1. Introduction
Gingival is soft tissue, which is part of periodontal tissue that covers the alveolar bone of mandibular and maxillary bones.1 Since the 1990s, biomaterial scaffolds such as acellular dermal matrix, collagen matrix, and enamel matrix derivative have been introduced as autogenous graft substitutions for gingival augmentation.2,3 Until today, the development of biomaterial scaffolds as autogenous grafts substituted for gingival augmentation has been increasing.4 Scaffolds are three-dimensional porous solid structure biomaterials that are synthesized to promote interaction between cells and biomaterials.5,6 Tissue regeneration using scaffold strategies provokes physical and biological support until new tissue formation occurs through cell proliferation, differentiation, and attachment processes.7 Biomaterial scaffolds for gingival regeneration should meet certain criteria, such as biocompatible, good adaptation and manipulation, space maintenance, clot stabilization, tissue integration, and promoting cell proliferation.4 The limitation of available biomaterial scaffolds is their stiffness, which may lead to poor adaptation on the root surface.8
One of the most promising scaffolds is hydrogel. It is a hydrophilic polymer that is crosslinked through covalent bonds or held together due to physical intramolecular and intermolecular attraction by physical or chemical methods.9,10 Hydrogels receive attention in tissue engineering because they have the capability to hold large amounts of water and have structural similarities to some human soft tissues.11,12 Hydrogels can be designed as scaffolds for soft regeneration because they are highly hydrated and can mimic native soft tissue. The hydrophilic networks of the hydrogels allow nutrients and oxygen to diffuse, therefore promoting cell growth.11,13 The shape adaptability characteristic of hydrogels is suitable for a minimally invasive approach in oral tissue engineering.1 The elasticity and soft nature of hydrogels will minimize irritation to the surrounding tissues.14
Natural polymers such as gelatin, collagen, hyaluronic acid, chitosan, and alginate are widely used for hydrogel fabrication due to their good biological properties.15 Despite collagen being known as the most abundant protein in the extracellular matrix (ECM), its helical structure and amino acid sequences appeared to initiate an antigenic and immunogenic response in vivo. Gelatin is a natural polymers obtained from the denaturation and hydrolysis of collagen, resulting in a lack of both tyrosine and tryptophan and very low levels of phenylalanine.16–18 Therefore, the potential of the antigenic response in vivo might be reduced since gelatin less has a smaller chance to form aromatic radicals.19 As a denaturation product of collagen, the gelatin structure consist of Gly-X-Y sequences (mainly proline and hydroxyproline) and arginine-glutamine aspartic acid (RGD) sequences, which play a role in integrin-mediated cell adhesion, as well as the target sequences of matrix metalloproteinase (MMP), which are suitable for remodelling the cells.20–22 There are two types of gelatin based on its extraction method: type A (acid-based), which is mainly obtained from pigs, poultry, and fish; and type B (alkaline-based), which is sourced from bovine.23,24 The physical characteristics of the gelatin allow the scaffold to form with good flexibility to fit deficient formations for the use in periodontal regeneration.25
Physical properties such as biodegradation rate, mechanical, and rheological properties can of the hydrogel can be modified by the crosslinking method to give hydrogels a solid structure through intermolecular bonds.26,27 Many crosslinking strategies can be performed in the preparation of gelatin hydrogels, such as physical methods through UV irradiation or dehydrothermal treatment and chemical methods such as glutaraldehyde, carbodiimide, genipin, and transglutamines.28 Glutaraldehyde is commonly used as a crosslinking agent to obtain a stable physical structure for the hydrogels.26,29 On the other hand, the cross-linking process of gelatin using methacryloyl produces GelMA (gelatin methacryloyl) hydrogel, which showed higher cytotoxicity compared to collagen hydrogel when oral mucosal fibroblast cells were encapsulated in it.17
Cross-linking strategies using carbodiimides such as 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) exhibit a good biological response because they are water-soluble zero-length crosslinkers.30,31 Unlike other crosslinker agents such as glutaraldehyde, EDC allows the formation of stable covalent bonds without becoming part of the crosslinked gelatin network, which can avoid the cytotoxic effect.26 EDC can be used for coupling polymers containing carboxyl groups and amines.32,33 N-hydroxysuccinimide (NHS) is a stabilizer reagent to enhance cross-linking efficiency with EDC.34 The crosslinking process of gelatin with EDC/NHS showed improvements in the rigidity, mechanical strength, and thermal stability of the hydrogels.32
Mimicking the physical properties of scaffolds with native tissue architecture at a macroscopic level is important for scaffold implantation and stimulating tissue regeneration.7 Once the physical properties of scaffolds are not matched with the tissue, the healing phase can result in poor functionality of the regenerated tissue and tissue loss.35 The aim of this study was to explore the potency of crosslinked gelatin hydrogels as scaffolds for gingival regeneration by investigating their architecture and physical characteristics.
2. Materials and Methods
2.1. Materials
Gelatin type B powder from bovine skin was purchased from Sigma Aldrich (USA). N-ethyl-N’-(3-dimethylaminopropyl)carbodiimide liquid (EDC; Mw: 155.24; Sigma Aldrich), N-hydroxysuccinimide powder (NHS; Mw: 115.09; Sigma Aldrich), and phosphate buffer saline (pH=7.4) from Sigma Aldrich. Distilled water and 95% ethanol were used throughout.
2.2. Preparation of Gelatin Hydrogels
Gelatin hydrogel was prepared by dissolving gelatin powder in distilled water at a concentration of 5% w/v in a stirrer at 40 0C until it reached a homogenous solution. To the gelatin solution, EDC and NHS were added drop by drop with a mass ratio of EDC:NHS:Gel = 1:1:12 (GelCL12) and 1:1:24 (GelCL24). The crosslinking process was performed by stirring the mixture in a magnetic stirrer at 4 OC until it was completely mixed. Uncrosslinked gelatin (GelUCL) was used as a control.
All the solution was then poured into tubes for 24 hours at 4 OC, resulting in the formation of hydrogel. To remove the residue of EDC/NHS, the hydrogel went through a triple-rinsing process using distilled water. Two-step freezing was conducted at -20 OC for 7 hours, subsequently followed by -80 OC for 24 hours. Frozen hydrogels were then transferred to a freeze-dryer (Gyrozen Hypercool) at -110 OC for 24 hours to get the lyophilized hydrogels.
2.3. Morphology and Chemical Analysis
The morphology and microstructure of the hydrogels were observed using a scanning electron microscope (SEM) (FEI Inspect F50, USA). Lyophilized hydrogel samples were cut and coated with gold for 10 seconds to provide a conductive surface for electrons prior to SEM. The cross-section of hydrogels was observed at x1000 magnification. Pores size, pores interconnectivity, and number of pores were analyzed using ImageJ. The liquid displacement method was used to calculate the porosity of the hydrogels.
The presence of typical gelatin and chemical bonds in uncrosslinked and crosslinked gelatin hydrogels was analyzed using fourier transform infrared spectroscopy (FTIR) spectra (Perken Elmer spectrometer). The lyophilized hydrogel samples were grinded then placed in a holder in the path of infrared sources. The spectra of all samples were recorded in the wavenumber range 4000-400 cm-1.
A DHR 1 rheometer (TA Instruments) with smart swap geometry was used for measuring the rheological properties of the hydrogels. Sample was placed on the plate for gap positioning, immobilized, and trimmed. Oscillatory mode at 37 °C was selected for rheological tests. We used amplitude and oscillatory sweep data from the linear viscoelastic area (γ =0.1–1000, ω=10Hz) and (ω=0.01–100 Hz, γ=100) respectively. Storage modulus (G’) and loss modulus (G″) used to evaluate viscosity were calculated from the linear viscoelastic region (LVR).
