Submitted:
26 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal Materials
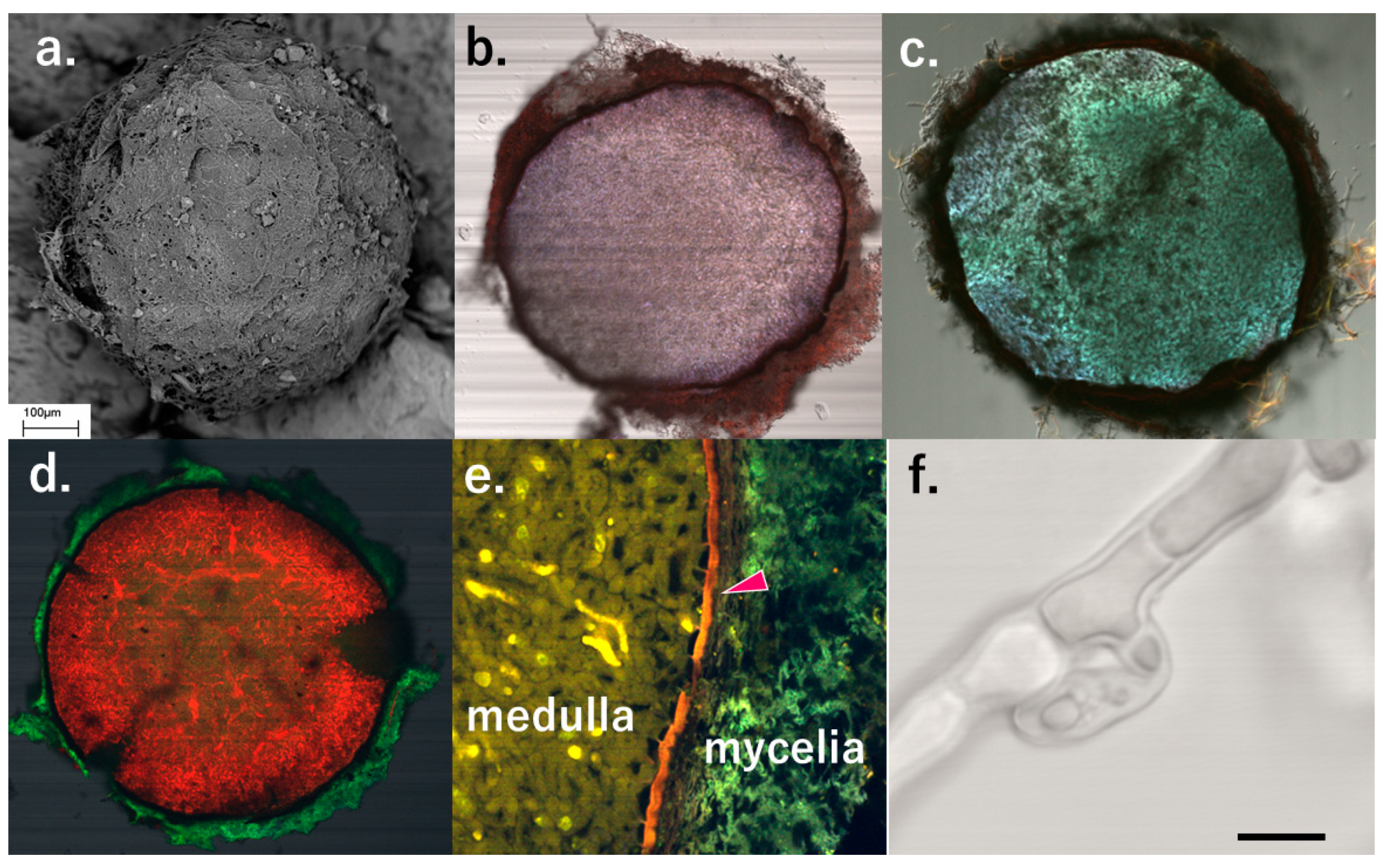
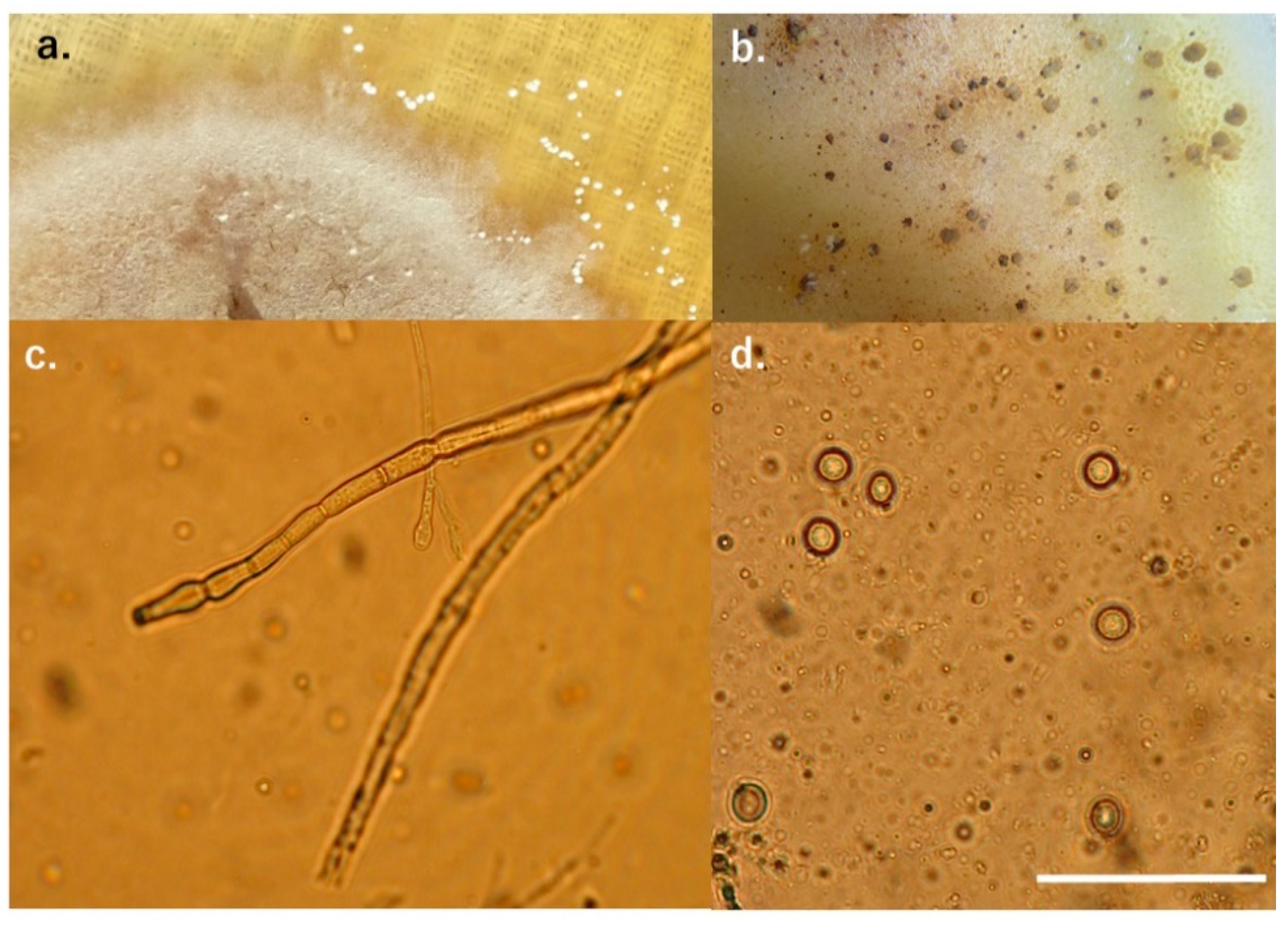
2.2. Morphological Observations
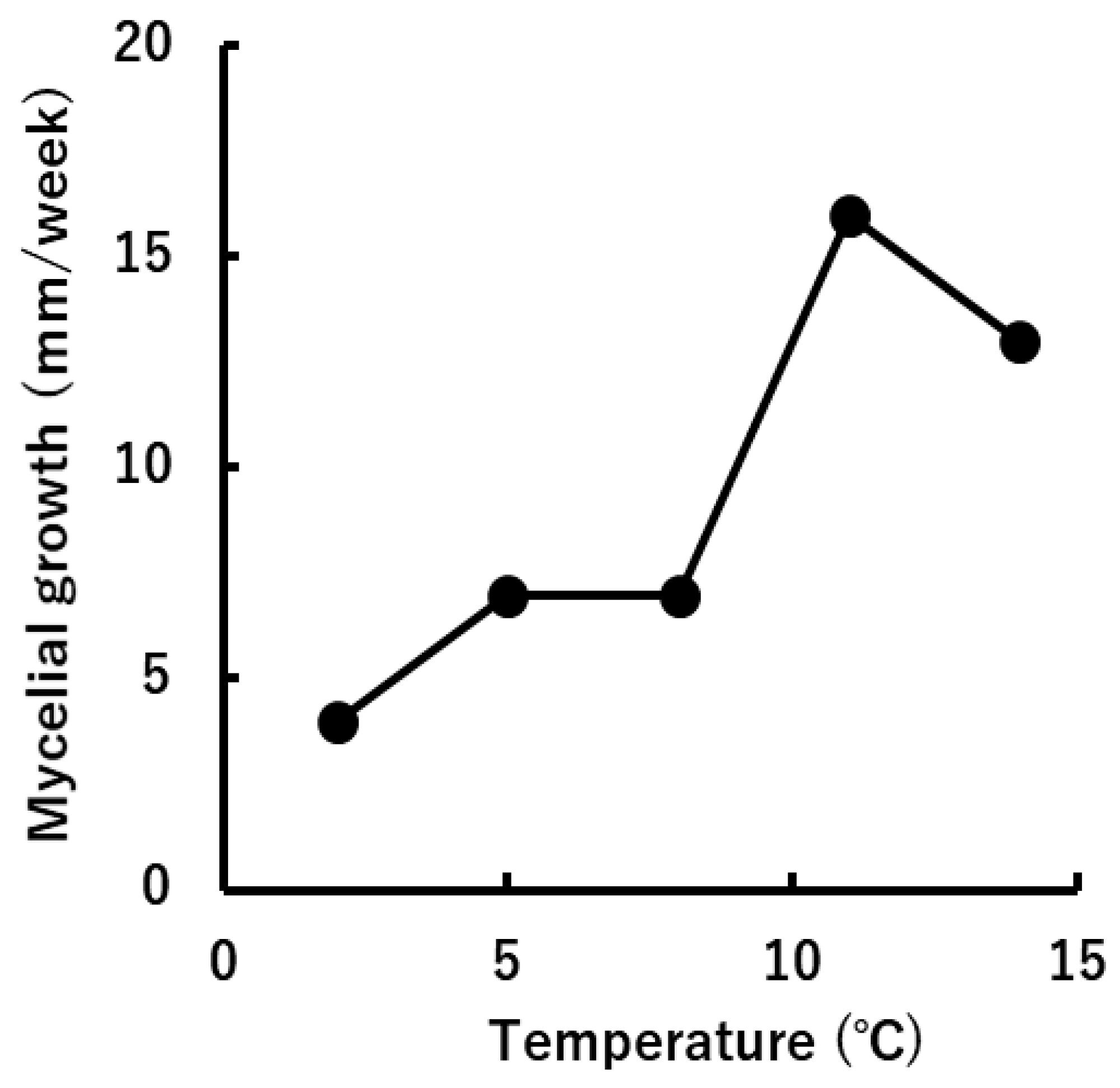
2.3. Mycelial Growth Temperature
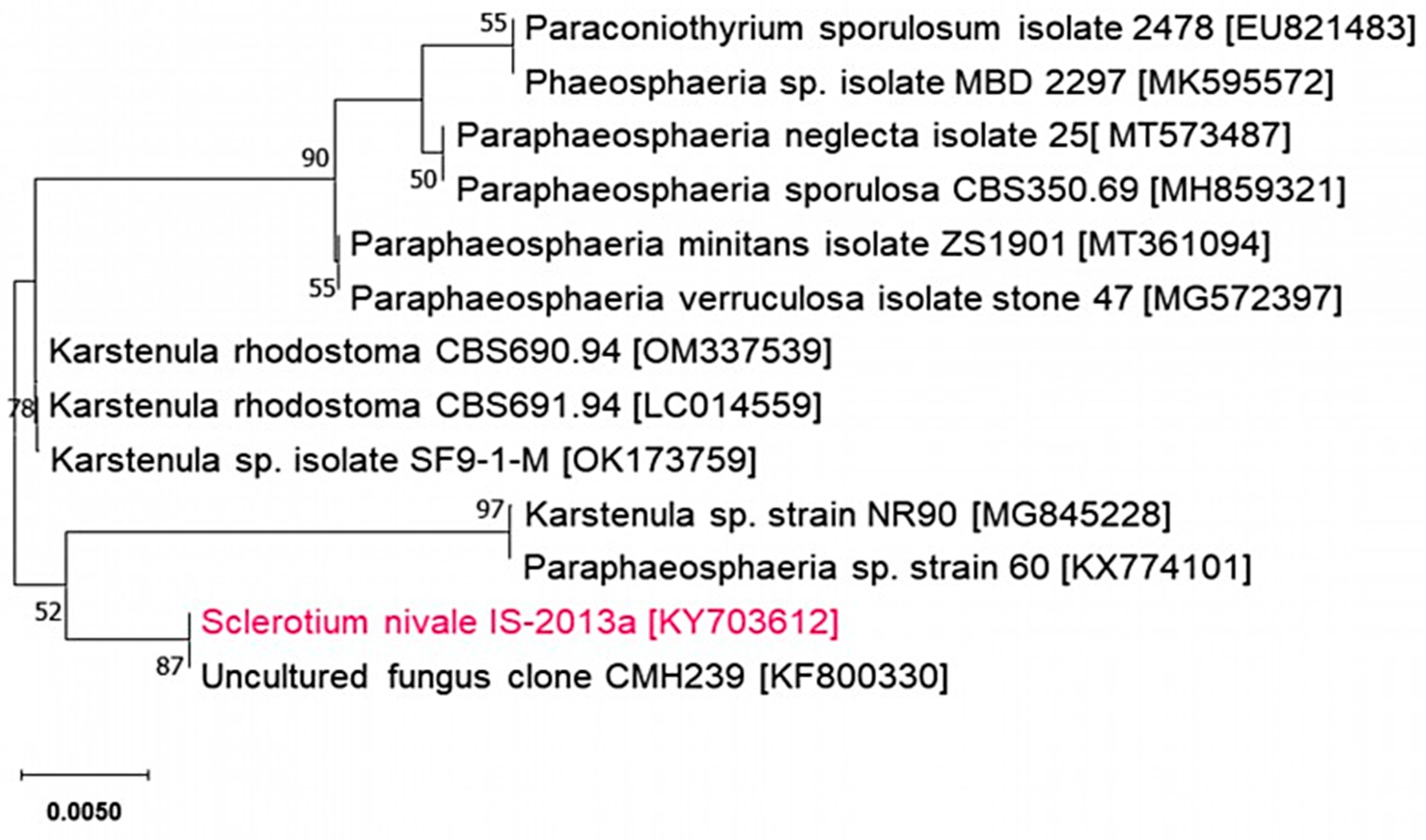
2.4. Phylogenic Analyses
3. Results
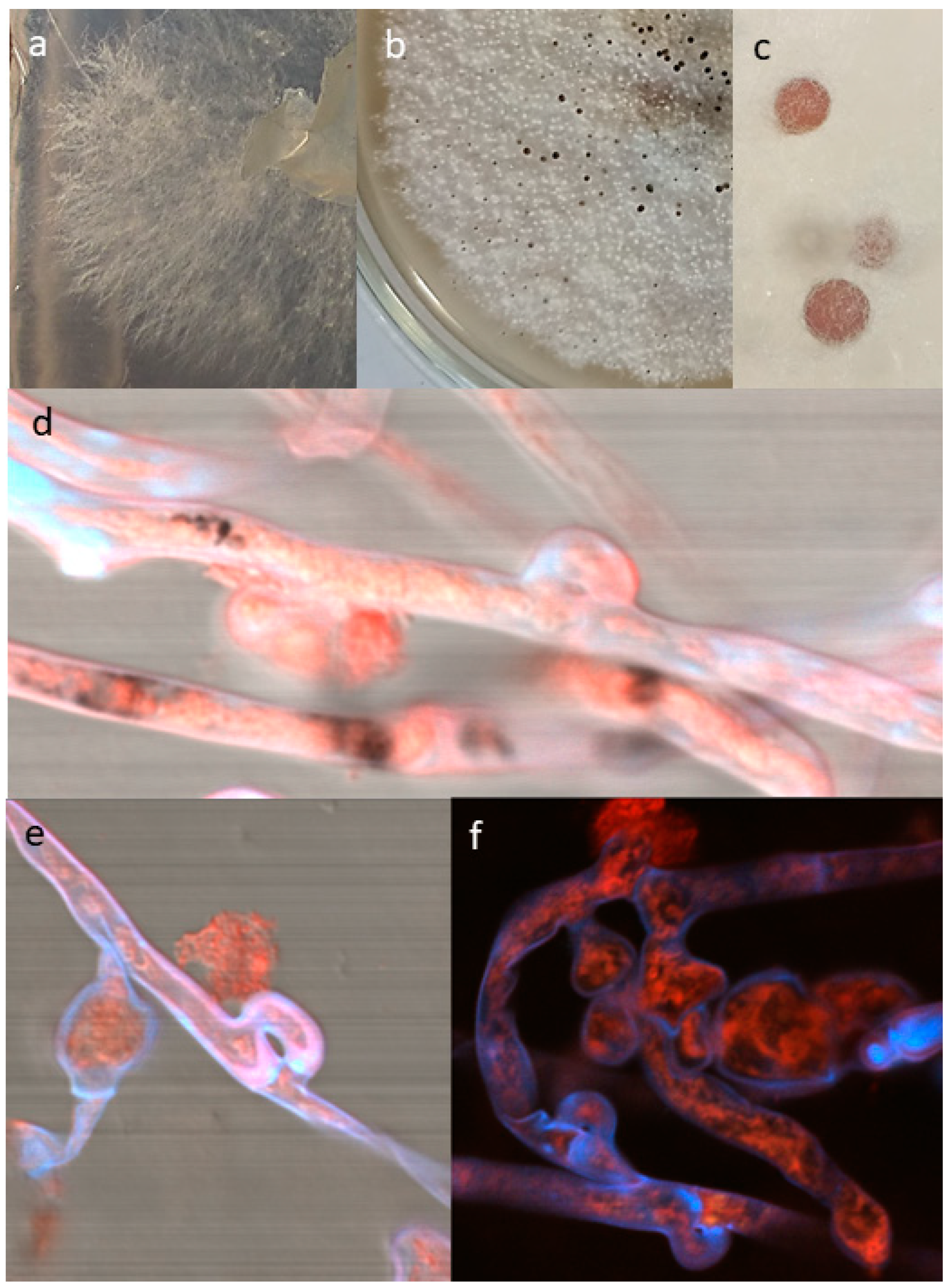
3.1. Morphological Characteristics of Sclerotium Nivale
3.2. Physiological Characteristics of “Sclerotium nivale”
