1. Introduction
Nowadays, attaching importance to the application of cognitive neuroscience results in the field of education has become a hot spot and trend [
1]. The discovery of Default Mode Network (DMN) has made people realize the importance of rest in learning, changed the cognition of human brain function and learning, and subverted the understanding of "rest". To focus well, one should learn to take breaks and "not to get focused." If the learning time exceeds a certain period, the effect will gradually decrease, and if the learning time is extended, it will be difficult to have an effect [
2]. Brain science research results show that one cannot learn efficiently without a good rest[
3], and rest promotes learning, as learning continues when people rest their brain. A scientific brain break will make learning easier and more enjoyable. Unable to get focused, one redoubles his effort and force himself to concentrate, he will have poor performance. Learning from dawn to dusk with tight schedule, one will be exhausted both physically and mentally, which results low efficiency; Taking advantage of every second to study without any distraction, one will fail to live up to his expectations. Mobilizing attention resources all the time requires the continuous working of the related brain areas, which results in brain "strike". Meanwhile, the brain is unable to switch to other functional areas for information integration and sorting. People who devote the longest time to studying are not necessarily high in efficiency, but the efficient performers all have one thing in common: they manage their study time well, take breaks, and complete necessary tasks while maintaining energy. Since the discovery of DMN, researchers have carried out a large number of studies, but more of them focus on the clinical application of mental diseases, rarely involving the field of learning. This paper concentrates on the aspect of learning and explains the neural mechanism of rest promoting learning from the perspective of DMN.
2. Default Mode Network and Learning
A number of studies have found that parts of the brain's association cortex are inhibited during tasks that require external attention, but become active during memory, imagining the future, and making social inferences. It is generally believed that the state of being awake, eyes closed or no specific cognitive task is the resting state. Louis Sokoloff et al. discovered that a fixed area of the brain is active in the resting state, and there is no difference between the metabolic rate of these areas and the task execution state, and there is continuous and active neural activity [
4]. Ingvar et al. found that frontal lobe activity reached a high level at rest and still carried out brain work, and the frontal lobe activity pattern corresponded to non-directional, spontaneous and conscious mental states [
5]. Raichle proposed the hypothesis of resting state activity based on the fact that in the waking resting state of the human brain, certain brain regions will present strong signals and have strong and regular activities [
6]. In 2001, Raichle et al. formally proposed the concept of brain default mode network [
7], and it was not until 2016 that Zhu et al. used fMRI technology to verify the existence of DMN for the first time [
8]. Shulman et al. found that the brain default mode network was almost inactive or showed negative activation (deactivation) under the condition of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) cognitive task experimental conditions, and the activity level of this brain region was higher in the resting state than in the task condition [
9]. The "default mode network" is a very important and continuously running organizational structure network of the brain in the resting state, which plays a very important role in the monitoring of internal and external environment, emotional control and emotional memory extraction [
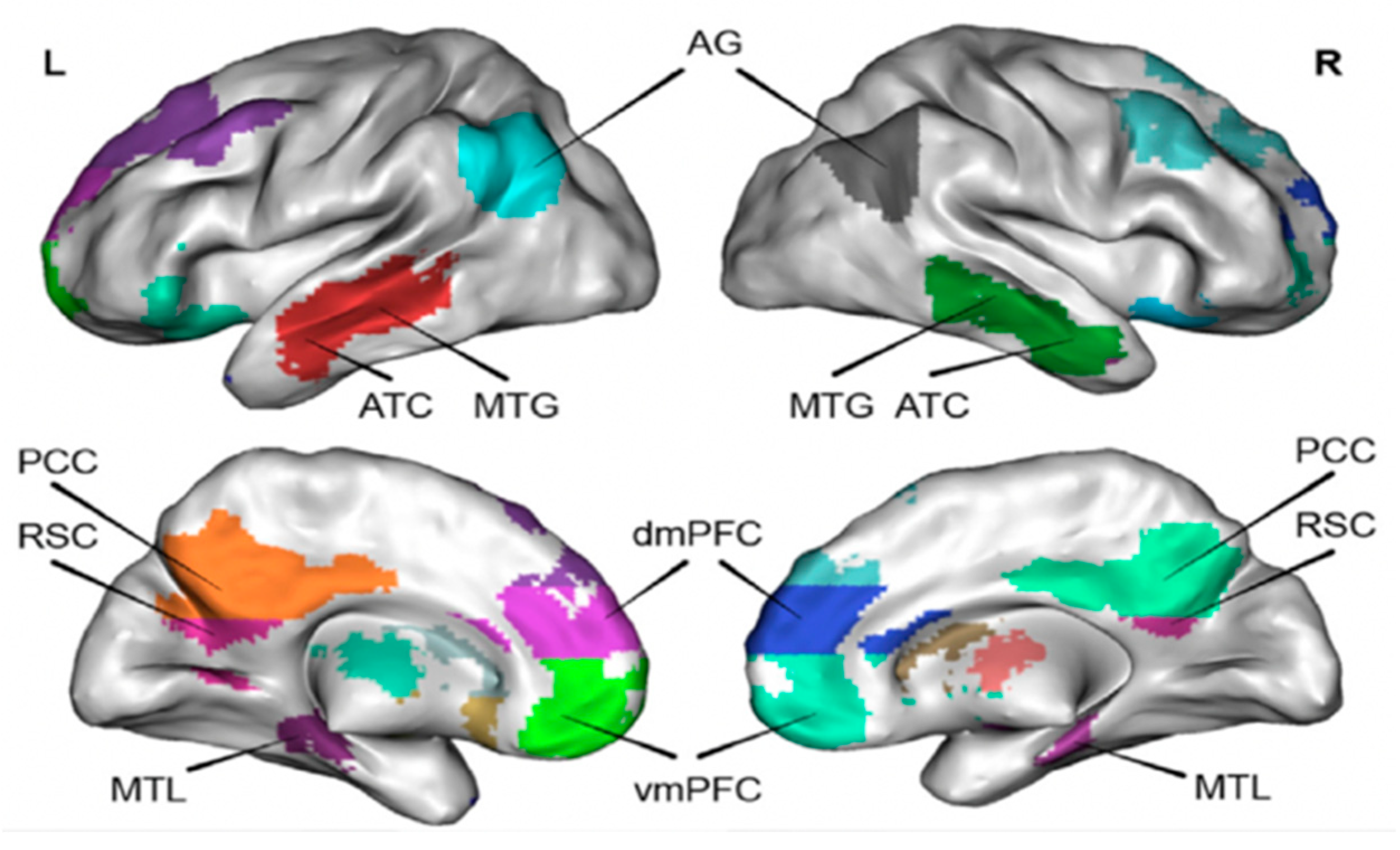
8]. The DMN is composed of discrete, bilaterally symmetrical brain regions, including the cingulate /Precuneus (PCC/Precuneus), dorsal-medial prefrontal cortex (dmPFC), ventromedial prefrontal cortex (vmPFC), angular gyrus (AG), retrosplenic cortex (RSC), pretemporal cortex (ATC), middle temporal gyrus (MTG), and hippocampus/medial temporal lobe (hippocampus/MTL) and other structures [
8,
9]. The brain default mode network structure and location are shown in
Figure 1.
