Submitted:
20 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
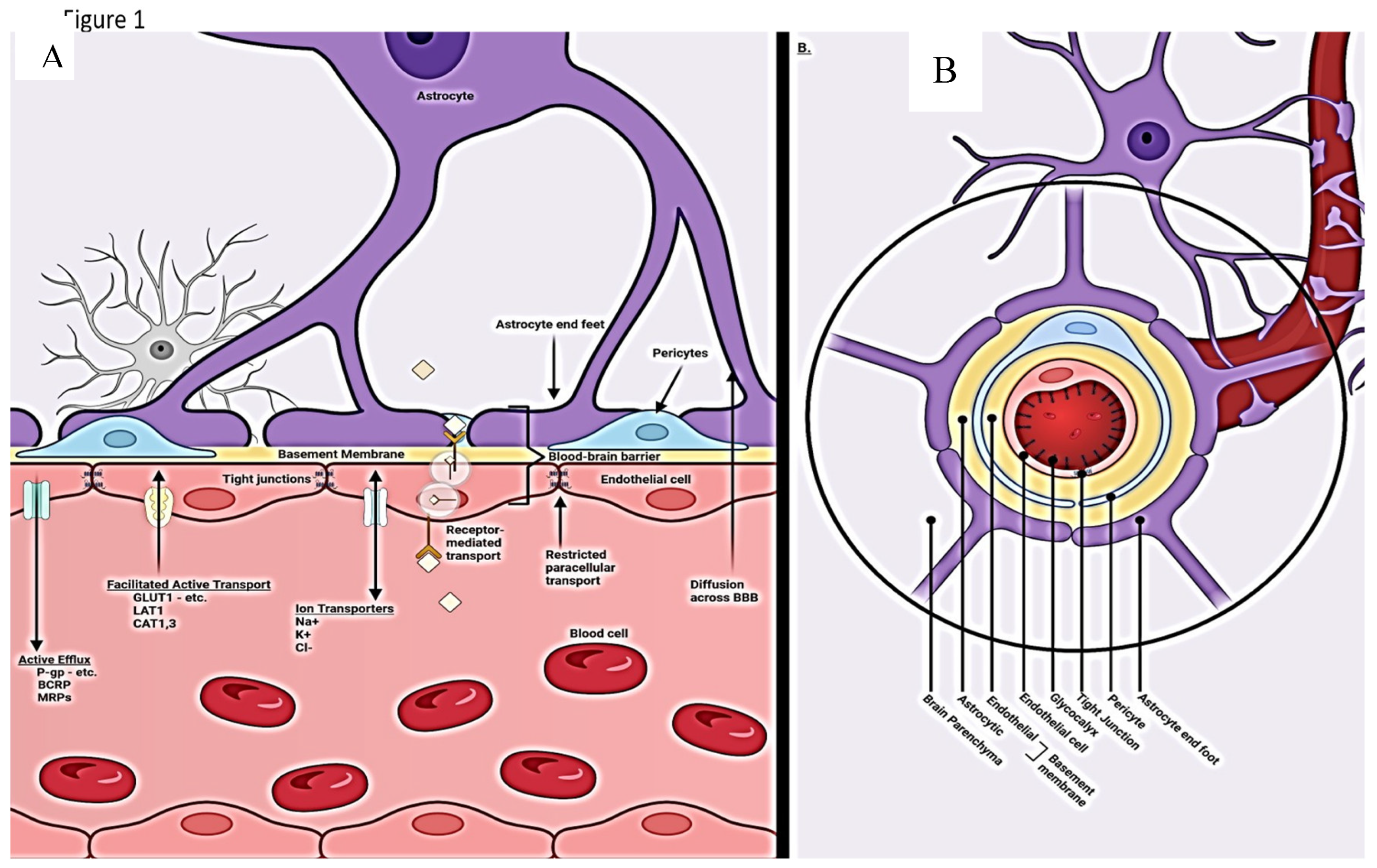
Normal BBB Physiology and regulation
Blood Brain Barrier Cellular makeup and their function
Capillary Endothelial cells
Pericytes
Tight junctions
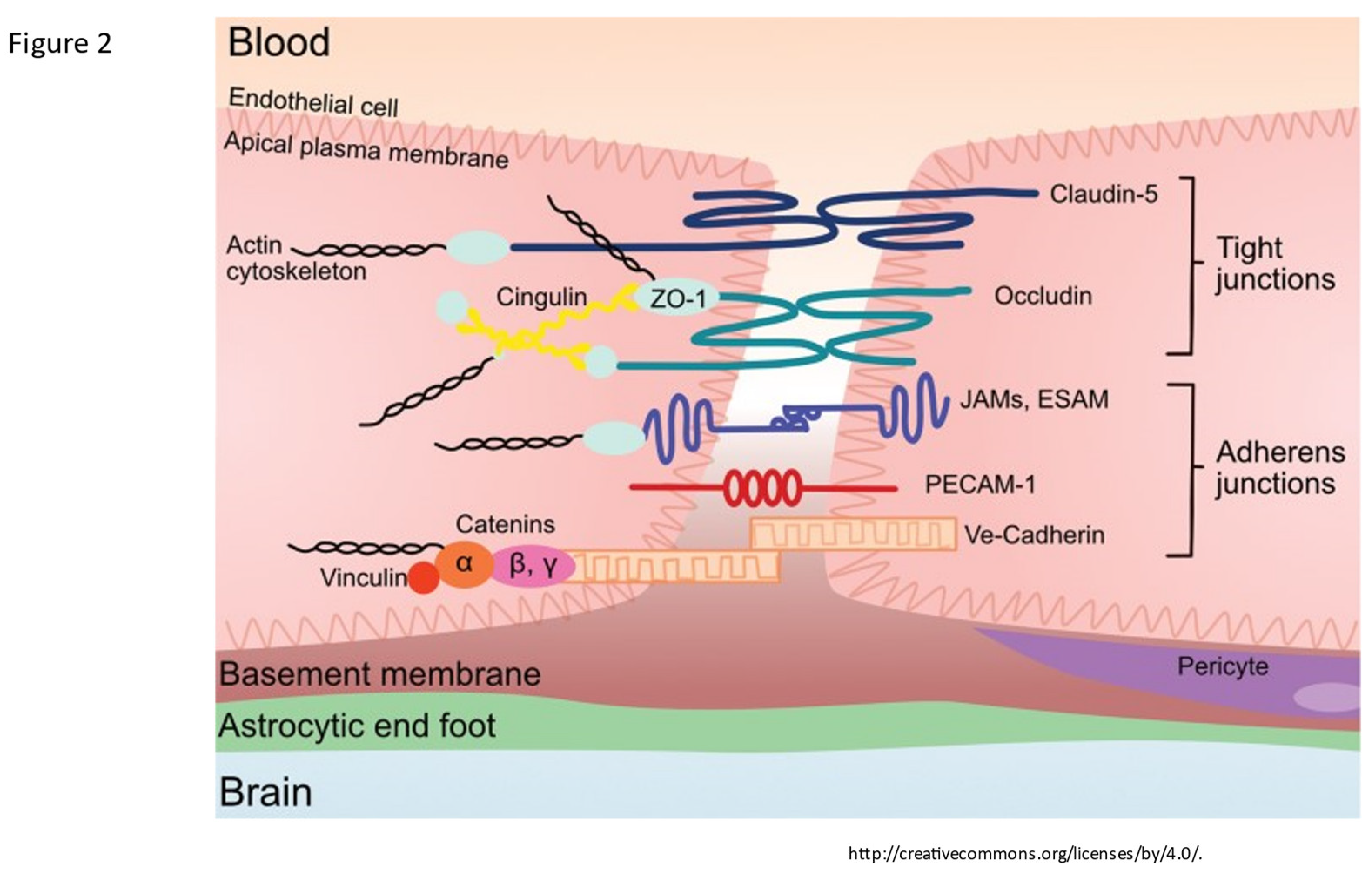
Astrocytes
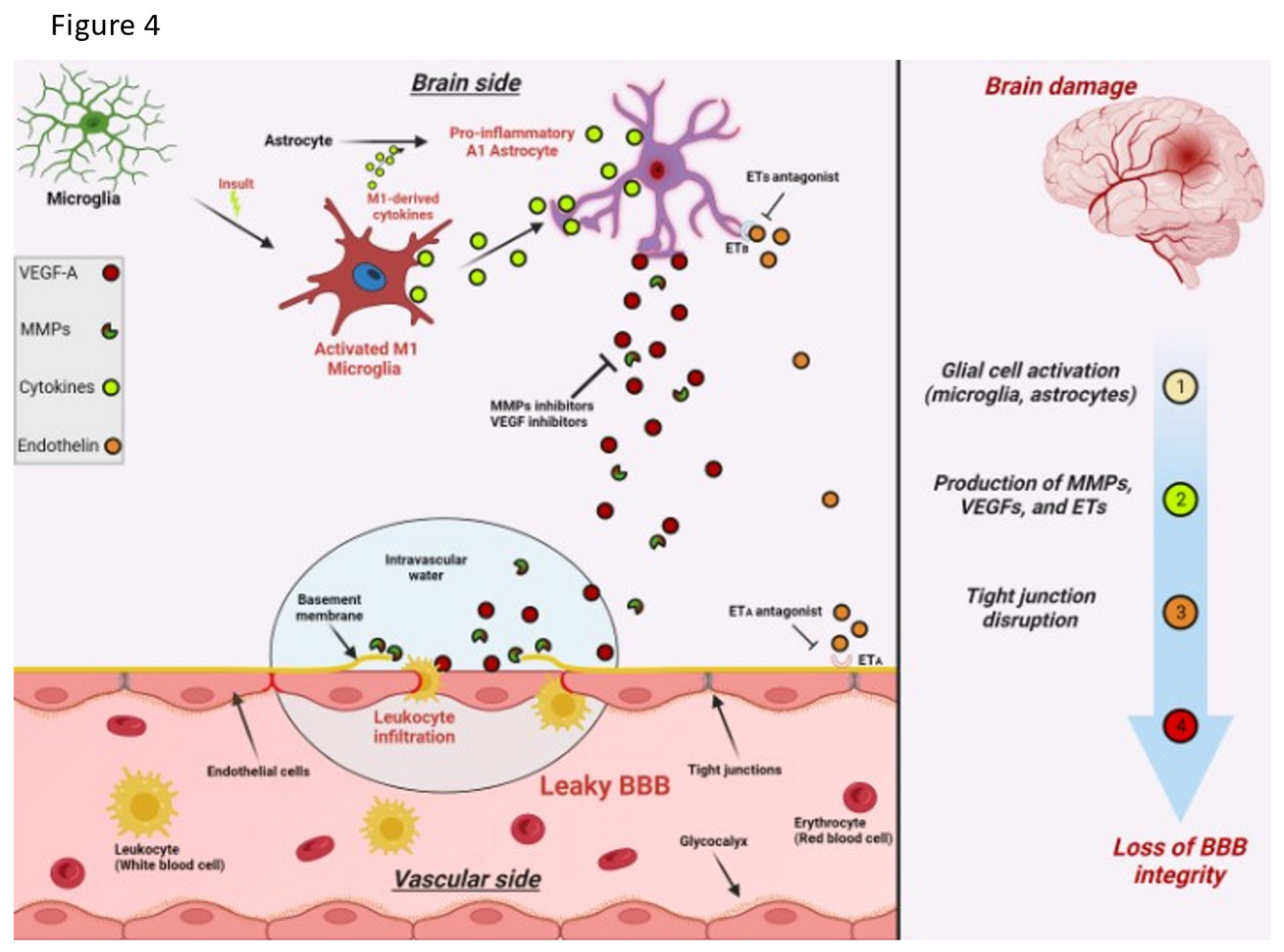
Microglia
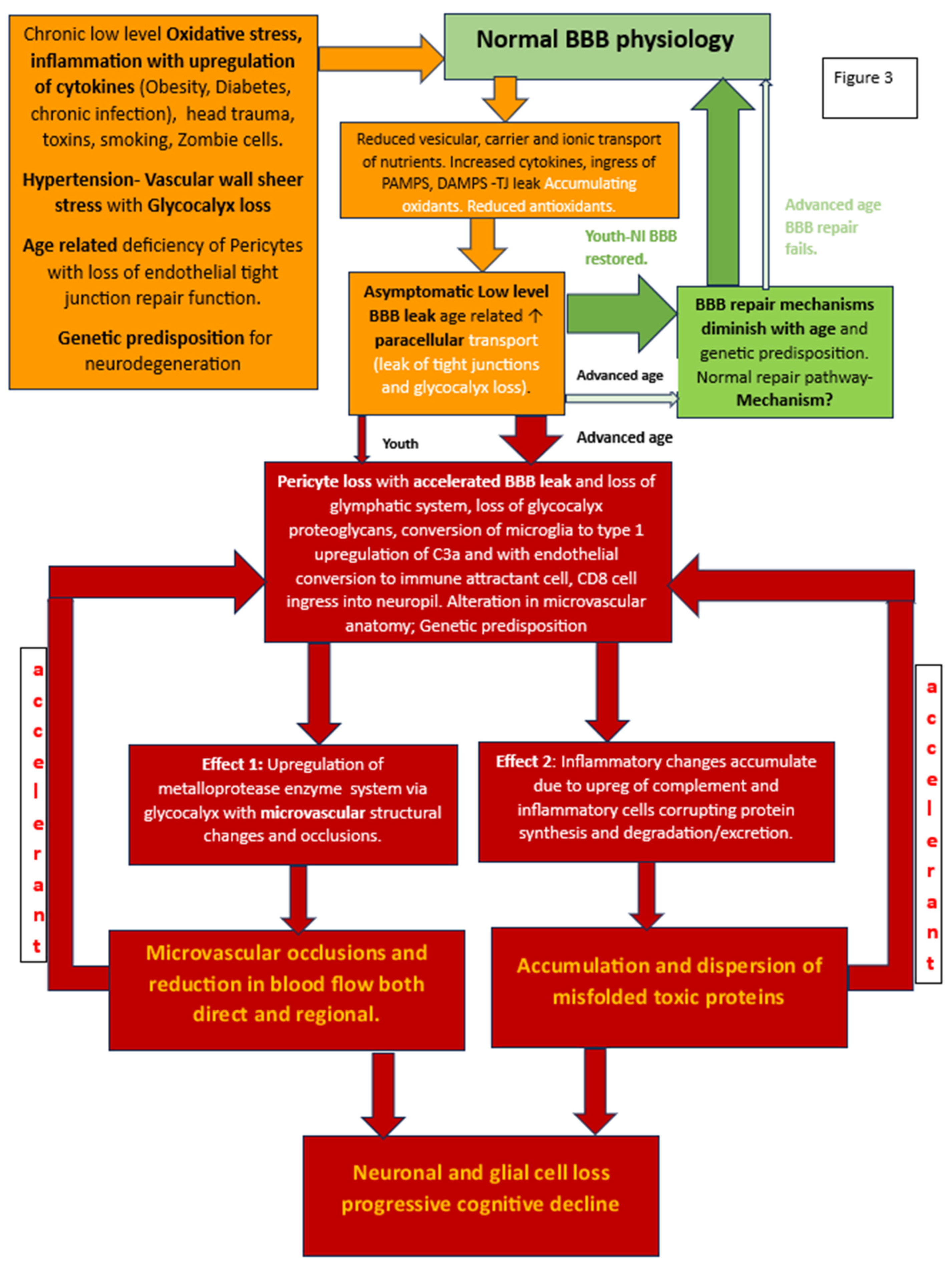
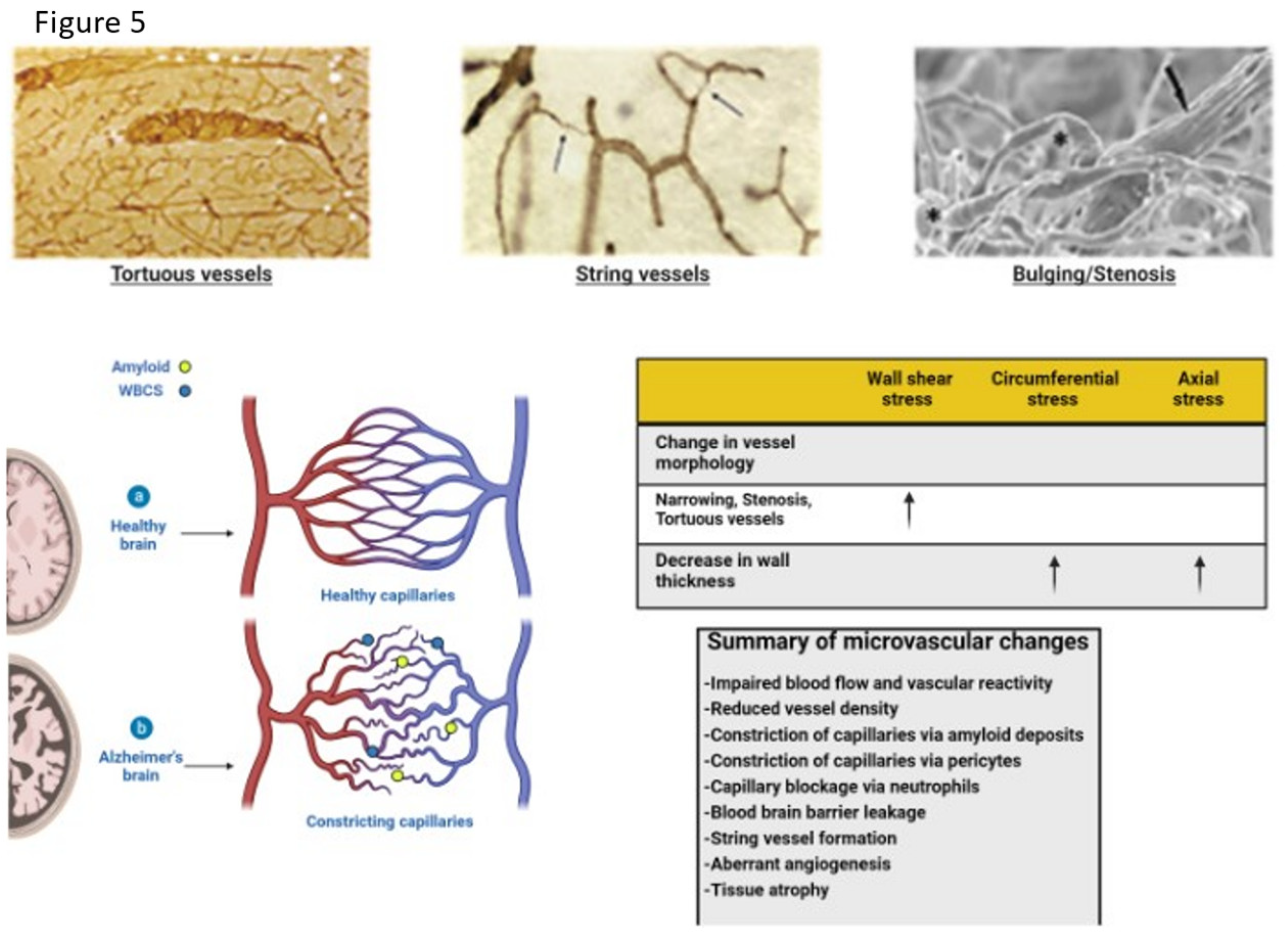
BBB dysfunction
Altered Fluid Dynamic
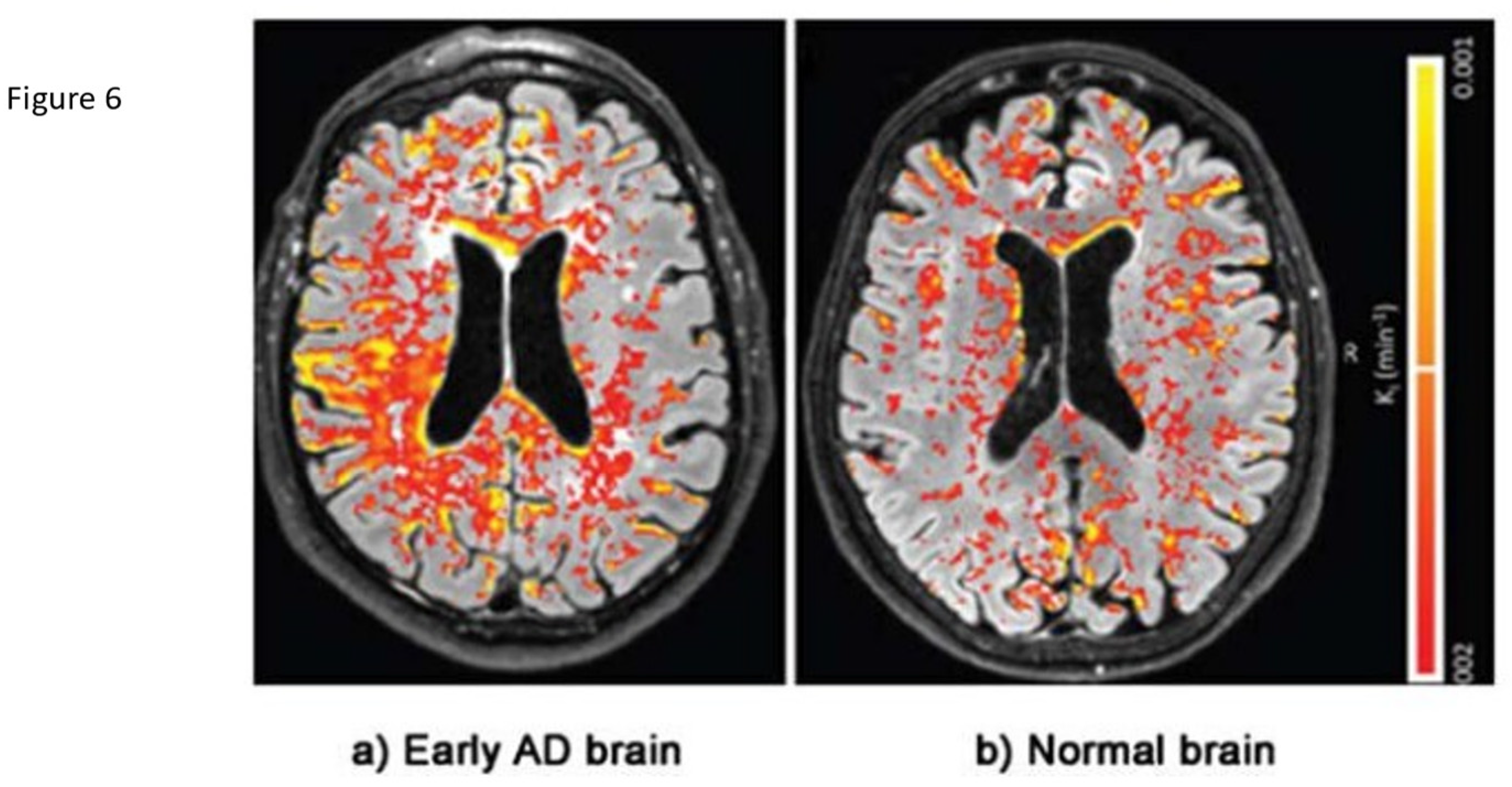
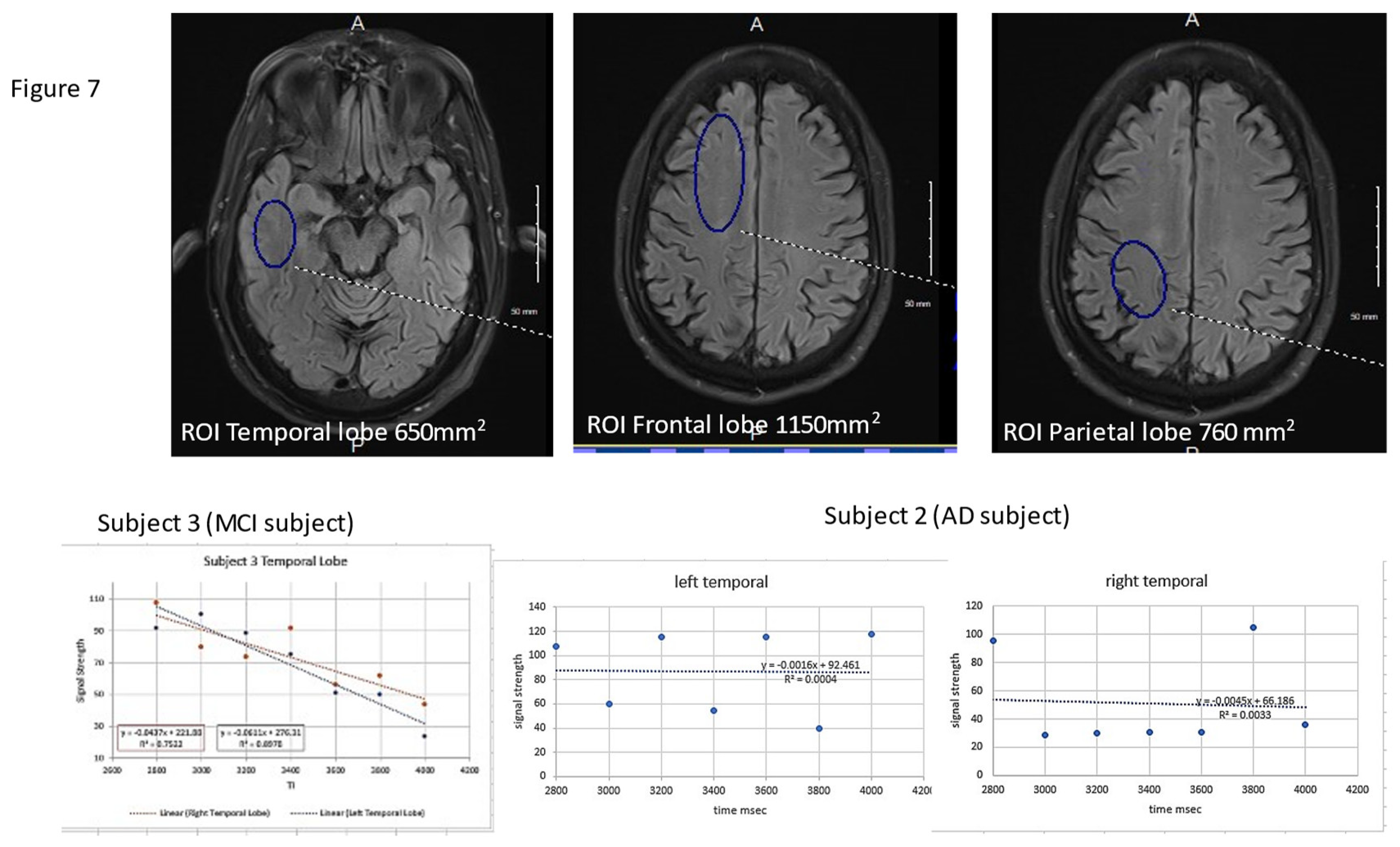
Early Identification of BBB Dysfunction