3.3. Phylogenic Analysis of “Sclerotium nivale”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumoto, N.; Hsiang, T. Snow mold. The battle under snow between fungal pathogens and their plant hosts; Springer: Singapore, Singapore, 2016; pp. 1–21. [Google Scholar]
- Hoshino, T.; Tkachenko, O.B.; Tojo, M.; Tronsmo, A.M.; Kasuya, T.; Matsumoto, N. Taxonomic revision of the Typhula ishikariensis complex. Mycoscience 2023, 63, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Årsvoll, K. Fungi causing winter damage on cultivated grasses in Norway. Meld. Norges Landbrukshøgsk 1975, 54, 1–49. [Google Scholar]
- Matsumoto, N. Autecology of the pathogenic species of Typhula. Res. Bull. Hokkaido Nat. Agr. Exp. Stn. 1989, 152, 91–162. [Google Scholar]
- Takio Ichitani Physiological injuries and infection disease of turfgrass – diagnosis and control strategy– (in Japanese), Soft Science: Tokyo, Japan, 2008; pp. 66–67.
- Diakova, G.A. Phytopathological dictionary Russian–English–German–French, Nauka: Moscow, USSR, 1969, pp. 40.
- Tkachenko, O.B. Snow mold fungi in Russia. In Plant and microbe adaptation to cold in changing world: proceedings