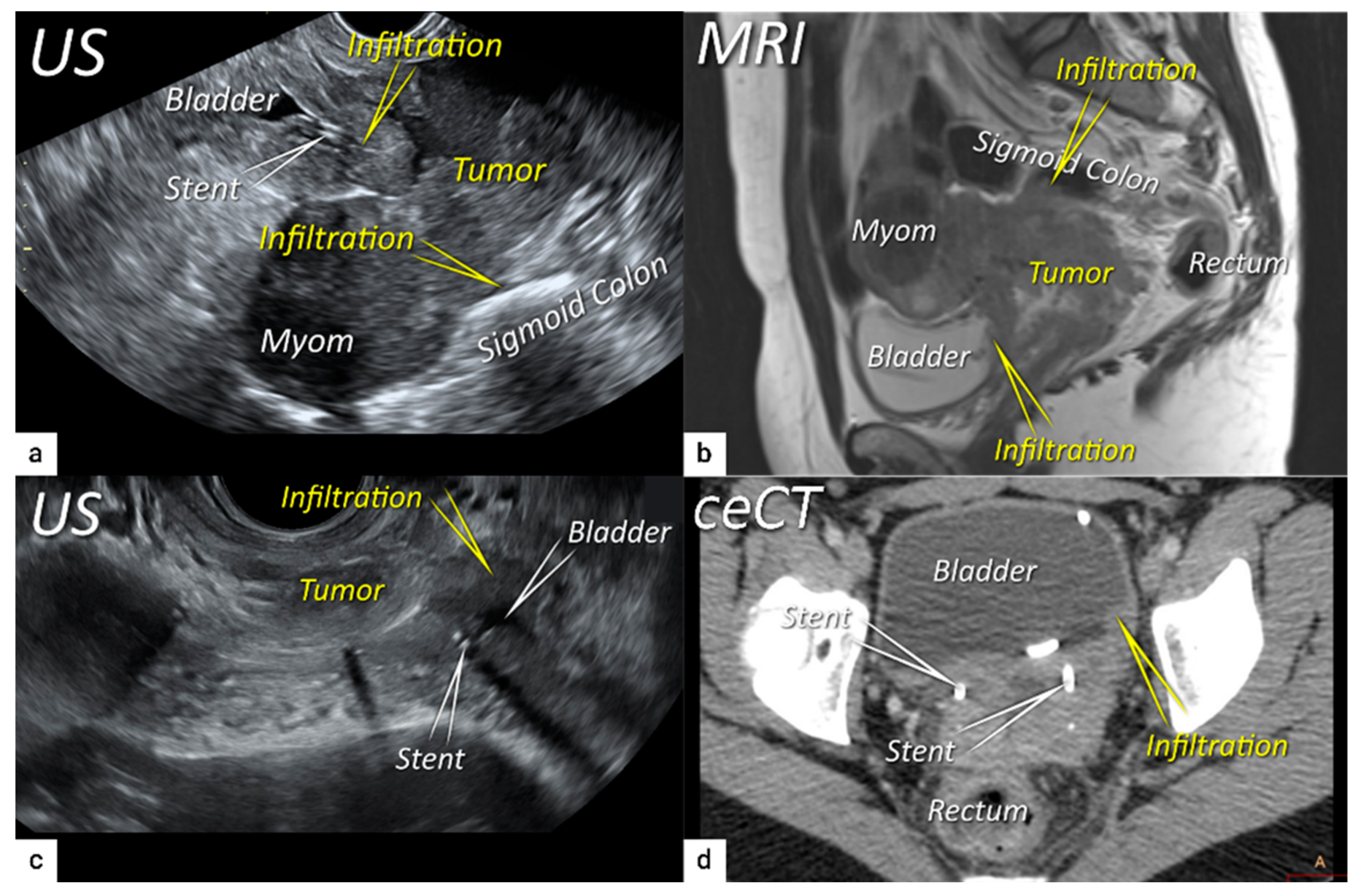
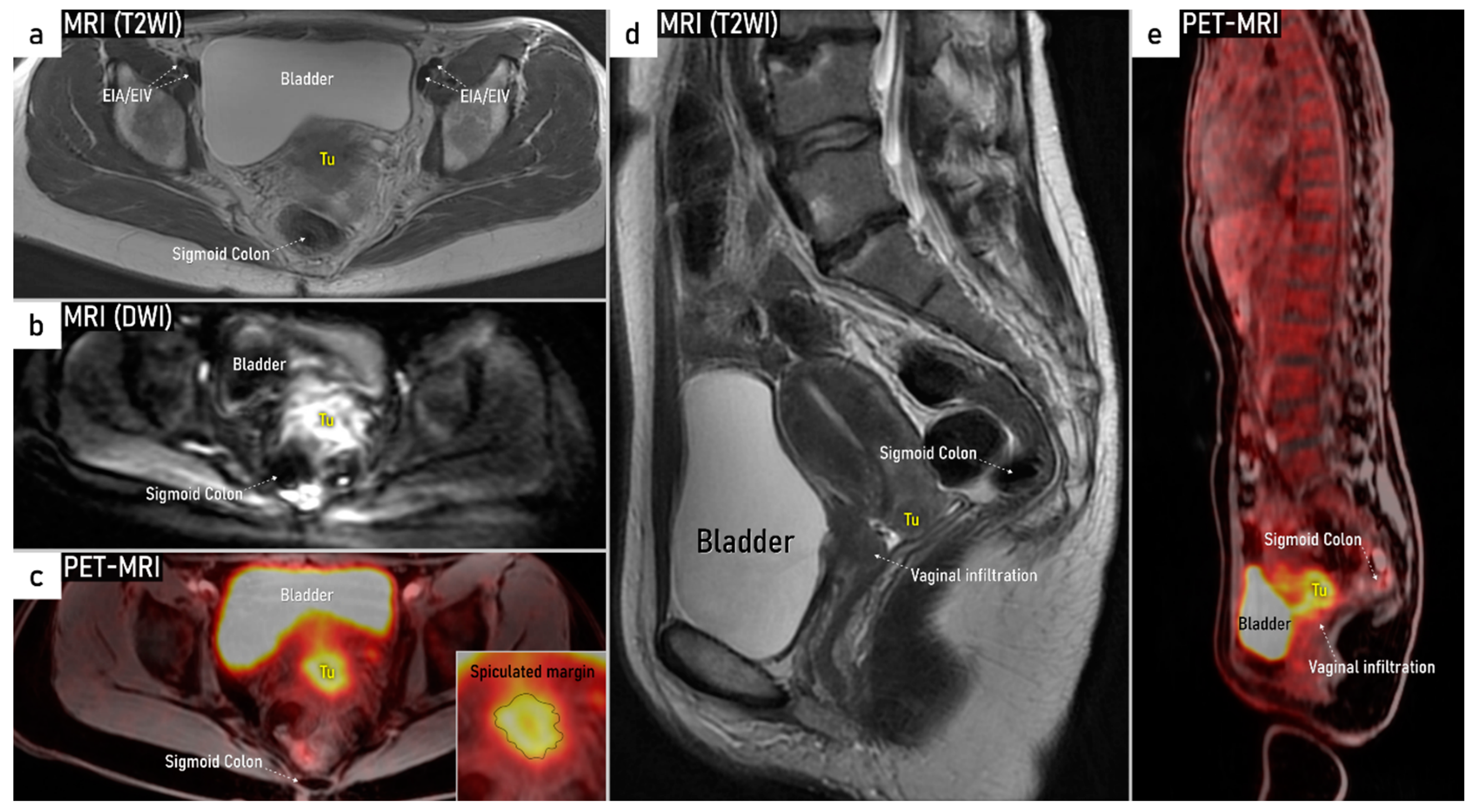
| T4f | IVAf | Tumour invasion into the mucosa of the bladder or rectum (biopsy-proven) or into adjacent organs. | US: Negative sliding sign, hypoechogenic tumour infiltration of bladder / rectal wall up to echogenic mucosa with polypoid tumor seen intraluminally. MRI: Focal or diffuse disruption of the normal T2-low signal intensity wall of the bladder/rectum, irregular or nodular wall, sometimes including an intraluminal tumour mass. Bulous edema sign, which is hyperintense thickening of the bladder mucosa on T2W images, is only an indirect sign of invasion and should not be regarded as T4 unless confirmed mucosal infiltration at cystoscopy. Infiltration of the posterior bladder wall without mucosal infiltration should not be regarded as T4a. |
| N (NODE) CATEGORYg | |||
| Nx | Regional lymph nodes cannot be assessed | US: Poor acoustic conditions in the pelvis or abdomen due to tumour infiltrating pelvic side wall or patient obesity. MRI/CT/PET-CT: Non-diagnostic image quality due to severe artefacts from e.g. hip implants or patient movement. |
|
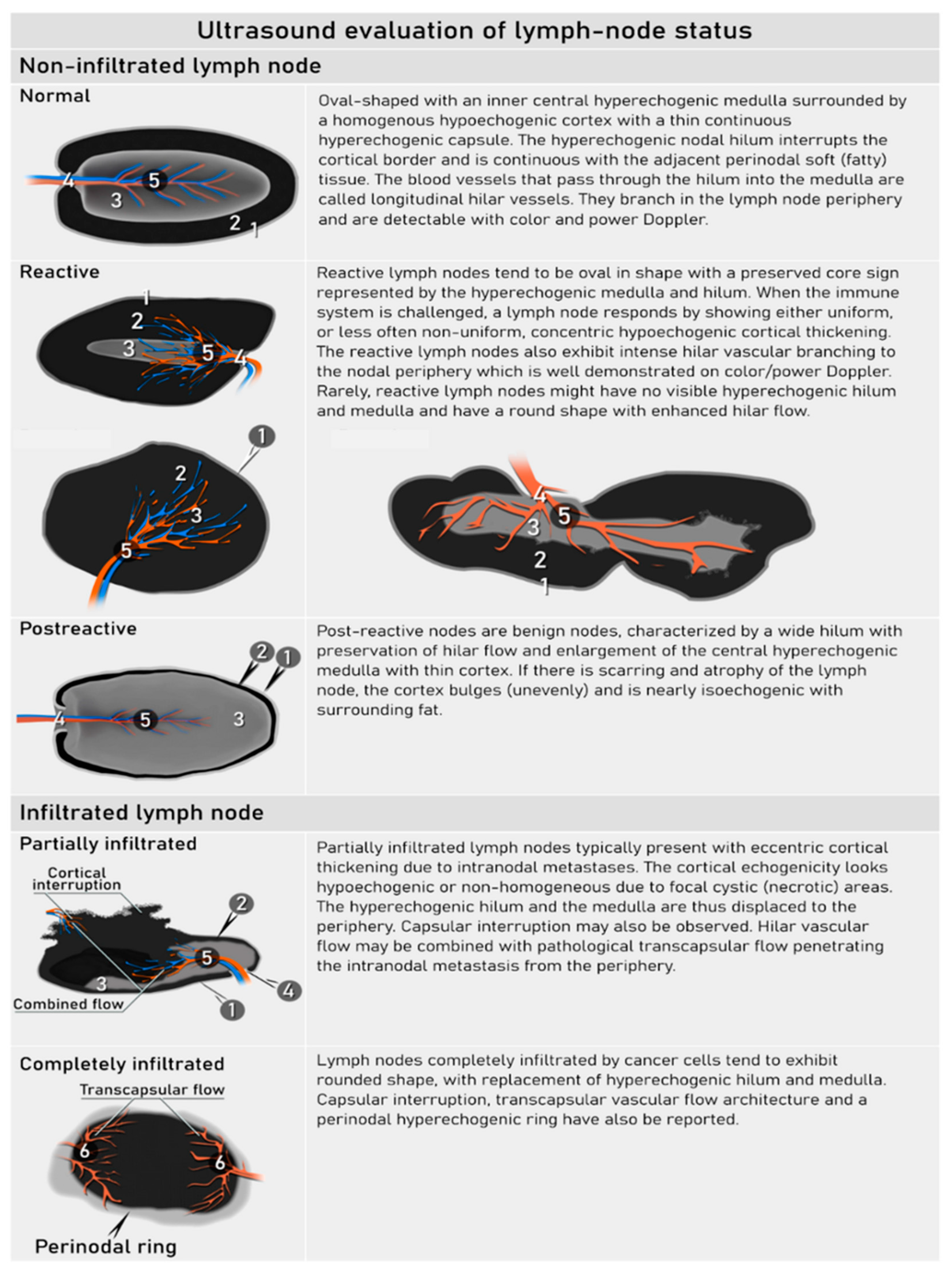
| N0 | No regional lymph node metastasis | Demonstration of paracervical, parailiac and paraaortic tissue without suspicious lymph node/-s. US: Oval-shaped nodes, the inner central hyperechogenic medulla and hilum (nodal-core sign) is surrounded by a homogeneous hypoechogenic cortex and thin continuous hyperechogenic capsule. Hilar longitudinal vessels may be visualized. In reactive lymph nodes due to the activation of the immune response, uniform concentric cortical thickening of the hypoechogenic cortex and intesified normal vascular tree from the central hilar region may be found. MRI/CT/PET-CT: No suspicion of malignant lymph nodes due to normal sized lymph nodes without irregular contours/signal or signs of restricted diffusion (MRI/DWI) or pathologic FDG-avidity (PET-CT) |
