Submitted:
23 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extract Preparation
2.2. In Vitro Insulin-Releasing Studies Using BRIN-BD11 Cells
2.3. In Vitro Insulin-Releasing Studies Using Isolated Mouse Islets
2.4. In Vitro Starch Digestion Using EEMI
2.5. In Vitro Glucose Diffusion Using EEMI
2.6. In Vitro DPPH Assay Using EEMI
2.7. Animals
2.8. Acute Effects of EEMI on Feeding Test
2.9. Acute Effects of EEMI Leaves on Oral Glucose Tolerance Test
2.10. Effect of EEMI on Body Weight and Fasting Blood Glucose
2.11. Effects of EEMI Leaves on Liver Glycogen Content
2.12. Effects of EEMI on Lipid Profile
2.13. Phytochemical Screening of EEMI
2.14. Statistical Analysis
3. Results
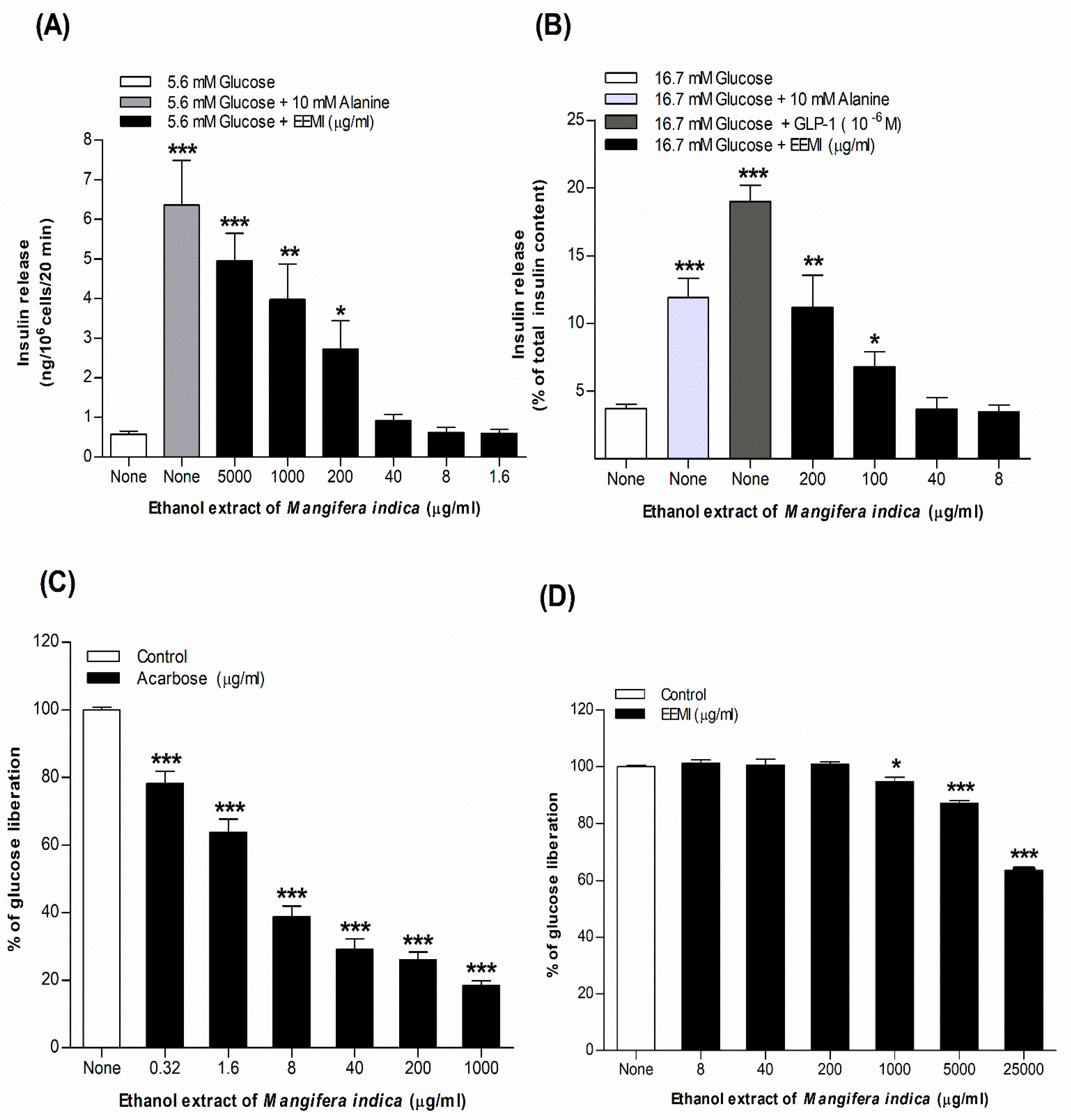
3.1. Insulin Release from BRIN-BD11 Cells Using EEMI
3.2. Insulin Release from Isolated Mouse Islets Using EEMI
3.3. Starch Digestion Using EEMI
3.4. DPPH Assay Using EEMI
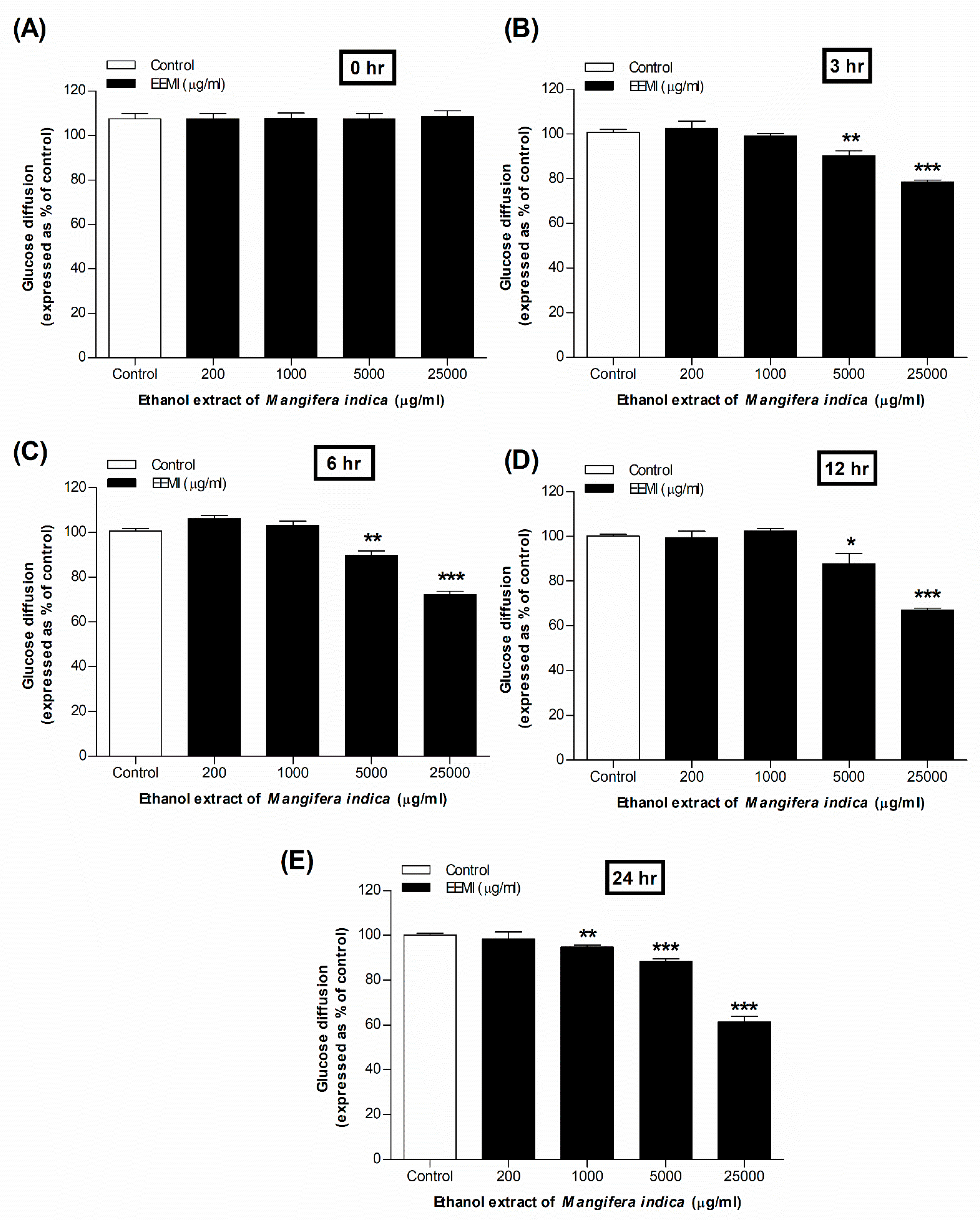
3.5. Glucose Diffusion Using EEMI
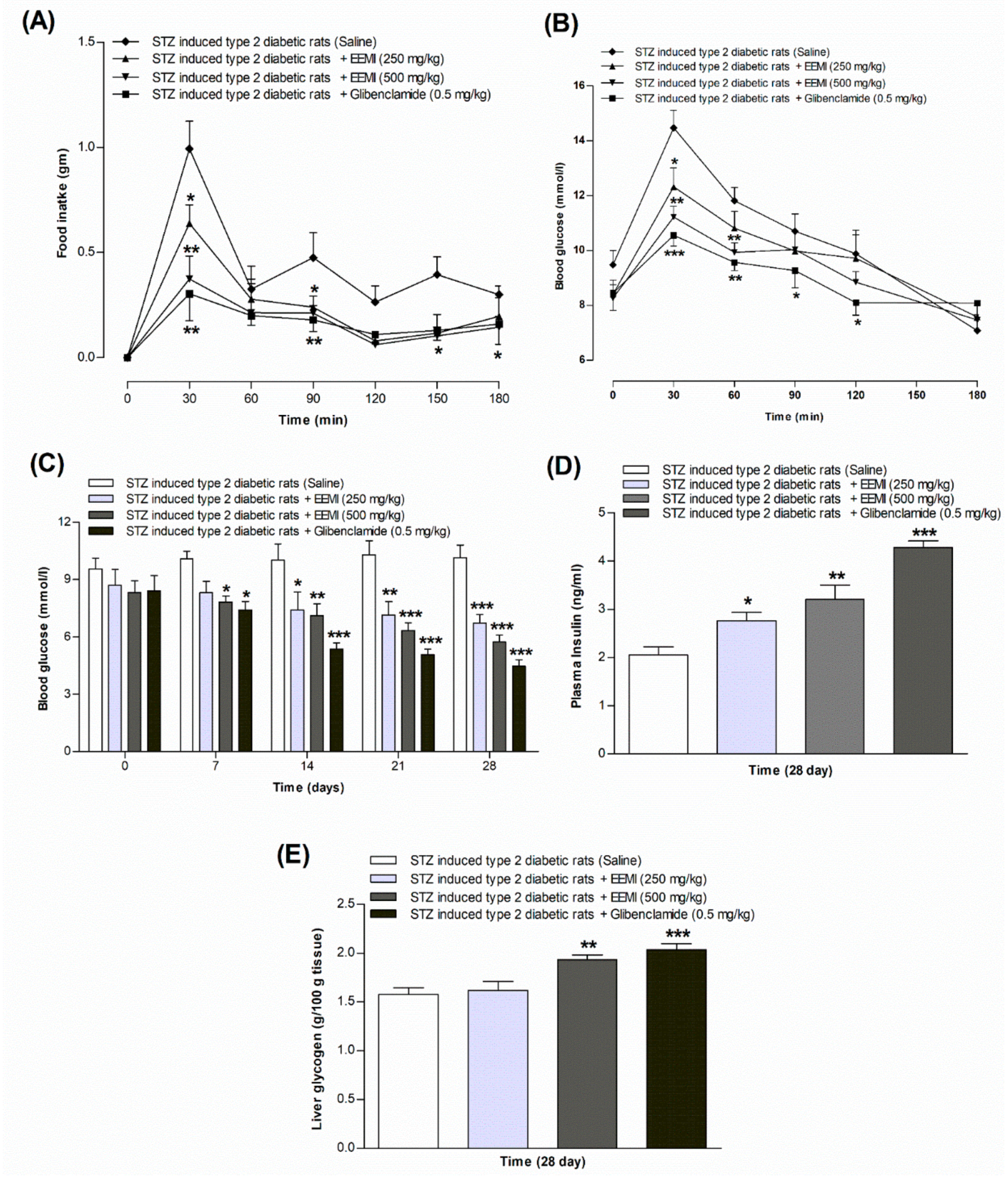
3.6. Feeding Test Using EEMI
3.7. Oral Glucose Tolerance Test Using EEMI
3.8. Fasting Blood Glucose, Plasma Insulin and Liver Glycogen Using EEMI
3.9. Chronic Effects Using EEMI
3.10. Phytochemical Screening Using EEMI
| Group | Results |
|---|---|
| Alkaloids | + |
| Tannins | + |
| Saponins | + |
| Steroids | + |
| Glycosides | + |
| Flavonoids | + |
| Reducing sugar | + |
| Anthraquinone | ‒ |
| (+) = Presence, (‒) = absence | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviation
| BRIN-BD11 | Clonal pancreatic BRIN-BD11 β-cells |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GLP-1 | Glucagon-Like Peptide 1 |
| HDL | High-Density Lipoprotein |
| KATP | ATP-sensitive K+ channel |
| LDL | Low-Density Lipoprotein |
| T2DM | Type 2 Diabetes Mellitus |
| VLDL | Very-Low-Density Lipoprotein |
References
- Ansari, P.; Tabasumma, N.; Snigdha, N. N.; Siam, N. H.; Panduru, R. V. N. R. S.; Azam, S.; Hannan, J. M. A.; Abdel-Wahab, Y. H. A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175. [Google Scholar] [CrossRef]