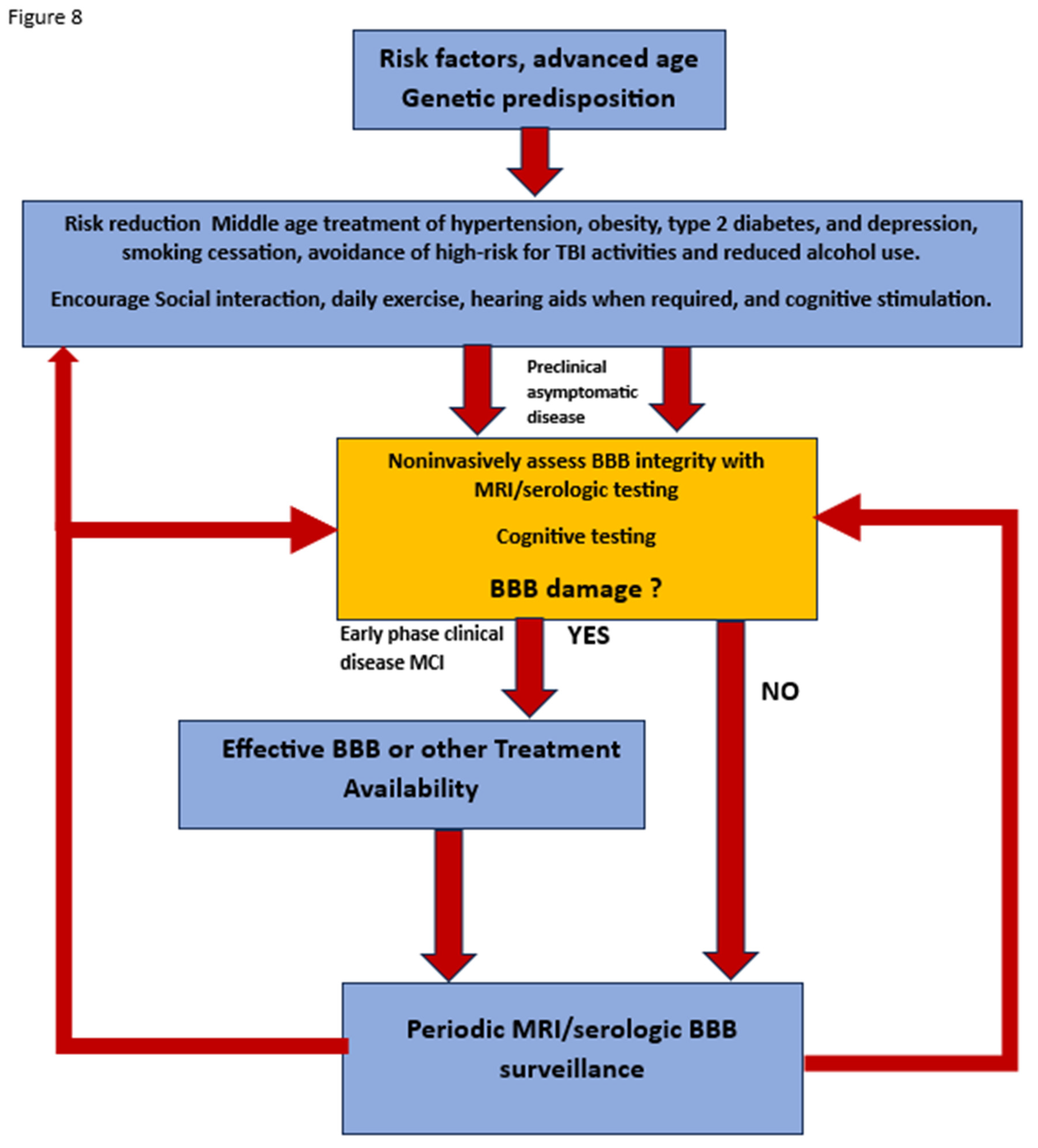
Current methods of risk reduction
Conclusion- where do we go from here?
Funding
Acknowledgements
References
- Van Dyck, Christopher H., et al. "Lecanemab in early Alzheimer’s disease." New England Journal of Medicine 388.1 (2023): 9-21. [CrossRef]
- Bateman, Randall J., et al. "Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease." New England Journal of Medicine 389.20 (2023): 1862-1876. [CrossRef]
- Huang, Zhangsen, et al. "Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease." Frontiers in neuroendocrinology 59 (2020): 100857. [CrossRef]