|
| N0(i+)h | Isolated tumour cells in regional lymph node(s) ≤0.2 mm or single cells or clusters of cells ≤200 cells in a single lymph node cross-section | ||
| N1 | IIIC1 | Regional lymph node metastasis to pelvic lymph nodes only | US: hypoechogenic rounded lymph node without preservation of typical architecture (loss of the nodal core-sign), inhomogeneous echogenicity due to cystic necrosis and calcifications, capsular interruption, grouping of metastatic lymph nodes and others. Hilar flow may still be preserved in a partial nodal involvement with or without transcapsular vascularisation (vessels penetrating the cortex from outside), the latter are usually found in an advanced stage of infiltration. MRI: lymph nodes with maximum transverse diameter>10 mm; capsule irregularity, rounded (as opposed to oval) shape, inhomogeneous signal with signs of necrosis on T2W images (MRI), restricted diffusion (DWI) or increased FDG-avidity (PET-CT) |
| N1mii | Regional lymph node metastasis (>0.2 mm but ≤2 mm in greatest dimension) to pelvic nodes | Limited resolution for imaging | |
| N1a | Regional lymph node metastasis (>2 mm in greatest dimension) to pelvic lymph nodes | Paracervical and parametrial nodes are first to be involved, followed by spread to external iliac nodes by lateral route, internal iliac nodes by hypogastric route, and lateral sacral and sacral promontory nodes by presacral route. All nodal groups drain to the common iliac nodes. |