2.4. Swelling Properties
Initially, lyophilized hydrogel samples were cut at 0,125 ml and weighed (Wo). Subsequently, the samples were soaked in a solution of 5 mL of PBS at a physiological temperature. The samples were taken out from PBS and rinsed with distilled water at pre-determined time intervals (1, 10, 30, 60, 120, and 240 minutes), blotted with filtration paper until no drop was left from the samples, and weighted as Wl.
The swelling ratio (SR) was calculated using the following equation:
2.5. Biodegradation Rate
Lyophilized hydrogels were cut into pieces until reach similar weight and weighted using analytical balance (Wo). The measurement of in-vitro biodegradation behavior was measured by immersing the lyophilized hydrogels in plastic pot contained 5 mL PBS at 370C. The precipitates were carefully extracted and rinsed with deionized water at specified time intervals of 1, 2, 7, and 14 days. The final weight was subsequently determined as Wt. PBS solution was replaced every three days.
The biodegradation ratio (BR) was calculated using the following equation:
2.6. Statistical Analyses
Each test was performed in triplicate. All data had parametric distribution and have been reported as mean ± standard deviation (SD). Statistical analysis was calculated using ANOVA Tukey post-hoc test for multiple comparisons using SPSS 26.0. The p value < 0.05 was considered as statistically significant for all comparisons.
3. Results and Discussion
3.1. Preparation of Gelatin Hydrogels
To make a gelatin hydrogel, we dissolved and stirred gelatin powder in hot water. Gelatin is insoluble in hot water with temperature above 30
oC.
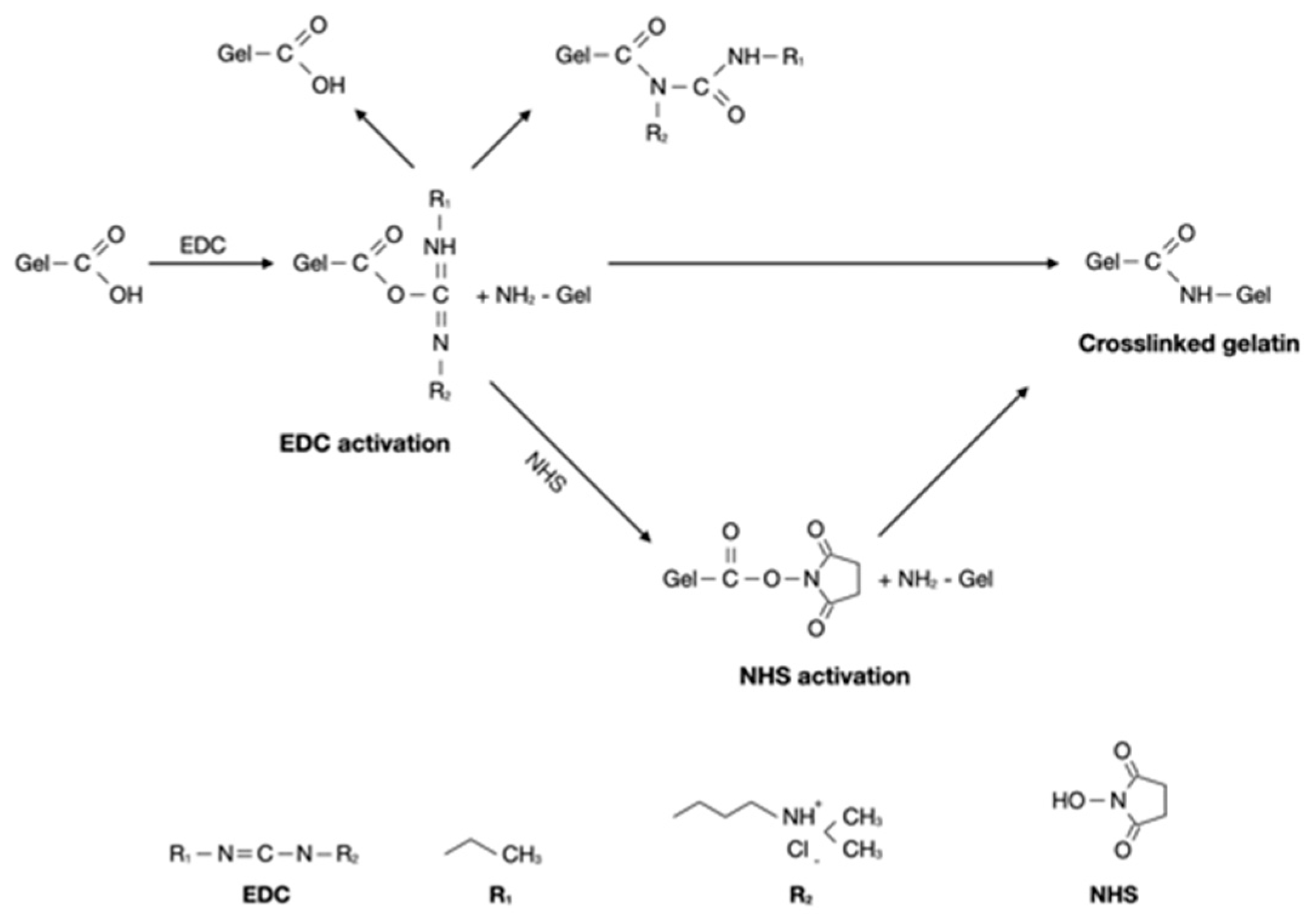
36 In attempt to increase mechanical stability and solubility of the gelatin hydrogels, we used EDC/NHS for the crosslinking strategy. As shown in
Figure 1, the addition of EDC/NHS into gelatin result in formation of short-range amide bonds between gelatin molecules. EDC activation form active O-urea which reacts with amino groups creating amide link and releasing isourea which can easily hydrolized. NHS was used to overcome this limitation, to form a more stable intermediate prior to amination.
37,38 Carbodiimide activates carboxylic acid residues EDC/NHS can link amino and carboxylic acid groups located within 1 nm from one another.
39 Previously, Goodarzi et al. prepared a stable gelatin-EDC/NHS hydrogels with mass ratio of Gel:EDC:NHS = 12:1:1.
40 The gelatin hydrogels underwent freeze-drying to induce porosity inside the hydrogel structure. The intention of this approach is to put the hydrogel to the circumstances of extreme cold and high pressure in order to facilitate the separation of water. Subsequently, the frozen water will melt through sublimation, resulting in the formation of a void/pore within the hydrogel structure.
41 Prior to the freeze-drying method, it is essential that the gelatin hydrogel is subjected to a frozen state. It has been established that freezing the hydrogel at temperatures of -20
0 and -80
0C creates a high degree of porosity within the hydrogel structure.