- Barisano, Giuseppe, et al. "Blood–brain barrier link to human cognitive impairment and Alzheimer’s disease." Nature cardiovascular research 1.2 (2022): 108-115.
- Hussain, Basharat, Cheng Fang, and Junlei Chang. "Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia." Frontiers in neuroscience 15 (2021): 688090. [CrossRef]
- Nehra, Geetika, Bjoern Bauer, and Anika MS Hartz. "Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance." Pharmacology & Therapeutics 234 (2022): 108119. [CrossRef]
- Sweeney, Melanie D., et al. "The role of brain vasculature in neurodegenerative disorders." Nature neuroscience 21.10 (2018): 1318-1331. [CrossRef]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Bloodbrain.
- barrier: from physiology to disease and back. Physiol Rev.2019;99:21–78. [CrossRef]
- Andjelkovic, Anuska V., et al. "Blood-brain barrier dysfunction in normal aging and neurodegeneration: mechanisms, impact, and treatments." Stroke 54.3 (2023): 661-672. [CrossRef]
- Selkoe, Dennis J. "Treatments for Alzheimer's disease emerge." Science 373.6555 (2021): 624-626. [CrossRef]
- Castro Dias, Mariana, et al. "Structure and junctional complexes of endothelial, epithelial and glial brain barriers." International journal of molecular sciences 20.21 (2019): 5372. [CrossRef]
- Zhang, Qianqian, et al. "Altered regional cerebral blood flow and brain function across the Alzheimer's disease spectrum: a potential biomarker." Frontiers in Aging Neuroscience 13 (2021): 630382. [CrossRef]
- Thrippleton, Michael J., et al. "Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations." Alzheimer's & Dementia 15.6 (2019): 840-858. [CrossRef]
- Sun, Huixin, et al. "Methods used for the measurement of blood-brain barrier integrity." Metabolic brain disease 36 (2021): 723-735. [CrossRef]
- Pereira, Joana B., et al. "Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease." Brain 144.11 (2021): 3505-3516. [CrossRef]
- Kadry, Hossam, Behnam Noorani, and Luca Cucullo. "A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity." Fluids and Barriers of the CNS 17.1 (2020): 1-24.
- Zhao, Lucy, et al. "Physiology of glymphatic solute transport and waste clearance from the brain." Physiology 37.6 (2022): 349-362. [CrossRef]
- Preston, Jane E., N. Joan Abbott, and David J. Begley. "Transcytosis of macromolecules at the blood–brain barrier." Advances in pharmacology 71 (2014): 147-163.