|
| N2 | IIIC2 | Regional lymph node metastasis to para-aortic lymph nodes, with or without positive pelvic lymph nodes. | |
| N2mii | Regional lymph node metastasis (>0.2 mm but ≤2.0 in greatest dimension) to para-aortic lymph nodes, with or without positive pelvic lymph nodes | Limited resolution for imaging | |
| N2a | Regional lymph node metastasis (>2.0 in greatest dimension) to para-aortic lymph nodes, with or without positive pelvic lymph nodes | Pelvic lymph nodes drain to the paraaortic nodes. | |
| M (METASTASIS) CATEGORY | |||
| M0 | No distant metastasis | US: Systematic abdominal scanning, evaluation of groins and supraclavicular lymph nodes without abnormal findings MRI/CT/PET-CT: No signs of metastatic lesions in the abdomen (MRI) or in the thorax/trunk/groins/head (whole-body PET-CT) |
|
| cM1j | IVB | Distant metastasis (clinical category) | US: non-regional lymph node infiltration (supraclavicular, inguinal, and other regions), hypoechogenic or target (hypoechogenic rim and echogenic center) liver lesion /-s, hypoechogenic infiltration of suprarenal glands, hypoechogenic peritoneal carcinomatosis and others. MRI, CT, PET-CT: distant spread of tumor to the liver, lung, bones, peritoneum, and soft tissue, and rarely adrenals, spleen, kidneys, pancreas, and gastrointestinal tract and others. |
| pM1j | IVB | Distant metastasis (pathologic category) | |
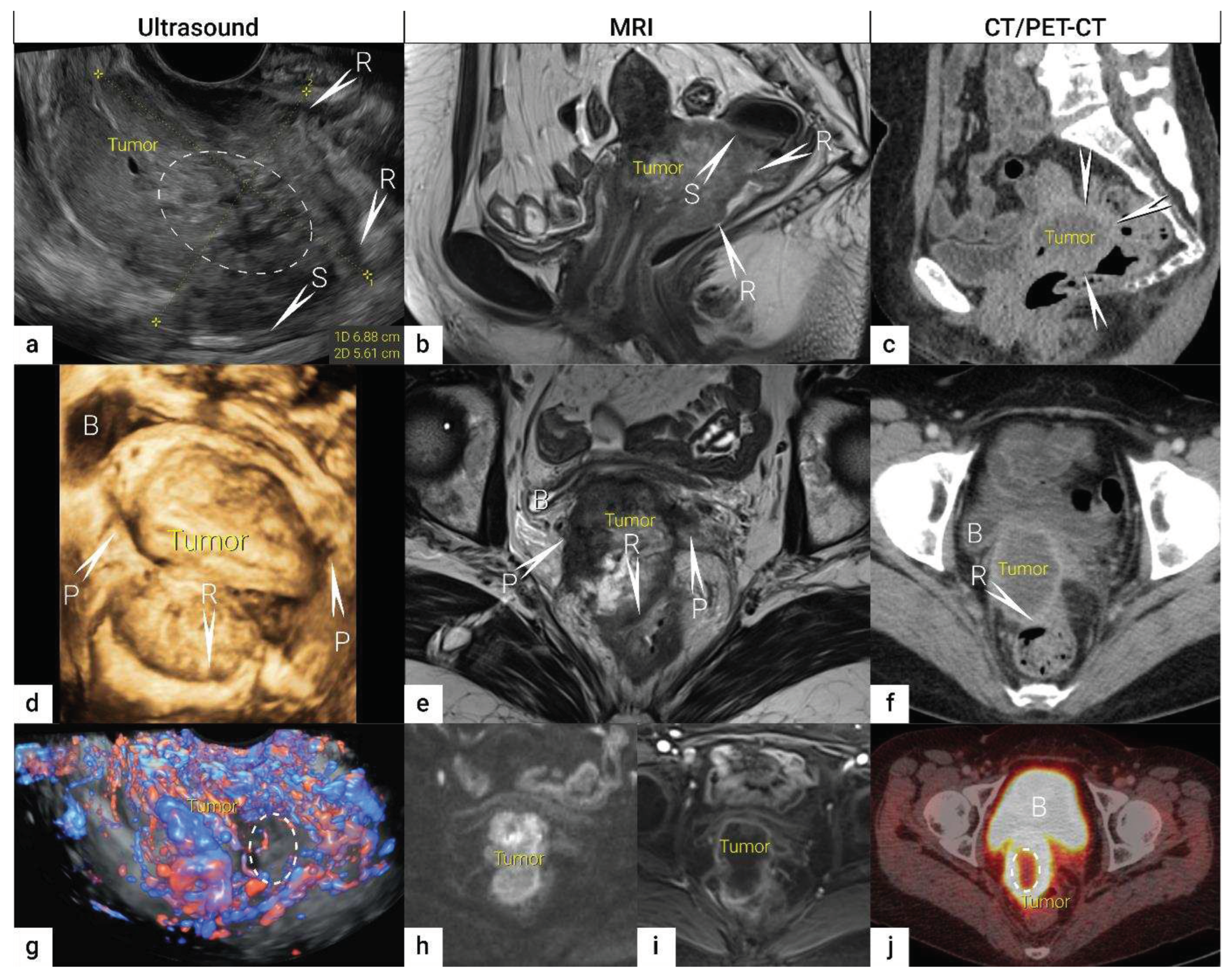
3. Local (pelvic) workup for different stages
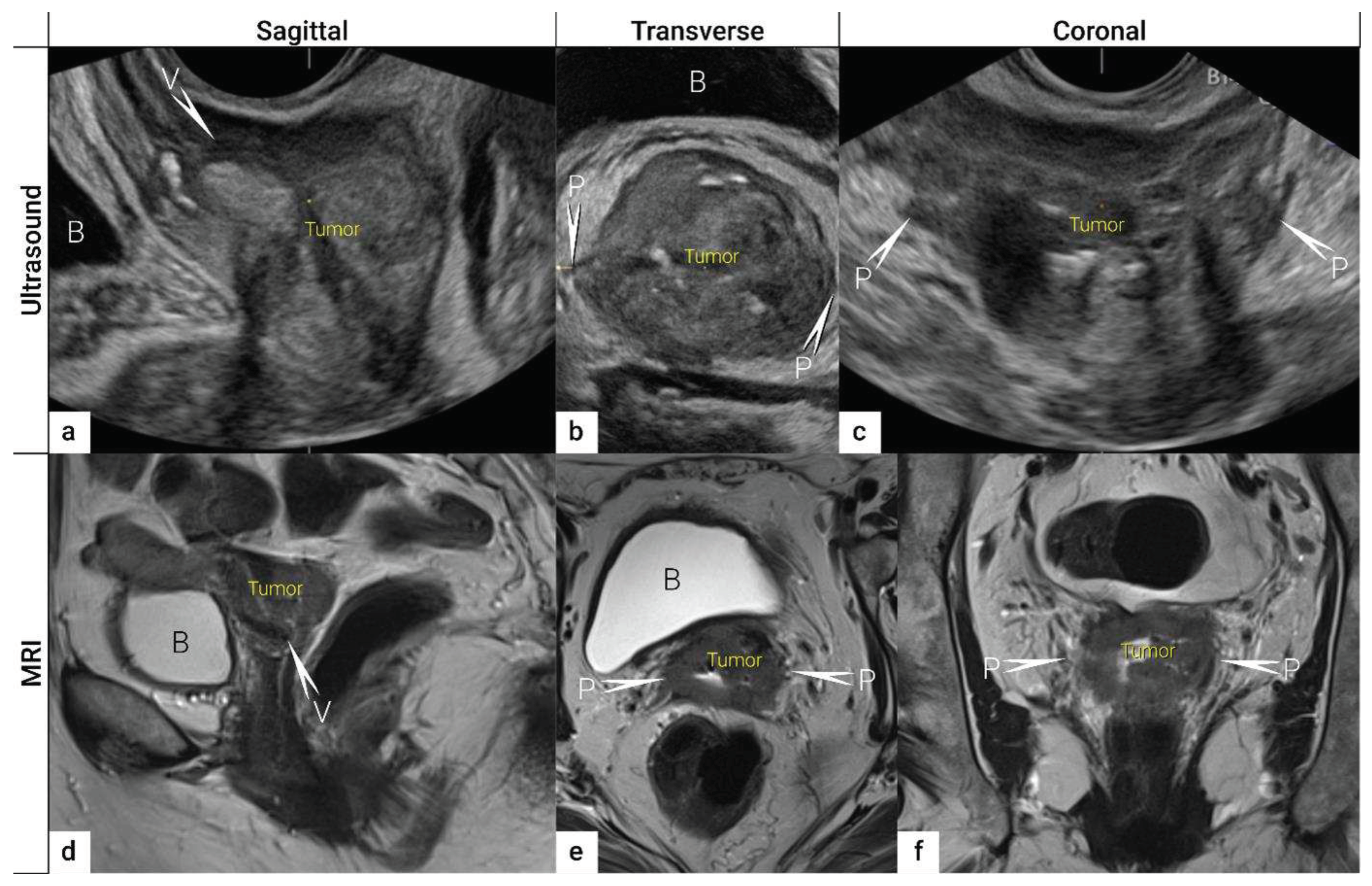
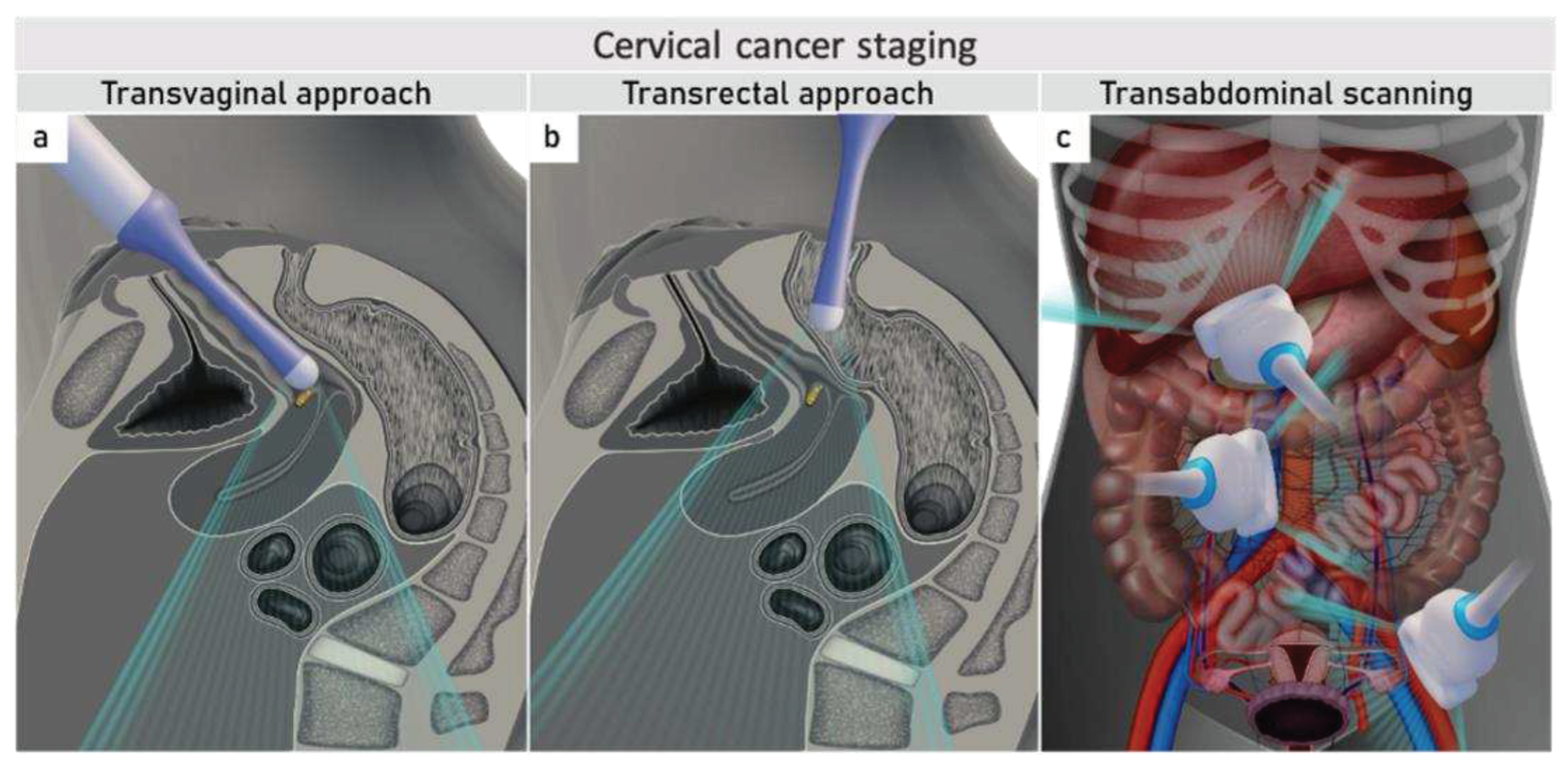
3.1. Tumor detection
3.2. Tumor delineation within cervix (tumour size, depth of stromal invasion, minimum of uninvolved stroma, and cranial tumour-free margin).