The finding of DMN indicates that the DMN brain region is activated at rest and learning does not stop. During task-free waking, resting state or rest, the default mode network brain area changes from being silent to active, and there is spontaneous neural activity, the level of which significantly surpasses other brain areas. At this time, the default mode network doesn’t rest but performs a specific brain function, namely learning related activities.
3. Default Mode Network Functions and Learning
The DMN functions are related to learning. It involves a set of brain regions that are functionally connected to each other [
10] and exhibit non-activation or negative activation in most external tasks [
9]. DMN is actively activated during internal psychological activities such as self, episodic memory, moral judgment and future imagination [
6,
11,
12,
13]. The DMN is the neural basis of the self, which senses the self, collects memories of events and facts about the individual, attributes and descriptions of the self, and reflects one's emotional state. DMN is also involved in episodic memory [
14] and absentmindedness [
15]. DMN has functions such as monitoring the internal and external environment of the brain, emotional processing, introspective, maintaining thinking and cognition, and extracting thought recall [
16]. DMN plays the role of connecting the past and the future, reviewing the past, thinking about the future, forming episodic memory, and deepening event understanding [
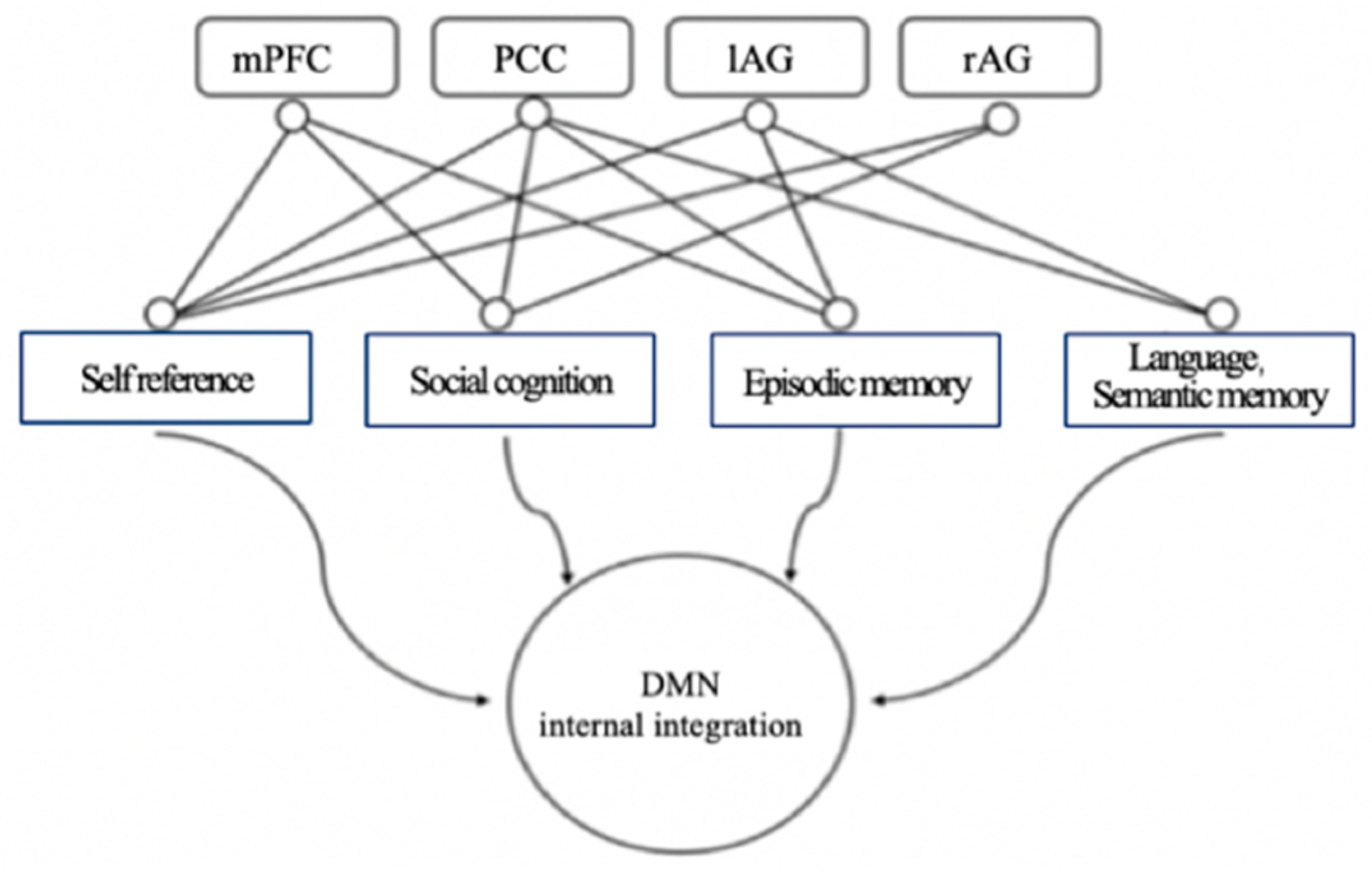
17]. See
Figure 2 below for DMN function diagramFunctional diagram of DMN (see above for symbols). Medial prefrontal cortex, bilateral angular gyrus, and cingulate/anterior cuneus are respectively related to social cognition, self-reference, episodic memory, language and semantic memory.