of the plants and microbe adaptation to cold conference 2012; Imai, R., Yoshida, M., Matsumoto, N., Eds.; Springer: New York, USA, 2013; pp. 293–303. [Google Scholar]
- Tkachenko, O.B. Снежные плесени (истoрия изучения, вoзбудители, их биoлoгические oсoбеннoсти) [Snezhnyye pleseni (istoriya izucheniya, vozbuditeli, ikh biologicheskiye osobennosti) (in Russian)], Russian Academy of Sciences: Moscow, Russia, 2017; pp. 12.
- Kask, K. Kõrreliste mükoosid, nende reservasioonikolded ja tõjevõimalused Eesti NSV-S (in Estonian). Eesti NSV Põllumajanduse Ministeerium Eesti Maaviljeluse ja Maaparanduse Teadusliku Uurimise Instituut: Saku, USSR, 1966, pp. 63–65, 164.
- Kask, K. Mikozy zlakov v Estonskoy SSR, ochagi ikh rezervatsii i vozmozhnosti bor’by s nimi (in Russian), Estonskaya Sel'skokhozyaystvennaya Akademiya: Tartu, USSR, 1967, pp. 11.
- Elenev, P. Mesures agricoles pour combattre la pourriture hibernale des céréales (in Russian). In La défense des Plantes, Bulletin du Bureau Permanent des Congrés Entomo-Phytopathologiques de Russie, Volume 3 (1): Leningrad, USSR, 1926; pp. 39–42.