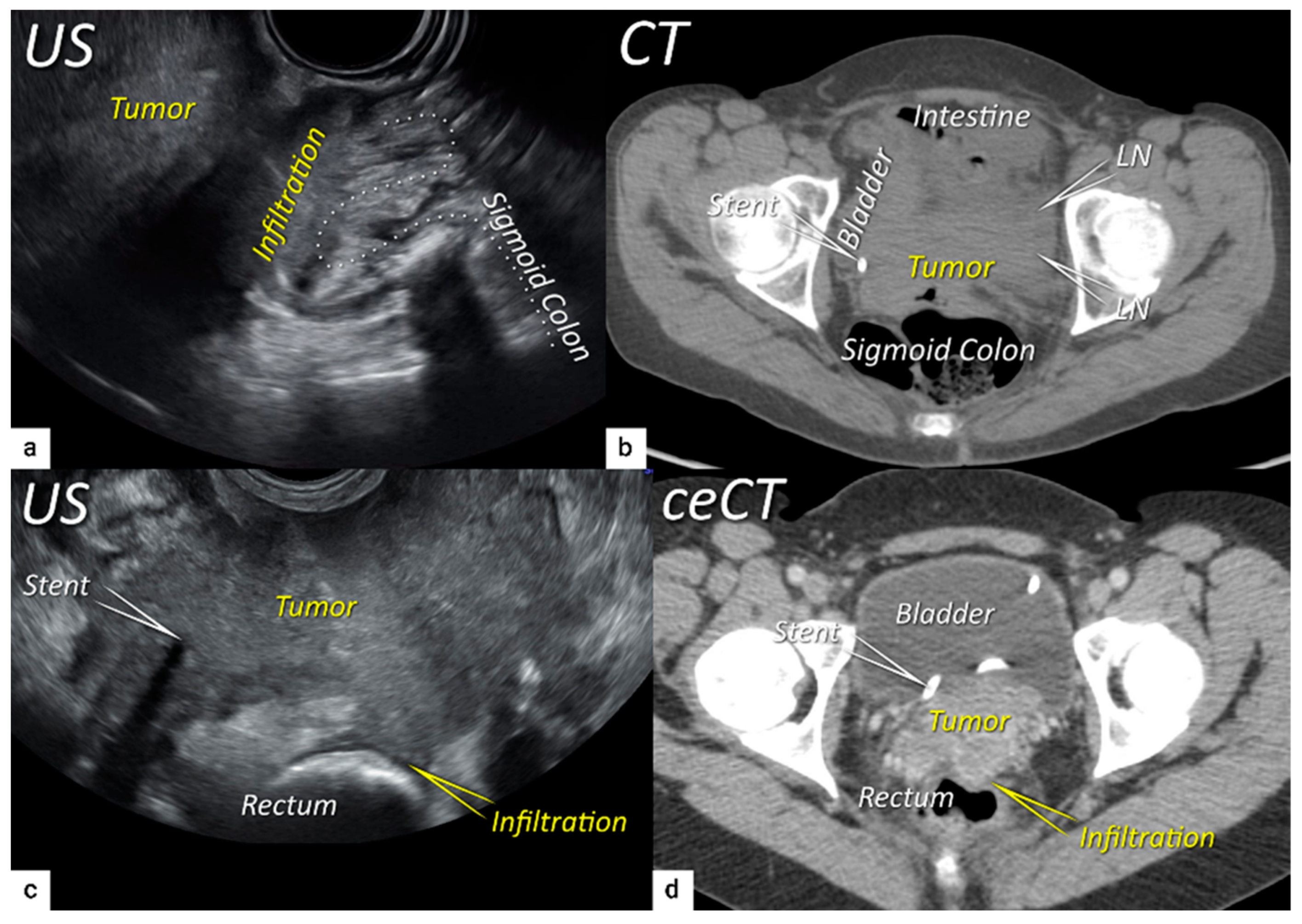
3.3. Extrauterine extension (vagina, parametria, pelvic side wall, hydronephrosis and others)
3.4. Extension to surrounding organs (bladder, rectum, sigmoid colon)
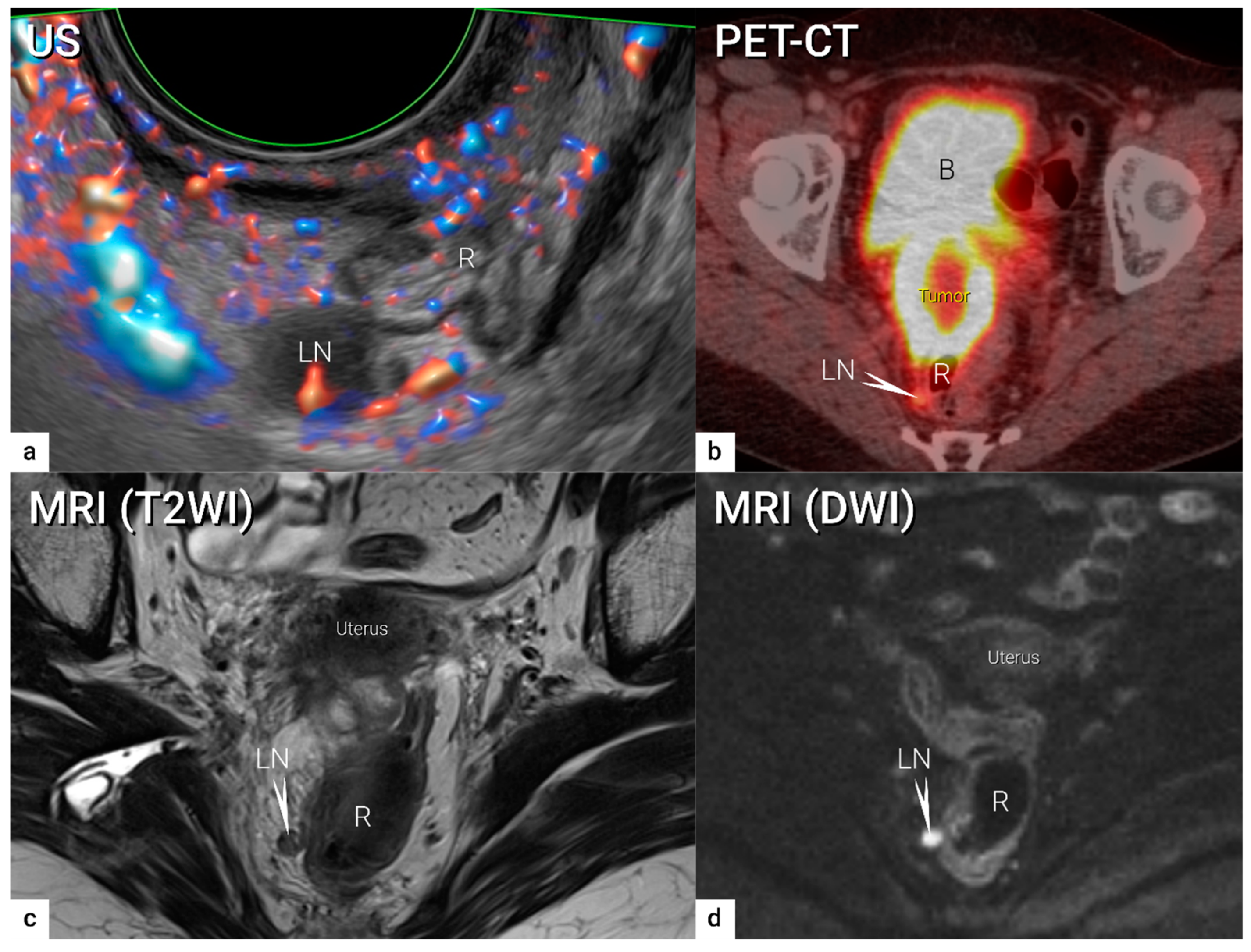
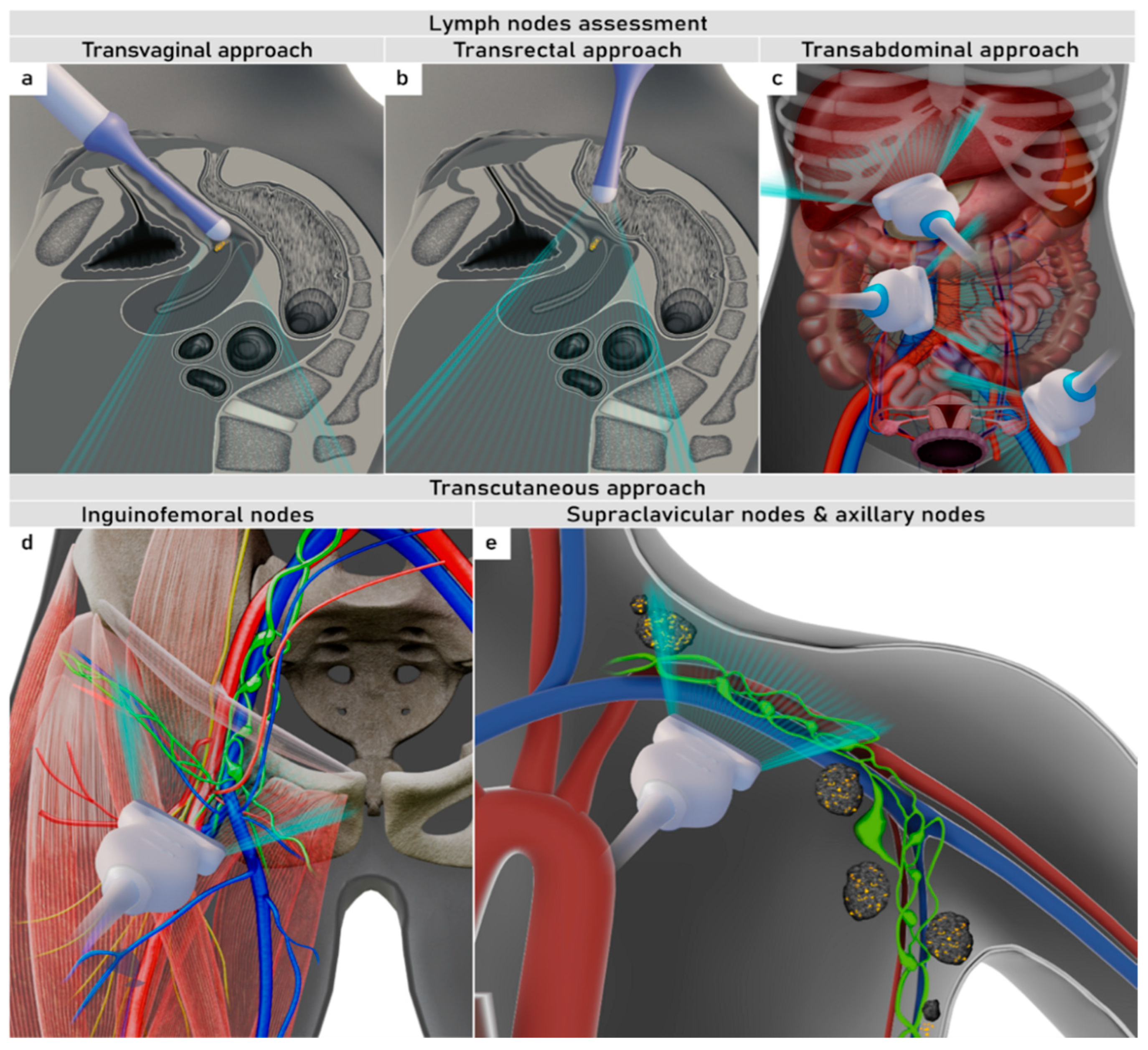
4. Nodal and distant diagnostic workup
5. Conclusions
Supplementary Materials
Author Contributions
- a)
- Conception of the work, acquisition and interpretation of literature data AND
- b)
- Drafting the work or revising it critically AND
- c)
- Final approve the version to be published AND
- d)
- Agree to be accountable for all aspects of the work in ensuring that any part of the work has been appropriately investigated.
Funding
Acknowledgments
Conflicts of Interest
References
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2006, 95 (Suppl. S1), S43–103. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S.; Zigliani, L.; Odicino, F. Revised FIGO staging for carcinoma of the cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2009, 105, 107–108. [Google Scholar] [CrossRef]
- Innocenti, P.; Pulli, F.; Savino, L.; Nicolucci, A.; Pandimiglio, A.; Menchi, I.; Massi, G. Staging of cervical cancer: reliability of transrectal US. Radiology 1992, 185, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G.; Snyder, B.; Coakley, F.; Reinhold, C.; Thomas, G.; Amendola, M.; Schwartz, L.H.; Woodward, P.; Pannu, H.; Hricak, H. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006, 24, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Hricak, H.; Gatsonis, C.; Chi, D.S.; Amendola, M.A.; Brandt, K.; Schwartz, L.H.; Koelliker, S.; Siegelman, E.S.; Brown, J.J.; McGhee, R.B., Jr.; et al. Role of imaging in pretreatment evaluation of early invasive cervical cancer: results of the intergroup study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005, 23, 9329–9337. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. European radiology 2013, 23, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Amendola, M.A.; Hricak, H.; Mitchell, D.G.; Snyder, B.; Chi, D.S.; Long, H.J., 3rd; Fiorica, J.V.; Gatsonis, C. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: results of intergroup protocol ACRIN 6651/GOG 183. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005, 23, 7454–7459. [Google Scholar] [CrossRef]
- Dostalek, L.; Avall-Lundqvist, E.; Creutzberg, C.L.; Kurdiani, D.; Ponce, J.; Dostalkova, I.; Cibula, D. ESGO Survey on Current Practice in the Management of Cervical Cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2018, 28, 1226–1231. [Google Scholar] [CrossRef]
- Cibula, D.; Potter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Kohler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2018, 28, 641–655. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023. Int J Gynecol Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin 2021, 71, 287–298. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef]
- Bhatla, N.; Denny, L. FIGO Cancer Report 2018. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2018, 143 (Suppl. S2), 2–3. [Google Scholar] [CrossRef]
- Corrigendum to "Revised FIGO staging for carcinoma of the cervix uteri" [Int J Gynecol Obstet 145(2019) 129-135]. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2019, 147, 279–280. [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef] [PubMed]
- Sponholtz, S.E.; Mogensen, O.; Hildebrandt, M.G.; Schledermann, D.; Parner, E.; Markauskas, A.; Frøding, L.P.; Fuglsang, K.; Holm, J.; Bjørnholt, S.M.; Jensen, P.T. From FIGO-2009 to FIGO-2018 in women with early-stage cervical cancer; Does the revised staging reflect risk groups? Gynecologic oncology 2021, 163, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.W.; Massad, L.S.; Mutch, D.G.; Powell, M.A.; Thaker, P.H.; McCourt, C.; Hagemann, A.; Fuh, K.; Kuroki, L.; Schwarz, J.K.; et al. FIGO 2018 staging criteria for cervical cancer: Impact on stage migration and survival. Gynecologic oncology 2020, 157, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Konishi, I.; Mikami, M. Validation of the 2018 FIGO cervical cancer staging system. Gynecologic oncology 2019, 152, 87–93. [Google Scholar] [CrossRef]
- McComas, K.N.; Torgeson, A.M.; Ager, B.J.; Hellekson, C.; Burt, L.M.; Maurer, K.A.; Werner, T.L.; Gaffney, D.K. The variable impact of positive lymph nodes in cervical cancer: Implications of the new FIGO staging system. Gynecologic oncology 2020, 156, 85–92. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.; Adam, J.A.; Buist, M.R.; van de Vijver, M.J.; Rasch, C.R.; Stoker, J.; Bipat, S.; Stalpers, L.J. Role of MRI in detecting involvement of the uterine internal os in uterine cervical cancer: systematic review of diagnostic test accuracy. European journal of radiology 2013, 82, e422–428. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Slama, J.; Dostálek, L.; Fischerová, D.; Germanova, A.; Frühauf, F.; Dundr, P.; Nemejcova, K.; Jarkovsky, J.; Sebestova, S.; et al. Tumour-free distance: a novel prognostic marker in patients with early-stage cervical cancer treated by primary surgery. British journal of cancer 2021, 124, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Pedone Anchora, L.; Zannoni, G.F.; Carbone, V.; Bruno, M.; Fedele, C.; Gallotta, V.; Chiantera, V.; Avesani, G.; Gui, B.; et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: a retrospective, single-centre, cohort study. British journal of cancer 2021, 125, 561–568. [Google Scholar] [CrossRef]
- Pradhan, T.S.; Duan, H.; Katsoulakis, E.; Salame, G.; Lee, Y.C.; Abulafia, O. Hydronephrosis as a prognostic indicator of survival in advanced cervix cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2011, 21, 1091–1096. [Google Scholar] [CrossRef]