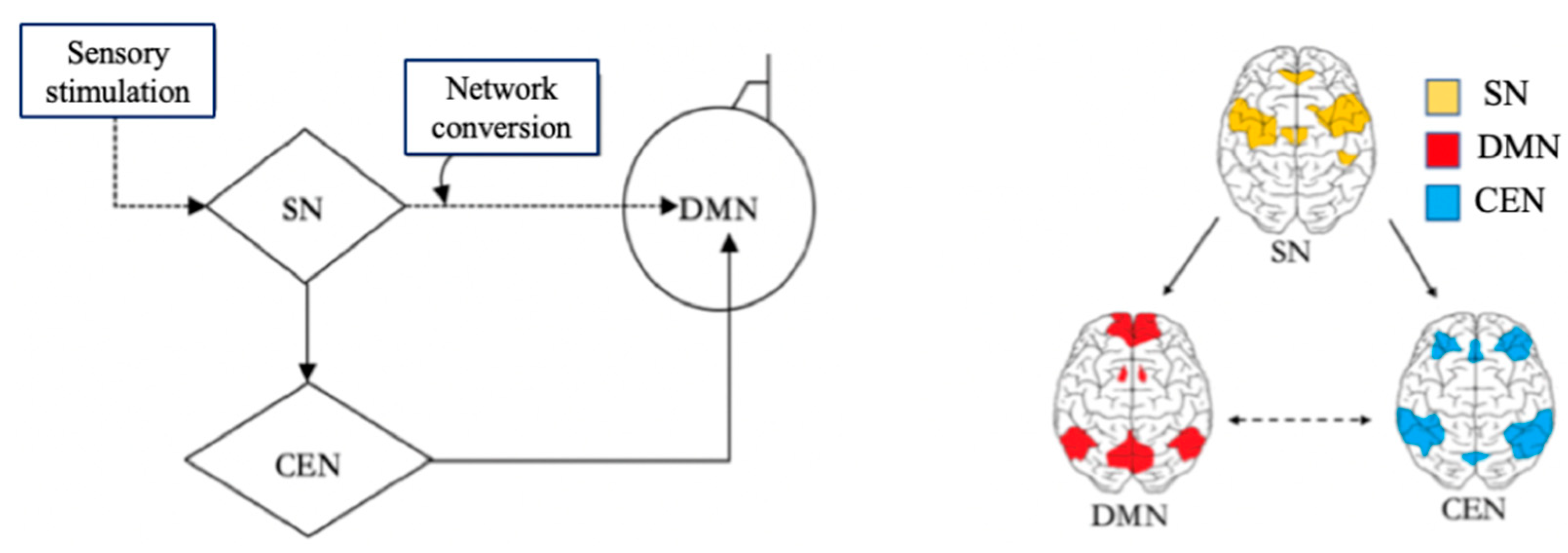
With the help of The DMN, Central Executive Network (CEN) and Salience Network (SN), the brain can switch freely between focused attention and divergent thinking, which helps the brain to get efficient rest. When the brain focuses attention, it is in the CEN state [
16]. When the brain does not focus attention, it will enter the DMN state. The CEN is a specific brain region that is activated during the completion of a task and is responsible for preventing interruptions and limiting irrelevant stimuli while people are engaged in work. When CEN is activated, people will quickly focus their attention on solving the immediate task, which helps to complete the task more attentively and efficiently. The brain turns on the DMN immediately after a task is completed or stopped. There is certain anticorrelation between the activities of DMN and CEN [
9]. CEN is negatively correlated with DMN, as one network is activated while the other will be dormant. After the brain has been in CEN for a long time, only if the brain is properly relaxed and the DMN in the
brain is fully invoked, the brain can think more flexibly, so that the brain can generate more different inspirations. Salience Network (SN) is responsible for regulating brain switching between CEN and DMN [
9]. SN is related to self-awareness, involved in detecting and filtering sensory stimuli, and mobilizing relevant functional networks [
18]. SN prompts the brain to convert CEN and DMN according to the needs of external or internal information processing. In the brain, SN is responsible for regulating the mechanism of switching DMN and CEN, which also indicates that the brain itself needs to constantly switch between work and rest, so as to alternate work with rest. The relationships among SN, CEN and DMN are shown in
Figure 3. Triple Network model.
4. Characteristics of Default Mode Network and Learning
The learning requirement leads to high energy consumption of DMN. Individuals have different learning tasks in different semesters, and the DMN function matches them accordingly. The DMN also adjusts to different activation states with different cognitive difficulty. Mental disease can lead to DMN dysfunction and disorder, which results in various learning problems. It is also proved from the characteristics of brain default mode network that DMN is closely related to learning.
