Submitted:
24 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Study Design
Population
Data Source
Primary Exposure
Primary Outcomes
Cut-Offs and Definitions
Statistical Analyses
Results
Demographics and Clinical Characteristics
Neighborhood Deprivation Index
Donors and Transplant
Causes of Death
Re-Transplantation
Complications
Demographic and Clinical Characteristics According to Di Strata
Complications According to Di Strata
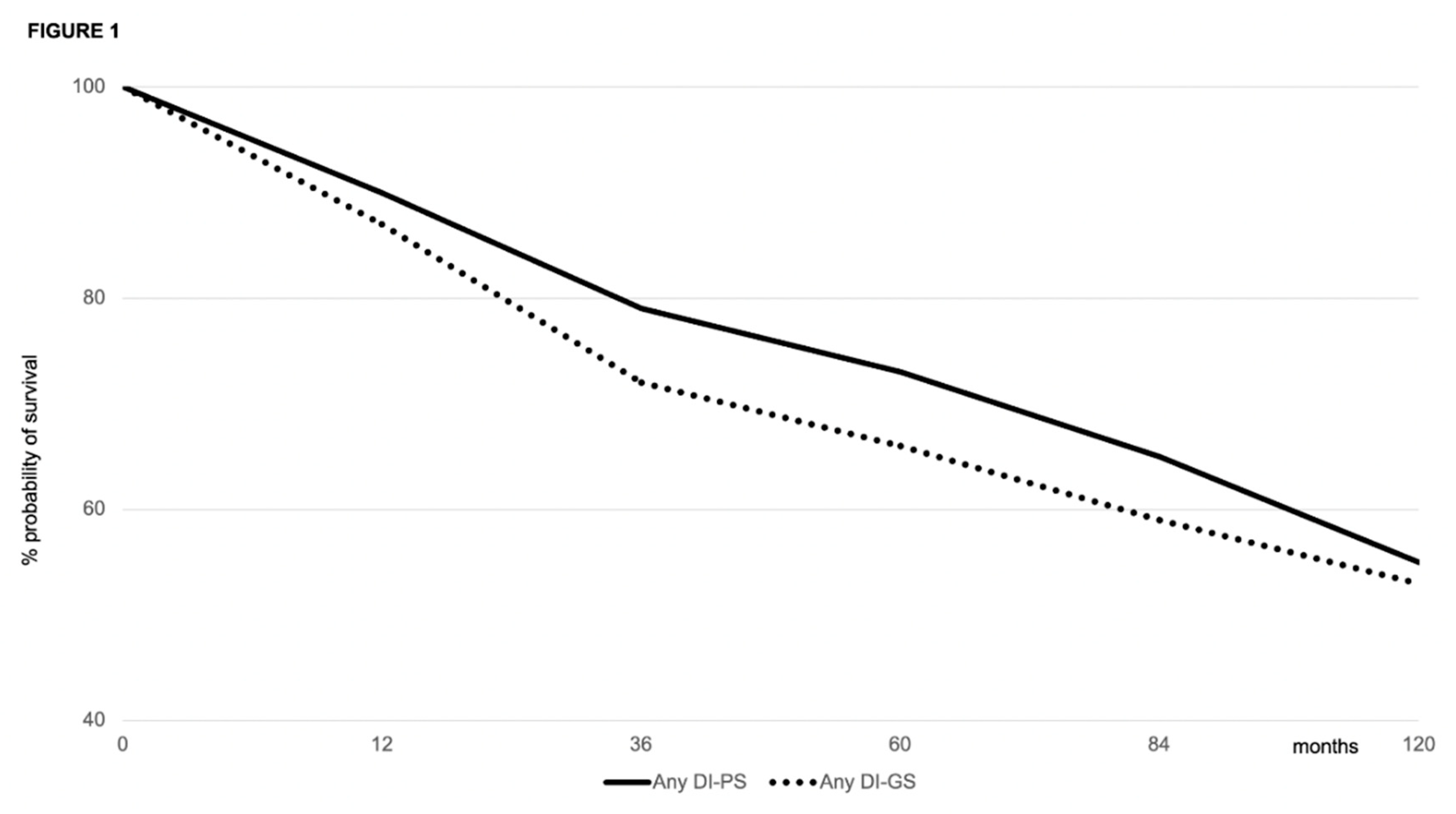
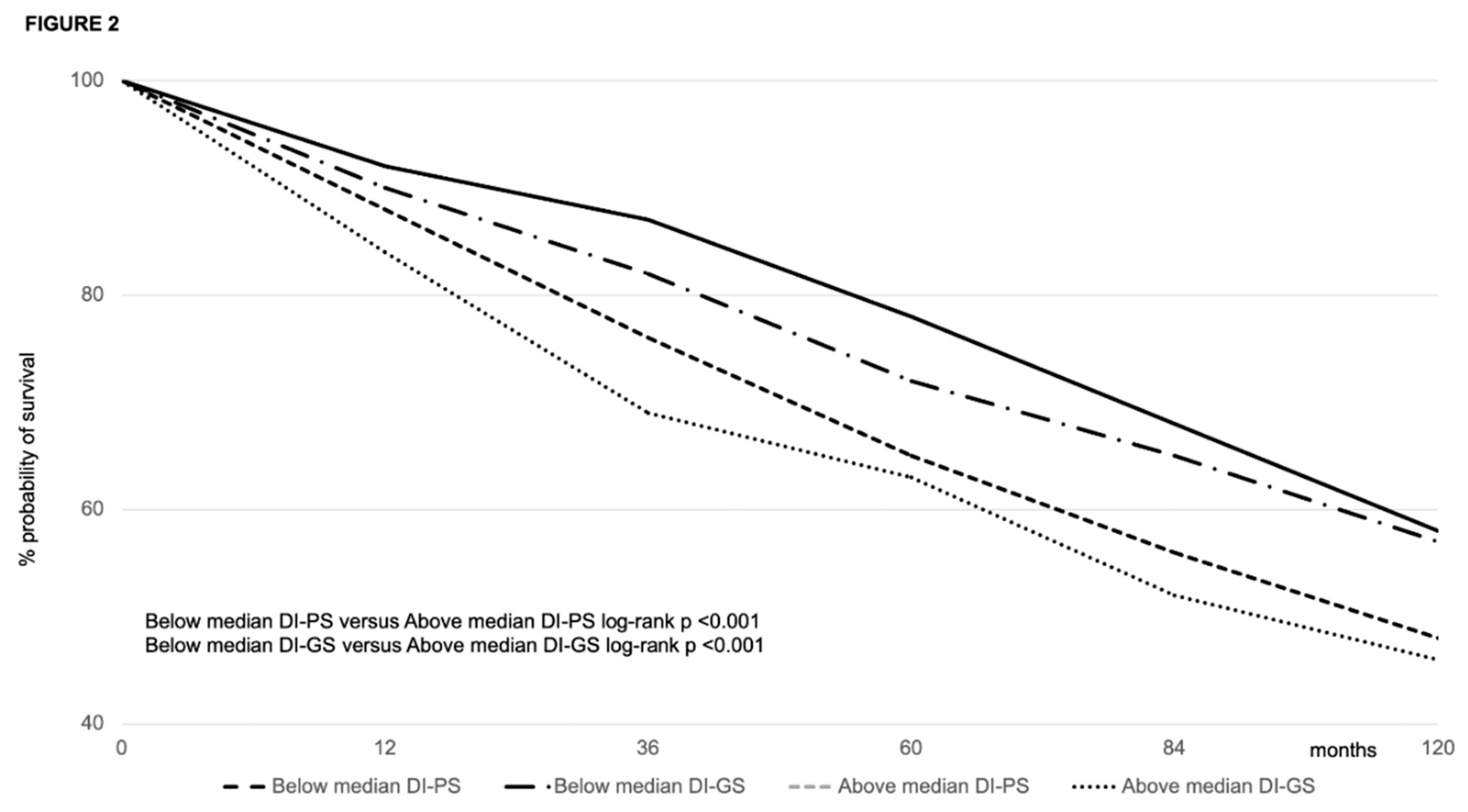
Patient and Graft Survival
Predictors of Survival
Discussion
Supplementary Materials
Authors’ contributions
Financial support
Acknowledgments
Conflicts of interest
Abbreviations
| ADA | American Diabetes Association |
| aHR | adjusted Hazard Ratio |
| AKI | acute kidney injury |
| CIT | cold ischemia time |
| CKD | chronic kidney dysfunction |
| DCD | donor after cardiocirculatory death |
| DI | deprivation index |
| DM | diabetes mellitus |
| EAD | early allograft dysfunction |
| eGFR | estimated GFR |
| GFR | glomerular filtration rate |
| HBsAg | Hepatitis B virus surface antigen |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HR | hazard ratio |
| IQR | interquartile range |
| ITBL | ischemia-type biliary lesion |
| KIDIGO | Kidney Disease: Improving Global Outcomes |
| LT | liver transplantation |
| MACE | major cardiovascular event |
| MDRD | modification of diet in renal disease |
| MELD | model for end-stage liver disease |
| MP | machine perfusion |
| PTDM | post-transplant diabetes mellitus |
| RR | relative risk |
| SED | socio-economic deprivation |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Zarrinpar, A., Busuttil, R. Liver transplantation: past, present, and future. Nat Rev Gastroenterol Hepatol 2013;10:434-440. [CrossRef]
