Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
3. Results & Discussion
3.1. Approximate phisico-chemical composition
| Parameters | Results, g per 100 g of sample |
|---|---|
| Carbohydrates | 75.80±0.28 |
| Ash content | 2.31±0.13 |
| Fiber | 21.50±1.84 |
| Protein | 1.27±0.10 |
| Lipids | 0.49±0.12 |
| Nutritional values | 355.65±1.07 |
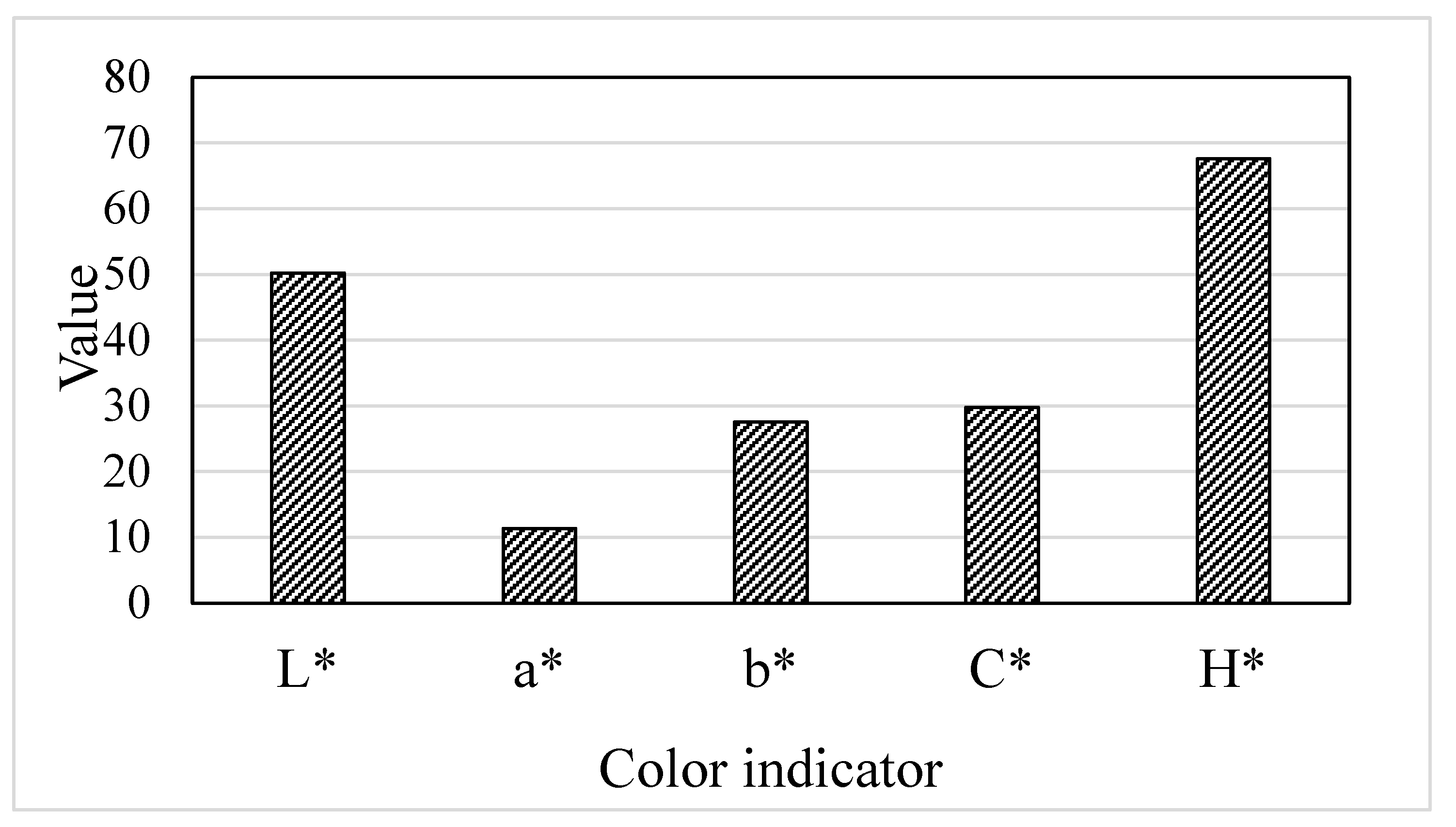
3.2. Color characteristics
3.3. Antioxidant activity of quince powder
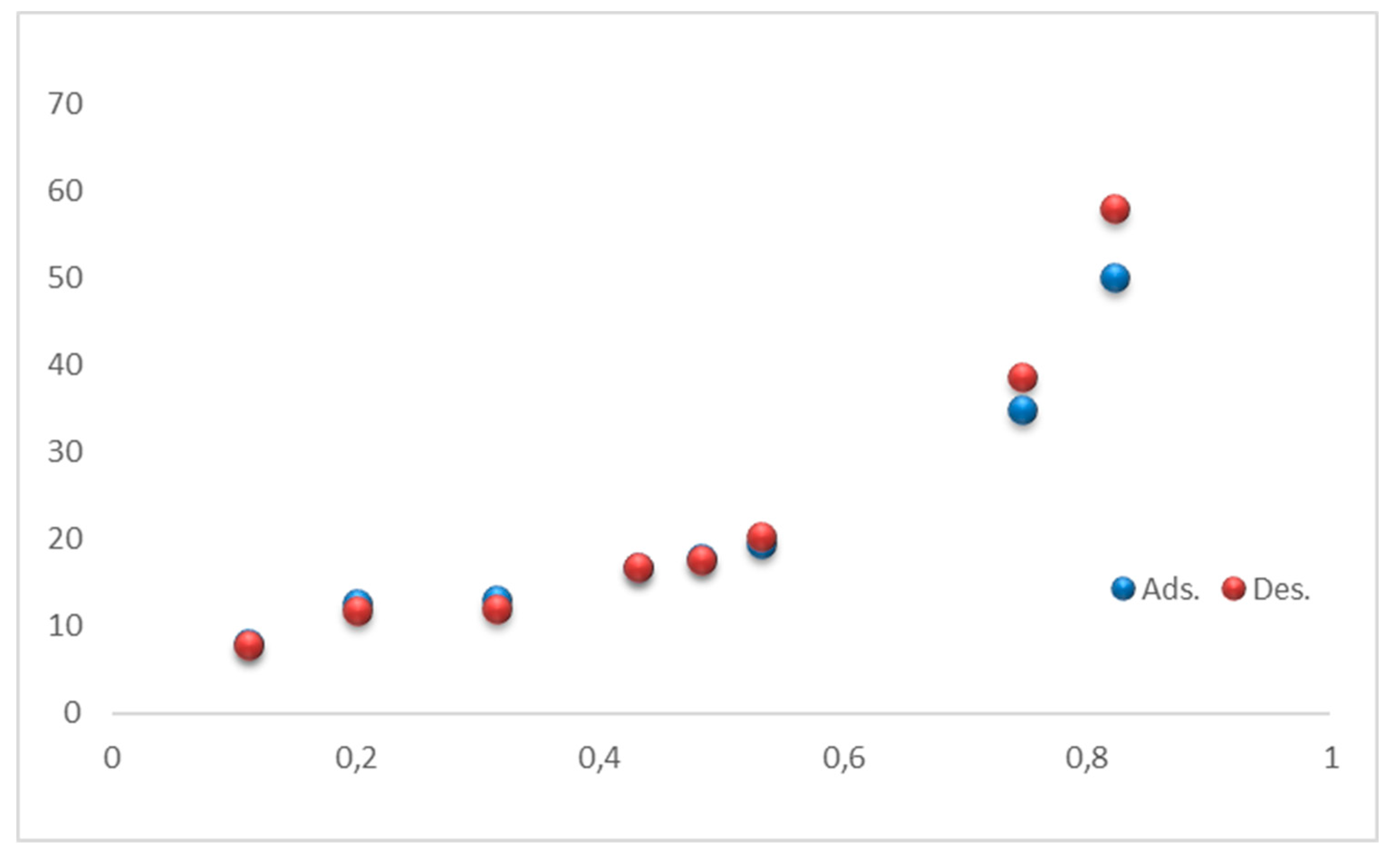
3.4. Sorption characteristics
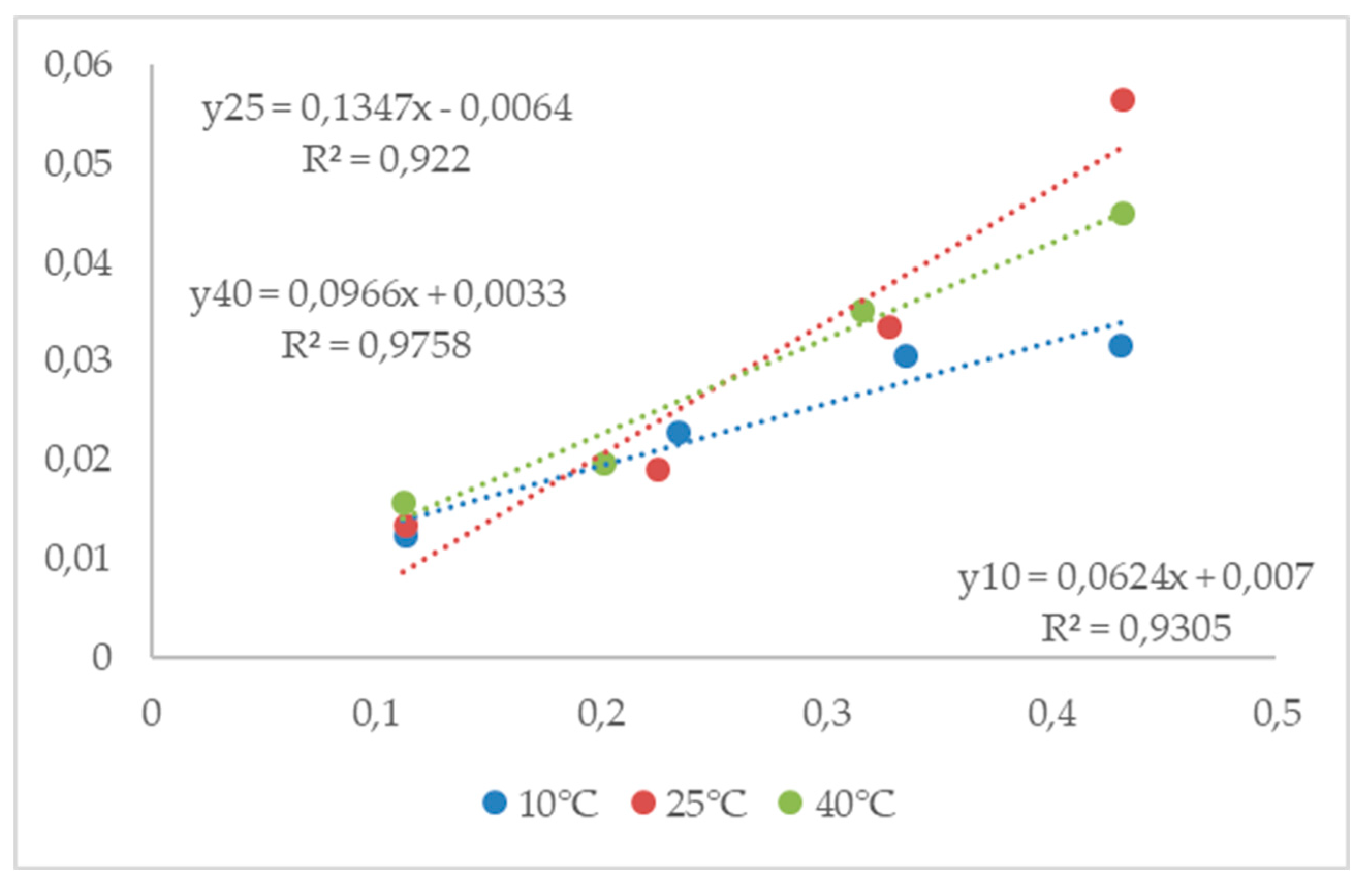
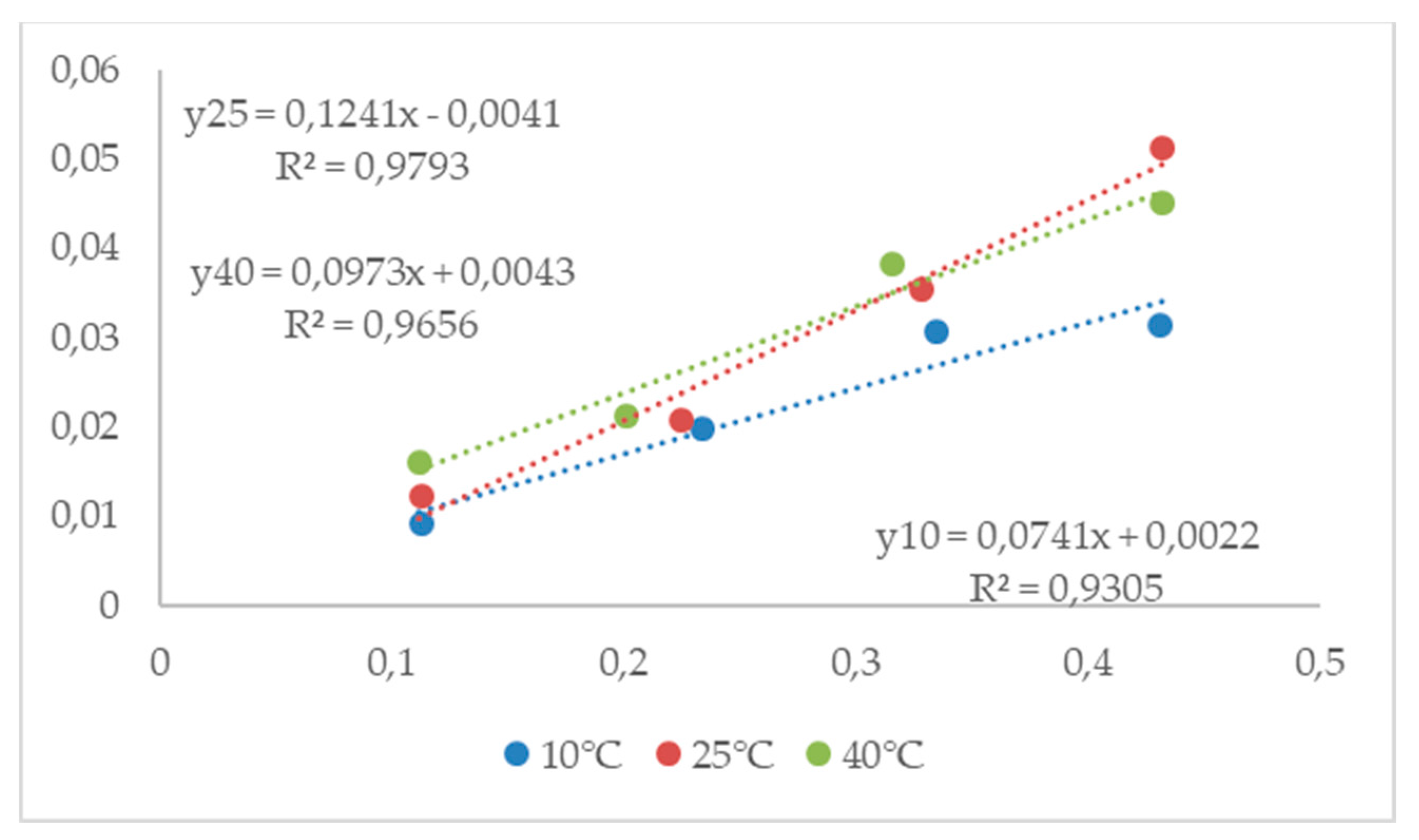