4.1. DMN’s High Energy Consumption to Meet the Needs of Learning
The energy consumption of the brain in the task state is only about 5% higher than that in the resting state [
20]. In the resting state, multiple brain regions of the DMN are always busy, and the energy consumption accounts for 60% to 80% of the total energy consumption of the brain, showing high metabolism in the internal cognitive process [
16]. The maximum energy consumption of the brain in the resting state comes from the DMN[
21]. Magnetic resonance imaging studies have found that DMN activity that enjoys internal activity consistency is significantly higher than other brain regions, which indicates that the default mode network has higher metabolism under resting conditions [
22,
23,
24,
25]. In the DMN state, the relevant brain regions are activated and run at high speed, and the learning activities are still in full motion, with large energy demand and high metabolism. Compared with other brain networks, the DMN has higher energy consumption to meet the needs of learning activities in the process of cognitive integration, memory, emotional processing and introspection and so on.
4.2. DMN Activation Automatic Adjustment According to the Difficulty of Learning Tasks
Specific target tasks can have an effect on the DMN, which is related to the degree of cognition required by the performed target task. When participating in simple manual and visual tasks, such as motor, sensory and perceptual tasks that do not require cognitive participation, the activity of the DMN is almost not affected. The greater the cognitive difficulty is, the more silent the DMN is, and there is a negative correlation between them. Task load is positively correlated with negative activation of DMN[
26]. The more difficult the external cognitive task is, the more attention is required, and the CEN operates at a high speed, at which time the DMN is more inhibited. At rest, the DMN needs more time to organize, further study, and digest previous cognitive content.
4.3. The DMN’s "Inverted U" Shape with Age: The Close Connection with Learning Activities at Different Ages
DMN is age-related [
27], and its functional development changes with age, from imperfect to perfect to degenerate to disappear [
26,
28,
29,
30,
31]. DMN changes with age and is stable at the same time. It is the organization mode of spontaneous activity of brain nerve cells, and is related to brain functions such as learning, memory and cognition [
31]. Fair found that compared with adults, the default mode network of children around 7 to 9 years old is not mature, and the functional connection strength between regions in the default mode network is not as strong as that of adults [
29,
32]. Supekar et al. found that the activity of medial prefrontal lobe (MPFC) was weak in children, the functional connectivity strength from cingulate gyrus (PCC) to MPFC was significantly lower in children than in adults, the gray mass of PCC and MPFC regions was greater than that of adults, the white mass was smaller than that of adults, and the anisotropy of nerve fibers was greater in adults than that in children[
33]. Thomason et al. found that compared with adults, the default mode network in children is not mature and is gradually developing to the normal adult mode [
32]. The default mode network in the brain of young children develops from insignificantly connected to gradually strengthened connection, and the network shows the most stable state in adulthood. Marsh et al. used the stroop paradigm to find that task-evoked default mode network negative activation was positively correlated with age, that is, the older the age, the greater the task-evoked negative activation [
34]. The functional connectivity of anterior and posterior parts of DMN decreases with age [
35,
36]. Younger people have more complete default mode network components than older people[
37], and different age composition will cause differences between DMN activated regions [
38]. People in childhood and old age study and work less, while those in adulthood are occupied with work, tasks and learning, which are all reflected in the development of DMN, so it shows an "inverted U-shaped" development, which is in line with the principle of evolution theory.
4.4. Strong Correlation between DMN and Disease: Mental Disease Affects DMN, and Then Learning
Alterations in the DMN have been widely observed in neurogenic disorders such as Alzheimer's disease (AD) [
39] and psychiatric disorders [
40,
41]. Attention deficit hyperactivity disorder (ADHD) is characterized by prolonged and persistent inattention, hyperactivity and poor impulse control. The functional connectivity between the dorsolateral anterior cingulate and other brain regions in the default mode network of ADHD patients is significantly enhanced [
42]. The functional activity of dorsal anterior cingulate cortex in default mode network decreases in normal children, but this characteristic does not exist in children with ADHD [
43]. ADHD may be related to the abnormal frontal-striatum -cerebellum pathway, and the changes in DMN functional connectivity cause related attention deficits and working memory disorders [
44]. The abnormal activity sites of DMN in patients with depression are not the same [
45,
46,
47], mainly concentrated in the prefrontal lobe, cingulate gyrus, hippocampus, angular gyrus and other parts, and the functional connectivity of different regions of DMN increases or decreases [
48,
49,
50]. The locations of DMN in depression patients are different, and their activities are abnormal [
45,
46,
47], mainly concentrated in the prefrontal lobe, cingulate gyrus, hippocampus, angular gyrus and other parts, and the functional connectivity of different regions of DMN increases or decreases [
48,
49,
50]. The decrease of DMN activation is the main feature of autism (ASD), and its symptoms occur more in the resting state than in the active moment. The negative activation of the DMN in ASD patients is abnormal during the stroop task [
51]. The functional connectivity of the medial prefrontal cortex and left angular gyrus of the DMN decreases in ASD patients, and no abnormality is observed during task activation [
52]. The DMN activity of ASD patients is abnormal [
53], and the functional connectivity of the anterior and posterior part of the DMN decreases. The abnormal default mode network of ASD may reflect the defects of theory of mind and self-reference processing ability [
52], and the regional consistency of bilateral PCC/Precuneus in ASD patients decreases [
54]. Mental disease leads to DMN dysfunction and disorder, which affects attention and behavior, thus causing learning activities to be affected. Abnormal activities of DMN exists in the brain of different types of mental diseases, resulting the failure of its normal functions, which explains why patients with mental diseases have a high incidence of learning problems in reality.Understanding the characteristics of the default mode network of mental disease is of great benefit to know the attention characteristics, learning interest and learning state of individuals.