- Ansari, P.; Samia, J. F.; Khan, J. T.; Rafi, M. R.; Rahman, M. S.; Rahman, A. B.; Abdel-Wahab, Y. H. A.; Seidel, V. Protective Effects of Medicinal Plant-Based Foods against Diabetes: A Review on Pharmacology, Phytochemistry, and Molecular Mechanisms. Nutrients 2023, 15, 3266. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Hannan, J. M. A.; Azam, S.; Jakaria, M. Challenges in Diabetic Micro-Complication Management: Focus on Diabetic Neuropathy. International Journal of Translational Medicine 2021, 1, 175–186. [Google Scholar] [CrossRef]
- George, M. M.; Copeland, K. C. Current Treatment Options for Type 2 Diabetes Mellitus in Youth: Today’s Realities and Lessons from the TODAY Study. Curr Diab Rep 2013, 13, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, A. M.; Talib, W. H. Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules 2021, 26, 742. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr J Tradit Complement Altern Med 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Paul, A. Devasagayam, T. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J Clin Biochem Nutr 2007, 40, 163–173. [Google Scholar] [CrossRef]
- Patel, D. K.; Prasad, S. K.; Kumar, R.; Hemalatha, S. An Overview on Antidiabetic Medicinal Plants Having Insulin Mimetic Property. Asian Pac J Trop Biomed 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Pang, G.-M.; Li, F.-X.; Yan, Y.; Zhang, Y.; Kong, L.-L.; Zhu, P.; Wang, K.-F.; Zhang, F.; Liu, B.; Lu, C. Herbal Medicine in the Treatment of Patients with Type 2 Diabetes Mellitus. Chin Med J (Engl) 2019, 132, 78–85. [Google Scholar] [CrossRef]
- Shah, K. A.; Patel, M. B.; Patel, R. J.; Parmar, P. K. Mangifera Indica (Mango). Pharmacogn Rev 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Shah, K. A.; Patel, M. B.; Patel, R. J.; Parmar, P. K. Mangifera Indica (Mango). Pharmacogn Rev 2010, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Hamid, A.; Khalil, A.; Ghaffar, A.; Tayyaba, N.; Saeed, A.; Ali, M.; Naveed, A. Review on Medicinal Uses, Pharmacological, Phytochemistry and Immunomodulatory Activity of Plants. Int J Immunopathol Pharmacol 2014, 27, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M. A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant Phenolic Compounds Recovery from Mangifera Indica L. by-Products by Supercritical Antisolvent Extraction. Journal of Food Engineering 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Saleem, M.; Tanvir, M.; Akhtar, M. F.; Iqbal, M.; Saleem, A. Antidiabetic Potential of Mangifera Indica L. Cv. Anwar Ratol Leaves: Medicinal Application of Food Wastes. Medicina (Kaunas) 2019, 55, 353. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y. H. A.; Power, G. J.; Flatt, P. R.; Woodhams, D. C.; Rollins-Smith, L. A.; Conlon, J. M. A Peptide of the Phylloseptin Family from the Skin of the Frog Hylomantis Lemur (Phyllomedusinae) with Potent in Vitro and in Vivo Insulin-Releasing Activity. Peptides 2008, 29, 2136–2143. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P. R.; Harriott, P.; Abdel-Wahab, Y. H. A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. Squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants (Basel) 2020, 9, 1348. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S. T.; Abdel-Wahab, Y. H. A. Insulin Secretory Actions of Ethanol Extract of Eucalyptus Citriodora Leaf, Including Plasma DPP-IV and GLP-1 Levels in High-Fat-Fed Rats, as Well as Characterization of Biologically Effective Phytoconstituents. Metabolites 2022, 12, 757. [Google Scholar] [CrossRef]
- Ansari, P.; Hannan, J. M. A.; Seidel, V.; Abdel-Wahab, Y. H. A. Polyphenol-Rich Leaf of Annona Squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats. Metabolites 2022, 12, 995. [Google Scholar] [CrossRef]
- Ansari, P.; Hannan, J. M. A.; Choudhury, S. T.; Islam, S. S.; Talukder, A.; Seidel, V.; Abdel-Wahab, Y. H. A. Antidiabetic Actions of Ethanol Extract of Camellia Sinensis Leaf Ameliorates Insulin Secretion, Inhibits the DPP-IV Enzyme, Improves Glucose Tolerance, and Increases Active GLP-1 (7-36) Levels in High-Fat-Diet-Fed Rats. Medicines (Basel) 2022, 9, 56. [Google Scholar] [CrossRef]
- Ansari, P.; Islam, S. S.; Akther, S.; Khan, J. T.; Shihab, J. A.; Abdel-Wahab, Y. H. A. Insulin Secretory Actions of Ethanolic Extract of Acacia Arabica Bark in High Fat-Fed Diet-Induced Obese Type 2 Diabetic Rats. Bioscience Reports 2023, 43, BSR20230329. [Google Scholar] [CrossRef]
- Ansari, P.; Hannan, J. A.; Abdel-Wahab, Y. H. A.; Flatt, P. R. Antidiabetic and Insulinotropic Properties of Bark of Heritiera Fomes: Inhibits Starch Digestion, Protein Glycation, DPP-IV Activity, and Glucose Absorption in Gut. Planta Medica 2021, 87, 1252–1252. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Seidel, V.; Abdel-Wahab, Y. H. A. In Vitro and in Vivo Antihyperglycemic Activity of the Ethanol Extract of Heritiera Fomes Bark and Characterization of Pharmacologically Active Phytomolecules. J Pharm Pharmacology 2022, rgac010. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 Medicinal Plant Extracts for Antioxidant Capacity and Total Phenols. Food Chemistry 2006, 94, 550–557. [Google Scholar] [CrossRef]