- Nam, H.; Huh, S.J.; Park, W.; Bae, D.S.; Kim, B.G.; Lee, J.H.; Kim, C.K.; Park, B.K. Prognostic significance of MRI-detected bladder muscle and/or serosal invasion in patients with cervical cancer treated with radiotherapy. Br J Radiol 2010, 83, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Jarkovsky, J.; Kocian, R.; Dundr, P.; Klat, J.; Zapardiel, I.; Landoni, L.; van Lonkhuijzen, L.; Frühauf, F.; Zikan, M.; et al. Magnetic resonance or expert ultrasound in preoperative local staging of patients with early-stage cervical cancer: final results of the SENTIX prospective, single-arm, international trial (CEEGOG CX-01; ENGOT-CX2). International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2023, 33, A2. [Google Scholar]
- Tsalafoutas, I.A.; Koukourakis, G.V. Patient dose considerations in computed tomography examinations. World journal of radiology 2010, 2, 262–268. [Google Scholar] [CrossRef]
- Bipat, S.; Glas, A.S.; van, d., V; Zwinderman, A.H.; Bossuyt, P.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol.Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Lura, N.; Blaakær, J.; Fischerova, D.; Werner, H.M.J. What Is the Role of Imaging at Primary Diagnostic Work-Up in Uterine Cervical Cancer? Current oncology reports 2019, 21, 77. [Google Scholar] [CrossRef]
- Lee, S.I.; Catalano, O.A.; Dehdashti, F. Evaluation of gynecologic cancer with MR imaging, 18F-FDG PET/CT, and PET/MR imaging. J Nucl Med 2015, 56, 436–443. [Google Scholar] [CrossRef]
- Lazzari, R.; Cecconi, A.; Jereczek-Fossa, B.A.; Travaini, L.L.; Dell' Acqua, V.; Cattani, F.; Rizzo, S.; Fodor, C.; Landoni, F.; Orecchia, R. The role of [(18)F]FDG-PET/CT in staging and treatment planning for volumetric modulated Rapidarc radiotherapy in cervical cancer: experience of the European Institute of Oncology, Milan, Italy. Ecancermedicalscience 2014, 8, 405. [Google Scholar] [CrossRef]
- Kaushik, A.; Jaimini, A.; Tripathi, M.; D'Souza, M.; Sharma, R.; Mondal, A.; Mishra, A.K.; Dwarakanath, B.S. Estimation of radiation dose to patients from (18) FDG whole body PET/CT investigations using dynamic PET scan protocol. The Indian journal of medical research 2015, 142, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. European radiology 2011, 21, 1102–1110. [Google Scholar] [CrossRef]
- Lura, N.; Wagner-Larsen, K.S.; Forsse, D.; Trovik, J.; Halle, M.K.; Bertelsen, B.I.; Salvesen, Ø.; Woie, K.; Krakstad, C.; Haldorsen, I.S. What MRI-based tumor size measurement is best for predicting long-term survival in uterine cervical cancer? Insights into imaging 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Lopes Dias, J.; Cunha, T.M. Added value of diffusion-weighted MRI in detection of cervical cancer recurrence: comparison with morphologic and dynamic contrast-enhanced MRI sequences. Diagn Interv Radiol 2015, 21, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. European radiology 2021, 31, 7802–7816. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.; Rangarajan, D.; Kubik-Huch, R.A. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. European journal of radiology 2010, 76, 367–385. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Kim, B. Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2-IIA cervical cancer. European radiology 2014, 24, 1081–1088. [Google Scholar] [CrossRef]
- Qu, J.R.; Qin, L.; Li, X.; Luo, J.P.; Li, J.; Zhang, H.K.; Wang, L.; Shao, N.N.; Zhang, S.N.; Li, Y.L.; et al. Predicting Parametrial Invasion in Cervical Carcinoma (Stages IB1, IB2, and IIA): Diagnostic Accuracy of T2-Weighted Imaging Combined With DWI at 3 T. AJR. American journal of roentgenology 2018, 210, 677–684. [Google Scholar] [CrossRef]
- Sarabhai, T.; Schaarschmidt, B.M.; Wetter, A.; Kirchner, J.; Aktas, B.; Forsting, M.; Ruhlmann, V.; Herrmann, K.; Umutlu, L.; Grueneisen, J. Comparison of (18)F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur J Nucl Med Mol Imaging 2018, 45, 67–76. [Google Scholar] [CrossRef]
- Chiappa, V.; Di Legge, A.; Valentini, A.L.; Gui, B.; Micco, M.; Ludovisi, M.; Giansiracusa, C.; Testa, A.C.; Valentin, L. Agreement of two-dimensional and three-dimensional transvaginal ultrasound with magnetic resonance imaging with regard to parametrial infiltration in cervical cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2014. [Google Scholar] [CrossRef]
- Fischerova, D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: a review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2011, 38, 246–266. [Google Scholar] [CrossRef]
- Fischerova, D.; Cibula, D.; Stenhova, H.; Vondrichova, H.; Calda, P.; Zikan, M.; Freitag, P.; Slama, J.; Dundr, P.; Belacek, J. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2008, 18, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Ludovisi, M.; Manfredi, R.; Zannoni, G.; Gui, B.; Basso, D.; Di Legge, A.; Licameli, A.; Di Bidino, R.; Scambia, G.; Ferrandina, G. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2009, 34, 335–344. [Google Scholar] [CrossRef]
- Epstein, E.; Testa, A.; Gaurilcikas, A.; Di Legge, A.; Ameye, L.; Atstupenaite, V.; Valentini, A.L.; Gui, B.; Wallengren, N.O.; Pudaric, S.; et al. Early-stage cervical cancer: tumor delineation by magnetic resonance imaging and ultrasound - a European multicenter trial. Gynecologic oncology 2013, 128, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Madár, I.; Hudelist, G.; Arányi, Z.; Turtóczki, K.; Rigó, J., Jr.; Ács, N.; Lipták, L.; Fancsovits, V.; Bokor, A. Visualization of sacral nerve roots and sacral plexus on gynecological transvaginal ultrasound: feasibility study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2023, 62, 290–299. [Google Scholar] [CrossRef]
- Fischerova, D.; Santos, G.; Wong, L.; Yulzari, V.; Bennett, R.J.; Dundr, P.; Burgetova, A.; Barsa, P.; Szabó, G.; Sousa, N.; et al. Imaging in gynecological disease (26): clinical and ultrasound characteristics of benign retroperitoneal pelvic peripheral-nerve-sheath tumors. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2023, 62, 727–738. [Google Scholar] [CrossRef]