42
3.2. Morphology
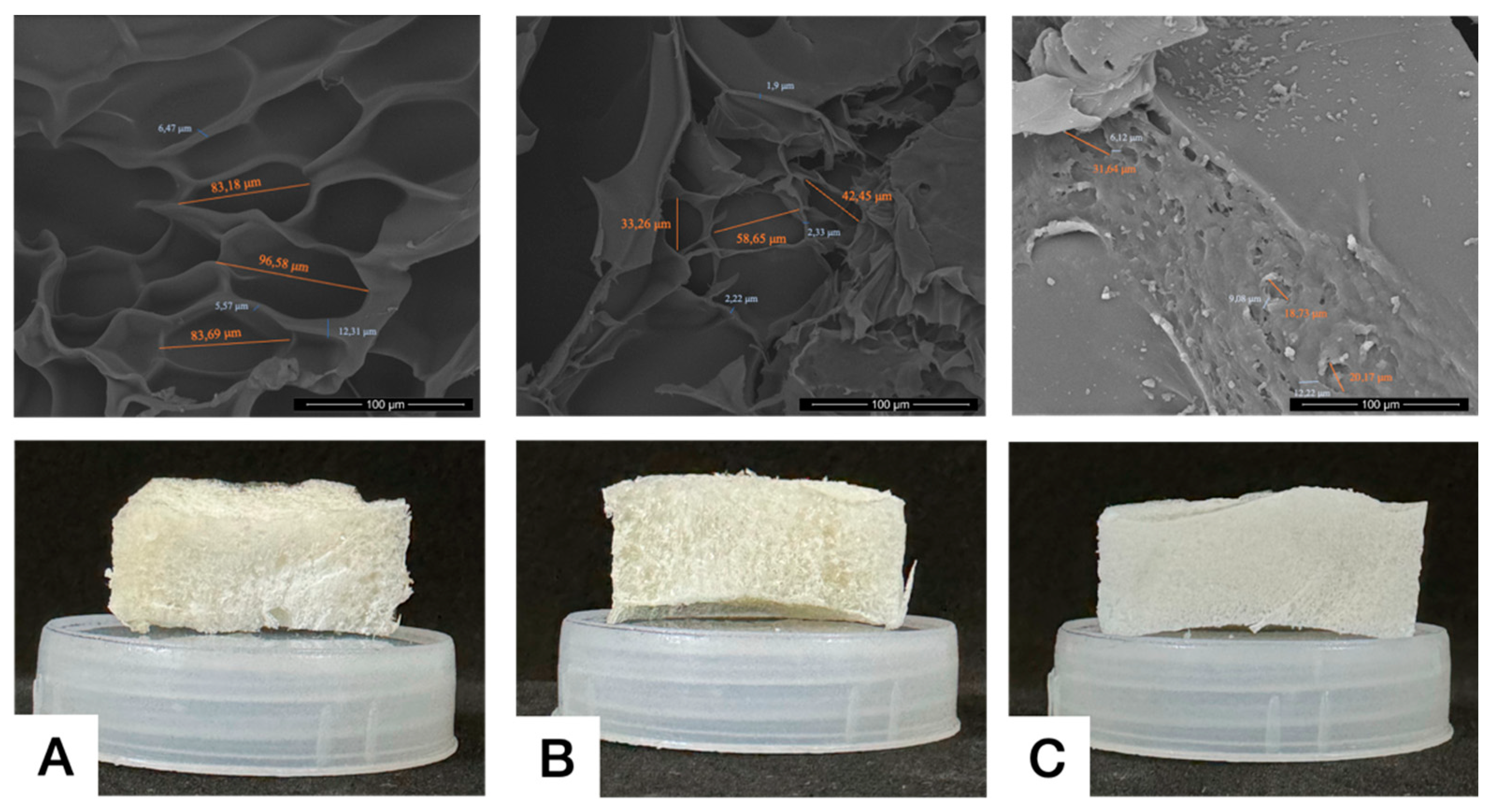
The crosslinked bond influences mechanical properties, swelling ability, and nutritional diffusion across the gel structure. Microarchitectural analysis, including pore diameter and interconnectivity is presented in
Figure 2. The pores observed in the crosslinked gelatin hydrogel are large with clear interconnectivity, while uncrosslinked gelatin exhibits irregular and non-homogeneous shapes with thin and narrow interconnectivity. There is still an ongoing debate regarding the ideal pore diameter for regenerative therapy. Previous studies reported that a pore diameter of 100-135 μm is ideal for facilitating cell attachment.
43 In this study, we found that GelCL12 showed biggest pore diameter (83,18-96,58 µm), whereas GelCL24 showed smallest diameter (18,73-31,64 µm). A pore diameter that is too small will cause cells to have difficulty migrating to the center of the of the pore, thus hampering the diffusion process of nutrients needed by cells to survive. A pore diameter that is too large will cause a decrease in the surface area required for cell adhesion.
44 The diffusion process of metabolites, oxygen, and growth factors will pass through the scaffold material so that the open tissue structure will facilitate cell survival and proliferation. The porous structure functions to encourage host cell infiltration and increase vascularization which provides nutrition to developing tissue.
45 Fibroblasts, which are the cells that contribute most to the formation of periodontal connective tissue, were reported to proliferate optimally at a pore diameter of 50-160 μm.
1 Interconnectivity is defined as the mean of the distance between adjacent pores. This facilitates the loading of cells into the scaffold while the inside of the pore wall acts as a vessel for cell attachment and also exchange of nutrient and waste.
46,47 Our study found that GelCL24 and GelCL12 exhibits a significantly higher range of 6,12-12,22 µm and 5,57-12,31 µm. GelUCL had narrower interconnectivity with range from 1,9-2,33 µm.
Figure 2.
Morphology of lyophilized hydrogels, (A) GelCL12, (B) GelUCL, (C) GelCL24.
Figure 2.
Morphology of lyophilized hydrogels, (A) GelCL12, (B) GelUCL, (C) GelCL24.
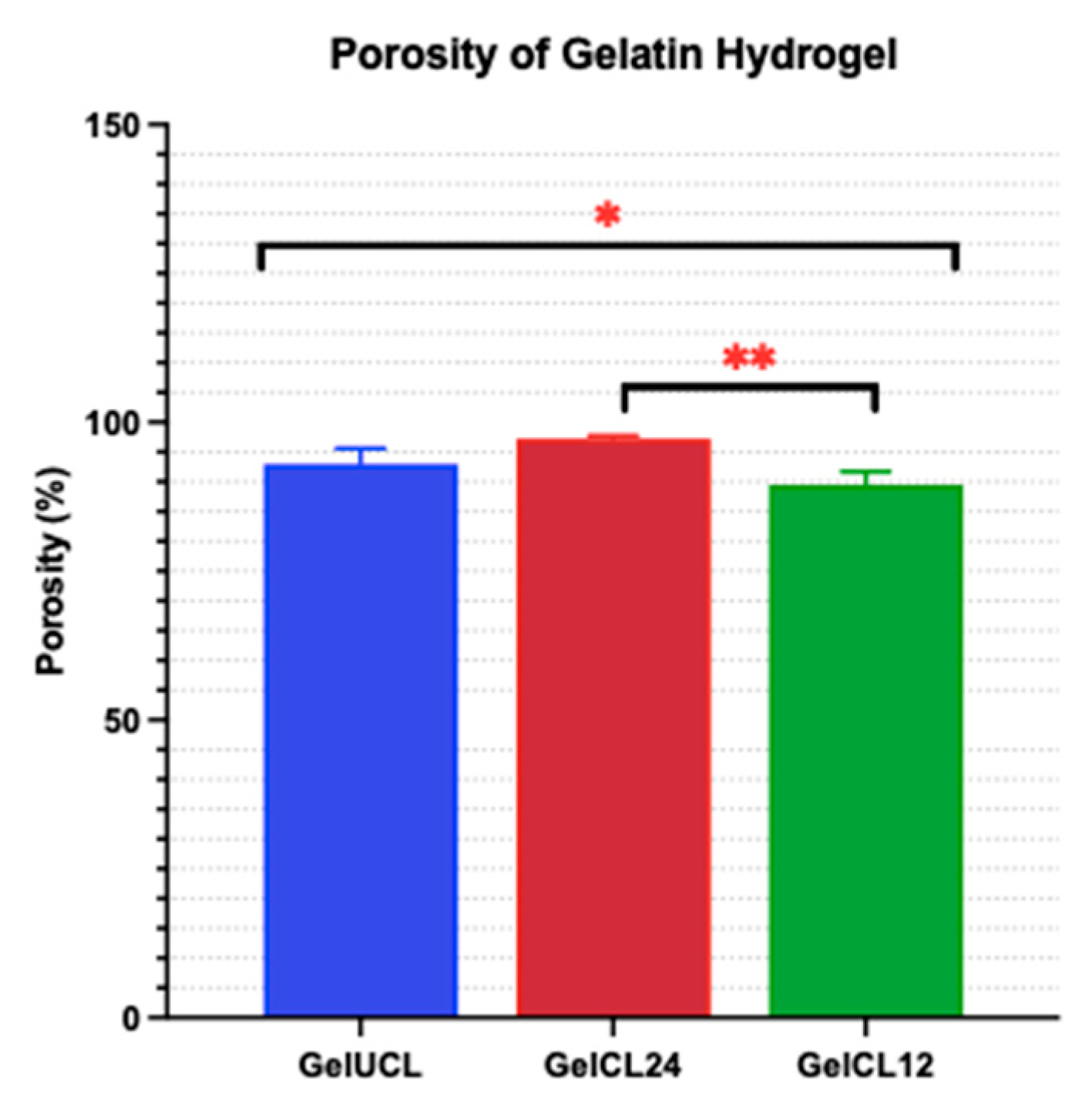
Figure 3.