- van Leeuwen, E., Hampton, M. B., and Smyth, L. C. D. (2020). Redox signaling and regulation of the blood-brain barrier. Int. J. Biochem. Cell Biol. 125:105794. [CrossRef]
- Proulx, Steven T. "Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics." Cellular and Molecular Life Sciences 78.6 (2021): 2429-2457.
- Lochhead, Jeffrey J., et al. "Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders." Frontiers in physiology 11 (2020): 914. [CrossRef]
- Hudson, Natalie, and Matthew Campbell. "Tight junctions of the neurovascular unit." Frontiers in Molecular Neuroscience 14 (2021): 752781. [CrossRef]
- Engelhardt, Britta, and Stefan Liebner. "Novel insights into the development and maintenance of the blood–brain barrier." Cell and tissue research 355.3 (2014): 687-699. [CrossRef]
- Iadecola, Costantino. "The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease." Neuron 96.1 (2017): 17-42. [CrossRef]
- Bhowmick, Saurav, et al. "Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury." Experimental neurology 317 (2019): 260-270. [CrossRef]
- Brassard, Patrice, et al. "Losing the dogmatic view of cerebral autoregulation." Physiological reports 9.15 (2021): e14982. [CrossRef]
- Boyé, Kevin, et al. "Endothelial Unc5B controls blood-brain barrier integrity." Nature Communications 13.1 (2022): 1169. [CrossRef]
- Santisteban, Monica M., et al. "Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension." Hypertension 76.3 (2020): 795-807. [CrossRef]
- Roudnicky, Filip, et al. "Inducers of the endothelial cell barrier identified through chemogenomic screening in genome-edited hPSC-endothelial cells." Proceedings of the National Academy of Sciences 117.33 (2020): 19854-19865. [CrossRef]
- Profaci, Caterina P., et al. "The blood–brain barrier in health and disease: Important unanswered questions." Journal of Experimental Medicine 217.4 (2020). [CrossRef]
- Knox, Emily G., et al. "The blood-brain barrier in aging and neurodegeneration." Molecular psychiatry 27.6 (2022): 2659-2673. [CrossRef]
- Yang, Rui, et al. "The Role of Heparin and Glycocalyx in Blood–Brain Barrier Dysfunction." Frontiers in Immunology 12 (2021): 754141. [CrossRef]
- Lendahl, Urban, Per Nilsson, and Christer Betsholtz. "Emerging links between cerebrovascular and neurodegenerative diseases—a special role for pericytes." EMBO reports 20.11 (2019): e48070. [CrossRef]
- Searson, Peter Charles, et al. "The influence of physiological and pathological perturbations on blood-brain barrier function." Frontiers in Neuroscience 17 (2023): 1289894. [CrossRef]
- Montagne, Axel, et al. "Blood-brain barrier breakdown in the aging human hippocampus." Neuron 85.2 (2015): 296-302. [CrossRef]
- Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood–brain barrier. Nat Aging. 2021;1:243–54.
- Verheggen, Inge CM, et al. "Increase in blood–brain barrier leakage in healthy, older adults." Geroscience 42 (2020): 1183-1193.
- Louveau, A., Smirnov, I., Keyes, T. J., Eccles, J. D., Rouhani, S. J., Peske, J. D., … Kipnis, J. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature. [CrossRef]
- Mestre, Humberto, Yuki Mori, and Maiken Nedergaard. "The brain’s glymphatic system: current controversies." Trends in neurosciences 43.7 (2020): 458-466.
- Ronaldson, Patrick T., and Thomas P. Davis. "Regulation of blood–brain barrier integrity by microglia in health and disease: a therapeutic opportunity." Journal of Cerebral Blood Flow & Metabolism 40.1_suppl (2020): S6-S24. [CrossRef]
- Iturria-Medina, Yasser, et al. "Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis." Nature communications 7.1 (2016): 11934. [CrossRef]
- Cash, Alison, and Michelle H. Theus. "Mechanisms of blood–brain barrier dysfunction in traumatic brain injury." International journal of molecular sciences 21.9 (2020): 3344. [CrossRef]
- Furman, David, et al. "Chronic inflammation in the etiology of disease across the life span." Nature medicine 25.12 (2019): 1822-1832. [CrossRef]
- Katsi, Vasiliki, et al. "Blood–brain barrier dysfunction: the undervalued frontier of hypertension." Journal of Human Hypertension 34.10 (2020): 682-691. [CrossRef]
- Lucas, Samuel JE, et al. "Influence of changes in blood pressure on cerebral perfusion and oxygenation." Hypertension 55.3 (2010): 698-705. [CrossRef]
- Klohs, Jan. "An integrated view on vascular dysfunction in Alzheimer’s disease." Neurodegenerative Diseases 19.3-4 (2020): 109-127. [CrossRef]
- Vázquez-Rosa, Edwin, et al. "P7C3-A20 treatment one year after TBI in mice repairs the blood–brain barrier, arrests chronic neurodegeneration, and restores cognition." Proceedings of the National Academy of Sciences 117.44 (2020): 27667-27675. [CrossRef]
- Kang, Jubin, et al. "Delay in Clearance of Labeled Protons in Patients with Acute Head Trauma Utilizing MRI 3D TGSE PASL MRI (Arterial Spin Labeling)(P8-1.007)." (2023). [CrossRef]
- Vigasova, Dana, et al. "Multi-pathogen infections and Alzheimer’s disease." Microbial Cell Factories 20.1 (2021): 1-13.