3.4.1. Equilibrium moisture content
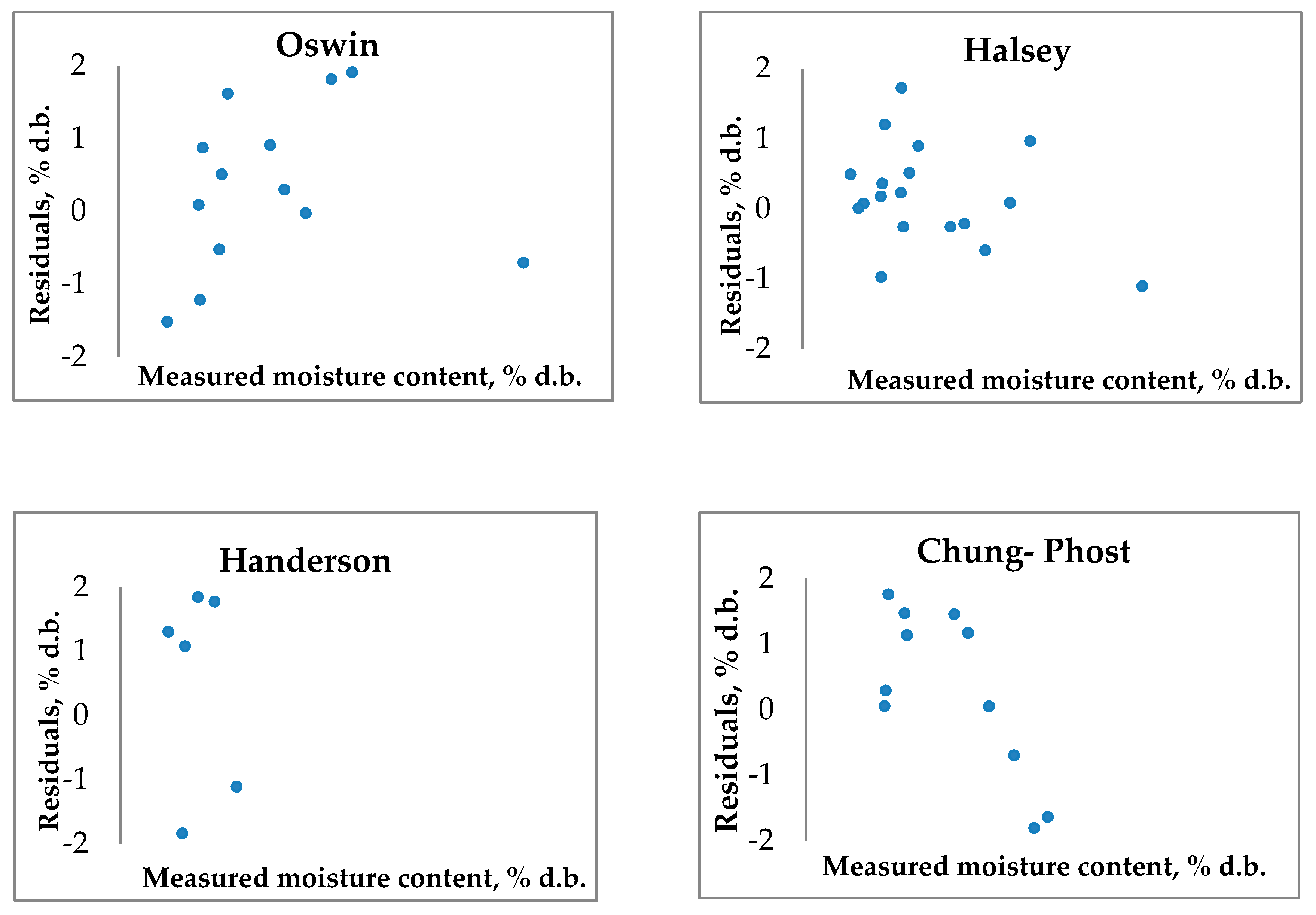
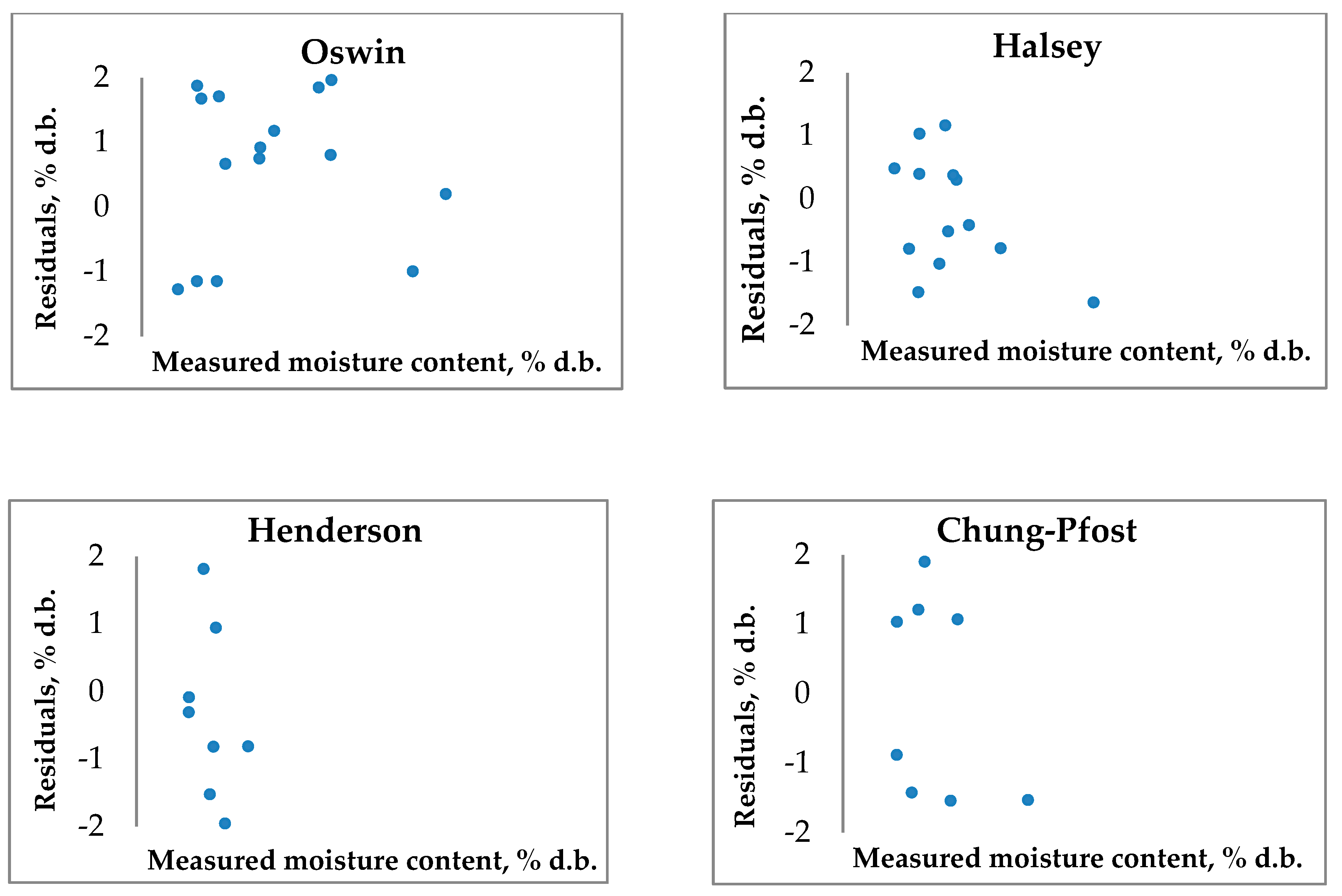
3.4.2. Modified three-parametrical models
3.4.3. Monolayer moisture content
4. Conclusions
References
- Abdollahi, H. A review on history, domestication and germplasm collections of quince (Cydonia oblonga Mill.) in the world. Genetic Resources and Crop Evolution, 2019, 66(5), 1041–1058. [Google Scholar] [CrossRef]
- Rather, G. A., Bhat, M. Y., Sana, S. S., Ali, A., Gul, M. Z., Nanda, A., & Hassan, M. Quince. Antioxidants in fruits: Properties and health benefits, 2020, 397-416. [CrossRef]