Porosity of the as-prepared hydrogels. Data is presented the mean ± SD of triplicate experiments; * and ** p < 0,05.
Figure 3.
Porosity of the as-prepared hydrogels. Data is presented the mean ± SD of triplicate experiments; * and ** p < 0,05.
Scaffold containing a high porosity degree (90%) enables better penetration for cells and nutrients; however, as the porosity increases, it will decrease the mechanical strength of the scaffold.41,48 Eventually, it is pertinent to find an ideal balance between porosity and the strength of the hydrogel. Materials with high porosity and open pore structure have demonstrated promising potential for the facilitation of host tissue repair and regeneration.49 The gelatin hydrogels underwent freeze-drying to induce porosity inside the hydrogel structure. The intention of this approach is to put the hydrogel to the circumstances of extreme cold and high pressure in order to facilitate the separation of water. Subsequently, the frozen water will melt through sublimation, resulting in the formation of a void orpore within the hydrogel structure.41 Prior to the freeze-drying method, it is essential that the gelatin hydrogel be in a frozen state. It has been established that freezing the hydrogel at temperatures of -20 0C and -80 0C creates a high degree of porosity within the hydrogel structure.42
Ideally, a scaffold with a porosity ranging from 60% to 90% would be more appropriate for tissue engineering needs. As the level of porosity in the scaffold escalates, a greater amount of empty space is created within the biomaterial, hence potentially compromising its mechanical strength.41 The porosity of GelUCL was measured to be 92.97% ± 2.65%. In comparison, the porosity of GelCL24 and GelCL12 was 97.24% ± 0.47% and 89.52% ± 2.19%, respectively. There was a statistically significant difference between GelCL24 and GelCL12 (p < 0.05). The data may describe that by utilizing the correct amount of the crosslinker, it is possible to attain a scaffold that maintains a somewhat porous structure while minimizing the possibility of damage to its mechanical strength. As a result, GelCL12 was identified as having the porosity closest to the ideal limits.
3.3. Chemical Analysis
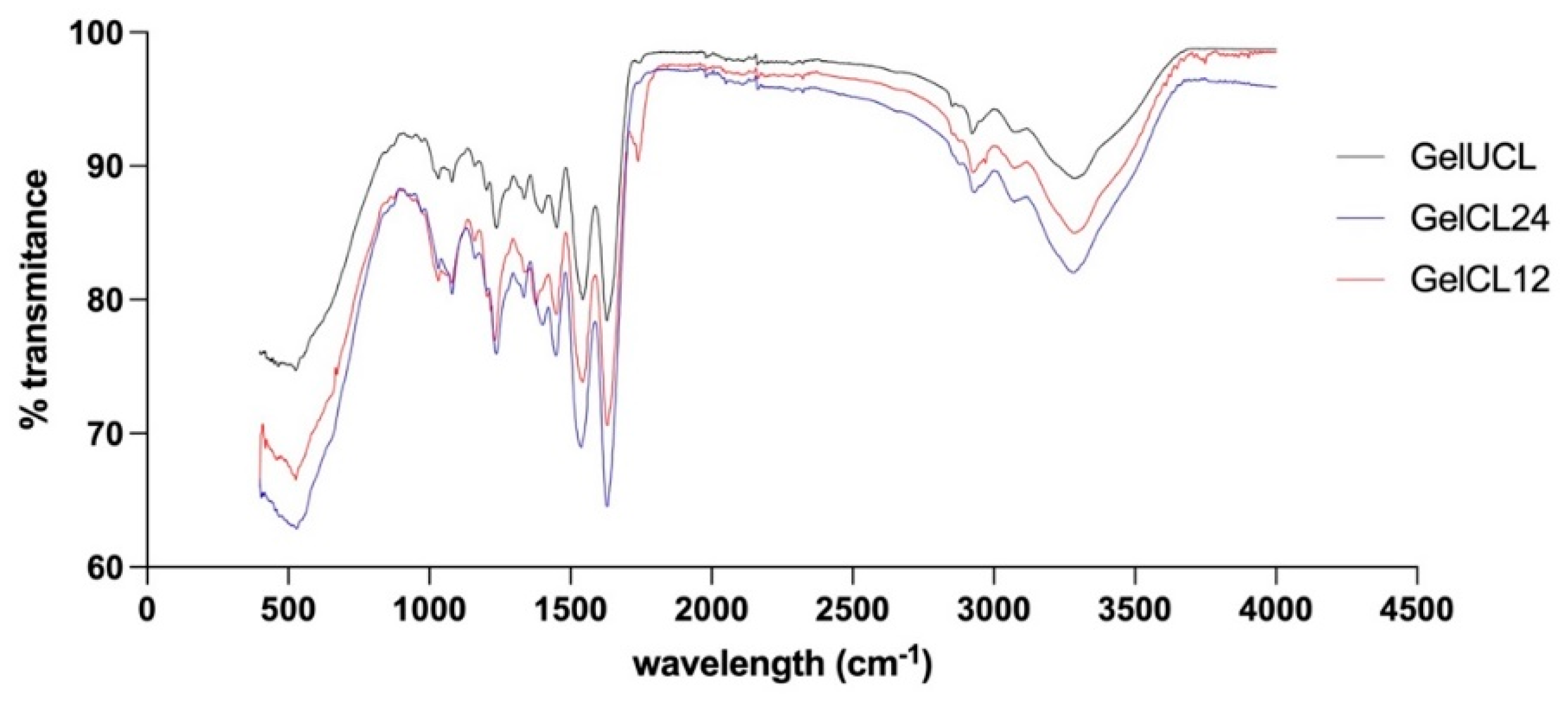
To investigate the influence of the crosslinking agent on the structure of gelatin hydrogels, FTIR analysis was conducted. FTIR spectra in
Figure 4. indicate that there are similar peptide bond characteristics between the three groups of gelatin hydrogels. The first peak was observed around 3287.00 cm
−1, confirming the O-H and N-H bonds. The second peak was observed at 2923.77-2926.20 cm
−1, confirming the -CH
3 bond. In all of the spectra observed, Amide I (1600-1700 cm
−1) and Amide II (1500-1590 cm
−1) were found. This indicates that the structure of the formed crosslinked gelatin hydrogels has not undergone any notable changes due to the crosslinking agents despite the concentration differences. This finding confirms a study by Hoon Lee et al. which reported the same results.
50 EDC/NHS classified as zero-length crosslinker where its addition will not change the main structure of the gelatin hydrogel.