- Iwamoto, K.; Kigawa, J.; Minagawa, Y.; Miura, H.; Terakawa, N. Transvaginal ultrasonographic diagnosis of bladder-wall invasion in patients with cervical cancer. Obstetrics and gynecology 1994, 83, 217–219. [Google Scholar] [PubMed]
- Huang, W.C.; Yang, J.M.; Yang, Y.C.; Yang, S.H. Ultrasonographic characteristics and cystoscopic correlates of bladder wall invasion by endophytic cervical cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2006, 27, 680–686. [Google Scholar] [CrossRef]
- Testa, A.C.; Ferrandina, G.; Moro, F.; Pasciuto, T.; Moruzzi, M.C.; De Blasis, I.; Mascilini, F.; Foti, E.; Autorino, R.; Collarino, A.; et al. PRospective Imaging of CErvical cancer and neoadjuvant treatment (PRICE) study: role of ultrasound to predict partial response in locally advanced cervical cancer patients undergoing chemoradiation and radical surgery. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2018, 51, 684–695. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Castillo, G.; Martinez-Monge, R.; Jurado, M. Transvaginal color Doppler sonography for predicting response to concurrent chemoradiotherapy for locally advanced cervical carcinoma. J Clin Ultrasound 2004, 32, 267–272. [Google Scholar] [CrossRef]
- Vanderpuye, V. Renal sonography in the diagnosis of renal obstruction or hydronephrosis in patients with cervical cancer. J Clin Ultrasound 2002, 30, 424–427. [Google Scholar] [CrossRef]
- Pálsdóttir, K.; Epstein, E. A Pilot Study on Diagnostic Performance of Contrast-Enhanced Ultrasonography for Detection of Early Cervical Cancer. Ultrasound in medicine & biology 2018, 44, 1664–1671. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med 2018, 39, e2–e44. [Google Scholar] [CrossRef] [PubMed]
- Hricak, H.; Gatsonis, C.; Coakley, F.V.; Snyder, B.; Reinhold, C.; Schwartz, L.H.; Woodward, P.J.; Pannu, H.K.; Amendola, M.; Mitchell, D.G. Early invasive cervical cancer: CT and MR imaging in preoperative evaluation - ACRIN/GOG comparative study of diagnostic performance and interobserver variability. Radiology 2007, 245, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Pálsdóttir, K.; Fridsten, S.; Blomqvist, L.; Alagic, Z.; Fischerova, D.; Gaurilcikas, A.; Hasselrot, K.; Jäderling, F.; Testa, A.C.; Sundin, A.; Epstein, E. Interobserver agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2021, 58, 773–779. [Google Scholar] [CrossRef]
- Federico, M.; Hernandez-Socorro, C.R.; Ribeiro, I.; Martin, J.G.; Oramas, M.D.R.; Saez-Bravo, M.L.; Jimenez, P.C.L. Prospective intra/inter-observer evaluation of pre-brachytherapy cervical cancer tumor width measured in TRUS and MR imaging. Radiation oncology (London, England) 2019, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Larsen, K.S.; Lura, N.; Salvesen, Ø.; Halle, M.K.; Forsse, D.; Trovik, J.; Smit, N.; Krakstad, C.; Haldorsen, I.S. Interobserver agreement and prognostic impact for MRI-based 2018 FIGO staging parameters in uterine cervical cancer. European radiology 2022, 32, 6444–6455. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Di Legge, A.; Masback, A.; Lindqvist, P.G.; Kannisto, P.; Testa, A.C. Sonographic characteristics of squamous cell cancer and adenocarcinoma of the uterine cervix. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2010, 36, 512–516. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tanaka, Y.O.; Nishida, M.; Tsunoda, H.; Yoshikawa, H.; Itai, Y. MR imaging of the uterine cervix: imaging-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc 2003, 23, 425–445, quiz 534-425. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Dresen, R.; De Keyzer, F. Novel imaging techniques in gynaecological cancer. Current opinion in oncology 2017, 29, 335–342. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, B.; Li, Y.; Lu, J.; Qiang, J. Diagnostic performance of MR imaging in evaluating prognostic factors in patients with cervical cancer: a meta-analysis. European radiology 2020, 30, 1405–1418. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Pinkavova, I.; Fruhauf, F.; Dundr, P.; Nemejcova, K.; Burgetova, A.; Cibula, D. The role of ultrasound in planning fertility sparing surgery and individual treatment in early stage cervical cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2012, 40, 51. [Google Scholar]
- Pinkavova, I.; Dundr, P.; Fischerova, D.; Zikan, M.; Slama, J.; Cibula, D. Intra-operative ultrasound in fertility sparing procedure for cervical cancer (OC24.04). Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2012, 40, 51. [Google Scholar]
- Pinkavova, I.; Fischerova, D.; Zikan, M.; Burgetova, A.; Slama, J.; Svarovsky, J.; Dundr, P.; Dusek, L.; Cibula, D. Transrectal Ultrasound and Magnetic Resonance Imaging in the Evaluation of Tumor Size after Application of Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2013. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Pinkavova, I.; Frühauf, F.; Dundr, P.; Nemejcova, K.; Burgetova, A.; Cibula, D. OC24. 05: The role of ultrasound in planning fertility sparing surgery and individual treatment in early stage cervical cancer. Ultrasound Obst Gyn 2012, 40, 51. [Google Scholar]
- Rizzo, S.; Calareso, G.; Maccagnoni, S.; Angileri, S.A.; Landoni, F.; Raimondi, S.; Pasquali, E.; Lazzari, R.; Bellomi, M. Pre-operative MR evaluation of features that indicate the need of adjuvant therapies in early stage cervical cancer patients. A single-centre experience. European journal of radiology 2014, 83, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Stukan, M.; Buderath, P.; Szulczyński, B.; Gębicki, J.; Kimmig, R. Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer-Analysis of Patients from the Prospective Study on Total Mesometrial Resection. Diagnostics (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Atun, R.; Ward, Z.J.; Scott, A.M.; Hricak, H.; Vargas, H.A. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: systematic review and meta-analysis. European radiology 2020, 30, 5560–5577. [Google Scholar] [CrossRef]