5. Neural Activity Occurring in Default Mode and Learning
Matthew Berman showed that people with high DMN activation completed tasks at least 10% faster than those with low DMN activation [
55]. John Trugakos et al. found that working or studying continuously for 52 minutes, followed by a 17-minute break, can maximize productivity [
56]. In the learning process, showing some pictures of baby animals to students for relaxation will make students pay more attention in the subsequent learning and greatly improve the learning efficiency [
57]. Taking a holiday or scheduling a break after busy work can reactivate cognitive skills and help improve your ability to solve problems in the future [
58]. The human brain is not designed to focus on study and work for a long time every day. In one study, a computer program was developed to remind individuals to take breaks at a fixed time. People who paid attention to breaks were 13% more accurate at work on average [
59]. Proper rest is not a waste of time, as the alternation between work with rest is the key to improve learning efficiency. The brain will consolidate and enhance the memory of the previously learned new knowledge through short breaks, which plays an important role in learning [
60]. A number of studies on learning have found that rest promotes learning. Here's a closer look at the neural activity that occurs in the brain during default mode to explain how rest promotes learning.
5.1. The Brain’s Frequent Use of Beta Waves for Rhythmic Regulation during Rest
The study found that the rhythm of beta in brain waves changes during rest in a neural network between the frontal lobes and the parietal lobe, known as the DMN. Enhanced beta waves can regulate individual behavior, help improve attention, enhance memory, and improve problem-solving ability [
60]. The brain uses beta waves to consciously switch between different pieces of information, and beta rhythm which serves as a control mechanism determines when information in working memory is read out or cleared, controls when information stored in working memory is expressed, and allows it to influence behavior. In beta rhythm, millions of neurons in the brain each generate their own signals, and these combined signals produce brain wave oscillations. When the δ wave rhythm increases, the β wave rhythm decreases and vice versa. Reasonable planning of learning and rest, which helps the brain switch to beta rhythm in time, is necessary to improve learning ability and to strengthen knowledge memory.
5.2. The Brain’s Spontaneous Initiation of the "Subconscious" Divergence Mode during Rest
The "conscious" focus mode and the "subconscious" divergence mode are the two modes of brain learning [
61]. The "conscious" focus mode is equivalent to the activation of CEN. When focusing on a certain thing or task, the prefrontal cortex automatically transmits signals along the neural pathways, transmitting information to various brain regions related to the task content and connecting them. However, in this mode, the answer is not necessarily found because it is not necessarily in the brain area of conscious attention. Therefore, this raises the need for "subconscious" divergence pattern, which switches into the DMN, taking the brain out of its original working area and allowing neurons to connect randomly to unrelated areas to find an answer that might solve the problem. In order to make the "subconscious" work, one must meet the condition that the awake “consciousness” is completely shut down to completely forget the original thing, that is, take a rest, and let the brain enter the DMN.
5.3. The Hippocampus’ Fully Engagement in Information Integration during Rest
At rest, the brain integrates information in various forms, checks for consistency, and "organizes" memories. This is when the hippocampus reviews the information and decides whether it is necessary or not. The bilateral hippocampus is the key brain region of DMN. Without the rest time, the hippocampus is not given the opportunity or time to sort out and select information, which will eventually be abandoned. At rest, the hippocampus helps the brain repair neurons and remove toxins and trash from the brain. As long as the information is not input into the brain, that is, when taking a rest, the hippocampus start processing information, which can increase memory and generate inspiration. At rest, the prefrontal lobe shuts down, but the hippocampus works all the time, transferring knowledge and experiences from previous short-term memory to long-term memory and consolidating them. Through the function of the hippocampus, the information that needs to be remembered is engraved into the cerebral cortex and transformed into long-term memory. Learning is not just the hard work with certain effective method, rest also plays an essential role in it. Without rest, the brain cannot enter the DMN, and the learning effect will be greatly reduced.
5.4. Recurrent Neural Replay Occurring in the Brain during Rest
Neural replay is used to enhance memory by activating activity in brain regions at rest, or in the same order as required for learning a skill. It has been shown that neural replay events occur in the medial temporal cortex and sensorimotor cortex in the DMN during rest. The medial temporal cortex includes the hippocampus and entorhinal cortex, which help encode memory for abstract information; The sensorimotor cortex includes brain regions that process sensory information,plan and perform movements. Neural replay events in the hippocampus and sensorimotor cortex can help consolidate memories of complex skills, integrate memories associated with abstract knowledge and motor tasks planning and execution. Neural replay is more frequent during rest, and the frequency of these neural replay events during rest correlates with the degree of improvement in task performance. Rapid and recurring neural replay events at rest after learning can strengthen the coordination between relevant brain regions, thereby consolidating memory [
62].
The above four kinds of neural activities in the brain at rest fully testifies that rest promotes learning. The DMN plays an important role in these neural activities occurring at rest. During rest, the brain waves in the DMN areas switched and changed rhythmicity, and the β-wave rhythm controlled behavior and improved attention, memory and problem-solving ability. The initiation of the "subconscious" divergent mode is actually a switch to the default mode of the brain, allowing neurons to connect freely. Hippocampus is the core region of DMN, which plays a key role in memory transformation and consolidation. Neural replay is the neural mechanism of the specific expression of hippocampal force. DMN is essential to learning. Rest can promote learning and truly achieve the combination of work and rest.
6. Discussion and Conclusions
The neural mechanism by which rest promotes learning is complex and it is also challenging for neuroscience. Based on the discovery, function and characteristics of DMN, this paper explores the close relationship between DMN and learning activities at rest, and proves that rest promotes learning from several neural activities related to DMN at rest. Future exploration of the mechanism of DMN, the function of different DMN regions, and the collaboration with other brain networks will further improve the comprehensive understanding of the DMN.
How to make better use of DMN to promote learning and how to help individuals switch to DMN are all worthy of study. For example, how to help individuals make plans for learning and rest, and how to make plans that are more consistent with the activity law of DMN, can better help improve learning efficiency. To explore these rules, the relationship between DMN and individual learning and development should be further revealed. The conclusion that the DMN develops in an inverted "U" shape with age is just a general trend of development, and the time node of development change is still unclear. The approximate age at which the DMN develops into the normal adult pattern and becomes aging is still unknown, and the normal level of DMN in different ages is still difficult to reveal. The relationship between DMN and individual psychological development also needs to be further studied, and the interaction mechanism between individual self-awareness development, ego and DMN is not clear.
