1. Introduction
Neurological diseases are the leading cause of disability worldwide [
1]. The most common neurological diseases are multiple sclerosis, Parkinson's disease and stroke. Patients with these conditions are at greater risk of falling than the general population. In fact, patients with Parkinson's disease have a high risk of falling, with a prevalence of 40 to 70% [
2,
3]. In patients who suffered a stroke, this risk can raise to 73% for falls, recorded within 6 months of discharge from hospital [
4]. According to a meta-analysis aiming at evaluating the risk of falls in patients with multiple sclerosis over a 3-month period, 56% had experienced one fall episode and 37% had experienced more than two episodes [
5].
The impact of these falls can be devastating for these already fragile patients, with consequences including fractures, injuries, fear of falling, balance problems and increased healthcare costs [
6,
7,
8,
9,
10,
11].
Prevention of falls should therefore be a major goal in neurological rehabilitation. The effectiveness of physical activity in preventing the risk of falls has been well established [
12,
13].
Furthermore, the literature describes many beneficial effects of physical activity on various functional parameters such as gait, balance, strength, and endurance [
14,
15,
16]. In addition to traditional physiotherapy and occupational therapy, many ways to improve and accelerate the rehabilitation process of patients exist, including innovative ways to improve physical function [
17,
18] Technological innovations are enabling the development of new rehabilitation methods that provide individualised programs and immediate feedback. Among these new technologies, the HUBER® (LPG Systems, Valence, France) has been developed as a training tool to improve the rehabilitation of different patient profiles. It is a motorised oval platform that performs rotary oscillatory movements with controlled amplitude and speed. It is also equipped with 2 large handgrips with force sensors mounted on a mobile column (
Figure 1).
Such a tool allows the simultaneous assessment of the patient's balance, core stability, core strength and overall body strength. Training can then be adjusted accordingly. The patient must continuously adjust his or her posture by performing isometric push and pull movements with the arms. This results in postural and muscular adaptation with visual feedback. One of the main aims of the HUBER® would therefore be to improve muscle strength and, at the same time, neuromuscular coordination.
We recently conducted a literature review including six different interventional studies using this device in a rehabilitation context [
19]. Different outcomes were measured in each study. At first, four studies [
20,
21,
22,
23] investigated the effect of the HUBER® rehabilitation platform on muscle strength. Markovic et al [
23] and Fabre et al. [
20] showed an improvement in muscle strength compared to the non-HUBER® groups. In contrary, Guiraud et al. [
22] and Couillandre et al. [
21] showed no benefit in terms of muscle strength after training with HUBER®.
Second, balance was also investigated in two studies [
21,
23]. According to Markovic et al. [
23], there was an improvement in static balance during a cognitive task in the HUBER® group compared to the Pilates group. The same result was shown by Couillandre et al. [
21], where balance was also improved in the HUBER® group.
Lastly, the improvement of quality of life has been evaluated in two studies following HUBER® training and resulted in contradictory results. Letafatkar et al. [
24] reported a significant improvement in quality of life in the HUBER® group, which was not found in the control group, while Guiraud et al [
22] reported no difference in quality of life between the groups.
In addition to aforementioned outcomes, various studies evaluated the effect of HUBER® on other events. For example, Tantôt et al [
25], reported a positive effect on pain and kinesiophobia in low back pain patients who benefited from HUBER® rehabilitation. According to Letafatkar et al [
24]. proprioception, control of lumbar movements and low back pain were improved after training with the HUBER®.
So, the overall positive effects of the HUBER® device on rehabilitation outcomes was shown in our latest review [
19]. Overall muscle strength is improved, as well as proprioception, balance, and cardiorespiratory tests.
The HUBER® instrument seems to be of interest for functional rehabilitation with positive effects on both physical (strength, proprioception, or balance) and psychological (quality of life) aspects.
We therefore wanted to carry out a randomised controlled trial, to compare mobile rehabilitation platform training with conventional physiotherapy, to assess the reduction in the risk of falls in a patient population requiring functional rehabilitation (= primary outcome). In addition to the risk of falls, the effect of such rehabilitation on balance, walking speed, physical performance and quality of life was assessed (= secondary outcomes).