- N, C.; A, S.; Jc, A. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants (Basel, Switzerland) 2020, 9. [Google Scholar] [CrossRef]
- Lenzen, S. The Mechanisms of Alloxan- and Streptozotocin-Induced Diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K. Revised Guide for the Care and Use of Laboratory Animals Available. American Physiological Society. Physiologist 1996, 39, 199, 208–211. [Google Scholar] [PubMed]
- Ansari, P.; Afroz, N.; Jalil, S.; Azad, S. B.; Mustakim, M. G.; Anwar, S.; Haque, S. M. N.; Hossain, S. M.; Tony, R. R.; Hannan, J. M. A. Anti-Hyperglycemic Activity of Aegle Marmelos (L.) Corr. Is Partly Mediated by Increased Insulin Secretion, α-Amylase Inhibition, and Retardation of Glucose Absorption. J Pediatr Endocrinol Metab 2017, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Azam, S.; Hannan, J. M. A.; Flatt, P. R.; Abdel Wahab, Y. H. A. Anti-Hyperglycaemic Activity of H. Rosa-Sinensis Leaves Is Partly Mediated by Inhibition of Carbohydrate Digestion and Absorption, and Enhancement of Insulin Secretion. J Ethnopharmacology 2020, 253, 112647. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J. M. A.; Ansari, P.; Azam, S.; Flatt, P. R.; Abdel Wahab, Y. H. A. Effects of Spirulina Platensis on Insulin Secretion, Dipeptidyl Peptidase IV Activity and Both Carbohydrate Digestion and Absorption Indicate Potential as an Adjunctive Therapy for Diabetes. Br J Nutr 2020, 124, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Badhan, S. S.; Azam, S.; Sultana, N.; Anwar, S.; Mohamed Abdurahman, M. S.; Hannan, J. M. A. Evaluation of Antinociceptive and Anti-Inflammatory Properties of Methanolic Crude Extract of Lophopetalum Javanicum (Bark). J Basic Clin Physiol Pharmacol 2016, 27, 379–385. [Google Scholar] [CrossRef]
- Harris, M.; Entmacher, P. Mortality from diabetes. Diabetes in America. 1985; 1–48. [Google Scholar]
- Ansari, P.; Akther, S.; Khan, J. T.; Islam, S. S.; Masud, M. S. R.; Rahman, A.; Seidel, V.; Abdel-Wahab, Y. H. A. Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies. Applied Sciences 2022, 12, 11777. [Google Scholar] [CrossRef]