There is still some debate about the function and assumptions of default mode network. Only by settling the assumption argument can we better understand its role in learning. Although the function of DMN has been revealed from the macro level, the exact function has not been completely defined so far. There are two functional theoretical hypotheses of DMN: internal mental processing hypothesis and vigilance hypothesis (Buckner et al., 2008). The internal mental processing hypothesis points out that the default mode network's function is to process self-reference, episodic memory, mental time travel and theory of mind, and the vigilance hypothesis holds that the default mode network's function is to maintain extensive attention to the external environment. In fact, the two theories are not mutually exclusive, and cognitive neuroscientists need to get better at bringing them together. The relationship between DMN and other networks such as visual network, auditory network, and language network remains at the simple switching level, and the specific relationship will be the focus of future research.
The application of DMN in non-disease fields should be strengthened, especially the application of DMN in learning field. However, the application of DMN is mainly clinical. A large number of studies have focused on revealing the relationship between DMN and mental diseases, such as depression, post-traumatic stress disorder, autism, attention deficit hyperactivity disorder and schizophrenia, and other diseases that are related to abnormal activity of default mode network. Less attention has been paid to the relationship between other non-disease behaviors and DMN, and there is a lack of specific research on the use of DMN. This paper attempts to explore the role of rest in learning from the perspective of DMN. More research is needed to reveal how to efficiently rest and switch to default mode.
Author Contributions
Conceptualization, W.L. and J.W.; project administration, W.L.; supervision, J.H; writing—original draft preparation, W.L.; writing—review and editing, W.L., B.L., Y.T., J.H., J.W., funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2021 Guangxi Education Modernization and Quality Monitoring Research Center Research Fund Project "Construction of Multi-dimensional Evaluation System for Informatization of Primary and Secondary Education in Guangxi" (project number JC2021005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank all participants who took part in the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mayer R, E. How Can Brain Research Inform Academic Learning and Instruction[J].Ed-ucational Psychology Review,2017,29(4):835-846. [CrossRef]
- Ericsson,K.A.,Krampe,R.T.,&Tesch-Römer,C.The role of deliberate practice in the acqu-isition of expert performance[J]..Psychological Review,1993,100(3),363-406. [CrossRef]
- Ethan, R. Buch,Leonardo Claudino,Romain Quentin,Marlene Bönstrup,Leonardo G.Cohen.Consolidation of human skill linked to waking hippocampo-neocortical replay[J]. Cell Reports,2021,35(10):109193. [CrossRef]
- Mangold,Renward,et al.The effects of sleep and lack of sleep on the cerebral circulation and.metabolism of normal young men[J]. The Journal of clinical investigation,1955,34(7):1092-1100.
- Buckner R L,Andrews-Hanna J R,Schacter D L.The brain's default network:Anato-my,function.and relevance to disease[J].Annals of the New York Academy of Sciences,2008,1124(1):1-38. [CrossRef] [PubMed]
- Raichle,Marcus E.,and Abraham Z.Snyder.A default mode of brain function:a brief his-tory of an evolving idea[J].Neuroimage,2007,37(4):1083-1090. [CrossRef] [PubMed]
- Raichle,M.E.,MacLeod,A.M.,Snyder,A.Z.,Powers,W.J.,Gusnard,D.A.,&Shulman,G.L.A default mode of brain function[J].Proceedings of theNational Academy of Sciences of the UnitedStates of America,2001,98(2):676-682. [CrossRef]
- Fox,M.D.,Snyder,A.Z.,Vincent, J.L.,Corbetta,M.,van Essen,D.C.,& Raichle,M.E.The hum-an brain is intrinsically organized into dynamic,anticorrelated functional networks[J].Pr-oceedings of the National Academy of Sciences of the United States of America,2005,102(27):9673-9678. [CrossRef] [PubMed]
- Shulman,G.L.,Fiez,J.A.,Corbetta,M.,Buckner,R.L.,Miezin,F.M.,Raichle,M.E.,& Peterse-n,S.E..Common blood flow changes across visualtasks.II.Decreases in cerebral cortex[J].Jo-urnal ofCognitive Neuroscience,1997,9(5):648-663.