2. Materials and Methods
2.1. Aims of the Study
1/ To evaluate the effectiveness of rehabilitation with HUBER 360® on the risk of falling in people in pathological situations requiring functional rehabilitation.
2/ Evaluation of the effectiveness of HUBER 360® rehabilitation on balance, walking speed, physical performance, and quality of life in people in a pathological situation requiring functional rehabilitation.
2.2. Research Hypotheses
HUBER 360® training, as proposed in our research protocol, would reduce the risk of falling, improve balance, walking speed and quality of life in the pathological group, significantly more than in the control group.
2.3. Type of Study
Interventional, randomised, not blinded study (patients and investigators know which group the patient belongs to). Two groups were created, an experimental group that benefit from training with the HUBER 360® in addition to conventional therapeutic rehabilitation, and a control group that will maintain its conventional therapeutic habits. This randomized control trial has been registered on clinicaltrials.gov under the number NCT04687293. An ethics committee agreement was given (number B7072020000079) and patients signed informed consent.
2.4. Population
Inclusion criteria:
- -
Patients benefiting from a physiotherapy rehabilitation program at the CHU Liège, site CNRF.
- -
Patients with a pathological situation justifying functional rehabilitation with the HUBER 360®.
- -
Patient with any pathology that does not constitute an exclusion criterion.
- -
Patient with informed consent for the study
Exclusion criteria (based on the exclusion criteria of the device).
Patients were excluded if they had one or more of these criteria:
- -
Cardiac, respiratory, neurological or rheumatological disease incompatible with physical activity.
- -
Arthritis.
- -
Rheumatic disease in acute phase.
- -
Recent trauma, infection of the musculoskeletal system.
- -
Fever.
- -
Venous thrombosis.
- -
Acute intervertebral disc disease.
- -
Neuropsychological problems that do not allow the integration of instructions or other serious psychological problems.
- -
Cardiovascular disease and any progressive, chronic disease that is not conducive to exercise.
- -
Major anatomical deformities.
2.5. Randomisation
Randomisation was carried out by randomly assigning patients to the experimental or control group. A numerical identifier was assigned to each enrolled person according to the order of enrolment (the first participant enrolled have the number 001, the second the number 002, etc.). Each numerical identifier has been previously linked to the experimental or control group assignment by a block of four-randomisation technique carried out in Excel.
2.6. Study Design/Intervention Protocol
Any patient attending the CNRF (CHU Liège) and meeting the inclusion criteria were invited to participate in the study. The patient was informed whether he/she participate in the study in the experimental group or in the control group. All patients underwent an assessment at the start of the study, consisting of the Timed Up and Go test and the Short Physical Performance Battery test. Patients were also complete a quality-of-life questionnaire, the Short-Form 36 (SF-36). These two physical assessments and the SF-36 questionnaire were also be administered/completed at the end of the study, after the 8week trial.
Experimental group: Each patient was trained on the HUBER 360® for 2 training sessions per week, spread over 2 non-consecutive days, 30 minutes per session, over a period of 8 weeks. Thus, each patient in the experimental group was received 16 sessions on the HUBER 360®. A training follow-up sheet was completed to monitor the patients' attendance. Each training session was supervised by a physiotherapy trainee who was present at the CHU Liège, throughout the experimental period. Thus, the instructions and the conditions of the training were given/carried out in a standardised way. Patients in the experimental group received their usual rehabilitation programme in addition to the HUBER 360® treatment. Patients were asked to maintain this conventional management regime during the 8-week study period.
Control group: The control group receives no additional training beyond that provided as part of their standard and usual management. Patients was asked to maintain this standard management regime for the 8-week study period.
2.7. Evaluation Criteria
Timed Up and Go test [
26]: The Timed Up and Go test is recognised as sensitive and specific for measuring the risk of falling [
26]. To perform this test, the patient is first asked to sit in an armchair with his back against the backrest. The patient is then asked to stand up, walk 3 metres, turn around (180°) and return to the seated position. The test is timed. The results correlate with the patient's walking speed, balance, and level of function. A time of more than 14 seconds indicates a high risk of falling.