- Yao L, Robert SA. The contributions of race, individual socioeconomic status, and neighborhood socioeconomic context on the self-rated health trajectories and mortality of older adults. Res Aging 2008;30(2):251-273. [CrossRef]
- Gaskin DJ, Roberts ET, Chan KS, McCleary R, Buttorff C, Delarmente BA. No man is an island: the impact of neighborhood disadvantage on mortality. Int J Environ Res Public Health 2019;16(7):1265. [CrossRef]
- Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav 2005;46(1):15-31. [CrossRef]
- Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav 2001;42(3):258-276.
- Wing JJ, Sánchez BN, Adar SD, Meurer WJ, Morgenstern LB, Smith MA, et al. Synergism of short-term air pollution exposures and neighborhood disadvantage on initial stroke severity. Stroke. 2017;48(11):3126-9. [CrossRef]
- Lusk JB, Hoffman MN, Clark AG, Bae J, Corsino L, Hammill BG. Neighborhood socioeconomic deprivation and 30-day mortality and readmission for patients admitted for diabetes management. Diabetes Care 2022;45(11):e169-170. [CrossRef]
- Lusk JB, Hoffman MN, Clark AG, Bae J, Luedke MW, Hammill BG. Association between neighborhood socioeconomic status and 30-day mortality and readmission for patients with common neurologic conditions. Neurology 2023. [CrossRef]
- Ebel NH, Lai JC, Bucuvalas JC, Wadhwani SI. A review of racial, socioeconomic, and geographic disparities in pediatric liver transplantation. Liver Transpl 2022;28(9):1520-1528. [CrossRef]
- Shifman HP, Huang CY, Beck AF, Bucuvalas J, Perito ER, Hsu EK, Ebel NH, Lai JC, Wadhwani SI. Association of state Medicaid expansion policies with pediatric liver transplant outcomes. Am J Transplant 2023 Sep 28:S1600-6135(23)00719-0. [CrossRef]
- Yalung JE, Shifman HP, Rasnick Manning E, Beck A, Bucuvalas J, Lai JC, Wadhwani SI. Ambient air pollution is associated with graft failure/death in pediatric liver transplant recipients. Am J Transplant 2023 Oct 26:S1600-6135(23)00817-1. [CrossRef]
