Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study areas
2.2. Sampling
2.3. Toxin analyses
2.4. Statistical analysis
3. Results
3.1. Mycotoxin levels in the liver of fallow deer hinds and foetuses
3.2. Spatial patterns of mycotoxin concentrations in the hinds and foetuses
3.3. Comparison of the mycotoxin levels between fallow deer hinds and foetuses
3.4. Difference in mycotoxin levels among the three age groups of foetuses
3.5. Relationships between the mycotoxin level of the hinds and their foetuses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall'Asta, C.; Dellafiora, L. Co-Occurrence and Combinatory Effects of Alternaria Mycotoxins and other Xenobiotics of Food Origin: Current Scenario and Future Perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef]
- Czéh, Á.; Mézes, M.; Mandy, F.; Szőke, Z.; Nagyéri, G.; Laufer, N.; Kőszegi, B.; Koczka, T.; Kunsági-Máté, S.; Lustyik, G. Flow cytometry based rapid duplexed immunoassay for fusarium mycotoxins. Cytometry A 2017, 1, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Czéh, Á.; Mandy, F.; Feher-Toth, S.; Torok, L.; Mike, Z.; Koszegi, B.; Lustyik, G. A flow cytometry based competitive fluorescent microsphere immunoassay (CFIA) system for detecting up to six mycotoxins. J Immunol. Methods. 2012, 384, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.T.; Gao, Y.J.; Zhang, W.J.; Bi, T.C.; Wang, X.; Ma, C.X.; Rong, R. Development a multi-immunoaffinity column LC-MS-MS method for comprehensive investigation of mycotoxins contamination and co-occurrence in traditional Chinese medicinal materials. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2021, 1178: 122730.
- Szőke, Z.; Babarczi, B.; Mézes, M.; Lakatos, I.; Poór, M.; Fliszár-Nyúl, E.; Oldal, M.; Czéh, Á.; Bodó, K.; Nagyéri, G.; Ferenczi, S. Analysis and Comparison of Rapid Methods for the Determination of Ochratoxin a Levels in Organs and Body Fluids Obtained from Exposed Mice. Toxins 2022, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Rossi, V.; Giorni, P.; Pietri, A.; Gualla, A.; Van der Fels-Klerx, H. J.; Booij, C.J.H.; Moretti, A.; Logrieco, A.; Miglietta, F.; Toscani, F.; Miraglia, M.; De Santis, B.; Brera, C. Modelling, predicting and mapping the emergence of aflatoxins in cereals in the EU due to climate change. EFSA support. publ. 2012, 9(1), 223E. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Milić, M., Jakšić, D., Šegvić Klarić, M., Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment Changes, Aflatoxins, and Health Issues, a Review. Int. J. Environ. Res. Public Health 2020, 17, 7850. [CrossRef]
- Russell, R.; Paterson, M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Farkas, J.; Bencze, J.; Szeitzné Szabó, M.; Kovács, M.; Varga, J.; Varga, L. Kárpát-medence éghajlat változásának kihatása élelmiszer-biztonságunkra. Magyar Tudomány 2013, 174(2), 147–158.
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am J Trop Med Hyg. 2017, 96(4), 770–776. [Google Scholar] [CrossRef] [PubMed]
- Lamplugh, S.M.; Hendrickse, R.G.; Apeagyei, F.; Mwanmut, D.D. Aflatoxins in breast milk, neonatal cord blood, and serum of pregnant women. Br Med J (Clin Res Ed). 1988, 296(6627), 968. [CrossRef]
- Abdulrazzaq, Y.M.; Osman, N.; Ibrahim, A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr. 2002, 22(1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Collinson, A.C.; Cheung, Y.B.; Gong, Y.; Hall, A.J.; Prentice, A.M; Wild, C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007, 36(5), 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.D.; Egner, P.A.; Schulze, K.J.; Wu, L.S.; Merrill, R.; Mehra, S.; Shamim, A.A.; Ali, H.; Shaikh, S.; Gernand, A.; Khatry, S.K.; LeClerq, S.C.; West, K.P.Jr.; Christian, P. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B₁-lysine albumin biomarkers. Food Chem Toxicol. 2014, 74, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J Toxicol. Environ Health B Crit Rev. 2000, 3(2), 109–143. [Google Scholar] [CrossRef] [PubMed]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Kovalsky Paris, M.P.; Schweiger, W., Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: a new masked mycotoxin. J Agric Food Chem. 2014, 62(5), 1181–1189. [CrossRef]
- Kriszt, R.; Winkler, Z.; Polyák, Á.; Kuti, D.; Molnár, C.; Hrabovszky, E., Kalló, I.; Szőke, Z., Ferenczi, S., Kovács, K.J. Xenoestrogens Ethinyl Estradiol and Zearalenone Cause Precocious Puberty in Female Rats via Central Kisspeptin Signaling. Endocrinology 2015, 156(11), 3996–4007. [CrossRef]