Short Physical Performance Battery test [
27]: The Short Physical Performance Battery test is a test to measure an individual's physical performance. A score (total score out of 12) is the sum of scores from three criteria: a balance test (3 balance positions, scored out of 4), a walking speed test (over 4 metres, timed and scored out of 4) and a chairlift test (5 consecutive chairlifts, timed and scored out of 4). This test is used as a whole (/12 points) and by subscale (/4 points each). A test of less than 6 points indicates poor performance, a test of less than 10 points indicates medium performance and a test of 10 points or more indicates good physical performance [
27]. In addition, data on walking speed (in metres/seconds) and the time taken to complete the chairlift test (in seconds) are also analysed quantitatively.
Short Form 36 (SF-36) [
28]: The SF-36 is a generic quality of life questionnaire. It is composed of 8 domains of quality of life (vitality, pain, physical functioning, mental functioning, limitations due to physical condition, limitations due to psychological condition, general health), which allow to obtain two composite scores: the mental subscale and the physical subscale.
2.8. Statistical Analyses
Statistical analyses were be performed using R software. The calculations were performed on the maximum number of observations available, in intention-to-treat. The analyses were performed in 3 steps:
1/ Comparison of the clinical characteristics of the two groups (age, sex, BMI, pathological profile, etc.). Depending on the normality of the quantitative variable, which was checked beforehand (test de Shapiro-Wilk, comparison median-mean, QQ plot et histograms), a Student's T or Mann-Whitney U test were used for quantitative variables. An X² test was used for qualitative variables.
2/ Evaluation of the effectiveness of the experimental protocol on the evaluation criteria. Two statistical tests were performed. The first was aim to determine the pre-post effect of the experimental protocol within each group. To this end, a paired Student's t-test or a Wilcoxon test were carried out (all the evaluation criteria were presented in the form of quantitative variables). The second test is to assess the difference between the groups, again using a Student's T or Mann-Whitney U test. If differences in clinical characteristics are observed between the two groups, an adjustment for the variables that differ significantly between the two groups were performed using logistic regression. The significance of changes between groups over time was assessed by a repeated measure analysis.