- Ansari, P.; Akther, S.; Hannan, J. M. A.; Seidel, V.; Nujat, N. J.; Abdel-Wahab, Y. H. A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R. K.; Dhumal, S.; Punia, S.; Amarowicz, R.; Mekhemar, M. Mango (Mangifera Indica L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Antioxidants (Basel) 2021, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V. M.; Rathod, V. K. Extraction of Mangiferin from Mangifera Indica Leaves Using Three Phase Partitioning Coupled with Ultrasound. Industrial Crops and Products 2014, 52, 292–297. [Google Scholar] [CrossRef]
- Henquin, J. C. Triggering and Amplifying Pathways of Regulation of Insulin Secretion by Glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Porte, D. Clinical Importance of Insulin Secretion and Its Interaction with Insulin Resistance in the Treatment of Type 2 Diabetes Mellitus and Its Complications. Diabetes Metab Res Rev 2001, 17, 181–188. [Google Scholar] [CrossRef]
- Kwon, D. Y.; Kim, Y. S.; Ryu, S. Y.; Choi, Y. H.; Cha, M.-R.; Yang, H. J.; Park, S. Platyconic Acid, a Saponin from Platycodi Radix, Improves Glucose Homeostasis by Enhancing Insulin Sensitivity in Vitro and in Vivo. Eur J Nutr 2012, 51, 529–540. [Google Scholar] [CrossRef]
- Kumari, Dr. M.; Jain, S. Tannin: An Antinutrient with Positive Effect to Manage Diabetes. Research Journal of Recent Sciences 2012, I. [Google Scholar]
- Muhammad, I.; Rahman, N.; Gul-E-Nayab; Nishan, U.; Shah, M. Antidiabetic Activities of Alkaloids Isolated from Medicinal Plants. Braz. J. Pharm. Sci. 2021, 57, e19130. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of Glucose Transporter Protein by Dietary Flavonoids in Type 2 Diabetes Mellitus. Int J Biol Sci 2015, 11, 508–524. [Google Scholar] [CrossRef]
- Slavin, J. L.; Savarino, V.; Paredes-Diaz, A.; Fotopoulos, G. A Review of the Role of Soluble Fiber in Health with Specific Reference to Wheat Dextrin. J Int Med Res 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ngo, D.-N.; Vo, T. T. N.; Vo, T. S. Mechanism of Action of Mangifera Indica Leaves for Anti-Diabetic Activity. Scientia Pharmaceutica 2019, 87, 13. [Google Scholar] [CrossRef]