- Peresypkin, V.F. Атласс бoлезней пoлевых культур [Atlas bolezney polevykh kul'tur (in Russian)], Urozhay: Kiev, USSR. 1987.
- Gutner, L.S.; Dobrozrakova, T.L.; Letov, L.S.; Stepanov, K.M. Определитель бoлезней растений пo внешним признакам [Opredelitel' bolezney rasteniy po vneshnim priznakam (in Russian)], Naumova, N.A. Ed. Sel’khozgiz gosudarstvennoye izdatel’stvo kolkhoznoy i sovkhoznoy literatury: Moscow, USSR, 1937, pp. 15, 291.
- Sokirko, V.P.; Gorkovenko, V.S.; Zazimko, M.I. Фитoпатoгенные грибы (Мoрфoлoгия и систематика) [Fitopatogennye griby (Morfologiya i sistematika) (in Russian)], Kuban State Agrarian University: Krasnodar, Russia, 2014, pp. 157–158.
- Anonymous. Royal Botanic Garden Edinburgh. Flora of British fungi, colour identification chart, Her Majesty’s Stationary Office, Edinburgh, UK, 1969.
- Ryabchenko, A.S.; Babosha, A.V. Using thermal compound as adhesive and heat-conducting composition when analysing biological samples of scanning electron microscope using freezing attachments. Russian patent RU 2 445 660 C2, 2012. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: a guide to methods and applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 18, 7213–7218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, T.; Ono, Y. Herpobasidium filicinum (Eocronartiaceae, Platygloeales) occurs on Dennstaedtia wilfordii (Dennstaedtiaceae) in Japan. Mycoscience 2018, 59, 443–448. [Google Scholar] [CrossRef]
- Huelsenbeck, J. P.; Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J. A. A. MrModeltest, ver. 3.7. Distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University, 2004.
- Rittenour, W.R.; Ciaccio, C.E.; Barnes, C.B.; Kashon, M.L.; Lemons, A.R.; Beezhold, D.H.; Green, B.J. Internal transcribed spacer rRNA sequencing analysis of fungal diversity in Kansas City indoor environments. Environ Sci.: Processes Impacts 2014, 16, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Townsend, B.B.; Willetts, H.J. The development of sclerotia of certain fungi. Trans. Br. Mycol. Soc. 1954, 37, 213–221. [Google Scholar] [CrossRef]
- Moromizato, Z. Effects of two or three physical factors on sclerotial longevity of Rhizoctonia solani Kühn and Sclerotinia sclerotiorum (Libert) de Bary (in Japanese, with English abstract). Sci. Bull. Coll. Agric. Univ. Ryukyus 1985, 32, 29–33. [Google Scholar]
- Matsuura, K.; Tanaka, C.; Nishida, T. Symbiosis of a termite and a sclerotium-forming fungus: Sclerotia mimic termite eggs. Ecol. Res. 2000, 15, 405–414. [Google Scholar] [CrossRef]
- Corner, E.J.H. A monograph of Clavaria and allied genera. Oxford University Press, 1950.
- Berthier, J. Monographie des Typhula Fr., Pistillaria Fr. et genres voisins. Numero Special Bull. Soc. Linn. Lyon 1976, 45, 1–195. [Google Scholar]
- Olariaga, I.; Salcedo, I. Two new species of Typhula from Iberian Peninsula: T. ochraceosclerotiata and T. schoeni. Mycol. Progress 2009, 8, 315–357. [Google Scholar] [CrossRef]
- Matsumoto, N.; Tronsmo, A.M.; Shimanuki, T. Genetic and biological characteristics of Typhula ishikariensis from Norway. Eur. J. Plant Pathol. 1996, 102, 431–439. [Google Scholar] [CrossRef]
- Matsumoto, N. Evolution and adaptation in snow mold fungi (in Japanese, with English abstract). Soil Microorg. 1997, 50, 13–19. [Google Scholar]
- Degawa, Y.; Hirooka, Y.; Hoshino, T.; Hosoya, T.; Inaba, S.; Iwamoto, S.; Katsumoto, K.; Kawakami, S.; Kiyuna, T.; Kurihara, Y.; Masaki, T.; Masuya, H.; Okada, G.; Tokiwa, T. Microfungi (Preliminary report) (in Japanese). In Results of the scientific research on the Tanzawa mountains, Appendix: A list of plants and animals; the research group of the Tanzawa mountains, Ed.; Hiraoka environmental science laboratory: Sagamihara, Kanagawa, Japan, 2007; pp. 461–472. [Google Scholar]