- Wang, H.; Zhao, X.; Ni, C.; Dai, Y.; Guo, Y. Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol Med Rep. 2018, 17(6), 7797–7806. [Google Scholar] [CrossRef] [PubMed]
- Afriyie-Gyawu, E.; Wiles, M.C.; Huebner, H.J.; Richardson, M.B.; Fickey, C.; Phillips, T.D. Prevention of Zearalenone-Induced Hyperestrogenism in Prepubertal Mice. J. Toxicol. Environ. Health Part A. 2005, 68, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit Rev Food Sci Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Dänicke, S.; Brüssow, K.P.; Valenta, H.; Ueberschär, K.H.; Tiemann, U. On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35-70 of gestation. Toxicol Lett. 2007, 171(1-2), 38–49. [CrossRef]
- Tiemann, U.; Brüssow, K.P.; Danicke, S.; Vanselow, J. Feeding of pregnant sows with mycotoxin-contaminated diets and their non-effect on foetal and maternal hepatic transcription of genes of the insulin-like growth factor system. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008, 25(11), 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.; Collins, R.L.; Sprando, T.N.; Black, N.; Olejnik, R.M.; Eppley, F.A.; Hines, et al., D.I. Ruggles Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem Toxicol., 2006, 44, 747–757. [CrossRef]
- Yu, M.; Chen, L.; Peng, Z.; Nüssler, A.K.; Wu, Q.; Liu, L.; Yang, W. Mechanism of deoxynivalenol effects on the reproductive system and fetus malformation: Current status and future challenges. Toxicol In Vitro. 2017, 41, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, P.; Cui, Y.; Xiao, B.; Liu, M.; Song, M.; Huang, W.; Li, Y. Review of the Reproductive Toxicity of T-2 Toxin. J Agric Food Chem. 2020, 68(3), 727–734. [Google Scholar] [CrossRef] [PubMed]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol. 2005, 73(7), 487–97. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Guitart, R.; Croubels, S.; Caloni, F.; Sachana, M.; Davanzo, F.; Vandenbroucke, V.; Berny, P. Animal poisoning in Europe. Part 1: Farm livestock and poultry. Vet. J. 2009, 183(3), 249–254. [CrossRef]
- Minervini, F.; Giannoccaro, A.; Fornelli, F.; Dell'Aquila, M.E.; Minoia, P.; Visconti, A. Influence of mycotoxin zearalenone and its derivatives (alpha and beta zearalenol) on apoptosis and proliferation of cultured granulosa cells from equine ovaries. Reprod Biol Endocrinol. 2006, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Mostrom, M.S.; Jacobsen, B.J. Ruminant Mycotoxicosis: An Update. Vet Clin North Am Food Anim Pract. 2020, 36(3), 745–774. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Khiaosa-ard, R.; Schmidt, M.; Bartl, E.-M.; Kehrer, J.; Nagl, V.; Faas, J.; Sulyok, M.; Krska, R.; Zebeli, Q. Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors. Toxins 2022, 14(7), 493. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.J.; Cendoya, E.; Zachetti, V.G.L.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Torres, A.M.; Chulze, S.N.; Ramirez, M.L. Mycotoxin profile of Fusarium armeniacum isolated from natural grasses intended for cattle feed. World Mycotoxin J. 2015, 8(4), 451–457. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Nagl, V.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Mixtures of mycotoxins, phytoestrogens and pesticides co-occurring in wet spent brewery grains (BSG) intended for dairy cattle feeding in Austria. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2022, 39(11), 1855–1877. [Google Scholar] [CrossRef] [PubMed]
- Csányi, S.; Márton, M.; Bőti, Sz; Schally, G. Vadgazdálkodási Adattár - 2021/2022. vadászati év. 2022, Országos Vadgazdálkodási Adattár, Gödöllő, 70 pp. ISSN 1417-4308.
- Sándor, Gy.; László, R.; Náhlik, A. Determination of time of conception of fallow deer in a Hungarian free range habitat. Folia Zoologica 2014, 63(2):122-126. 2014. [CrossRef]
- Ács, K. and Lanszky, J. Pre-, postnatal growth and maternal condition in a free ranging fallow deer population. Folia Zoologica 2017, 66(1) : 72-78. [CrossRef]
- Zar, J.H. Biostatistical analysis. 2010 Prentice-Hall, New Jersey. 944 pp.
- Wood, S.N. Fast stable direct fitting and smoothness selection for generalized additive models. J. Roy. Stat. Soc. Ser. B-Stat. Met. 2008, 70, 495–518. [CrossRef]
- Wood, S.N.; Augustin, N.H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 2002, 157(2–3), 157–177. [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2023 Vienna, Austria.
- Sokal, R.R.; Rohlf, F.J. Biomety: the Principles and Practice of Statistics in Biological Research. 1995 3rd ed. New York, USA: W. H. Freeman and Company.