- Irondi, E. A.; Oboh, G.; Akindahunsi, A. A. Antidiabetic Effects of Mangifera Indica Kernel Flour-supplemented Diet in Streptozotocin-induced Type 2 Diabetes in Rats. Food Sci Nutr 2016, 4, 828–839. [Google Scholar] [CrossRef]
- Gondi, M.; Basha, S. A.; Bhaskar, J. J.; Salimath, P. V.; Rao, U. J. S. P. Anti-Diabetic Effect of Dietary Mango (Mangifera Indica L.) Peel in Streptozotocin-Induced Diabetic Rats. J Sci Food Agric 2015, 95, 991–999. [Google Scholar] [CrossRef]
- Maritim, A. C.; Sanders, R. A.; Watkins, J. B. Diabetes, Oxidative Stress, and Antioxidants: A Review. J Biochem Mol Toxicol 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Van den Oever, I. A. M.; Raterman, H. G.; Nurmohamed, M. T.; Simsek, S. Endothelial Dysfunction, Inflammation, and Apoptosis in Diabetes Mellitus. Mediators Inflamm 2010, 2010, 792393. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R. P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Laurindo, L. F.; Machado, N. M.; Duarte, L. G.; Guiguer, E. L.; Araujo, A. C.; Dias, J. A.; Lamas, C. B.; Nunes, Y. C.; Bechara, M. D.; Baldi Júnior, E.; Gimenes, F. B.; Barbalho, S. M. Mangifera Indica L., By-Products, and Mangiferin on Cardio-Metabolic and Other Health Conditions: A Systematic Review. Life 2023, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Villas Boas, G. R.; Rodrigues Lemos, J. M.; de Oliveira, M. W.; dos Santos, R. C.; Stefanello da Silveira, A. P.; Barbieri Bacha, F.; Ito, C. N. A.; Bortolotte Cornelius, E.; Brioli Lima, F.; Sachilarid Rodrigues, A. M.; Belmal Costa, N.; Francisco Bittencourt, F.; Freitas de Lima, F.; Meirelles Paes, M.; Gubert, P.; Oesterreich, S. A. Aqueous Extract from Mangifera Indica Linn. (Anacardiaceae) Leaves Exerts Long-Term Hypoglycemic Effect, Increases Insulin Sensitivity and Plasma Insulin Levels on Diabetic Wistar Rats. PLoS One 2020, 15, e0227105. [Google Scholar] [CrossRef] [PubMed]
- Anila, L.; Vijayalakshmi, N. R. Flavonoids from Emblica Officinalis and Mangifera Indica—Effectiveness for Dyslipidemia. Journal of Ethnopharmacology 2002, 79, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pranakhon, R.; Aromdee, C.; Pannangpetch, P. Effects of Iriflophenone 3-C-β-Glucoside on Fasting Blood Glucose Level and Glucose Uptake. Pharmacogn Mag 2015, 11, 82–89. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R. P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, D.; Kamble, V. A. Phytochemical Screening of Ethanolic Extracts of Stem, Leaves, Flower and Seed Kernel of Mangifera Indica L. International Journal of Pharma and Bio Sciences 2013, 4, P383–P389. [Google Scholar]