- Greicius,M.D.,Krasnow,B.,Reiss,A.L.,& Menon,V.Functional Connectivity in the RestingBrain:A Network Analysis of the Default Mode Hypothesis[J].Proceedings of the Nati-onal Academy of Sciences of the United States of America,2003,100:253-258. [CrossRef]
- Zhu, H. ,Zhou P.,Alcauter S.,Chen Y.,Cao H.,Tian M.,Ming D.,Qi H.,Wang X.,Zh-ao X.,He F.,Ni H.,and Gao W.Changes of intranetwork and internetwork functional connecti-vity in alzheimer's disease and mild cognitive impairment[J].Journal of Neural Engine-ering,2016,13(4):046008. [CrossRef] [PubMed]
- Andrews-Hanna, JR. The Brain’s Default Network and Its Adaptive Role in Internal.M-entation[J].The Neuroscientist,2012;18(3):251-270. [CrossRef] [PubMed]
- Foster,B.L.,Dastjerdi,M., & Parvizi,J..Neural populations in human posteromedial cortexdisplay opposing responses during memory and numerical processing[J].Proceedings of the National Academy of Sciences of the United States of America,2012,109(38):15514-15519. [CrossRef]
- Buckner,R.L.,Snyder,A.Z.,Shannon,B.J.,LaRossa,G.,Sachs,R.,Fotenos,A.F.,... Mintun,M.A.Molecular,structural,and functional characterization of Alzheimer's disease:Evidence for a relationship between default activity,amyloid and memory[J].Journal of Neuroscience,2005,25(34):7709-7717. [CrossRef] [PubMed]
- Mason, M.F. ,Norton M.I.,Van Horn J.D.,Wegner D.M.,GraftonS.T.,and Macrae C.N.Wan-dering minds:the defaultnetwork and stimulus-independent thought[J].Science,2007,315(5810):393-395. [CrossRef]
- Yeo BT,Krienen FM,Sepulcre J,et al.The organization of the human cerebral cortex es-timated by intrinsic functional connectivity[J].J Neurophysiol,2011,106(3):1125-1165. [CrossRef] [PubMed]
- Andrews-Hanna, JR. The Brain’s Default Network and Its Adaptive Role in Internal.M-entation[J].The Neuroscientist,2012;18(3):251-270. [CrossRef] [PubMed]
- Peters,SK.,Dunlop,K.,Downar,J.Cortico-Striatal-Thalamic Loop Circuits of the Salience Network:A Central Pathway in Psychiatric Disease and Treatment.Frontiers in Systems Neuroscience.2016,10:104. [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model.Trends Cogn Sci.2011,15(10):483-506. [CrossRef] [PubMed]
- Montos,Palvas,Voipio J,et al.Very slow EEG fluctuations predict the dynamics of stim-ulus detection and oscillation amplitudes in humans[J].Journal of Neuroscience,2008,28(33):8268-8272. [CrossRef]
- Fox MD,Raichile ME.Spontaneous fluctuations in brain activity observed with functio-nal magneticresonance imaging[J].Nature Reviews Neuroscience,2007,8(9):700-711. [CrossRef]
- Zang,Y.F.,He,Y.,Zhu,C.Z.,Cao,Q.J.,Sui,M.Q.,Liang,M.,... Wang,Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI[J].Brain and Development,2007,29(2):83-91. [CrossRef]
- Zang,Y.F.,Jiang,T.Z.,Lu,Y.L.,He,Y.,& Tian,L.X.Regional homogeneity approach to fMRI data analysis. Neuroimage[J],2004,22(1):394-400. [CrossRef]
- Long,X.Y.,Zuo,X.N.,Kiviniemi,V.,Yang,Y.H.,Zou,Q.H.,Zhu,C.Z.,...Zang,Y.F.Defaultmode n-etwork as revealed with multiple methods for resting-state functional MRI analysis[J]. Journal of Neuroscience Methods,2008,171(2):349-355. [CrossRef]
- Zou,Q.H.,Zhu,C.Z.,Yang,Y.H.,Zuo,X.N.,Long,X.Y.,Cao,Q.J.,...Zang,Y.F.An improved appr-oach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI:fractional ALFF[J].Journal of Neuroscience Methods,2008,172(1):137-141. [CrossRef]
- Supekar,K.,Uddin,L.Q.,Prater,K.,Amin,H.,Greicius,M.D., & Menon,V..Developmentof fun-ctional and structural connectivity within the default mode network in young children[J].Neuroimage,2010,52(1):290-301. [CrossRef]
- Meunier D,Achard S,Morcom A,et al.Age-related changesin modular organization of human brain functional net-works[J].Neuroimage,2009,44(3):715-723. [CrossRef]
- Fransson,P.,& Marrelec,G.The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network:Evidence from a partial correlation network analysis[J].N-euroimage,2008,42(3):1178-1184. [CrossRef]
- Fair,D.A.,Cohen,A.L.,Dosenbach,N.U.,Church,J.A.,Miezin,F.M.,Barch,D.M.,...Schlaggar,B.L..The maturing architecture of he brain's default network[J].Proceedings of the NationalAcademy of Sciences of he United States of America,2008,105(10):4028-4032. [CrossRef]
- Gao,W.,Zhu,H.T.,Giovanello,K.S.,Smith,J.K.,Shen,D.G.,Gilmore,J.H.,&Lin,W.L..Evidenceo-n the emergence of the brain's default network from 2-week-old to 2-year-old healthypediatric subjects[J].Proceedingsof the National Academy of Sciences of the United St-ates of America,2009,106(16):6790-6795. [CrossRef]
- Boly,M.,Tshibanda,L.,Vanhaudenhuyse,A.,Noirhomme,Q.,Schnakers,C.,Ledoux,D.,...Laurey-s,S..Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient[J].Human Brain Mapping,2009,30(8):2393-2400. [CrossRef] [PubMed]
- Thomason,M.E.,Chang,C.E.,Glover,G.H.,Gabrieli,J.D.,Greicius,M.D.,& Gotlib,I.H.De-faultmode function and task-induced deactivation have overlapping brain substrates in chil-dren[J].Neuroimage,2008,41(4):1493-1503. [CrossRef]
- Supekar,K.,Uddin,L.Q.,Prater,K.,Amin,H.,Greicius,M.D.,& Menon,V.Development offunct-ional and structural connectivity within the default mode network in young children[J].Neuroimage,2010,52(1):290-301. [CrossRef]
- Marsh,R.,Zhu,H.,Schultz,R.T.,Quackenbush,G.,Royal,J.,Skudlarski,P.,& Peterson,B.S..A.de-velopmental fMRI study of self-regulatory control[J].Human Brain Mapping,2006,27(11):848-863. [CrossRef]
- Damoiseaux JS,Beckmann CF,Arigita EJ,et al.Reduced rest-ing-state brain activity in t-he "default network" in normal aging[J].Cereb Cortex,2008,18(8):1856-1864. [CrossRef]
- Tartaglia MC,Rosen HJ,Miller BL.Neuroimaging in Dementia[M].Berlin,Heidelber-g:Spr-inger,2011.