- Toutounchi, N.S.; Braber, S.; Land, B.V.; Thijssen, S.; Garssen, J.; Folkerts, G. Hogenkamp A. Deoxynivalenol exposure during pregnancy has adverse effects on placental structure and immunity in mice model. Reprod Toxicol. 2022, 112, 109–118. [CrossRef]
- Huston, J.E.; Rector, B.S.; Ellis, W.C. and Allen, M.L. Dynamics of digestion in cattle, sheep, goats and deer. Journal of Animal Science 1986, 62(1), 208–215. [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Shackelford, M.E.; Laborde, J.B.; Hansen, D.K.; Eppley, R.M.; Trucksess, M.W.; Howard, P.C.; Bryant, M.A.; Ruggles, D.I.; Olejnik, N.; Rorie, J.I. Effects of fumonisin B1 in pregnant rats. Part 2. Food Chem Toxicol. 1998, 36(8), 673–685. [Google Scholar] [CrossRef] [PubMed]
- Obremski, K.; Zalewski, K.; Gajęcka, M.; Giżejewski, Z.; Zielonka, Ł.; Gajęcki, M.; Nitkiewicz, B. Zearalenone intoxication of game animals. Polish Journal of Natural Science 2006, 21. 1099-1106.
- Thompson, C.; Henke, S.E. Effect of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J. Wild. Dis. 2000, 36, 172–179. [Google Scholar] [CrossRef]
- Mesterházy Szieberth, D.; Szabó, B.; Berényi, A.; Tóth, B. Mycotoxin contamination of maize (Zea mays L.) samples in Hungary, 2012–2017. Cereal Research Communications 2022, 50(4), 1065–1073. [CrossRef]
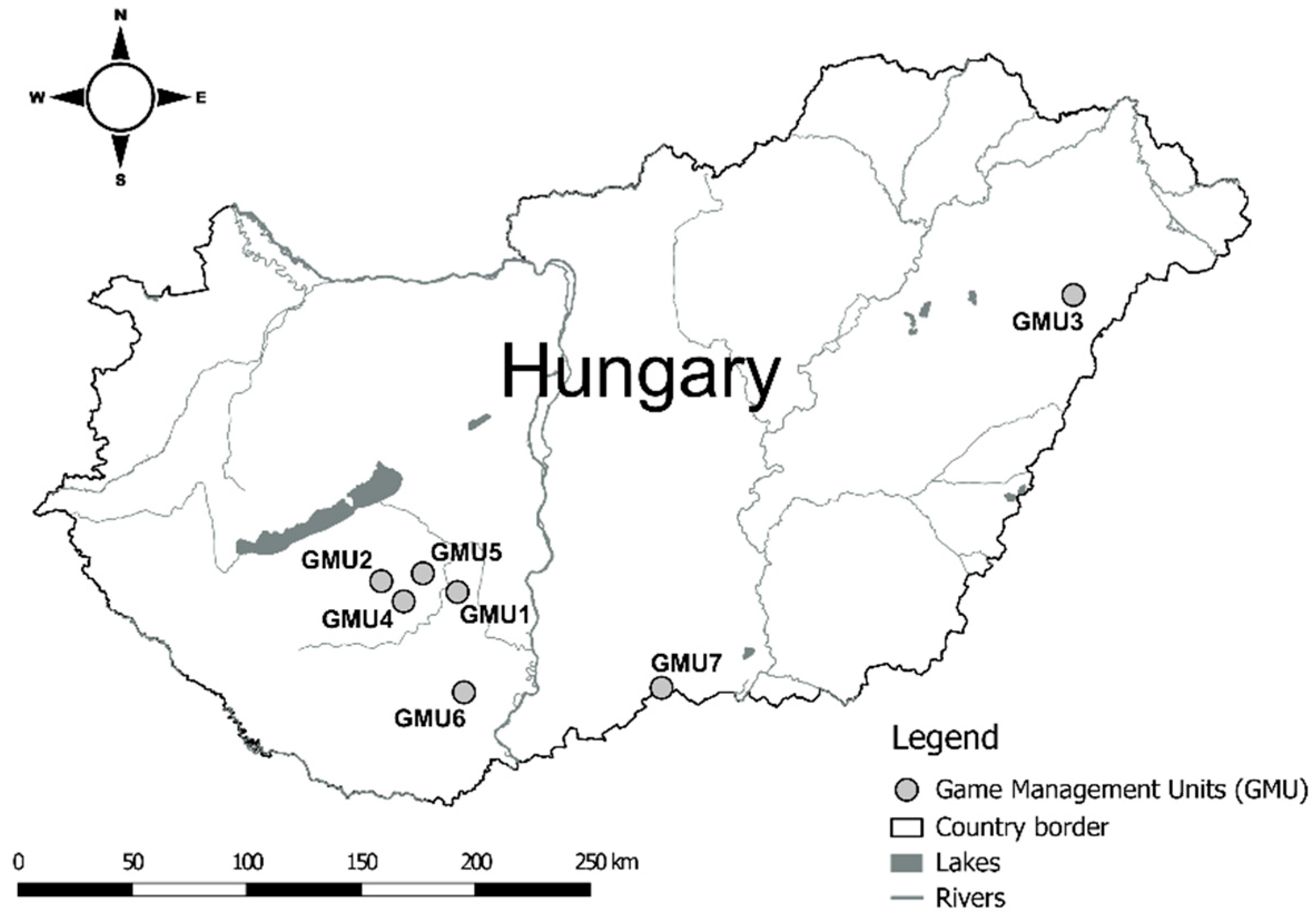
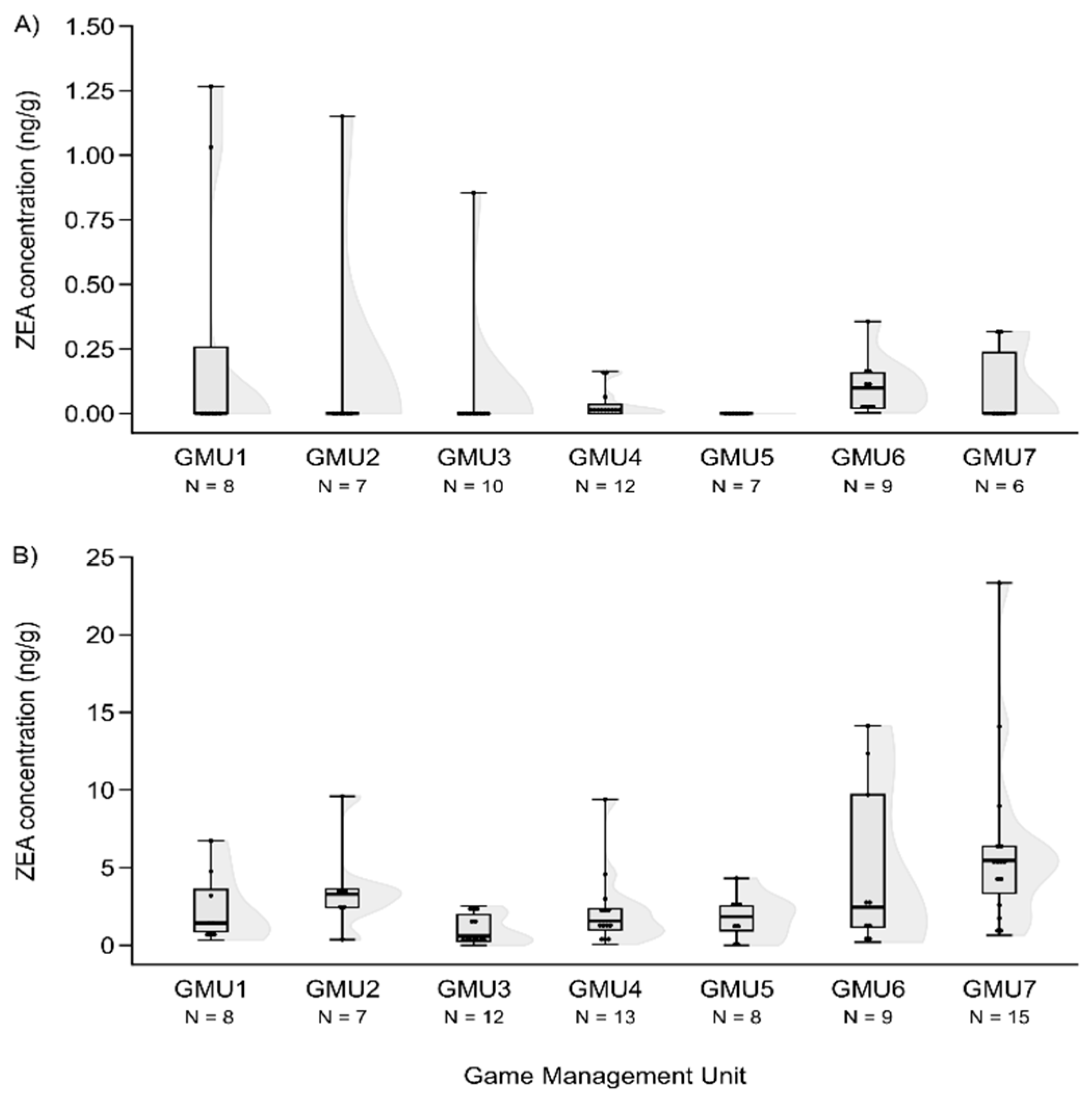
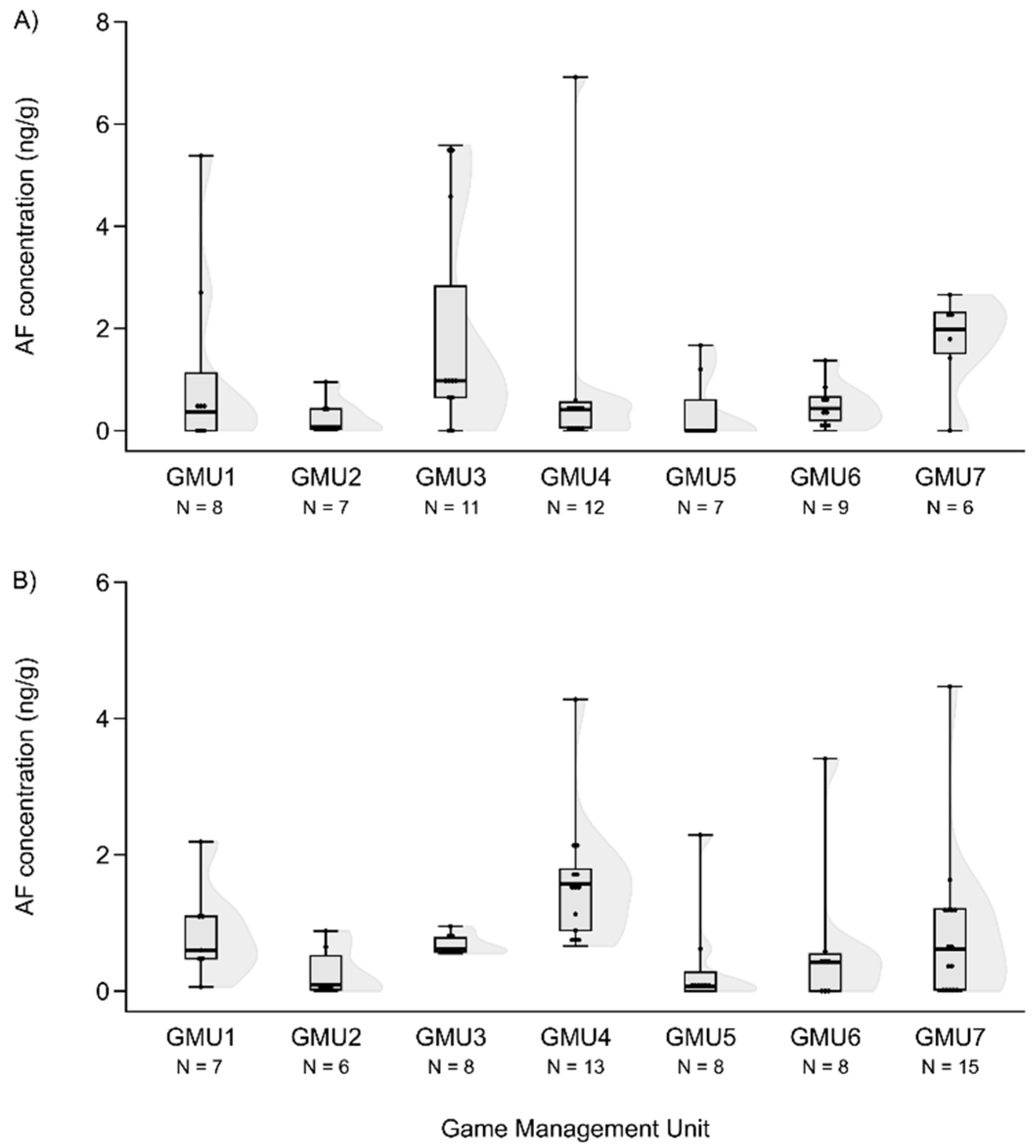