- Olasehinde G. I., S. K. J.; Ajayi J. B., A. O. O. & A. A. A. Phytochemical and Antimicrobial Properties of Mangifera Indica Leaf Extracts. Covenant Journal of Physical and Life Sciences 2018, 6.
- Bbosa, G. S.; Kyegombe, D. B.; Ogwal-Okeng, J.; Bukenya-Ziraba, R.; Odyek, O.; Waako, P. Antibacterial Activity of Mangifera Indica (L.). African Journal of Ecology 2007, 45, 13–16. [Google Scholar] [CrossRef]
- Muthusamy, V. S.; Anand, S.; Sangeetha, K. N.; Sujatha, S.; Arun, B.; Lakshmi, B. S. Tannins Present in Cichorium Intybus Enhance Glucose Uptake and Inhibit Adipogenesis in 3T3-L1 Adipocytes through PTP1B Inhibition. Chem Biol Interact 2008, 174, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, J.; Wu, Y.; Cui, J.; Jia, N.; Xi, M.; Wen, A. Identification of AMPK Activator from Twelve Pure Compounds Isolated from Aralia Taibaiensis: Implication in Antihyperglycemic and Hypolipidemic Activities. Korean J Physiol Pharmacol 2017, 21, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Perez G., R. M.; Zavala S., M. A.; Perez G., S.; Perez G., C. Antidiabetic Effect of Compounds Isolated from Plants. Phytomedicine 1998, 5, 55–75. [Google Scholar] [CrossRef]
- Shigemasa, H. Partial purification of crude gymnemic acids by affinity chromatography and effects of purified fractions on the oral glucose tolerance test in rats. Yonago Igaku Zasshi 1992, 43, 350–364. [Google Scholar]
- Esmaeili, M. A.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teucrium Polium on Beta-Cell Destruction in a Model of Streptozotocin-Induced Diabetes. Planta Med 2009, 75, 1418–1420. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S. T.; Seidel, V.; Rahman, A. B.; Aziz, M. A.; Richi, A. E.; Rahman, A.; Jafrin, U. H.; Hannan, J. M. A.; Abdel-Wahab, Y. H. A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
| Concentration (µg/ml) | Ascorbic acid (% inhibition) | EEMI (% inhibition) |
|---|---|---|
| 1.6 | 10.82 ± 1.32 ** | 9.95 ± 1.15 ** |
| 8 | 32.91 ± 1.15 *** | 30.42 ± 1.17 *** |
| 40 | 70.47 ± 1.85 *** | 47.25 ± 2.07 *** |
| 200 | 87.11 ± 1.61 *** | 62.14 ± 2.15 *** |