Greenhouse-Geisser intrasubject effects test for time x group analysis was reported.
Analyses were adjusted on age.
2.9. Statistical Power
Because literature was scared on the effect of HUBER on the risk of falls, we were unable to measure an a priori statistical power calculation. Instead, we decided to include as much patients as possible during a two-year recruitment period.
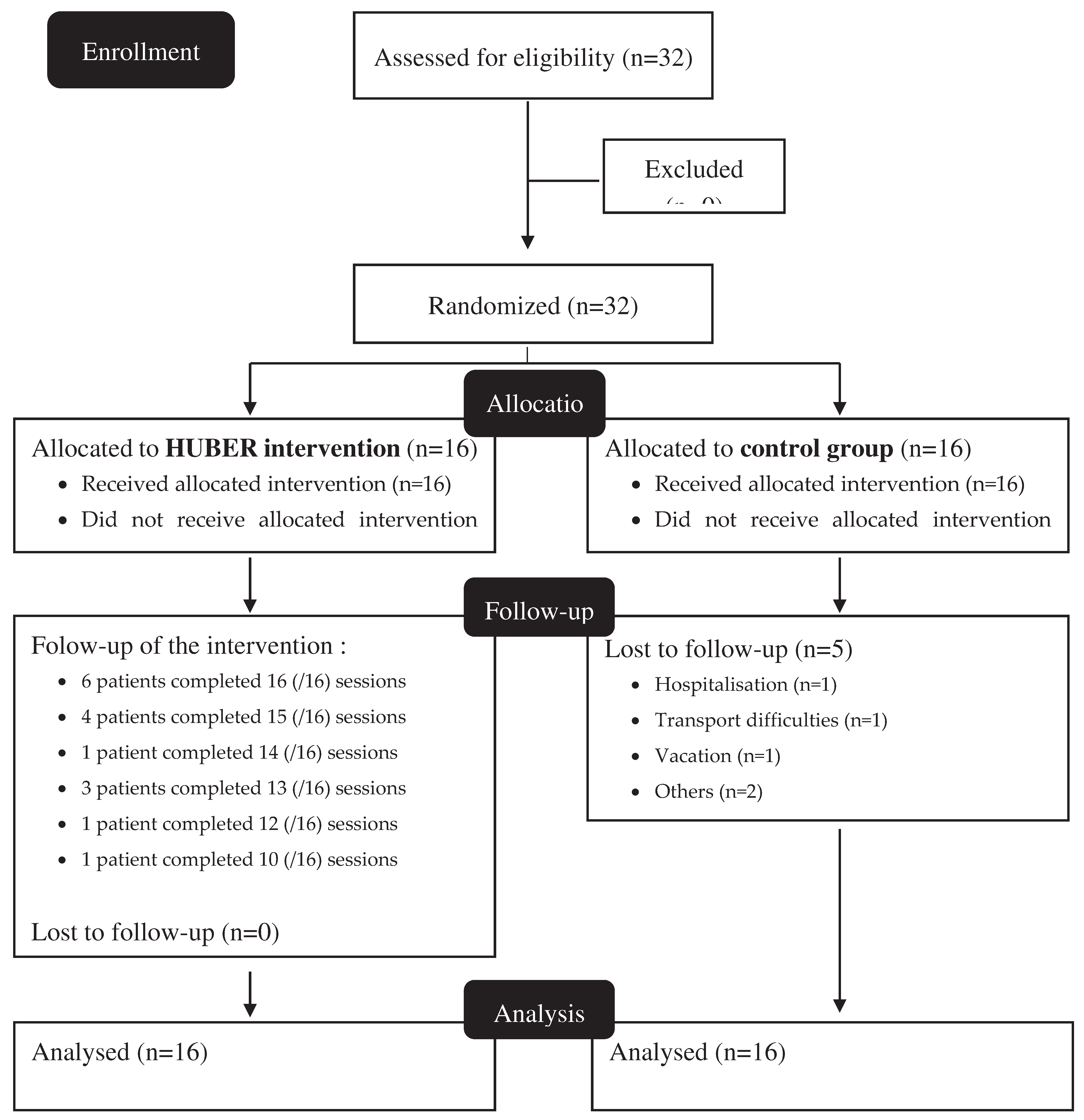
3. Results
32 participants were included in the study, 16 in both groups (
Figure 2). Five participants withdrew before study completion (all from the control group). Compliance to HUBER® sessions was of 90.2%, with 6 patients fully compliant to all the 16 sessions.
Among participants, 12 women were included (37.5%) and the mean age was 61.4 ± 10.4 years. No significant difference was found between groups in regards of gender balance, body mass index, pathologies or the need to use a walking aid. Nevertheless, the HUBER® groups appear to be older than the control group (mean age 65.4 ± 8.44 for the HUBER® group vs 57.4 ± 10.9 for the Control group, p=0.03) (
Table 1).
No significant difference in regards of physical measurements (i.e., SPBB test and TUG test) or in regards of health-related quality of life measurements (i.e., EQ5D utility scores, EQVAS, SF36 PCS or SF-36 MCS) were found at baseline between groups. However, a the 8-week followup time point, the HUBER® group showed significant higher values for HRQoL measurements compared to the control group (i.e., for the EQ5D utility score, the EQVAS and the SF-36 PCS) (
Table 2 and
Table 3).
Between baseline and the 8-week follow-up time point, patients in the HUBER® group significantly improved the SPPB Chair Rising test (mean difference of +0.63 ± 0.88 points, p=0.013), the SPBB total test (mean difference of +0.75 ± 0.77 points, p=0.002), the EQ5D utility score (mean difference of +0.09 ± 0.16, p=0.04), the EQVAS score (mean difference of 8.12 ± 9.81 points, p=0;005) and the SF-36 MCS (mean difference of +5.32 ± 7.41, p=0.012). Patients in the control group only improved the EQVAS (mean difference of +4.68 ± 6.45, p=0.01) during the study period.