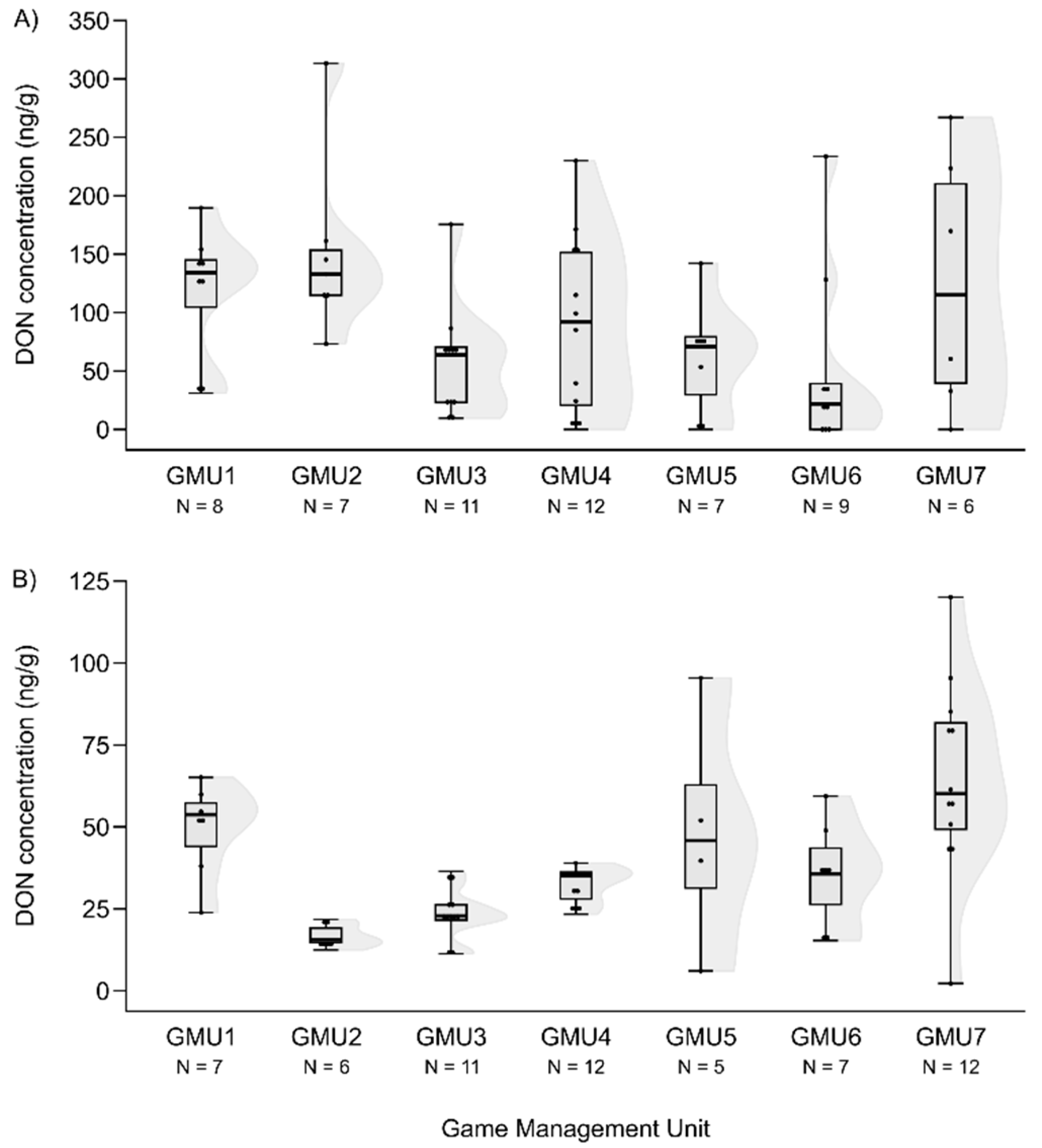
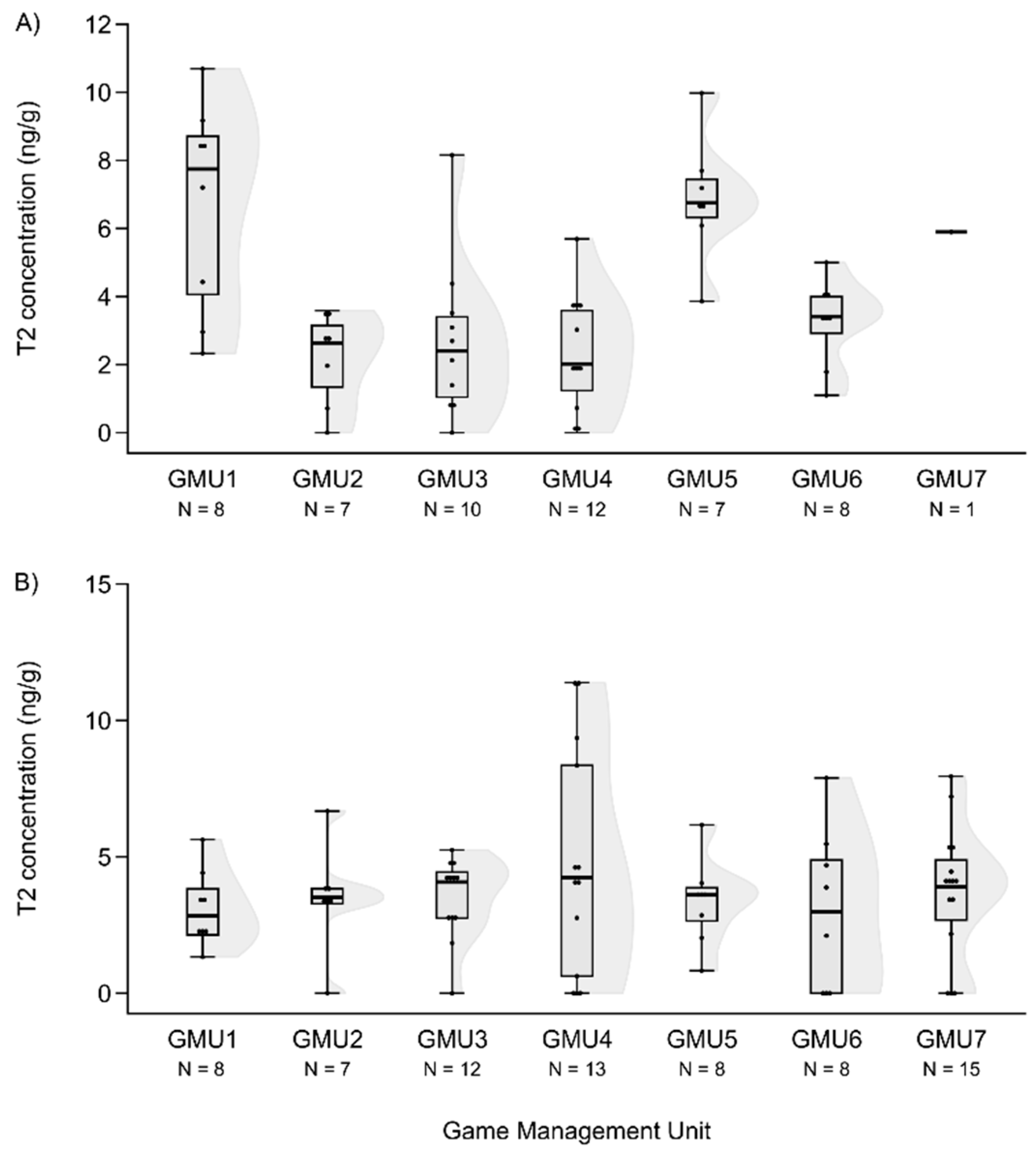
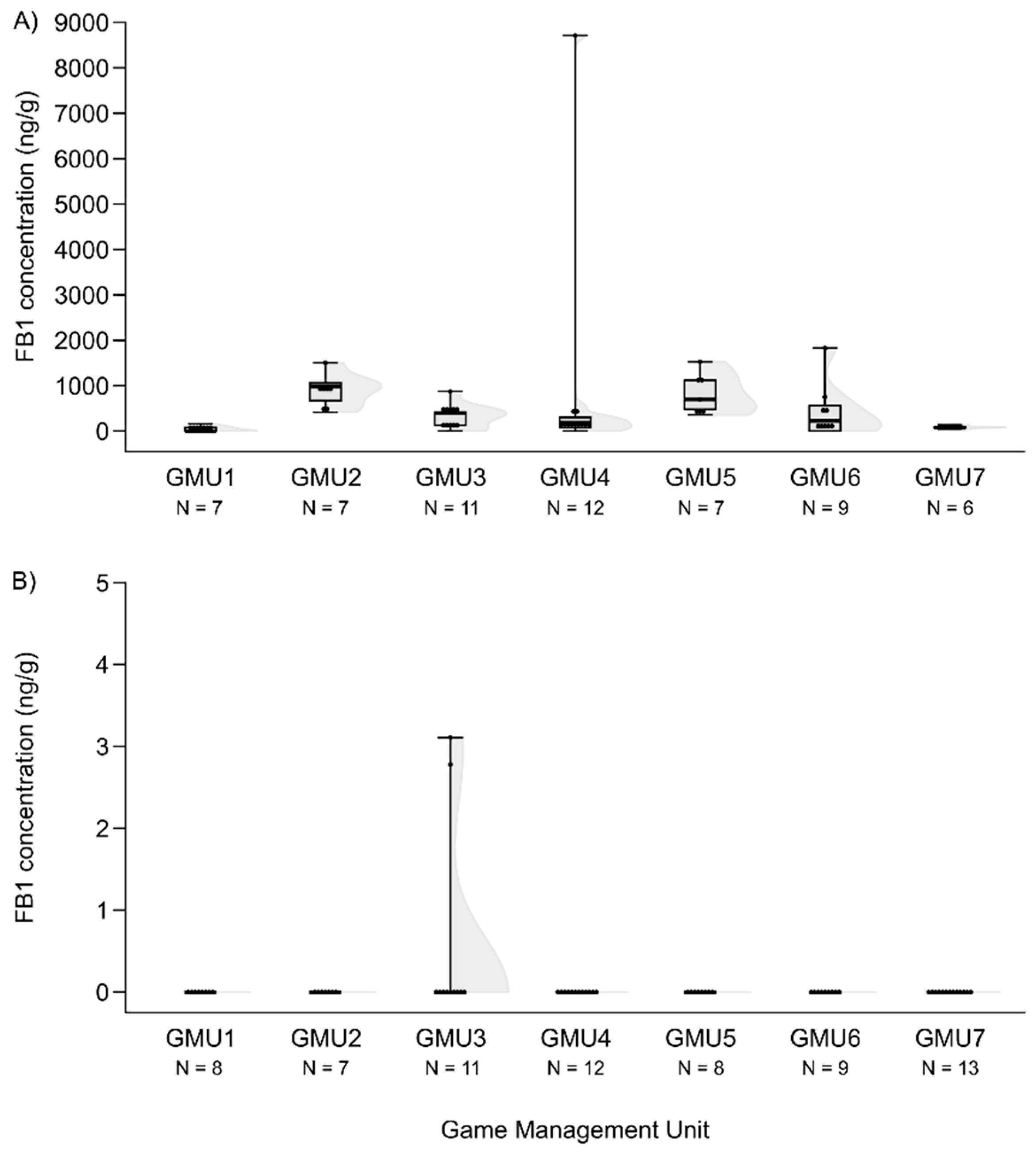
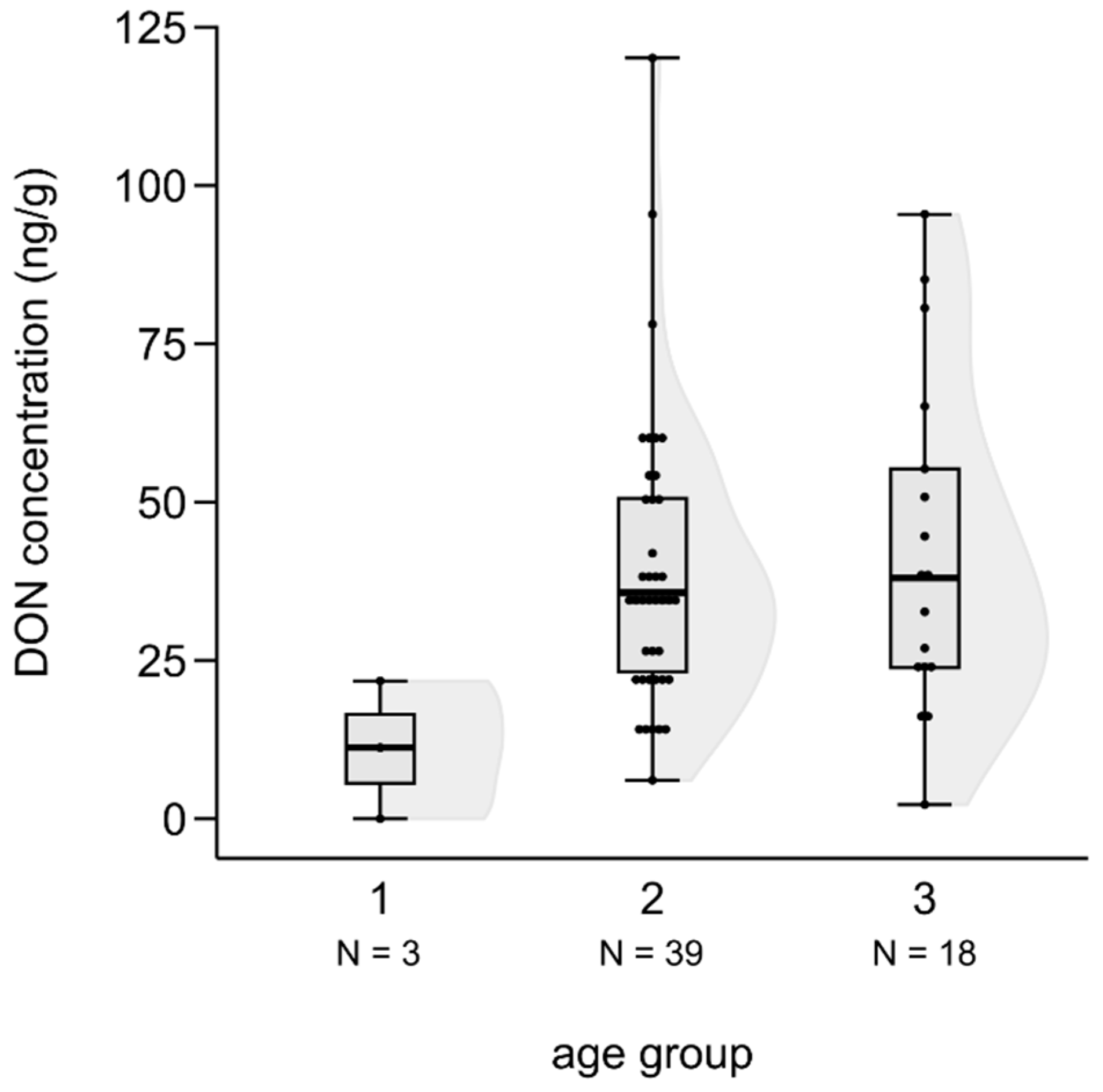
| Mycotoxin | Valid N | Median | Minimum | Maximum | number of “0” concentration |
|---|---|---|---|---|---|
| AF | 60 | 0.475 | 0 | 6.918 | 18 |
| ZEA | 59 | 0.000 | 0 | 1.267 | 35 |
| DON | 60 | 72.740 | 0 | 313.240 | 6 |
| T2-toxin | 53 | 3.426 | 0 | 16.850 | 2 |
| FB1 | 59 | 256.987 | 0 | 8711.522 | 10 |