- Wu JT,Wu HZ,Yan CG,et al.Aging-related changes in thedefault mode network and its anti-correlated networks:a rest-ing-state fMRI study[J].Neurosci Lett,2011,504(1):6267. [CrossRef]
- Shi Qingli,Bi Yanchao,Chen Weikang,et al.Resting default network study on mild cog-nitive impairment in cerebral white matter osteoporosis[J].Chinese Rehabilitation Theo-ry and Practice,2014,20(12):1133-1139.
- Greicius,M.D.,Srivastava,G.,Reiss,A.L.,& Menon,V..Default mode network activitydistinguishes Alzheimer's disease from healthy aging:Evidence from functional MRI[J].Proceedings of the National Academy of Sciences of the United States of America,2004,101(13):4637-4642. [CrossRef]
- Greicius,M.Resting-state functional connectivity in neuropsychiatric disorders[J].Current Opinion in Neurology,2008,21(4):424-430. [CrossRef]
- Broyd,Samantha J.,et al.Default-mode brain dysfunction in mental disorders:a systemat-ic review[J].Neuroscience & biobehavioral reviews,2009,33(3):279-296. [CrossRef]
- Tian L,Jiang T,Wang Y,et al. Altered resting-statefunctional connectivity patterns of an teriorcingulate cortex inadolescents with attention deficit hyperactivity disorder. Neurosci Lett 2006, 400, 39-43. [CrossRef]
- Sun L,Cao Q,Long X,et al.Abnormal functional connectivity between the anterior cingulate and the default mode network in drugnaive boys with attention deficit hyperacti-vity disorder[J].Psychiatry Res,2012,201(2):120-127. [CrossRef] [PubMed]
- Uddin LQ,Kelly AMC,Biswal BB,et al.a Network homogeneity reveals decreased inte-grity of default-mode network in ADHD[J].J Neurosci Methods,2008,169:249-254. [CrossRef] [PubMed]
- Greicius MD,Flores BH,Menon V,et al.Resting-state functional connectivity in major.d-epression:abnormally increased contributions from subgenual cingulate cortex and thala-nlu8[J].Biol Psychiatry,2007,62(5):429-437. [CrossRef] [PubMed]
- Wichers, M. ,Geschwind N.,Van O.J.and Peeters F.Scarsin depression is a conceptual.shi-ft necessary to solve the puzzle,Psychological Medicine,2010,40(3):359-365. [CrossRef] [PubMed]
- Zhang, B. ,Li S.,Zhuo C.,Li M.,Safron A.,Genz A.,Qin W.,Yu C.,and Walter M.Altered task specific deactivation in the default mode network depends on valence in patients with major depressive disorder[J].Journal of Affective Disorders,2016,207:377-383. [CrossRef] [PubMed]
- Greicius,M.D.,Flores,B.H.,Menon,V.,Glover,G.H.,Solvason,H.B.,Kenna,H.,...Schatzbe-rg,A.F.Resting-state functional connectivity in major depression:Abnormally increased contri-butions from subgenual cingulate cortex and thalamus[J].Biological Psychiatry,2007,6.2(5):429-437. [CrossRef]
- Sun Jun,Liu Hanqiu,Sun Huaping,et al.Resting fMRI study of first-episode depression patients before and after treatment [J].Chinese Journal of Medical Computer Imaging, 2011,17(3):212-216.
- Zhu Xueling,Wang Xiang,Xiao Jing.A study on the resting default network of firsteveruntreated depression [J].Chinese Journal of Clinical Psychology,2011,34(2):146-148.
- Kennedy DP,Courchesne E.The intrinsic functional organi-sation of the brain is altered in autism[J].Neuroimage,2008,39(4):1877-1885. [CrossRef] [PubMed]
- Washington,S.D.,Gordon,E.M.,Brar,J.,Warburton,S.,Sawyer,A.T.,Wolfe,A.,...Vanmeter,J.W.Dysmaturation of the default mode network in autism[J].Human Brain Mapping,2014,35(4):1284-1296. [CrossRef]
- Funakoshi, Y. ,Harada M.,Otsuka H.,Mori K.,Ito H.,and Iwanaga T.Default mode netwo-rk abnormalities in children with autism spectrum disorder detected by restingstate fu-nctional magnetic resonance imaging[J].J.Med.Invest.,2016,63(3-4):204-208. [CrossRef]
- Shukla,D.K.,Keehn,B.,& Müller,R.A.Regional homogeneity of fMRI time series inautis-m spectrum disorders[J].Neuroscience Letters,2010,476(1):46–51. [CrossRef]
- Matthew Lieberman, Jia Yongmin.Social Nature:Three Driving Forces of Human Soci-alism[M].Hangzhou:Zhejiang People's Publishing House,2016:100.
-
https://www.themuse.com/advice/the-rule-of-52-and-17-its-random-but-it-ups-your-productivity.
- Nittono,Hiroshi,et al."The power of kawaii:Viewing cute images promotes a ca-reful behavior and narrows attentional focus."PloS one 7.9(2012):e46362. [CrossRef]
-
https://qz.com/267823/the-perfect-recipe-for-productivity-rest-17-minutes-every-52/.
-
http://news.cornell.edu/stories/1999/09/onscreen-break-reminder-boosts-productivity.
- Marlene Bönstrup,Iñaki Iturrate,Ryan Thompson,Gabriel Cruciani,Nitzan Censor, Leonardo G.Cohen.A Rapid Form of Offline Consolidation in Skill Learning.Current Biology,2019.
- Josh Witzkin.The Way to Learning [M].Translated by Su Hongyan and Xie Ji-ngxiu.Beijing:China Youth Publishing House,2016:138.
- Eichenlaub,J.B.,Jarosiewicz,B.,Saab, J.,Franco,B.,Kelemen,J.,Halgren,E.,...& Cash,S.S.Re-play of Learned Neural Firing Sequences during Rest in Human Motor Cortex. 2020,CellReports,31(5),107581. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).