32 The FTIR analysis provides a quantitative indicator of the influence of EDC/NHS crosslinking on gelatin hydrogel when compared to the uncrosslinked group. It can be concluded that, despite differences in the crosslinking process, the main chemical structure of the gelatin hydrogel remains similar, with hydrogen bonds and key peptide groups playing a central role in its properties. The interpretation of this FTIR spectrum serves not only as an analytical tool but also as a research instrument to comprehend the structural characteristics of both types of gelatin hydrogel, which can have significant implications in gingival tissue regeneration applications.
3.4. Rheological Properties
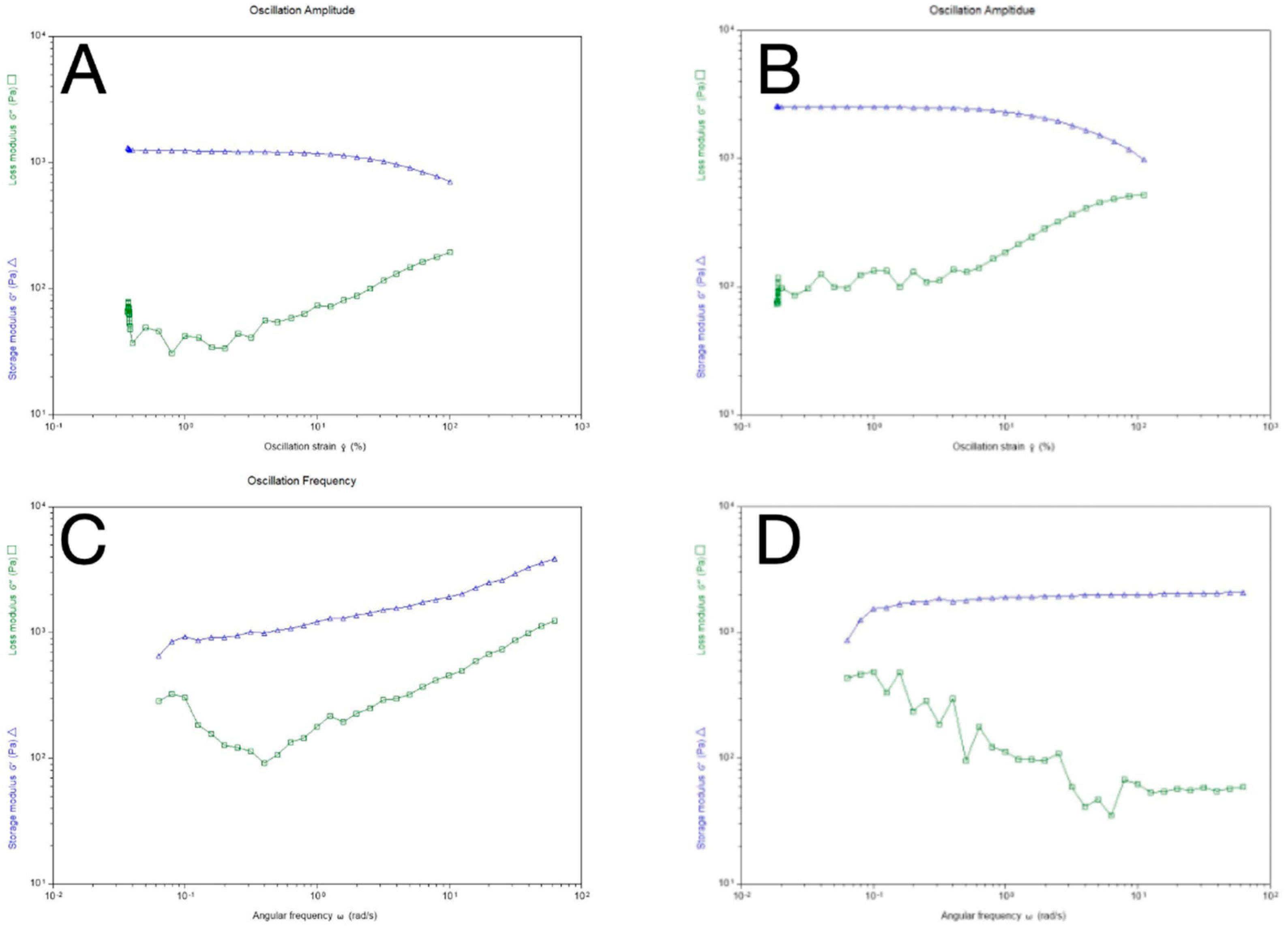
The frequency sweep and strain sweeps were used as rheology measurements for this study and are considered critical to the full characterization of viscoelastic material. LVR viscosity analysis was carried out to analyze the relationship between oscillation strain and hydrogel shape which is influenced by storage modulus (G’) and loss modulus (G").
36 The graph on
Figure 5 shows that the loss modulus of the GelCL12 and GelCL24 were below the storage modulus curve, which means that the crosslinked gelatin hydrogel is in a viscoelastic form when a strain of 10
-1 -10
2 is applied. Sample deformation was seen at 10% critical strain, marking a non-linear line. A rapid increase in loss modulus occurred after the critical strain which marked a change in the properties of the crosslinked gelatin hydrogel. The sample increasingly resembles a fluid-like material and its ability to store energy elastically decreases. In this experimental study, it was found that the GelUCL experienced shape degradation when heated to a temperature of 37 °C even before the expected rheological tests were carried out. This shows consistent results with previous research and proves that crosslinked gelatin hydrogel with EDC-NHS has superior thermal stability and structural strength when compared with GelUCL.
44 In the LVR and Oscillation Frequency test results (
Figure 5 A and B) the crosslinked gelatin hydrogel consistently exhibited lower loss modulus (G") values compared to the storage modulus (G’), indicating superior elastic energy absorption or storage over viscosity. A critical strain point was identified at a 10% strain, initiating deformations with a decrease in storage modulus. This critical strain determination is crucial, as data beyond it does not reflect equilibrium conditions. In the LVR test, the loss modulus reached a linear stage post 5% strain, possibly due to low strain settings causing initial data overlap. Further structural characterization involved a frequency sweep test, revealing constant storage modulus (G’) in
Figure 5 A&B, which confirmed the characteristics of structured materials. The loss modulus decreased in the 0.1-0.5 rad/s frequency range, suggesting a fluid-like property of the crosslinked gelatin hydrogel. We found that an unstable trend in the loss modulus, possibly linked to factors such as gelatin concentration, temperature sensitivity, and changes in shear stress rates. Ensuring consistent sample preparation and calibrating the rheometer machine are crucial to controlling experimental conditions, optimizing reliability, and ensuring reproducibility in rheological analysis.
Hydrogel is a potential biomaterial because of its similarity to the extracellular matrix, which plays an important role in the function of cell development and homeostasis. Hydrogels have unique viscoelastic characteristics and a very low modulus when compared to solid materials. Analysis of the mechanical and rheological properties of hydrogels is necessary to understand cell mechanotransduction.51 The frequency sweep test is carried out by changing the given frequency progressively while keeping the amplitude constant, while the strain sweep test provides a fixed frequency but a progressive strain range. The gelation process of gelatin is the result of conformational changes (coil-to-helix transition) and the aggregation of protein chains.12 The new chemical structure created during solution cooling is stabilized by hydrogen bonds. Hydrogen bonds that are easily broken when heated cause gelatin hydrogel to be thermally reversible and have poor mechanical properties.
3.5. Swelling Properties
Hydrogels hydration makes will result in relaxation of the polymer chain and the hydrogels will be expanded due to osmotic forces.
52 Swelling ratio is frequently used as a parameter to show the scaffold’s reaction to its surrounding environment.
53 The appearance of swelling can be associated to the formation of hydrogen bonds between the free -OH groups and the molecules that exist in the aqueous solution. These hydrogen bonds facilitate the hydrogel gelatin in retaining water within its structure, ultimately resulting in the apparent swelling.