Repeated measurement analysis of outcome measures at each visit showed a time x group improvement in favour of the HUBER® group for the SPBB chair rising test (p=0.021) and the SPPB total (p=0.003). No other significant time x group difference was observed for physical measurements.
However, significant higher HRQoL measurements were found for the HUBER® group, compared to the control groups, after 8 weeks of study period, for the EQ5D utility scores, for the EQVAS as well as for the SF-36 PCS (p<0.05).
4. Discussion
The aim of our study was to determine whether a group of patients trained with the HUBER® training for eight weeks showed a reduction in the risk of falling, an improvement in balance and walking speed and an increase in quality of life compared to a control group.
Physical performance was assessed using the TUG, SPPB chair stand test, SPPB walking, SPPB balance and SPPB global. The TUG, SPPB walking and SPPB balance showed no significant difference between the two groups at the end of the eight weeks of rehabilitation. There was also no significant difference compared to the control group. The scores obtained in the SPPB balance and SPPB walking tests were already close to maximum performance in the pre-rehabilitation test and improvement at the end of rehabilitation was therefore difficult to achieve because the tests were not challenging enough for this group of patients. More challenging tests might be of interest to give patients a greater margin of progress. In comparison, SPPB chairlift and global SPPB were significantly improved in the mobile rehabilitation platform group at the end of rehabilitation. This improvement was not seen in the control group, resulting in a significant difference between the two groups at the end of rehabilitation. Our results correspond to those of Markovic et al [
23] and Couillandre et al [
21] who showed an improvement in balance after training on the mobile rehabilitation platform.
Performance improved globally in the HUBER® group for all tests, although not always significantly. In the control group, however, performance tended to decline during the eight weeks of rehabilitation, especially for the SPPB walking, SPPB chair raising and SPPB global. The patients in the study suffer from degenerative neurological diseases where a decline in performance over time is to be expected. Stability or improvement in performance, as observed in the HUBER® group for the various physical tests assessed, can be considered a benefit of the mobile rehabilitation platform device.
None of the tests used in our study allow for an assessment of the quality of walking, only speed is considered. Further research would be needed to understand and quantify stride length, number of strides per minute, gait endurance, and possible stamping, mowing or lameness as poor gait quality may be a factor in the fall.
We also did not evaluate specifically muscular strength but several studies [
20,
21,
22,
23] reported an improvement in muscle strength after training on mobile rehabilitation platform.
In this paper, quality of life was assessed using four parameters: the SF-36 mental, the SF-36 physical, the EQ-5D total and the EQ-VAS. There was a significant improvement in the EQ-5D, EQ-VAS and SF-36 physical scores in the HUBER® group at the end of the eight weeks of training. In comparison, only the EQ-VAS improved significantly in the control group. The positive impacts of HUBER® on quality of life is not yet fully understood as Letafatkar's et al [
24] also reported an improvement, whereas Guiraud et al [
22] observed no differences between the groups.
As compared to recent studies, the main strength of our study is the presence of a control group combined with randomisation into two groups There were no significant differences between the characteristics of the two groups at enrolment, except for a slightly older population in the HUBER® group. We do not expect that this difference would have an impact on the results of this study. In addition, the high participation rate is also encouraging, with few dropouts during the study in the control group and in the HUBER® group. Patients therefore seemed to enjoy their rehabilitation sessions on the HUBER®.
However, there are some limitation to this study. The lack of double-blinding could influence the results of the tests due to the examiner who knows which group the patient belongs to. In order to make the study more reliable, it would have been interesting to have the various tests carried out before, during and after the study by an external examiner who did not know which group the patient belonged to. The time of the day when the test is performed could also influence the results. In fact, in certain degenerative diseases, including MS and Parkinson's disease, physical performance can vary throughout the day, depending on fatigue or the timing of medication, for example. Another limitation if the timing of our study, we investigated the impact of HUBER® for a period of eight weeks but a new series of tests could have been carried out some time after the last training session with HUBER® to evaluate the duration of the positive effects in the longer term. Furthermore, as mentioned above, some of the tests performed prior to the start of the study showed near maximum scores. It was therefore unrealistic to expect a significant improvement in these tests. Therefore, a better selection of more discriminating tests should be considered in a future study, in combination to gait quality which is known to prevent falls. As a limitation, we can also mention that no power size calculation was performed prior the study.