- Wadhwani SI, Ge J, Gottlieb L, Lyles C, Beck AF, Bucuvalas J, Neuhaus J, Kotagal U, Lai JC. Racial/ethnic disparities in wait-list outcomes are only partly explained by socioeconomic deprivation among children awaiting liver transplantation. Hepatology 2022;75(1):115-124. [CrossRef]
- Wadhwani SI, Huang CY, Gottlieb L, Beck AF, Bucuvalas J, Kotagal U, Lyles C, Lai JC. Center variation in long-term outcomes for socioeconomically deprived children. Am J Transplant 2021;21(9):3123-3132. [CrossRef]
- Wadhwani SI, Gottlieb L, Bucuvalas JC, Lyles C, Lai JC. Addressing social adversity to improve outcomes for children after liver transplant. Hepatology 2021;74(5):2824-2830. Epub 2021 Oct 4. [CrossRef]
- Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant 2020;20(6):1597-1605. [CrossRef]
- Wadhwani SI, Bucuvalas JC, Brokamp C, Anand R, Gupta A, Taylor S, Shemesh E, Beck AF. Association between neighborhood-level socioeconomic deprivation and the medication level variability for children following liver transplantation. Transplantation 2020;104(11):2346-2353. [CrossRef]
- Yilma M, Cogan R, Shui AM, Neuhaus JM, Light C, Braun H, Mehta N, Hirose R. Community-level social vulnerability and individual socioeconomic status on liver transplant referral outcome. Hepatol Commun 2023;7(7):e00196. eCollection 2023 Jul 1. [CrossRef]
- Cullaro G, Ge J, Lee BP, Lai JC, Wadhwani SI. Association between neighborhood-based material deprivation and liver transplant waitlist registrants demographics and mortality. Clin Transplant 2023 Nov 8:e15189. Online ahead of print. [CrossRef]
- Sgrò A, Cambridge WA, McLean KA, Drake TM, Camilleri-Brennan J, Knight SR, Pius R, Wu DA, Wigmore SJ, Harrison EM. Is socioeconomic deprivation associated with worse quality of life, anxiety and depression in liver transplant recipients? A cross-sectional study in a national transplantation programme. BMJ Open 2023;13(8):e070422.