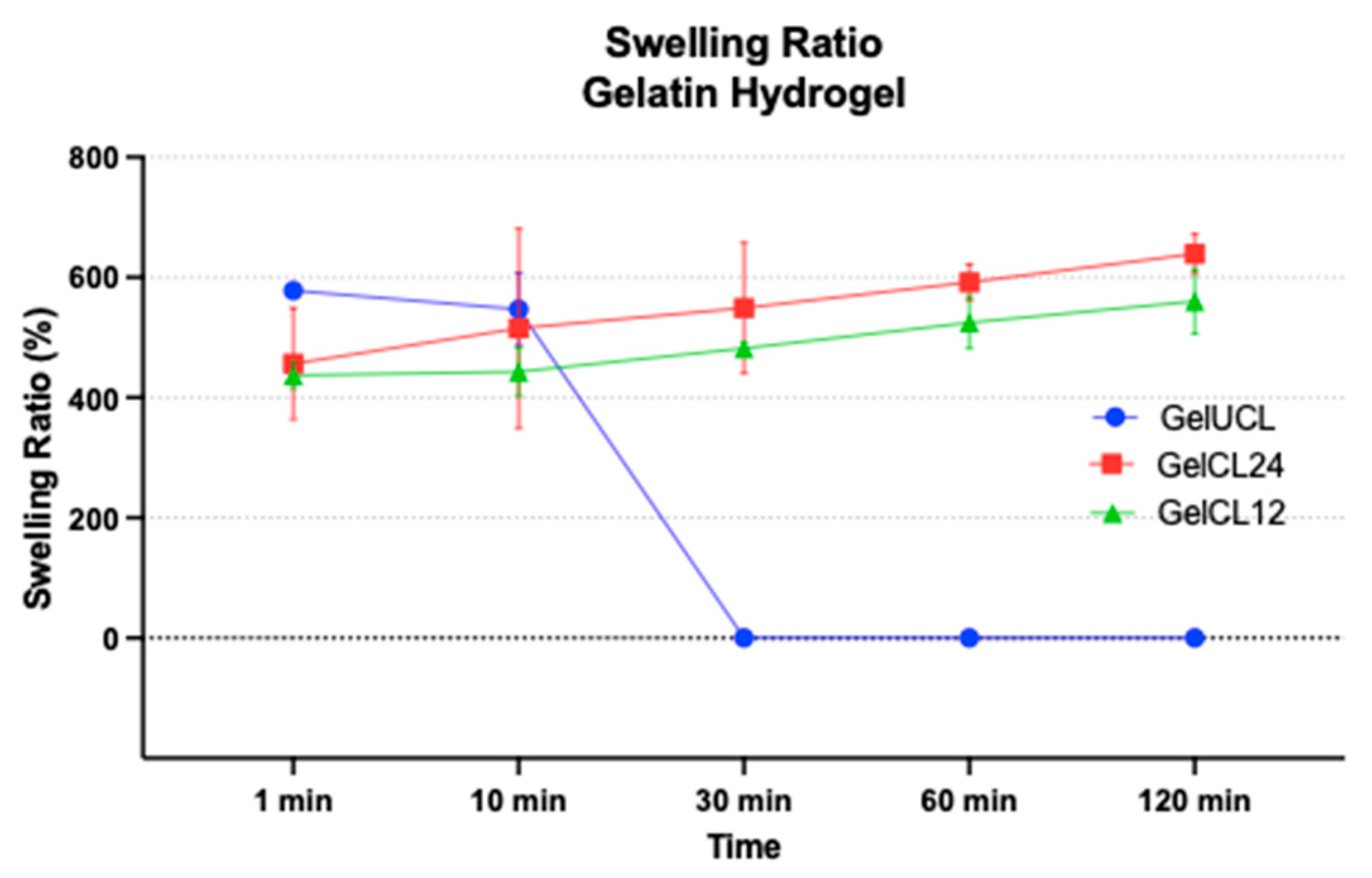
54 Figure 6 displays the swelling ratio of all samples. Almost all the swelling ratios revealed significant differences across all groups (
p<0.05), with the exception of the 1-minute and 10-minute immersion in PBS. GelUCL hydrogel had the highest swelling ratio during the first minute (578.03±7.71), followed by Gel CL24 with 456.45% ± 92.49%, and Gel CL12 was the lowest with 436.86% ± 21.82%. However, Ge UCL has no longer held its shape for 30 minutes or more, so the measurement of its swelling ratio was unfeasible. Overall, Gel CL12 had a lower swelling ratio compared to Gel CL24 (p<0,05), as a result of the more rigid network with the increased amount of crosslinker.
Previous studies have shown that hydrogel exhibits a high swelling rate. Scaffolds with a high degree of swelling, more than 150%, have been found to possess advantageous properties for the purpose of tissue regeneration and as drug delivery agents.55 Careful control of the rate of swelling is necessary to avoid compromising the mechanical strength of hydrogel.56,57 The presence of crosslinker agents in hydrogel gelatin leads to a reduction in the swelling ratio, resulting in enhanced stability and rigidity of the material.58 As the amount of crosslinker agents went up, the amount of amino and carboxyl group hydrogel gelatin went down; hence, the ratio of swelling went down as well.59 More than that, upon exposure to an aqueous solution, hydrogel gelatin also experiences a transformation in consistency that resembles the structure of rubber. This alteration leads to a reduction in interfacial tension with other biological fluids, thereby mimicking the characteristics of living tissue.60
3.6. Biodegradation Rate
Biodegradation capability of the hydrogels was done as a simulation when scaffolds were implanted
in-vivo.
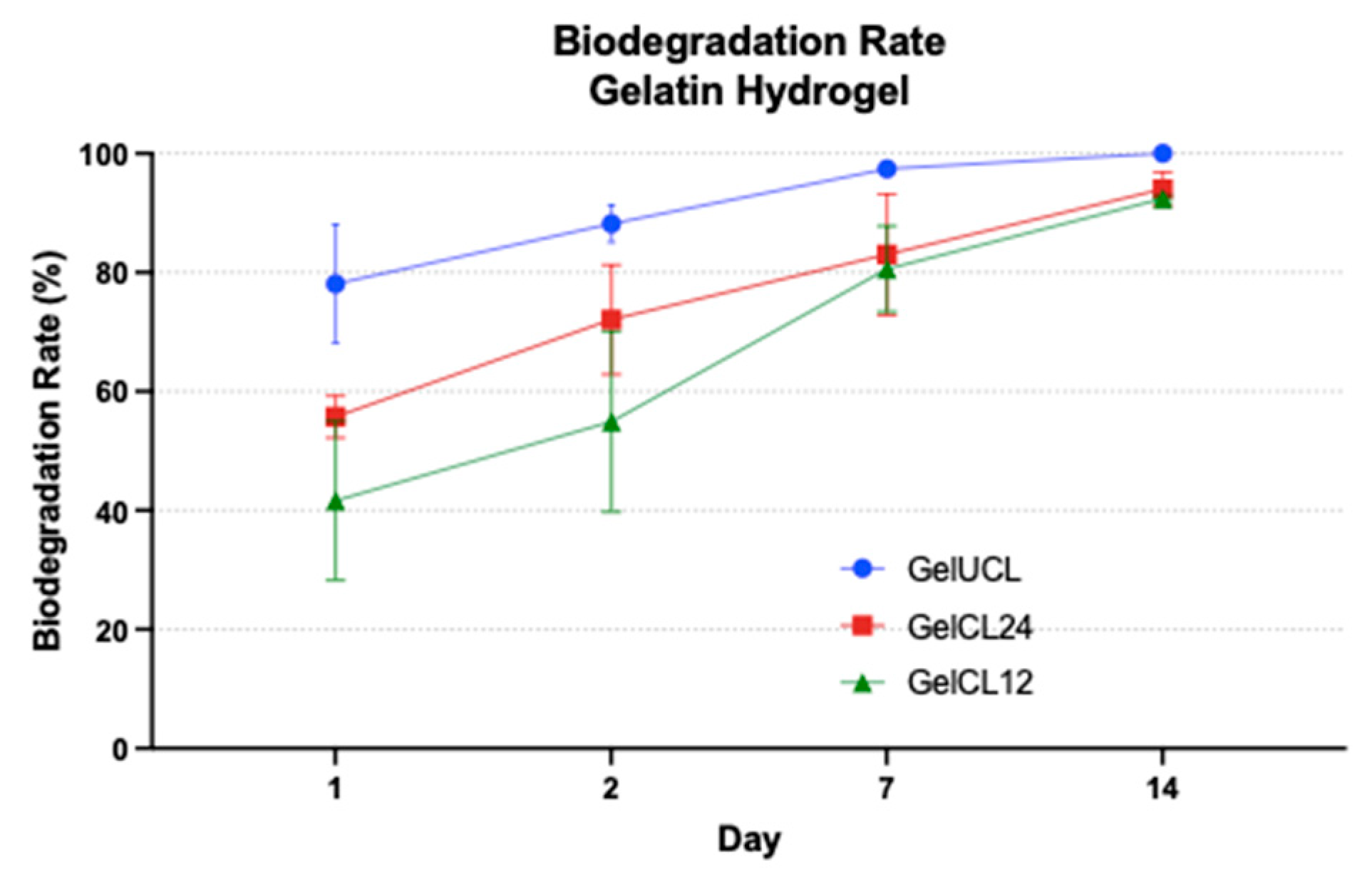
Figure 7 describes the hydrolytic degradation by soaking the hydrogel in PBS under physiological temperature.