However, statistical power was assessed post hoc, using the results obtained to the SPBB outcome (i.e. effect size of 1.2). Using an alpha of 0.5, a beta of 0.8 and this effect size of 1.2, it appears that our sample size of 32 patients was sufficient to draw statistically significant conclusions. We also did not evaluate the beneficial effects of the mobile rehabilitation platform in relation to the different pathologies. It would have been interesting to do this to determinate if HUBER had a better effect on a specific pathology. Finally, it could be interesting to compare the results obtained after training on HUBER® to a group of patients refusing any rehabilitation. Indeed, all the patients participating in this study benefited from a classic physiotherapy management.
As a summary, the advantages and innovative features of the HUBER® rehabilitation machine seem to be numerous and promising. However, to increase the impact of future studies, it would be interesting if they could include further physical tests and, in particular, an analysis of the quality of walking, including a measurement of walking endurance. Secondly, a single-blinded study should be carried out with an evaluator who is unaware of which group the patient belongs to, to confirm the results observed here. An analysis by specific pathology would also be interesting. Finally, to evaluate the effectiveness of HUBER® in the longer term, future studies should also include follow-up testing, after 8-weeks period.
5. Conclusions
The results of our study are promising for the HUBER® device. The patients who received the two mobile rehabilitation platform training sessions per week for eight weeks in addition to their conventional rehabilitation showed a greater improvement in their scores on certain physical (including the risk of falling) and quality of life tests than the control group. There was no decline in performance on any of the tests. Further studies could be conducted in this area, with adaptations based on the limitations identified in our study. This could eventually lead to the routine use of HUBER® training in the functional rehabilitation of patients with neurological disorders. On the other hand, it seems clear that the HUBER® tool alone will never replace the functional rehabilitation treatment by physiotherapy that our patients currently benefit from and must be used in addition to it.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. CONSORT-2010-Checklist.
Author Contributions
CB: CL and JFK conceived the study and developed hypotheses and protocol. CB conceived the survey questionnaires with the approval of CL, JFK and CB was responsible for data collection. CB were responsible for data management and data analyses. CL wrote the drafts of the article under the supervision of CB, JS and JFK. All authors have read, reviewed and approved the final manuscript. Conceptualization, Charlotte Laurent, Charlotte Beaudart and Jean-François Kaux; Formal analysis, Charlotte Beaudart; Investigation, Charlotte Laurent; Methodology, Charlotte Laurent, Charlotte Beaudart and Jean-François Kaux; Supervision, Charlotte Beaudart, Justine Slomian and Jean-François Kaux; Writing – original draft, Charlotte Laurent; Writing – review & editing, Charlotte Beaudart, Justine Slomian and Jean-François Kaux.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee “Comité d’éthique Hospitalo-Facultaire du CHU de Liège” (number B7072020000079).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dorsey ER, Sherer T, Okun MS, Bloemd BR. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis [Internet]. 2018 [cited 2023 May 1];8(s1):S3–8. Available from:. [CrossRef]
- Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol [Internet]. 2001 [cited 2023 May 1];248(11):950–8. Available from: https://pubmed.ncbi.nlm.nih.gov/11757958/. [CrossRef]
- Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disabil Rehabil [Internet]. 2008 [cited 2023 May 1];30(16):1205–12. Available from: https://pubmed.ncbi.nlm.nih.gov/18608387/. [CrossRef]
- Eng JJ, Pang MYC, Ashe MC. Balance, falls, and bone health: role of exercise in reducing fracture risk after stroke. J Rehabil Res Dev [Internet]. 2008 [cited 2023 May 1];45(2):297–314. Available from: https://pubmed.ncbi.nlm.nih.gov/18566947/. [CrossRef]