- Kim, M.J.; Chung, J.J.; Lee, Y.H.; Lee, J.T.; Yoo, H.S. Comparison of the use of the transrectal surface coil and the pelvic phased-array coil in MR imaging for preoperative evaluation of uterine cervical carcinoma. AJR. American journal of roentgenology 1997, 168, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.K.; Hricak, H.; Subak, L.L.; Zaloudek, C.J.; Powell, C.B. Preoperative staging of cervical carcinoma: phased array coil fast spin-echo versus body coil spin-echo T2-weighted MR imaging. AJR. American journal of roentgenology 1998, 171, 707–711. [Google Scholar] [CrossRef]
- Fischerova, D.; Ballaschova, T.; Dostalek, L.; Frühauf, F.; Zikan, M.; Slama, J.; Kocian, R.; Germanova, A.; Němejcova, K.; Jarkovsky, J.; Cibula, D. Diagnostic performance of ultrasound in the assessment of parametrial involvement before surgery in cervical cancer patients (diagnostic accuracy study). International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2019, 29. [Google Scholar]
- Alcazar, J.L.; García, E.; Machuca, M.; Quintana, R.; Escrig, J.; Chacón, E.; Mínguez, J.A.; Chiva, L. Magnetic resonance imaging and ultrasound for assessing parametrial infiltration in cervical cancer. A systematic review and meta-analysis. Medical ultrasonography 2020, 22, 85–91. [Google Scholar] [CrossRef]
- Moro, F.; Zermano, S.; Ianieri, M.M.; De Cicco Nardone, A.; Carfagna, P.; Ciccarone, F.; Ercoli, A.; Querleu, D.; Scambia, G.; Testa, A.C. Dynamic transvaginal ultrasound examination for assessing anatomy of parametrium. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2023, 62, 904–906. [Google Scholar] [CrossRef]
- Nicolet, V.; Carignan, L.; Bourdon, F.; Prosmanne, O. MR imaging of cervical carcinoma: a practical staging approach. Radiographics : a review publication of the Radiological Society of North America, Inc 2000, 20, 1539–1549. [Google Scholar] [CrossRef]
- Rockall, A.G.; Ghosh, S.; Alexander-Sefre, F.; Babar, S.; Younis, M.T.; Naz, S.; Jacobs, I.J.; Reznek, R.H. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecologic oncology 2006, 101, 244–249. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, T.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, definitions and measurements to describe sonographic features of lymph nodes: consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2021, 57, 861–879. [Google Scholar] [CrossRef]
- Fischerova, D.e.a. Terms, definitions and measurements to describe the so-nographic features of inguinal lymph nodes in patients with vulvar cancer: a consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obstet Gynecol 2021. [CrossRef]
- Shen, G.; Zhou, H.; Jia, Z.; Deng, H. Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: a systematic review and meta-analysis. Br J Radiol 2015, 88, 20150063. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N.; Amant, F.; Michielsen, K.; De Keyzer, F.; Fieuws, S.; Van Calsteren, K.; Dresen, R.C.; Gziri, M.M.; Vandecaveye, V. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. European radiology 2018, 28, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; McCook, B.M.; Torok, F.S. An introduction to PET-CT imaging. Radiographics : a review publication of the Radiological Society of North America, Inc 2004, 24, 523–543. [Google Scholar] [CrossRef]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Kaji, Y.; Sugimura, K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. European radiology 2009, 19, 1529–1536. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, H.J.; Park, S.Y.; Lee, H.Y.; Seo, S.S.; Yoo, C.W.; Jung, D.C.; Kang, S.; Cho, K.S. Additional value of MR/PET fusion compared with PET/CT in the detection of lymph node metastases in cervical cancer patients. European journal of cancer 2009, 45, 2103–2109. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, J.; Gao, J.; Guo, L.; Zhou, H.; Hu, Y.; Zhu, C.; Li, Q.; Ma, X. Diagnostic role of 18F-FDG PET/MRI in patients with gynecological malignancies of the pelvis: A systematic review and meta-analysis. PloS one 2017, 12, e0175401. [Google Scholar] [CrossRef]
- Choi, H.J.; Ju, W.; Myung, S.K.; Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci 2010, 101, 1471–1479. [Google Scholar] [CrossRef]
- Tian, Y.; Luo, H. Diagnostic accuracy of transvaginal ultrasound examination for local staging of cervical cancer: a systematic review and meta-analysis. Medical ultrasonography 2022, 24, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.K.; Chung, D.C.; Seo, S.S.; Kim, J.Y.; Nam, B.H.; Park, S.Y. Diagnostic value of (18)F-FDG PET for evaluation of paraaortic nodal metastasis in patients with cervical carcinoma: a metaanalysis. J Nucl Med 2010, 51, 360–367. [Google Scholar] [CrossRef] [PubMed]
- James, R.M.; Cruickshank, M.E.; Siddiqui, N.; Guideline Development, G. Management of cervical cancer: summary of SIGN guidelines. Bmj 2008, 336, 41–43. [Google Scholar] [CrossRef]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, M.; Guerra, L.; Montanelli, L.; Crivellaro, C.; Buda, A.; Dell'Anna, T.; Picchio, M.; Milani, R.; Fruscio, R.; Messa, C. Preoperative staging of cervical cancer: is 18-FDG-PET/CT really effective in patients with early stage disease? Gynecologic oncology 2011, 123, 236–240. [Google Scholar] [CrossRef]
- Selman, T.J.; Mann, C.; Zamora, J.; Appleyard, T.L.; Khan, K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: a systematic review and meta-analysis. CMAJ 2008, 178, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, S.; Li, S. A Comprehensive Comparison of CT, MRI, Positron Emission Tomography or Positron Emission Tomography/CT, and Diffusion Weighted Imaging-MRI for Detecting the Lymph Nodes Metastases in Patients with Cervical Cancer: A Meta-Analysis Based on 67 Studies. Gynecol Obstet Invest 2017, 82, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhang, Y.; Ren, H. Meta-analysis of PET/CT Detect Lymph Nodes Metastases of Cervical Cancer. Open medicine (Warsaw, Poland) 2018, 13, 436–442. [Google Scholar] [CrossRef]