- Hegedűs, A., Papp, N., & Stefanovits-Bányai, É. Review of nutritional value and putative health-effects of quince (Cydonia oblonga Mill.) fruit. International Journal of Horticultural Science, 2013, 19(3-4), 29-32. [CrossRef]
- Hussain, S. Z., Naseer, B., Qadri, T., Fatima, T., & Bhat, T. A. Quince (Cydonia oblonga)—Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas: Nutritional and Health Benefits, 2021, (pp. 49-62). Cham: Springer International Publishing. [CrossRef]
- Stojanović, B. T., Mitić, S. S., Stojanović, G. S., Mitić, M. N., Kostić, D. A., Paunović, D. Ɖ., ... & Pavlović, A. N. (2017). Phenolic profiles and metal ions analyses of pulp and peel of fruits and seeds of quince (Cydonia oblonga Mill.). Food Chemistry 2017, 232, 466–475. [CrossRef]
- Essafi-Benkhadir, K., Refai, A., Riahi, I., Fattouch, S., Karoui, H., & Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochemical and biophysical research communications 2012, 418(1), 180–185. [CrossRef] [PubMed]
- Owfi, R. E. Natural products and botanical medicines of Iran. 2020, CRC Press.
- Razna, K. Cyanogenic glycosides-their role and potential in plant food resources. Journal of microbiology, biotechnology and food sciences, 2021, 11(3), e4771–e4771. [Google Scholar] [CrossRef]