- Strauss AT, Moughames E, Jackson JW, Malinsky D, Segev DL, Hamilton JP, Garonzik-Wang J, Gurakar A, Cameron A, Dean L, Klein E, Levin S, Purnell TS. Critical interactions between race and the highly granular area deprivation index in liver transplant evaluation. Clin Transplant 2023 May;37(5):e14938. Epub 2023 Feb 27. [CrossRef]
- Henson JB, Chan NW, Wilder JM, Muir AJ, McElroy LM. Characterization of social determinants of health of a liver transplant referral population. Liver Transpl 2023;29(11):1161-1171. [CrossRef]
- Menahem B, Dejardin O, Alves A, Launay L, Lubrano J, Duvoux C, Laurent A, Launoy AG; French Liver Transplantation Group. Socioeconomic deprivation does not impact liver transplantation outcome for HCC: A survival analysis from a national database. Transplantation 2021;105(5):1061-1068. [CrossRef]
- Quillin RC III, Wilson GC, Wima K, Hohmann SF, Sutton JM, Shaw JJ, et al. Neighborhood level effects of socioeconomic status on liver transplant selection and recipient survival. Clin Gastroenterol Hepatol 2014;12:1934-1941. [CrossRef]
- Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl 2004;10:235-243. [CrossRef]
- Ross-Driscoll K, Kramer M, Lynch R, Plantiga L, Wedd J, Patzer R. Variation in racial disparities in liver transplant outcomes across transplant centers in the United States. Liver Transpl 2021;27(4):558-567. [CrossRef]
- Caranci N, Biggeri A, Grisotto L, Pacelli B, Spadea T, Costa G. L’indice di deprivazione italiano a livello di sezione di censimento: definizione, descrizione e associazione con la mortalità [The Italian deprivation index at census block level: definition, description and association with general mortality]. Epidemiol Prev 2010;34(4):167-176. [CrossRef]
- Rosano A, Pacelli B, Zengarini N, Costa G, Cislaghi C, Caranci N. Aggiornamento e revisione dell’indice di deprivazione italiano 2011 a livello di sezione di censimento [Update and review of the 2011 Italian deprivation index calculated at the census section level]. Epidemiol Prev 202;44(2-3):162-170.
- Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010;16(8):943-949.
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements 2012;2(1):1-141. [CrossRef]
- Tonon M, Rosi S, Gambino CG, Piano S, Calvino V, Romano A, Martini A, Pontisso P, Angeli P. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol 2021;74(3):578-583. [CrossRef]
- Diabetes Standards of Care: ADA guidelines (2018). Available at: http://diabetesed.net/wp-content/uploads/2017/12/2018-ADA-Standards-of-Care.pdf. Accessed Sep, 1st 2023.