- Itzhaki, Ruth F., et al. "Do infections have a role in the pathogenesis of Alzheimer disease?." Nature Reviews Neurology 16.4 (2020): 193-197. [CrossRef]
- Jacob, Alexander, and Jessy John Alexander. "Complement and blood–brain barrier integrity." Molecular immunology 61.2 (2014): 149-152. [CrossRef]
- Li, Zhilian, et al. "Correlation of serum complement factor 5a level with inflammatory response and cognitive function in patients with Alzheimer’s disease of different severity." BMC neurology 23.1 (2023): 319.
- Eadon, M.T., Jacob, A., Cunningham, P.N., Quigg, R.J., Garcia, J.G., Alexander, J.J. Transcriptional profiling reveals that C5a alters miRNA in brain endothelial cells. Immunology. 2014 May 6. (epub ahead of print). [CrossRef]
- Propson, N. E., Roy, E. R., Litvinchuk, A., Köhl, J., & Zheng, H. (2021). Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. The Journal of Clinical Investigation, 131(1). [CrossRef]
- Shah, Akash, Uday Kishore, and Abhishek Shastri. "Complement system in Alzheimer’s disease." International Journal of Molecular Sciences 22.24 (2021): 13647. [CrossRef]
- Krance, Saffire H., et al. "The complement cascade in Alzheimer’s disease: a systematic review and meta-analysis." Molecular Psychiatry 26.10 (2021): 5532-5541. [CrossRef]
- Elschot, Elles P., et al. "A comprehensive view on MRI techniques for imaging blood-brain barrier integrity." Investigative Radiology 56.1 (2021): 10-19. [CrossRef]
- Nation, Daniel A., et al. "Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction." Nature medicine 25.2 (2019): 270-276. [CrossRef]
- Chang, Junlei, et al. "Gpr124 is essential for blood–brain barrier integrity in central nervous system disease." Nature medicine 23.4 (2017): 450-460. [CrossRef]
- Travagli, R. Alberto, Kirsteen N. Browning, and Michael Camilleri. "Parkinson disease and the gut: new insights into pathogenesis and clinical relevance." Nature Reviews Gastroenterology & Hepatology 17.11 (2020): 673-685. [CrossRef]
- Liu, Shan, et al. "Microbiota-gut-brain axis and Alzheimer’s disease: implications of the blood-brain barrier as an intervention target." Mechanisms of Ageing and Development 199 (2021): 111560. [CrossRef]
- Ahumada-Castro, Ulises, et al. "Keeping zombies alive: The ER-mitochondria Ca2+ transfer in cellular senescence." Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1868.11 (2021): 119099. [CrossRef]
- Takata, Fuyuko, et al. "Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction." Frontiers in cellular neuroscience 15 (2021): 661838. [CrossRef]
- Yoshiura, Takashi, et al. "Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease." American Journal of Neuroradiology 30.7 (2009): 1388-1393. [CrossRef]
- Binnewijzend, Maja AA, et al. "Cerebral perfusion in the predementia stages of Alzheimer’s disease." European radiology 26 (2016): 506-514. [CrossRef]
- Zheng, Weimin, et al. "Disrupted regional cerebral blood flow, functional activity and connectivity in Alzheimer’s disease: a combined ASL perfusion and resting state fMRI study." Frontiers in Neuroscience 13 (2019): 738. [CrossRef]
- Chowdhary, Neerja, et al. "Reducing the risk of cognitive decline and dementia: WHO recommendations." Frontiers in neurology 12 (2022): 765584. [CrossRef]
- Livingston, Gill, et al. "Dementia prevention, intervention, and care: 2020 report of the Lancet Commission." The Lancet 396.10248 (2020): 413-446. [CrossRef]
- Archie, Sabrina Rahman, Abdullah Al Shoyaib, and Luca Cucullo. "Blood-brain barrier dysfunction in CNS disorders and putative therapeutic targets: an overview." Pharmaceutics 13.11 (2021): 1779. [CrossRef]
- Greenberg, Steven M., et al. "Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways." Nature Reviews Neurology 16.1 (2020): 30-42.
- Jäkel, Lieke, et al. "Prevalence of cerebral amyloid angiopathy: a systematic review and meta-analysis." Alzheimer's & Dementia 18.1 (2022): 10-28. [CrossRef]
- Fazlollahi, Amir, et al. "Increased cerebral blood flow with increased amyloid burden in the preclinical phase of alzheimer's disease." Journal of Magnetic Resonance Imaging 51.2 (2020): 505-513. [CrossRef]
- Van De Haar, Harm J., et al. "Blood-brain barrier leakage in patients with early Alzheimer disease." Radiology 281.2 (2016): 527-535. [CrossRef]
- Montagne, Axel, et al. "Imaging subtle leaks in the blood–brain barrier in the aging human brain: potential pitfalls, challenges, and possible solutions." GeroScience 44.3 (2022): 1339-1351.
- Joseph, C. R., Benhatzel, C. M., Stern, L. J., Hopper, O. M., & Lockwood, M. D. (2020). Pilot study utilizing MRI 3D TGSE PASL (arterial spin labeling) differentiating clearance rates of labeled protons in the CNS of patients with early Alzheimer disease from normal subjects. Magnetic Resonance Materials in Physics, Biology and Medicine, 33(4), 559–568. [CrossRef]
- Joseph, Charles R., et al. "Utilizing Reduced Labeled Proton Clearance to Identify Preclinical Alzheimer Disease with 3D ASL MRI." Case Reports in Neurology 15.1 (2023): 177. [CrossRef]
- Mahapatra, Manoj K., Muthukumar Karuppasamy, and Biswa M. Sahoo. "Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: a narrative review." Pharmaceutical Research 39.6 (2022): 1233-1248. [CrossRef]
- Gonzales, Mitzi M., et al. "Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): a pilot clinical trial." The journal of prevention of Alzheimer's disease (2022): 1-8.
- Zhang, P., Kishimoto, Y., Grammatikakis, I. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22, 719–728 (2019). [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