| Mycotoxin | Valid N | Median | Minimum | Maximum | number of “0” concentration |
|---|---|---|---|---|---|
| AF | 65 | 0.610 | 0 | 4.470 | 12 |
| ZEA | 72 | 2.217 | 0 | 23.343 | 3 |
| DON | 60 | 35.685 | 0 | 120.120 | 1 |
| T2-toxin | 71 | 3.810 | 0 | 11.392 | 11 |
| FB1 | 68 | 0.000 | 0 | 3.110 | 66 |
| Mycotoxin | ZEA | AF | DON | T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling area | U | Z | U | Z | U | Z | U | Z | |||
| GMU1 | 6 | 2.678** | 20 | n.s. | 11 | n.s. | 10 | -2.258* | |||
| GMU2 | — | — | 20 | n.s. | 0 | -2.929** | 11.5 | n.s. | |||
| GMU3 | 16 | 2.868** | 24 | n.s. | 37 | n.s. | 38.5 | n.s. | |||
| GMU4 | 3 | 4.052*** | 13 | 3.508*** | 47 | n.s. | 63.5 | n.s. | |||
| GMU5 | 4 | 2.720** | 24 | n.s. | 12.5 | n.s. | 3 | -2.835** | |||
| GMU6 | 1 | 3.444*** | 30.5 | n.s. | 24 | n.s. | 30 | n.s. | |||
| GMU7 | 0 | 3.464*** | 19.5 | n.s. | 29 | n.s. | — | — | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