- Sato, K., Kobayashi, S., Yamaguchi, M., Sakata, R., Sasaki, Y., Murayama, C., & Kondo, N. Working from home and dietary changes during the COVID-19 pandemic: A longitudinal study of health app (CALO mama) users. Appetite 2021, 165, 105323.
- Ayati, N., Saiyarsarai, P., & Nikfar, S. Short and long term impacts of COVID-19 on the pharmaceutical sector. DARU Journal of Pharmaceutical Sciences, 2020, 28, 799–805. [CrossRef] [PubMed]
- Minich, D. M. A review of the science of colorful, plant-based food and practical strategies for “eating the rainbow”. Journal of Nutrition and Metabolism, 2019. [Google Scholar]
- Blumfield, M., Mayr, H., De Vlieger, N., Abbott, K., Starck, C., Fayet-Moore, F., & Marshall, S. Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules, 2022, 27(13), 4061. [CrossRef]
- AOAC, C. Official methods of analysis of the Association of Analytical Chemists International. Official Methods: Gaithersburg, 2005, MD, USA.
- Ivanov, I., Vrancheva, R., Marchev, A., Petkova, N., Aneva, I., Denev, P., ... & Pavlov, A. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. International Journal of Current Microbiology and Applied Sciences, 2014, 3(2), 296–306.