- Hoshino, T.; Yajima, Y.; Degawa, Y.; Kume, A.; Tkachenko, O.B.; Matsumoto, N. Life cycle plasticity in Typhula and Pistillaria n Arctic and the temperate zone. Microorganisms 2023, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Asef, M.R.; Fujiwara, M.; Yumoto, I.; Zare, R. One of the southern limits of geographical distribution of sclerotium forming snow mould fungi: First records of Typhula species from Iran. Rostaniha 2007, 8, 35–45. [Google Scholar]
- Hoshino, T.; Nakagawa, T.; Yajima, Y.; Uchida, M.; Tojo, M. Note on a snow mold and fungus-like microbe from Kuujjuarapik-Whapmagoostui, Quebec, subarctic Canada. Polar Sci. 2021, 27, 100559. [Google Scholar] [CrossRef]
- Yajima, Y.; Tojo, M.; Chen, B.; Hoshino, T. Typhula cf. subvariabilis, new snow mold in Antarctica. Mycology 2017, 8, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Olariaga, I.; Salcedo, I. Contribución al género Typhula Fr. (Fungi) en la Peninsula Ibérica. An. Biol. 2005, 27, 39–51. [Google Scholar]
- Gerhardt, E. Checkliste der Großpilze von Berlin (West) 1970-1990. Englera 1990, 13, 3–251. [Google Scholar] [CrossRef]
- Dynowska, M. Typhula subvariabilis – nowy gatunek w mikoflorze Polski. Acta Mycol. 1991–1992, 27, 31–32. [Google Scholar] [CrossRef]
- Shiryaev, A.; Iršėnaitė, R. Contribution to clavarioid fungi of Lithuania. Bot. Lith. 2009, 15, 117–127. [Google Scholar]
- Shiryaev, A. Diversity and distribution of clavarioid fungi in Estonia. Folia Cryptog. Estonica, Fasc. 2009, 45, 65–80. [Google Scholar]
- Shiryaev, A. Clavarioid fungi of the Urals. II. The nemoral zone. Karstenia 2007, 47, 5–16. [Google Scholar] [CrossRef]
- Gafforov, Y.; Hoshino, T. Remarks on Typhula sp. in Uzbekistan. Mycoscience 2015, 56, 109–113. [Google Scholar] [CrossRef]
- Hoshino, T.; Tojo, M.; Herrero, M.L.; Tronsmo, A.M.; Kiriaki, M.; Yokotota, Y.; Yumoto, I. Chilling resistances of isolates of Pythium ultimum var. ultimum from the Arctic and Temperate Zones. CryoLett. 2002, 23, 151–156. [Google Scholar]
- Oniki, M.; Ogoshi, A.; Araki, T. Ceratobasidium setariae, C. cornigerum, and C. gramineum, the teleomorphs of the pathogenic binucleate Rhizoctonia fungi from gramineous plants. Transactions of the Mycological Society of Japan 1986, 27, 147–158. [Google Scholar]
- Takamatsu, S. A new snow mold of wheat and barley caused by foot rot fungus, Ceratobasidium gramineum. Ann. Phytopath. Soc. Jpn. 1989, 55, 233–237. [Google Scholar] [CrossRef]
- Cheng, D.; Igarashi, T. Comparison of susceptibility to Racodium therryanum infection of seed and seedlings of four coniferous species (in Japanese). Res. Bull. Exp. For. Hokkaido Univ. 1990, 47, 125–136. [Google Scholar]
- Hoshino, T.; Terami, F.; Tkachenko, O.B.; Tojo, M.; Matsumoto, N. Mycelial growth of snow mold fungus, Sclerotinia borealis, improved at low water potentials: an adaptation to frozen environment. Mycoscience 2010, 51, 98–103. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Wanner, L.A.; Hageskal, G.; Henriksen, B.; Klemsdal, S.S.; Tronsmo, A.M. Isolates of Microdochium nivale and M majus differentiated by pathogenicity on perennial ryegrass (Lolium perenne L.) and in vitro growth at low temperature. J. Phytopathol. 2006, 154, 267–274. [Google Scholar] [CrossRef]
- Gagkaeva, T.Yu.; Orina, A.S.; Gavrilova, O.P.; Gogina, N.N. Evidence of Microdochium fungi associated with cereal grains in Russia. Microorganisms 2020, 8, 340. [Google Scholar] [CrossRef]
- Bockus, W.W.; Appel, J.A.; Bowden, R.L.; Fritz, A.K.; Gill, B.S.; Martin, T.J.; Brown-Guedira, G.L.; Eversmeyer, M.G. Success stories: breeding for wheat disease resistance in Kansas. Plant Dis. 2001, 85, 453–461. [Google Scholar] [CrossRef] [PubMed]
- K-state extension, Agronomy e-Update, Number 64, January 12, 2007. Available online: https://www.agronomy.k-state.edu/documents/eupdates/eupdate011207.pdf (accessed on 13 December 2023).