| 1000 | 95.04 ± 1.55 *** | 73.32 ± 2.25 *** |
| 5000 | 97.24 ± 1.10 *** | 80.99 ± 1.35 *** |
| Days | Treatment group | Body wt. (gm) | HDL (mg/dl) |
LDL (mg/dl) |
TG (mg/dl) |
Total Cholesterol (mg/dl) |
|---|---|---|---|---|---|---|
| 0 days | Diabetic control | 170.0 ± 0.8 | 35.92 ± 2.8 | 57.26 ± 3.1 | 93.22 ± 2.1 | 92.00 ± 1.2 |
| EEMI (250 mg/kg) | 169.3 ± 2.1 | 31.68 ± 2.7 | 46.83 ± 6.1 | 87.17 ± 6.1 | 89.10 ± 2.7 | |
| EEMI (500 mg/kg) | 164.9 ± 2.3 | 33.17 ± 1.7 | 52.78 ± 4.5 | 79.57 ± 5.7 | 85.97 ± 3.0 | |
| Glibenclamide (0.5 mg/kg) | 158.8 ± 5.2 | 34.05 ± 2.1 | 55.54 ± 3.0 | 85.00 ± 2.9 | 94.77 ± 2.4 | |
| 7 days | Diabetic control | 176.1 ± 3.1 | 29.92 ± 2.8 | 77.55 ± 6.9 | 118.0 ± 6.9 | 83.67 ± 3.9 |
| EEMI (250 mg/kg) | 166.2 ± 2.7 | 34.39 ± 2.9 | 66.69 ± 6.1 | 107.0 ± 6.1 | 79.31 ± 1.5 | |
| EEMI (500 mg/kg) | 167.2 ± 1.0 | 42.10 ± 1.7 | 76.32 ± 2.3 | 105.1 ± 4.5 | 72.29 ± 2.8 | |
| Glibenclamide (0.5 mg/kg) | 166.9 ± 2.0 | 62.81 ± 2.6*** | 36.99 ± 3.1** | 47.55 ± 3.1** | 76.51 ± 2.9 | |
| 14 days | Diabetic control | 171.6 ± 0.9 | 33.91 ± 2.7 | 57.57 ± 4.1 | 98.00 ± 4.1 | 88.14 ± 2.2 |
| EEMI (250 mg/kg) | 167.2 ± 1.9 | 33.61 ± 1.7 | 75.94 ± 6.1 | 116.3 ± 6.1 | 72.18 ± 1.0** | |
| EEMI (500 mg/kg) | 164.5 ± 3.5 | 36.05 ± 2.9 | 58.61 ± 4.5 | 84.05 ± 4.5 | 63.43 ± 4.2** | |
| Glibenclamide (0.5 mg/kg) | 167.8 ± 1.1 | 70.79 ± 2.9*** | 39.09 ± 3.8* | 49.65 ± 3.8*** | 53.72 ± 1.7*** | |
| 21 days | Diabetic control | 191.4 ± 1.5 | 37.29 ± 3.2 | 82.05 ± 3.4 | 122.5 ± 3.4 | 100.6 ± 2.5 |
| EEMI (250 mg/kg) | 160.8 ± 4.4** | 26.78 ± 1.7 | 72.91 ± 6.1 | 113.2 ± 6.1 | 57.45 ± 5.2** | |
| EEMI (500 mg/kg) | 154.1 ± 2.3*** | 41.05 ± 2.0* | 61.45 ± 4.5* | 86.89 ± 4.5** | 63.12 ± 2.0*** | |
| Glibenclamide (0.5 mg/kg) | 155.4 ± 2.7*** | 85.28 ± 2.5** | 39.64 ± 0.1*** | 46.87 ± 3.4*** | 48.10 ± 1.8*** | |
| 28 days | Diabetic control | 196.4 ± 2.1 | 23.19 ± 2.5 | 95.88 ± 3.3 | 136.3 ± 3.3 | 105.6 ± 2.7 |
| EEMI (250 mg/kg) | 154.9 ± 2.3*** | 31.62 ± 1.7 | 59.69 ± 6.1** | 100.0 ± 6.1** | 60.96 ± 3.8*** | |
| EEMI (500 mg/kg) | 150.0 ± 3.1*** | 42.67 ± 1.9** | 57.27 ± 4.5** | 82.71 ± 4.5*** | 57.44 ± 3.6*** | |
| Glibenclamide (0.5 mg/kg) | 153.1 ± 3.0*** | 92.34 ± 1.9*** | 44.37 ± 1.0*** | 41.59 ± 2.8*** | 41.66 ± 2.3*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