- Bogoeva, A. L., Durakova, A. G., Pavlov, A. I., Yanakieva, V. B., Vrancheva, R. Z., Bozadzhiev, B. V., & Choroleeva, K. B. Antioxidant activity and storage regime of defatted grape seeds flour. Wine Studies 2017, 6(1). [Google Scholar]
- Bogoeva, A. Sorption characteristics of flour mixtures enriched with grape seeds flour of Bulgarian and French raw materials. Journal of Central European Agriculture, 2020, 21(3), 609–617. [Google Scholar] [CrossRef]
- Durakova, A. Sorption Characteristics of Bulgarian Penny Buns (Boletus Edulis). In E3S Web of Conferences, 2020, (Vol. 180, p. 03008). EDP Sciences. [CrossRef]
- Abboud, K. Y., da Luz, B. B., Dallazen, J. L., de Paula Werner, M. F., Cazarin, C. B. B., Junior, M. R. M., ... & Cordeiro, L. M. Gastroprotective effect of soluble dietary fibres from yellow passion fruit (Passiflora edulis f. flavicarpa) peel against ethanol-induced ulcer in rats. Journal of Functional Foods, 2019, 54, 552-558. [CrossRef]
- European Parliament & Council of the European Union, Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union, 2006, 304, 18–63.
- Murugesan, R., & Orsat, V. Spray drying of elderberry (Sambucus nigra L.) juice to maintain its phenolic content. Drying Technology 2011, 29(14), 1729–1740. [CrossRef]
- Santhalakshmy, S., Bosco, S. J. D., Francis, S., & Sabeena, M. Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technology 2015, 274, 37–43. [CrossRef]
- Salehi, F., Kamran, H. R., & Goharpour, K. Production and evaluation of total phenolics, antioxidant activity, viscosity,color, and sensory attributes of quince tea infusion: Effects of drying method, sonication, and brewing process. Ultrasonics Sonochemistry, 2023, 99, 106591. [CrossRef] [PubMed]
- Costa, R. M., Magalhães, A. S., Pereira, J. A., Andrade, P. B., Valentão, P., Carvalho, M., & Silva, B. M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis). Food and Chemical toxicology, 2009, 47(4), 860–865. [CrossRef]
- Fattouch, S., Caboni, P., Coroneo, V., Tuberoso, C. I., Angioni, A., Dessi, S., ... & Cabras, P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. Journal of Agricultural and Food Chemistry, 2007, 55(3), 963–969. [CrossRef]
- Gheisari, H. R., & Abhari, K. H. Drying method effects on the antioxidant activity of quince (Cydonia oblonga Miller) tea. Acta Scientiarum Polonorum Technologia Alimentaria, 2014, 13(2), 129–134. [Google Scholar] [CrossRef]
- Mir, S. A., Wani, S. M., Wani, T. A., Ahmad, M., Gani, A., Masoodi, F. A., & Nazir, A. Comparative evaluation of the proximate composition and antioxidant properties of processed products of quince (Cydonia oblonga Miller). International Food Research Journal 2016, 23(2).
- Szychowski, P. J., Lech, K., Sendra-Nadal, E., Hernández, F., Figiel, A., Wojdyło, A., & Carbonell-Barrachina, Á. A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food chemistry 2018, 255, 157–164. [CrossRef]
- Al-Zughbi, I., & Krayem, M. Quince fruit Cydonia oblonga Mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: A review. Food Chemistry, 2022, 393, 133362. [Google Scholar] [CrossRef]
- Benahmed Djilali, A., Mehraz, R., Bouacem, K., Benseddik, A., Moualek, I., Nabiev, M., & Benzara, A. Bioactive substances of Cydonia oblonga fruit: Insecticidal effect of tannins on Tribuliumm confusum. International Journal of Fruit Science, 2021, 21(1), 721–731. [Google Scholar] [CrossRef]
- Sut, S., Dall'Acqua, S., Poloniato, G., Maggi, F., & Malagoli, M. Preliminary evaluation of quince (Cydonia oblonga Mill.) fruit as extraction source of antioxidant phytoconstituents for nutraceutical and functional food applications. Journal of the Science of Food and Agriculture 2019, 99(3), 1046–1054. [CrossRef] [PubMed]
- Kapsalis, J.G. Influences of hysteresis and temperature on moisture sorption isotherms. In Water activity, 2017, (pp. 173-213). Routledge.