61 Following the first day of immersion in the PBS, Gel UCL exhibits a preservation rate of less than 20% of the original dry weight, while Gel CL 24 and Gel CL12% have a preservation rate over 50% of their original dry weights. Those scores are statistically significant difference with a p value < 0,05. On the 7
th day, the precipitate of Gel UCL had almost gone with 97.41%±1.48%. By the 14
th day, the precipitate had completely vanished without leaving any detectable residue. However, samples with EDC/NHS, exhibit uncompleted degradation even after a duration of 14 days. The degradation rate for Gel CL24 and Gel CL12 was 94.00%±2.80% and 92.43% ±0.43%, with no significant difference (p>0,05). This phenomenon suggests that the presence of EDC/NHS may enhance the stability of the hydrogel.
The degradation of the scaffold should, ideally, be synchronised with the formation of the targeted new tissue.57 In gingival regeneration, a duration of 14 days is considered sufficient for facilitating the development of new tissue, infiltration, and proliferation of cells.61,62 Moreover, the rate of degradation cannot be too slow, as it might cause infection of the surrounding tissue and leads to nutrient and oxygen deficiencies in the growing tissue.41,63 From the result, we found that GelCL12 had slower biodegradation followed by GelCL24 and GelUCL. It might be concluded that higher crosslinker concentration resulted in more stable hydrogels during degradation process. This phenomenon might happens because of the loose structure of gelatin-based hydrogel, which facilitates the rapid infiltration of water molecules into its internal structure. As a consequence, both the internal and external bonds of the hydrogel are obstructed at the same time, leading to quick breakdown of the scaffold by bulk erosion.64 In GelCL24 and GelCL12, the possible scenario was that both of them underwent surface erosion on their initial day of immersion in PBS. Surface erosion occurs due to the minimal access of water molecules into the scaffold’s internal structure, resulting in the initial breakage of surface bonds followed by subsequent breakage of inner bonds. The rate of surface erosion is influenced by the degree of crosslinking; a higher crosslinking density restricts the infiltration of water molecules into the internal framework of the scaffold, resulting in a slower degradation process.64 This could potentially describe the slower rate of degradation observed in GelCL12 when compared to other groups.
4. Conclusion
The aim of this study was to investigate the effect of EDC/NHS concentration to physical and chemical characteristic in gelatin hydrogels as a potential scaffold for gingival tissue regeneration. The crosslinking strategy using EDC/NHS appeared to improve the physical stability of hydrogels without compromising its chemical structure. Moreover, by employing the correct amount of crosslinker ratio, it becomes possible to achieve the optimal balance between the desired characteristics and mechanical strength. This study found that GelCL12 which has higher crosslinker degree had superior result compared to GelCL24 and GelUCL. For future investigation, it is important to analyzed the biological characteristic of the hydrogels whether in vitro or in vivo.
Author Contributions
Conceptualization, D.I.H., Y.S, E.W.B, and E.G.G; methodology, D.I.H., Y.S, and E.G.G.; formal analysis, D.I.H., D.F.S., E.W.B., Y.S. and L.A.; data curation, D.I.H., F.A.G., K.K., Y.S., F.M.T., A.W., and E.G.G.; writing— original draft preparation, D.I.H., F.A.G., K.K., Y.S., and E.G.G.; writing—review and editing, Y.S., E.W.B, F.M.T, E.G.G; supervision, Y.S., E.W.B., F.M.T, A.D, N.H., R.L., and E.G.G; funding acquisition, Y.S., F.M.T., and D.I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Directorate of Research and Development, Universitas Indonesia under Hibah PUTI 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hutomo DI, Amir L, Suniarti DF, Bachtiar EW, Soeroso Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers (Basel). 2023, 15, 2591. [Google Scholar] [CrossRef] [PubMed]
- AlSarhan MA, Al Jasser R, Tarish MA, AlHuzaimi AI, Alzoman H. Xenogeneic collagen matrix versus connective tissue graft for the treatment of multiple gingival recessions: A systematic review and meta-analysis. Clin Exp Dent Res. 2019, 5, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Chambrone L, Tatakis DN. Long-Term Outcomes of Untreated Buccal Gingival Recessions: A Systematic Review and Meta-Analysis. J Periodontol. 2016, 87, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Tavelli L, McGuire MK, Zucchelli G, et al. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Toledano M, Toledano-Osorio M, Carrasco-Carmona Á, et al. State of the art on biomaterials for soft tissue augmentation in the oral cavity. Part I: Natural polymers-based biomaterials. Polymers (Basel). 2020, 12. [Google Scholar] [CrossRef]
- Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: A review. Int J Polym Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Sawadkar P, Mandakhbayar N, Patel KD, et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J Tissue Eng. 2021, 12. [Google Scholar] [CrossRef]
- Ayala-Ham A, López-Gutierrez J, Bermúdez M, et al. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Front Mater. 2021, 8, 1–26. [Google Scholar] [CrossRef]
- El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract. 2013, 2013, 38. [Google Scholar] [CrossRef]
- Lee Y, Gou Y, Pan X, Gu Z, Xie H. Advances of multifunctional hydrogels for periodontal disease. Smart Mater Med. 2023, 4, 460–467. [Google Scholar] [CrossRef]
- Nacu I, Bercea M, Niță LE, Peptu CA, Butnaru M, Vereștiuc L. 3D bioprinted scaffolds based on functionalized gelatin for soft tissue engineering. React Funct Polym. 2023, 190. [Google Scholar] [CrossRef]
- Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3. [CrossRef]
- Spicer, CD. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels … A review. Saudi Pharmaceutical Journal. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zhao L, Zhou Y, Zhang J, Liang H, Chen X, Tan H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15. [CrossRef]
- Ghassemi Z, Slaughter G. Storage stability of electrospun pure gelatin stabilized with EDC/Sulfo-NHS. Biopolymers 2018, 109. [CrossRef]
- Tabatabaei F, Moharamzadeh K, Tayebi L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J Oral Biol Craniofac Res. 2020, 10, 573–577. [Google Scholar] [CrossRef]
- Echave MC, Hernáez-Moya R, Iturriaga L, et al. Recent advances in gelatin-based therapeutics. Expert Opin Biol Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Chou SF, Luo LJ, Lai JY, Ma DHK. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Materials Science and Engineering C. 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Lukin I, Erezuma I, Maeso L, et al. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics. 2022, 14. [Google Scholar] [CrossRef]
- Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Wang X, Ao Q, Tian X, et al. Gelatin-based hydrogels for organ 3D bioprinting. Polymers (Basel) 2017, 9. [CrossRef]