- Breslow NE, Day NE, Halvorsen KT, Prentice RL, Sabai C. Estimation of multiple relative risk functions in matched case-control studies. Am J Epidemio 1978;108(4)299-307. [CrossRef]
- Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl 2004;10:235-243. [CrossRef]
- Quillin RC III, Wilson GC, Wima K, Hanseman DJ, Sutton JM, Shaw JJ, et al. Independent effect of Black recipient race on short term outcomes after liver transplantation. Surgery 2015;157:774-784. [CrossRef]
- Hong JC, Kosari K, Benjamin E, Duffy JP, Ghobrial RM, Farmer DG, et al. Does race influence outcomes after primary liver transplantation? A 23-year experience with 2,700 patients. J Am Coll Surg 2008;206:1009-1016; discussion 1016-1008. [CrossRef]
- OPTN/SRTR 2016 annual data report: preface. Am J Transplant 2018;18 (suppl 1):1-9.
- Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the MELD era. Am J Gastroenterol 2008;103:901-910. [CrossRef]
- Ross-Driscoll K, Kramer M, Lynch R, Plantiga L, Wedd J, Patzer R. Variation in racial disparities in liver transplant outcomes across transplant centers in the United States. Liver Transpl 2021;27(4):558-567. [CrossRef]
- Townsend P. Poverty in the United Kingdom: A survey of household resources and standards of living. University of California Press, 1979.
- Fabrizi E, Mussida C, Parisi ML. Comparing material and social deprivation indicators: identification of deprived populations. Soc Indic Res 2023;165:999-1020. [CrossRef]
- Alkire S, Foster J. Counting and multidimensional poverty measurement. J Pub Econ 2011;95(7-8):476-487. [CrossRef]
- Fusco A, Guio AC, Marlier E. Building a material deprivation index in a multinational context: lessons from the EU experience: Lessons from the EU experience. In: Berenger V, Bresson F (eds) Poverty and social exclusion around the Mediterranean Sea. Economic studies in inequality, social exclusion, and well-being, vol 9. Springer, Boston, MA. [CrossRef]
- Dhongde S, Haveman R. Multi-dimensional deprivation in the US. Soc Indic Res 2017;133:477-500. [CrossRef]
- United Nations. Transforming our world: The 2030 Agenda for sustainable development. Retrieved January 2nd, 2024 20 from https:://sustainabledevelopment.un.org/post2015/transformingourworld.
| Variable | Value |
|---|---|
|
RECIPIENT 2,568 | |
| Male sex, n (%) | 1,872 (72.5) |
| Age at transplant (median, IQR), years | 56 (10) |
| Indication to transplant, n (%) HCV HBV (±HDV) HCV-HBV(±HDV) Alcohol MASLD Autoimmune/PSC Other Presence of HCC, n (%) |
1,253 (48.7) 668 (26.0) 95 (3.7) 282 (10.9) 102 (3.9) 133 (5.2) 35 (1.3) 1052 (40.9) |
| Lab-MELD at transplant (median, IQR) | 12 (6) |
| DM at transplant, n (%) | 626 (24.4) |
| CKD at transplant, n (%) | 149 (5.8) |