- Bell, L. N. Moisture effects on food's chemical stability. Water activity in foods: fundamentals and applications, 2020, 227-253. [CrossRef]
- Labuza, T. P., & Altunakar, B. Water activity prediction and moisture sorption isotherms. Water activity in foods: fundamentals and applications, 2020, 161-205. [CrossRef]
- Brunauer, S., Deming, L. S., Deming, W. E., & Teller, E. On a theory of the van der Waals adsorption of gases. Journal of the American Chemical society, 1940, 62(7), 1723–1732. [Google Scholar] [CrossRef]
- Brunauer, S., Emmett, P. H., & Teller, E. Adsorption of gases in multimolecular layers. Journal of the American chemical society, 1938, 60(2), 309–319. [Google Scholar] [CrossRef]
| Method | mMTE/g extract | mMTE/g dry weight |
|---|---|---|
| DPPH | 65.09±6.80 | 45.57±4.76 |
| ABTS | 8.35±0.07 | 5.84±0.05 |
| FRAP | 4.54±0.33 | 3.18±0.23 |
| CUPRAC | 7.94±1.01 | 5.56±0.71 |
| Sel | 10°C | 25°C | 40°C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aw | M* | sd** | aw | M* | sd** | aw | M* | sd** | |
| LiCl | 0.113 | 10.25 | 0.33 | 0.113 | 9.37 | 0.25 | 0.112 | 8.00 | 0.08 |
| CH3COOK | 0.234 | 13.37 | 0.30 | 0.225 | 13.22 | 0.24 | 0.201 | 12.77 | 0.17 |
| MgCl2 | 0.335 | 16.53 | 0.32 | 0.328 | 13.82 | 0.14 | 0.316 | 13.16 | 0.05 |
| K2CO3 | 0.431 | 24.00 | 0.06 | 0.432 | 16.61 | 0.20 | 0.432 | 16.91 | 0.06 |
| MgNO3 | 0.574 | 27.19 | 0.11 | 0.529 | 20.17 | 0.28 | 0.484 | 17.93 | 0.22 |
| NaBr | 0.622 | 30.70 | 0.15 | 0.576 | 24.87 | 0.28 | 0.532 | 19.45 | 0.16 |
| NaCl | 0.757 | 40.54 | 0.08 | 0.753 | 38.29 | 0.33 | 0.747 | 34.89 | 0.13 |
| KCl | 0.868 | 66.38 | 0.17 | 0.843 | 57.16 | 0.15 | 0.823 | 50.15 | 0.17 |
| Sel | 10°C | 25°C | 40°C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aw | M* | sd** | aw | M* | sd** | aw | M* | sd** | |
| LiCl | 0.113 | 13.76 | 0.09 | 0.113 | 10.34 | 0.22 | 0.112 | 7.90 | 0.09 |
| CH3COOK | 0.234 | 15.43 | 0.45 | 0.225 | 12.05 | 0.27 | 0.201 | 11.89 | 0.29 |
| MgCl2 | 0.335 | 16.40 | 0.48 | 0.328 | 13.02 | 0.13 | 0.316 | 12.09 | 0.20 |
| K2CO3 | 0.431 | 24.11 | 0.44 | 0.432 | 18.29 | 0.08 | 0.432 | 16.88 | 0.37 |
| MgNO3 | 0.574 | 25.91 | 1.09 | 0.529 | 19.85 | 0.21 | 0.484 | 17.74 | 0.11 |
| NaBr | 0.622 | 28.92 | 0.13 | 0.576 | 25.70 | 0.24 | 0.532 | 20.39 | 0.21 |
| NaCl | 0.757 | 41.46 | 0.71 | 0.753 | 41.28 | 0.35 | 0.747 | 38.68 | 0.88 |
| KCl | 0.868 | 66.49 | 1.39 | 0.843 | 59.25 | 0.75 | 0.823 | 58.06 | 1.08 |
| Model | А | В | С | Р | SEM |
| Oswin | 24.3597 | -0.1028 | 0,5495 | 11.13 | 2.39 |
| Halsey | 4.19682 | -0.0091 | 1.4246 | 5.79 | 2.03 |
| Henderson | 0.00017 | 2.6988 | 1.6486 | 26.03 | 9.30 |
| Chung-Pfost | 356.522 | 0.0655 | 90.338 | 17.89 | 6.19 |
| Model | А | В | С | Р | SEM |
| Oswin | 22.8885 | -0.0282 | 0.5744 | 14.66 | 4.27 |
| Halsey | 4.31774 | -0.0122 | 1.4329 | 9.89 | 3.09 |
| Henderson | 0.00014 | 9.2395 | 1.6159 | 21.99 | 9.71 |
| Chung-Pfost | 318.699 | 0.0637 | 79.833 | 21.29 | 7.29 |
| t (°C) | Adsorption | Desorption |
|---|---|---|
| 10 | 14.41% d.b. | 13.11% d.b. |
| 25 | 7.09% d.b. | 7.80% d.b. |
| 40 | 10.01% d.b. | 9.84% d.b. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