- Song, Q.; Yu, Y.; Zhang, X.; Zhu, Y.; Luo, Y.; Yu, T.; Sun, J.; Liu, F.; Dong, Y. Value of MRI and diffusion-weighted imaging in diagnosing normal-sized pelvic lymph nodes metastases in patients with cervical cancer. Br J Radiol 2022, 95, 20200203. [Google Scholar] [CrossRef] [PubMed]
- Fruhauf, F.; Zikan, M.; Kocian, R.; Cibula, D.; Fischerova, D. OC11.05: Diagnostic accuracy of ultrasound in preoperative assessment of lymph nodes in cervical cancer. Ultrasound Obst Gyn 2022, 60, 16–18. [Google Scholar]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. European radiology 2018, 28, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Q.; Dong, L.; Jia, Y.; Hua, C.; Mi, F.; Li, C. Different imaging techniques for the detection of pelvic lymph nodes metastasis from gynecological malignancies: a systematic review and meta-analysis. Oncotarget 2017, 8, 14107–14125. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Kou, C.; Bai, W.; Yu, X.; Duan, R.; Zhu, B.; Li, Y.; Hua, W.; Ren, X.; Yang, Y. The diagnostic performance of PET/CT scans for the detection of para-aortic metastatic lymph nodes in patients with cervical cancer: A meta-analysis. PloS one 2019, 14, e0220080. [Google Scholar] [CrossRef]
- He, T.; Sun, J.; Wu, J.; Wang, H.; Liang, C.; Wang, H.; Li, S.; Su, S. PET-CT versus MRI in the diagnosis of lymph node metastasis of cervical cancer: A meta-analysis. Microsc Res Tech 2022, 85, 1791–1798. [Google Scholar] [CrossRef]
- Cosin, J.A.; Fowler, J.M.; Chen, M.D.; Paley, P.J.; Carson, L.F.; Twiggs, L.B. Pretreatment surgical staging of patients with cervical carcinoma: the case for lymph node debulking. Cancer 1998, 82, 2241–2248. [Google Scholar] [CrossRef]
- Marnitz, S.; Kohler, C.; Roth, C.; Fuller, J.; Hinkelbein, W.; Schneider, A. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecologic oncology 2005, 99, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, E.; Narducci, F.; Frumovitz, M.; Lesoin, A.; Castelain, B.; Baranzelli, M.C.; Taieb, S.; Fournier, C.; Querleu, D. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecologic oncology 2007, 105, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, A.T.; Marnitz, S.; Soares Nunes, J.; Mattos de Cunha Andrade, C.E.; Scapulatempo Neto, C.; Blohmer, J.U.; Herrmann, J.; Kerr, L.M.; Martus, P.; Schneider, A.; et al. Incidence of Histologically Proven Pelvic and Para-Aortic Lymph Node Metastases and Rate of Upstaging in Patients with Locally Advanced Cervical Cancer: Results of a Prospective Randomized Trial. Oncology 2017, 92, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Tsunoda, A.T.; Martus, P.; Vieira, M.; Affonso Junior, R.J.; Nunes, J.; Budach, V.; Hertel, H.; Mustea, A.; Sehouli, J.; et al. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB-IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2020, 30, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Marnitz-Schulze, S.; Tsunoda, A.; Martus, P.; Vieira, M.V.; Affonso, R.; Nunes, J.S.; Budach, V.; Schneider, A.; Hertel, H.; Mustea, A.; et al. UTERUS-11 STUDY: A randomized clinical trial on surgical staging versus ct-staging prior to primary chemoradiation in patients with FIGO2009 stages IIB-IVA cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2019, 29, A1–A197. [Google Scholar]
- Dabi, Y.; Simon, V.; Carcopino, X.; Bendifallah, S.; Ouldamer, L.; Lavoue, V.; Canlorbe, G.; Raimond, E.; Coutant, C.; Graesslin, O.; et al. Therapeutic value of surgical paraaortic staging in locally advanced cervical cancer: a multicenter cohort analysis from the FRANCOGYN study group. Journal of translational medicine 2018, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Cibula, D.; Dundr, P.; Zikan, M.; Calda, P.; Freitag, P.; Slama, J. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2008, 18, 833–837. [Google Scholar] [CrossRef]
- Zikan, M.; Fischerova, D.; Pinkavova, I.; Dundr, P.; Cibula, D. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2010, 36, 767–772. [Google Scholar] [CrossRef]
- Meva, J.; Chaudhary, R.K.; Bhaduri, D.; Bhatia, M.; Hatti, S.; Ba, R. Lacunae in International Federation of Gynecology and Obstetrics (FIGO) classification for cervical carcinoma: observational study using TNM classification as comparator. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2013, 23, 1071–1077. [Google Scholar] [CrossRef]
| Expert Ultrasound | MRI | CECT | FDG-PET-CT | |
|---|---|---|---|---|
| Cost | 1x | 4x | 2x | 6x |
| Availability | Specialized centers | Most hospitals | Most hospitals | Specialized centers |
| Range of examination | Abdomen and pelvis, peripheral lymph nodes | Whole body | Whole body | Whole body |
| Examination duration (minutes) | 15-30# | 30-45 (pelvic) 60 (whole-body) ~15 (reading time) |
5 ~10 (reading time) |
30 ~20 (reading time) |
| Dynamic evaluation* | Yes | No | No | No |
| Preparation before imaging | None | Antiperistaltic agents | None | 4h fasting and 1h rest prior to scanning |
| Contrast agent | None** | Gadolinium-based | Iodine-based | FDG-radiotracer and iodine-based |
| Radiation exposure | None | None | 10-20 mSvß | 20-25 mSvß |
| Limitations for application and factors impacting diagnostic quality | No contraindications. Limited depiction of abdominal deeper structures when overlying bowel gas/air. | Contraindication if severe claustrophobia, and for some metal- or cochlear implants/ cardiac pacemakers. Image artefacts from implants. |
Contraindication for iodine-based contrast agent: - renal insufficiency& - hyperthyroidism - severe iodine allergy Image artefacts from implants. |
Contraindication for iodine-based contrast agent: - renal insufficiency& - hyperthyroidism - severe iodine allergy Image artefacts from implants. |
| Dependence of expertise | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