| Hypertension at transplant, n (%) | 382 (14.9) |
| Neighborhood DI rank, n (%) 1 (very low) 2 (low) 3 (moderate) 4 (high) 5 (very high) Median (IQR) DI rank |
572 (22.3) 572 (22.3) 788 (30.7) 424 (16.5) 212 (8.3) 3 (1) |
|
DONOR 2,568 | |
| Male sex, n (%) | 1,624 (63.2) |
| Age, median (IQR) | 63 (11) |
| ICU stay, median (IQR) days | 3 (4) |
| CVA as cause of death, n (%) |
1,926 (75) |
| Anti-HCV-positive, n (%) | 22 (0.85) |
| Anti-HBc-positive, n (%) | 334 (13.0) |
| Cardiac arrest episodes, n (%) | 282 (10.9) |
| Use of inotropes, n (%) | 2174 (84.7) |
|
TRANSPLANTATION |
2,568 |
| CIT, median (IQR) (min) | 424 (89) |
| MP, n (%) | 19 (0.7) |
| Re-transplantation, n (%) | 156 (6.1) |
| TAC, n (%) | 1,445 (56.3) |
| EVR, n (%) | 847 (32.9) |
| DM at 1 year, n (%) | 539 (20.9) |
| Attended follow-up visits*, n Scheduled follow-up visits, n Attended/scheduled follow-up visits, n (%) |
43,224 (i.e. 2.08 per patient-year) 51,630 (i.e. 2.2 per patient-year) 83.7% |
| Variable | Value Total patients = 2,568 |
|---|---|
| Death, n (%) HCV recurrence De novo malignancy HCC recurrence Infection/sepsis MACE Recurrent liver disease Stroke |
810 (31.5) 319 (12.4) 129 (5.0) 115 (4.5) 113 (4.4) 83 (3.2) 32 (1.2) 19 (0.7) |
| Re-transplantation, n (%) HAT/HAS PNF/EAD Ischemic cholangiopathy HCV recurrence) VBDS/Chronic rejection |
156 (6.1) 77 (2.9) 51 (2.0) 23 (0.9) 3 (0.11) 2 (0.08) |
| Variable | Value Total patients = 2,568 |
|---|---|
| Hypertension, n (%) | 1,592 (61.9) |
| HCV recurrence, n (%) | 1,127 (43.8) |
| Biliary complications, n (%) Ischemic cholangiopathy |
590 (22.9) 493 (19.2) |
| Obesity, n (%) | 467 (18.1) |
| DM, n (%) | 462 (18.0) |
| t/BPAR, n (%) | 436 (16.9) |
| CKD, n (%) | 378 (14.7) |
| De novo malignancies*, n (%) PTLD Colon Kidney Prostate Laryngeal Breast Lung Uterus Anal Melanoma Cholangiocarcinoma Testis Of unspecified origin |
361 (14.0) 186 (7.2) 45 (1.7) 38 (1.5) 35 (1.3) 15 (0.6) 14 (0.5) 13 (0.5) 5 (0.2) 3 (0.1) 3 (0.1) 2 (0.06) 1 (0.03) 1 (0.03) |
| MACE, n (%) | 334 (13.0) |
| HCC recurrence, n (%) | 152 (5.9) |
| Neurologic, n (%) | 102 (3.9) |
| VBDS/chronic rejection, n (%) | 7 (0.2) |
| Patient with >1 complication, | 1,901 (74) |
| Variable | DI=1 (#572) | DI=2 (#572) |
DI=3 (#788) |
DI=4 (#424) |
DI=5 (212) |
P |
|---|---|---|---|---|---|---|
| RECIPIENT | ||||||
| Male sex, (%) |
412 (72.0) |
422 (73.7) |
560 (71.1) |
305 (71.9) |
173 (81.6) | 0.32 |
| Age at transplantation (median, IQR), years |
56 (9) |
58 (9) |
59 (10) |
57 (9) |
57 (8) |
0.78 |
| HCV, n (%) | 280 (48.9) | 291 (50.8) | 386 (48.9) | 206 (48.6) | 90 (42.4) | 0.13 |
| HCC, n (%) | 235 (41.1) | 242 (42.3) | 338 (42.8) | 178 (41.9) | 59 (27.8) | 0.001 |