- Nilsagard Y, Gunn H, Freeman J, Hoang P, Lord S, Mazumder R, et al. Falls in people with MS--an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult Scler [Internet]. 2015 Jan 14 [cited 2023 May 1];21(1):92–100. Available from: https://pubmed.ncbi.nlm.nih.gov/24948687/. [CrossRef]
- Callaly EL, Ni Chroinin D, Hannon N, Sheehan O, Marnane M, Merwick A, et al. Falls and fractures 2 years after acute stroke: The North Dublin Population Stroke Study. Age Ageing [Internet]. 2015 Sep 1 [cited 2023 May 1];44(5):882–6. Available from: https://jhu.pure.elsevier.com/en/publications/fallsand-fractures-2-years-after-acute-stroke-the-north-dublin-p-3. [CrossRef]
- Liu TW, Ng GYF, Ng SSM. Effectiveness of a combination of cognitive behavioral therapy and taskoriented balance training in reducing the fear of falling in patients with chronic stroke: Study protocol for a randomized controlled trial. Trials [Internet]. 2018 Mar 7 [cited 2023 May 1];19(1):1–10. Available from: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2549-z. [CrossRef]
- Paul SS, Sherrington C, Canning CG, Fung VSC, Close JCT, Lord SR. The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: a large prospective cohort study. Neurorehabil Neural Repair [Internet]. 2014 Mar [cited 2023 May 1];28(3):282–90. Available from: https://pubmed.ncbi.nlm.nih.gov/24243915/. [CrossRef]
- Paul SS, Harvey L, Canning CG, Boufous S, Lord SR, Close JCT, et al. Fall-related hospitalization in people with Parkinson’s disease. Eur J Neurol [Internet]. 2017 Jan 24 [cited 2023 May 1];24(3):523–9. Available from: https://europepmc.org/article/MED/28117538. [CrossRef]
- Cameron MH, Nilsagard Y. Balance, gait, and falls in multiple sclerosis. Handb Clin Neurol [Internet]. 2018 Jan 1 [cited 2023 May 1];159:237–50. Available from: https://pubmed.ncbi.nlm.nih.gov/30482317/. [CrossRef]
- Coote S, Comber L, Quinn G, Santoyo-Medina C, Kalron A, Gunn H. Falls in People with Multiple Sclerosis: Risk Identification, Intervention, and Future Directions. Int J MS Care [Internet]. 2020 Nov 1 [cited 2023 May 1];22(6):247–55. Available from: https://pubmed.ncbi.nlm.nih.gov/33424479/. [CrossRef]
- Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med [Internet]. 2017 Dec 1 [cited 2023 May 1];51(24):1749–57. Available from: https://pubmed.ncbi.nlm.nih.gov/27707740/. [CrossRef]
- De Souto Barreto P, Rolland Y, Vellas B, Maltais M. Association of Long-term Exercise Training With Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Metaanalysis. JAMA Intern Med [Internet]. 2019 Mar 1 [cited 2023 May 1];179(3):394–405. Available from: https://pubmed.ncbi.nlm.nih.gov/30592475/. [CrossRef]
- Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res [Internet]. 2013 Apr 1 [cited 2023 May 1];16(2):105–14. Available from: https://pubmed.ncbi.nlm.nih.gov/23327448/. [CrossRef]
- De Souto Barreto P, Rolland Y, Vellas B, Maltais M. Association of Long-term Exercise Training With Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Metaanalysis. JAMA Intern Med [Internet]. 2019 Mar 1 [cited 2023 May 1];179(3):394–405. Available from: https://pubmed.ncbi.nlm.nih.gov/30592475/. [CrossRef]
- Pereira CLN, Vogelaere P, Baptista F. Role of physical activity in the prevention of falls and their consequences in the elderly. European Review of Aging and Physical Activity [Internet]. 2008 Apr 23 [cited 2023 May 1];5(1):51–8. Available from: https://eurapa.biomedcentral.com/articles/10.1007/s11556-008-0031-8. [CrossRef]
- Saywell N, Taylor N, Rodgers E, Skinner L, Boocock M. Play-based interventions improve physical function for people with adult-acquired brain injury: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil [Internet]. 2017 Feb 1 [cited 2023 May 1];31(2):145–57. Available from: https://pubmed.ncbi.nlm.nih.gov/26869595/. [CrossRef]
- McGlinchey MP, James J, McKevitt C, Douiri A, Sackley C. The effect of rehabilitation interventions on physical function and immobility-related complications in severe stroke: a systematic review. BMJ Open [Internet]. 2020 Feb 5 [cited 2023 May 1];10(2). Available from: https://pubmed.ncbi.nlm.nih.gov/32029489/. [CrossRef]
- Laurent C, Beaudart C, Léonard Y, Maertens B, Laurent L, Kaux J-F. Rééducation globale à l’aide du système HUBER® en kinésithérapie : une revue systématique de la littérature. Revue médicale de Liège septembre 2023 :78 (9):490-495.