- Baydin T, Aarstad OA, Dille MJ, Hattrem MN, Draget KI. Long-term storage stability of type A and type B gelatin gels: The effect of Bloom strength and co-solutes. Food Hydrocoll 2022, 127. [CrossRef]
- Nakajima D, Tabata Y, Sato S. Periodontal tissue regeneration with PRP incorporated gelatin hydrogel sponges. Biomedical Materials (Bristol). 2015, 10. [Google Scholar] [CrossRef]
- Campiglio CE, Negrini NC, Farè S, Draghi L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12. [CrossRef]
- Miranda DG, Malmonge SM, Campos DM, Attik NG, Grosgogeat B, Gritsch K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J Biomed Mater Res B Appl Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, A. Non-toxic crosslinking of electrospun gelatin nanofibers for tissue engineering and biomedicine—a review. Polymers (Basel). 2021, 13. [Google Scholar] [CrossRef]
- Cañas AI, Delgado JP, Gartner C. Biocompatible scaffolds composed of chemically crosslinked chitosan and gelatin for tissue engineering. J Appl Polym Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ahmad Z, Shepherd JH, Shepherd D V, et al. Effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide concentrations on the mechanical and biological characteristics of cross-linked collagen fibres for tendon repair. Regen Biomater. 2015, 2, 77–85. [Google Scholar] [CrossRef]
- Li S, Wu P, Ji Z, et al. In vitro biocompatibility study of EDC/NHS cross-linked silk fibroin scaffold with olfactory ensheathing cells. J Biomater Sci Polym Ed. 2023, 34, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska J, Tuszynska M, Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Cammarata CR, Hughes ME, Ofner CM. Carbodiimide induced cross-linking, ligand addition, and degradation in gelatin. Mol Pharm. 2015, 12, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Usha R, Sreeram KJ, Rajaram A. Stabilization of collagen with EDC/NHS in the presence of l-lysine: A comprehensive study. Colloids Surf B Biointerfaces. 2012, 90, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar AK, Singh I, Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- He L, Li S, Xu C, et al. A New Method of Gelatin Modified Collagen and Viscoelastic Study of Gelatin-Collagen Composite Hydrogel. Macromol Res. 2020, 28, 861–868. [Google Scholar] [CrossRef]
- Wickramathilaka MP, Tao BY. Characterization of covalent crosslinking strategies for synthesizing DNA-based bioconjugates. J Biol Eng 2019, 13. [CrossRef]
- Jarquin Yanez K, Arenas Alatorre J. Structural Effect of Different EDC Crosslinker Concentration in Gelatin- Hyaluronic Acid Scaffolds. J Bioeng Biomed Sci 2016, 06. [CrossRef]
- Rafat M, Li F, Fagerholm P, et al. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials. 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Goodarzi H, Jadidi K, Pourmotabed S, Sharifi E, Aghamollaei H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int J Biol Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Annabi N, Nichol JW, Zhong X, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Somo SI, Akar B, Bayrak ES, et al. Pore Interconnectivity Influences Growth Factor-Mediated Vascularization in Sphere-Templated Hydrogels. Tissue Eng Part C Methods. 2015, 21, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Murphy CM, O’Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao F, Lacroix D, Ito K, van Rietbergen B, Hofmann S. Changes in scaffold porosity during bone tissue engineering in perfusion bioreactors considerably affect cellular mechanical stimulation for mineralization. Bone Rep. 2020, 12. [Google Scholar] [CrossRef]
- Jia G, Huang H, Niu J, et al. Exploring the interconnectivity of biomimetic hierarchical porous Mg scaffolds for bone tissue engineering: Effects of pore size distribution on mechanical properties, degradation behavior and cell migration ability. Journal of Magnesium and Alloys. 2021, 9, 1954–1966. [Google Scholar] [CrossRef]
- Goodarzi H, Jadidi K, Pourmotabed S, Sharifi E, Aghamollaei H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int J Biol Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Teng W, Long TJ, Zhang Q, Yao K, Shen TT, Ratner BD. A tough, precision-porous hydrogel scaffold: Ophthalmologic applications. Biomaterials. 2014, 35, 8916–8926. [Google Scholar] [CrossRef]
- Lee DH, Tamura A, Arisaka Y, Seo JH, Yui N. Mechanically reinforced gelatin hydrogels by introducing slidable supramolecular cross-linkers. Polymers (Basel) 2019, 11. [CrossRef]
- Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Pinelli F, Magagnin L, Rossi F. Progress in hydrogels for sensing applications: a review. Mater Today Chem 2020, 17. [CrossRef]
- Nurfriana P, Widiyanti P, Rudyarjo DI. Effect of collagen-chitosan-glycerol composition in scaffold for gingival recession therapy. Journal of Biomimetics, Biomaterials and Biomedical Engineering. 2019, 40, 101–108. [Google Scholar] [CrossRef]
- Mushtaq F, Raza ZA, Batool SR, et al. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int J Biol Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Bartnikowski M, Wellard RM, Woodruff M, Klein T. Tailoring hydrogel viscoelasticity with physical and chemical crosslinking. Polymers (Basel). 2015, 7, 2650–2669. [Google Scholar] [CrossRef]
- Jin X, Wei C, Wu C, Zhang W. Gastric fluid-induced double network hydrogel with high swelling ratio and long-term mechanical stability. Compos B Eng 2022, 236. [CrossRef]
- Hutomo DI, Amir L, Suniarti DF, Bachtiar EW, Soeroso Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers (Basel). 2023, 15, 2591. [Google Scholar] [CrossRef]
- Xing Q, Yates K, Vogt C, Qian Z, Frost MC, Zhao F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci Rep. 2014, 4. [Google Scholar] [CrossRef]
- Zhao L, Wu Y, Chen S, Xing T. Preparation and characterization of cross-linked carboxymethyl chitin porous membrane scaffold for biomedical applications. Carbohydr Polym. 2015, 126, 150–155. [Google Scholar] [CrossRef]
- Gyles DA, Castro LD, Silva JOC, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur Polym J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Vallecillo C, Toledano-Osorio M, Vallecillo-Rivas M, Toledano M, Osorio R. In vitro biodegradation pattern of collagen matrices for soft tissue augmentation. Polymers (Basel). 2021, 13. [Google Scholar] [CrossRef]
- Rodrigues AZ, de Oliveira PT, Novaes AB, Maia LP, de Souza SLS, Palioto DB. Evaluation of In Vitro human gingival fibroblast seeding on acellular dermal matrix. Braz Dent J. 2010, 21, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Soheilifar S, Bidgoli M, Faradmal J, Soheilifar S, Bidgoli M. Effect of Periodontal Dressing on Wound Healing and Patient Satisfaction Following Periodontal Flap Surgery. Vol 12.; 2015. www.jdt.tums.ac.ir.
- Liu HW, Su WT, Liu CY, Huang CC. Highly Organized Porous Gelatin-Based Scaffold by Microfluidic 3D-Foaming Technology and Dynamic Culture for Cartilage Tissue Engineering. Int J Mol Sci 2022, 23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).