| Lab-MELD at transplant (median, IQR) | 11 (7) | 13 (6) | 12 (5) | 13 (7) | 12 (5) | 0.37 |
| DM at transplant, n (%) | 137 (23.9) | 142 (24.8) | 176 (22.3) | 101 (23.8) | 70 (33.1) | 0.86 |
| CKD at transplant, n (%) | 35 (6.1) | 42 (7.3) | 39 (4.9) | 25 (5.9) | 8 (3.7) | 0.26 |
| Hypertension at transplant, n (%) | 52 (9.1) | 58 (10.1) | 94 (11.9) | 125 (29.4) | 53 (25.0) | <0.0001 |
| DONOR | ||||||
| Male sex, n (%) | 360 (63.0) | 372 (65.0) | 504 (63.9) | 272 (64.1) | 116 (54.7) | 0.11 |
| Age, median (IQR) | 62 (10) | 64 (12) | 63 (10) | 64 (9) | 63 (11) | 0.43 |
| ICU stay, median (IQR) days | 3 (3) | 4 (3) | 3 (4) | 4 (3) | 4 (3) | 0.89 |
| CVA as cause of death, n (%) | 425 (74.3) | 434 (75.9) | 598 (75.8) | 313 (73.8) | 156 (73.6) | 0.86 |
| Anti-HCV-positive, n (%) | 4 (0.7) | 3 (0.5) | 7 (0.9) | 6 (1.4) | 2 (0.9) | 0.64 |
| Anti-HBc-positive, n (%) | 75 (13.1) | 77 (13.4) | 102 (12.9) | 55 (12.9) | 25 (11.8) | 0.98 |
| Cardiac arrest episodes, n (%) | 70 (12.2) | 62 (10.8) | 85 (10.8) | 45 (10.6) | 20 (9.4) | 0.81 |
| Use of inotropes, n (%) | 483 (84.4) | 486 (84.9) | 661 (83.8) | 364 (85.8) | 180 (84.9) | 0.92 |
| TRANSPLANTATION | ||||||
| CIT, median (IQR) min | 434 (94) | 414 (88) | 412 (96) | 401 (87) | 422 (82) | 0.47 |
| MP, n (%) | 3 (0.5) | 6 (1.0) | 8 (1.0) | 1 (0.2) | 1 (0.5) | 0.46 |
| TAC, n (%) | 314 (54.9) | 326 (56.9) | 433 (54.9) | 245 (57.8) | 127 (59.9) | 0.82 |
| EVR, n (%) | 183 (31.9) | 177 (30.9) | 253 (32.1) | 153 (36.1) | 81 (38.2) | 0.79 |
| Attended/scheduled follow-up visits (%) | 92.2 | 94.3 |
87.8 |
76.1 |
68.2 | <0.0001 |
| Variable | DI=1 (#572) | DI=2 (#572) |
DI=3 (#788) |
DI=4 (#424) |
DI=5 (212) |
P |
|---|---|---|---|---|---|---|
| Death, n (%) HCV recurrence, n (%) De novo malignancy, n (%) HCC recurrence, n (%) Infection/sepsis, n (%) MACE, n (%) Recurrent liver disease, n (%) Stroke, n (%) Total |
69 (12.0) 20 (3.4) 23 (4.0) 22 (3.8) 8 (1.4) 5 (0.9) 4 (0.7) 151 (26.3) |
75 (13.1) 17 (2.9) 24 (4.2) 21 (3.6) 7 (1.2) 3 (0.5) 3 (0.5) 150 (26.2) |
86 (10.9) 37 (4.7) 44 (5.6) 45 (5.7) 25 (3.2) 12 (1.5) 7 (0.9) 256 (32.5) |
52 (12.2) 27 (6.4) 13 (3.1) 16 (3.8) 25 (5.9) 6 (1.4) 2 (0.5) 141 (33.2) |
37 (17.4) 28 (13.2) 11 (5.2) 9 (4.2) 18 (8.5) 5 (2.3) 3 (1.4) 110 (51.8) |
0.86 <0.0001 0.30 0.31 <0.0001 0.20 0.67 <0.0001 |
| Re-transplantation, n (%) HAT/HAS, n % PNF/EAD, n (%) Ischemic cholangiopathy, n (%) HCV recurrence, n (%) Chronic rejection, n (%) Total |
14 (2.4) 11 (1.9) 4 (0.7) 1 (0.2) 0 (0) 30 (5.2) |
18 (3.1) 12 (2.1) 6 (1.0) 2 (0.4) 1 (0.2) 39 (6.8) |
26 (3.2) 18 (2.3) 9 (1.1) 0 (0) 1 (0.12) 54 (6.8) |
10 (2.3) 7 (1.6) 3 (0.7) 0 (0) 0 (0) 20 (4.7) |
9 (4.2) 3 (1.4) 1 (0.5) 0 (0) 0 (0) 13 (6.1) |
0.62 0.90 0.82 0.99 0.99 0.48 |