- Fabre, J.B., Martin, V., Borelli, G., Fritsch, N. , Theurel, J. Effects of a whole-body strength training program on metabolic responses and body composition. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2014 January-February; 173 (1-2):47-56. | Request PDF [Internet]. [cited 2023 May 2]. Available from: https://www.researchgate.net/publication/357484414_Effects_of_a_wholebody_strength_training_program_on_metabolic_responses_and_body_composition_FABRE_JB_ET_AL _Gazzetta_Medica_Italiana_Archivio_per_le_Scienze_Mediche_2014_January-February_173_1-247-56.
- Couillandre A, Duque Ribeiro MJ, Thoumie P, Portero P. Changes in balance and strength parameters induced by training on a motorised rotating platform: a study on healthy subjects. Ann Readapt Med Phys [Internet]. 2008 Mar [cited 2023 May 2];51(2):67–73. Available from: https://pubmed.ncbi.nlm.nih.gov/18207276/. [CrossRef]
- Guiraud T, Labrunée M, Besnier F, Sénard JM, Pillard F, Rivière D, et al. Whole-body strength training with Huber Motion Lab and traditional strength training in cardiac rehabilitation: A randomized controlled study. Ann Phys Rehabil Med [Internet]. 2017 Jan 1 [cited 2023 May 2];60(1):20–6. Available from: https://pubmed.ncbi.nlm.nih.gov/27650531/. [CrossRef]
- Markovic G, Sarabon N, Greblo Z, Krizanic V. Effects of feedback-based balance and core resistance training vs. Pilates training on balance and muscle function in older women: a randomized-controlled trial. Arch Gerontol Geriatr [Internet]. 2015 Sep 1 [cited 2023 May 2];61(2):117–23. Available from: https://pubmed.ncbi.nlm.nih.gov/26036209/. [CrossRef]
- Letafatkar A, Nazarzadeh M, Hadadnezhad M, Farivar N. The efficacy of a HUBER exercise system mediated sensorimotor training protocol on proprioceptive system, lumbar movement control and quality of life in patients with chronic non-specific low back pain. J Back Musculoskelet Rehabil [Internet]. 2017 [cited 2023 May 2];30(4):767–78. Available from: https://pubmed.ncbi.nlm.nih.gov/28453452/. [CrossRef]
- Tantot M, Le Moal V, Mévellec É, Nouy-Trollé I, Lemoine-Josse E, Besnier F, et al. Effects of an Intensive 6-Week Rehabilitation Program with the HUBER Platform in the Treatment of Non-Specific Chronic Low Back Pain: A Pilot Study. Clin Pract [Internet]. 2022 Aug 1 [cited 2023 May 2];12(4):609–18. Available from: https://pubmed.ncbi.nlm.nih.gov/36005067/. [CrossRef]
- D P, S R. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc [Internet]. 1991 [cited 2023 May 2];39(2):142–8. Available from: https://pubmed.ncbi.nlm.nih.gov/1991946/. [CrossRef]
- Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med [Internet]. 2016 Dec 22 [cited 2023 May 2];14(1). Available from: https://pubmed.ncbi.nlm.nih.gov/28003033/. [CrossRef]
- Ware JE, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol [Internet]. 1998 Nov [cited 2023 May 2];51(11):903–12. Available from: https://pubmed.ncbi.nlm.nih.gov/9817107/. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).