| Hypertension, n (%) | 314 (54.9) | 323 (56.4) | 464 (58.8) | 312 (73.6) | 179 (84.4) | <0.0001 |
| HCV recurrence, n (%) | 276 (48.2) | 248 (43.3) | 323 (40.9) | 185 (43.6) | 95 (44.8) | 0.12 |
| Ischemic cholangiopathy, n (%) | 114 (19.9) | 119 (20.8) | 143 (18.1) | 85 (20.0) | 32 (15.1) | 0.37 |
| Obesity, n (%) | 82 (14.3) | 91 (15.9) | 157 (19.9) | 94 (22.2) | 43 (20.3) | 0.02 |
| DM, n (%) | 81 (14.2) | 92 (16.1) | 152 (19.3) | 93 (21.9) | 44 (20.7) | 0.02 |
| t/BPAR, n (%) | 67 (11.7) | 78 (13.6) | 143 (18.1) | 87 (20.5) | 61 (28.7) | <0.0001 |
| CKD | 50 (9.9) | 58 (11.9) | 107 (15.7) | 63 (16.7) | 56 (27.3) | <0.0001 |
| De novo malignancies*, n (%) | 64 (11.2) | 68 (11.8) | 117 (14.8) | 61 (14.4) | 51 (24.1) | <0.0001 |
| MACE, n (%) | 56 (8.7) | 61 (10.6) | 103 (13.1) | 68 (16.0) | 56 (26.4) | <0.0001 |
| HCC recurrence, n (%) | 31 (5.4) | 34 (5.9) | 47 (6.0) | 24 (5.6) | 16 (7.5) | 0.85 |
| Neurologic, n (%) | 23 (4.0) | 25 (4.4) | 27 (3.4) | 19 (4.5) | 8 (3.8) | 0.91 |
| VBDS/chronic rejection, n (%) | 3 (0.5) | 2 (0.3) | 0 (0) | 1 (0.2) | 0 (0) | 0.99 |
| Variable | Coefficients (95%CI) |
SE | z | HR | p | |
|---|---|---|---|---|---|---|
| Pre-transplant | Patient female sex | 1.67 (-0.03;3.36) | 0.87 | 1.92 | 0.89 | 0.04 |
| Patient age | -0.01 (-0.00; 0.11) | 0.06 | 0.14 | 1.18 | 0.02 | |
| HCV versus HBV | -2.34 (-4.6; 0.07) | 1.15 | 2.02 | 1.32 | <0.01 | |
| HCC (yes/no) | 0.42 (-0.02; 0.81) | 0.2 | 2.06 | 1.22 | 0.02 | |
| Higher deprivation area | 0.82 (-0.78; 2.42) | 0.82 | 1.01 | 1.14 | 0.02 | |
| High lab-MELD | -1.18 (-1.63; 0.72) | 0.23 | 5.11 | 1.13 | 0.02 | |
| DM at transplant (yes/no) | -1.02 (-1.64; 0.78) | 1.2 | 2.01 | 1.14 | 0.03 | |
| CKD at transplant (yes/no) | -0.78 (-0.06; 1.23) | 0.34 | 2.23 | 1.08 | 0.06 | |
| Hypertension at transplant (yes/no) | -1.02 (-0.87; 1.13) | 1.1 | 2.24 | 1.02 | 0.75 | |
| Donor | Donor male sex | -0.05 (-0.09; 0.6) | 0.03 | 3.12 | 1.05 | 0.75 |
| Donor age | 0.11 (-0.01; 0.22) | 0.05 | 2.11 | 1.21 | 0.03 | |
| Longer ICU stay (days) | -0.04 (-0.08; 0.02) | 0.03 | 1.12 | 1.03 | 0.67 | |
| Longer CIT (min) | -1.09 (-3.24; 1.06) | 1.1 | 1 | 1.16 | 0.03 | |
| Donor cause of death (CVA versus other) | -0.06 (-0.09; 0.12) | 0.05 | 2.89 | 1.04 | 0.89 | |
| Donor cardiac arrest (yes/no) | -0.56 (-0.08; 1.13) | 0.44 | 2.12 | 1.08 | 0.07 | |
| Post-transplant | Post-transplant DM (yes/no) | -0.88 (-1.13; 0.56) | 1.08 | 1.78 | 1.05 | 0.06 |
| DM at 1 year (yes/no) | -0.98 (-1.23; 0.88) | 1.15 | 2.06 | 1.13 | 0.01 | |
| CKD (yes/no) | -0.03 (-0.00; 0.12) | 0.06 | 0.14 | 1.18 | 0.76 | |
| TAC versus CyA | 0.01 (0; 0.01) | 0 | 2.52 | 0.68 | 0.01 | |
| EVR | -0.88 (1.2; -0.46) | 0.21 | 3.66 | 0.66 | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