- Saccardo, P.A. Saccardo’s Sylloge Fungorum XIV; Edwards, J.W., Ed.; Edwards Brothers, Inc.: Ann Arbor, MI, USA, 1899; pp. 1141–1154. [Google Scholar]
- Hoshino, T.; Xiao, N.; Tkachenko, O.B. Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 2009, 50, 26–36. [Google Scholar] [CrossRef]
- Hoshino, T.; Yajima, Y.; Tkachenko, O.B.; Degawa, Y.; Tojo, M.; Matsumoto, N. Diversity and evolution of fungal phytopathogens associated with snow. In Advanced in Medicine Biology; Berhardt, L.V., Ed.; Nova Science Publishers: New York, USA, 2013; Volume 69, pp. 69–82. [Google Scholar]
- Smith, M.; Henkel, T.W.; Rollins, J.A. How many fungi make sclerotia? Fungal Ecol. 2014, 13, 211–220. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirayama, K.; Yonezawa, H.; Sato, G.; Toriyabe, A.; Hashimoto, A.; Matsumura, M.; Harada, Y.; Kurihara, Y.; Shirouzu, T.; Hosoya, T. Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud. Mycol. 2015, 82, 75–136. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Gareth Jones, E.B.; Liu, J.-K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.-Q.; Diederich, P.; Dissanayake, A.; Doilom, M.; Doveri, F.; Hongsanan, S.; Jayawardena, R.; Lawrey, J.D.; Li, Y.-M.; Liu, Y.-X.; Lücking, R.; Monkai, J.; Muggia, L.; Nelsen, M.P.; Pang, K.-L.; Phookamsak, R.; Senanayake, I.C.; Shearer, C.A.; Suetrong, S.; Tanaka, K.; Thambugala, K.M.; Wijayawardene, N.W.; Wikee, S.; Wu, H.-X.; Zhang, Y.; Aguirre-Hudson, B.; Alias, A.; Aptroot, A.; Bahkali, A.H.; Bezerra, J.L.; Bhat, D.J.; Camporesi, E.; Chukeatirote, E.; Gueidan, C.; Hawksworth, D.L.; Hirayama, K.; De Hoog, S.; Kang, J.C.; Knudsen, K.; Li, W.-J.; Li, X.-H.; Liu, Z.-Y.; Mapook, A.; McKenzie, E.H.C.; Miller, A.N.; Mortimer, P.E.; Phillips, A.J.L.; Raja, H.A.; Scheuer, C.; Schumm, F.; Taylor, J.E.; Tian, Q.; Tibpromma, S.; Wanasinghe, D.N.; Wang, Y.; Xu, J.-C.; Yacharoen, S.; Yan, J.-Y.; Zhang, M. Families of Dothideomycetes. Fung. Div. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Campbell, W.A. A new species of Coniothyrium parasitic on sclerotia. Mycologia 1947, 39, 190–195. [Google Scholar] [CrossRef]
- Matsumoto, N. Germination inactivity of Typhula ishikariensis Biotype A sclerotia in late fall. Jpn. J. Phytopathol. 2008, 74, 212. [Google Scholar]
- Matsumoto, N. Autecology of the pathogenic species of Typhula (in Japanese, with English abstract). Res. Bull. Hokkaido Natl. Agric. Exp. Stn. 1989, 152, 91–162. [Google Scholar]
- Woodbridge, B.; Coley-Smith, J.R.; Reid, D.A. A new species of Cylindrobasidium parasitic on sclerotia of Typhula incarnata. Trans. Br. Mycol. Soc. 1988, 91, 166–169. [Google Scholar] [CrossRef]
- Kohn, L.M.; Nagasawa, E. The genus Sclerotimitrula (Sclerotiniaceae), Episclerotium gen. nov. (Leotiaceae) and allied stipitate-capitate species with reduced ectal excipula. Trans. Mycol. Soc. Jpn. 1984, 25, 127–148. [Google Scholar]
- Saito, I.; Hoshino, T.; Yumoto, I. Cultural features of Episclerotium sclerotipus parasitizing sclerotia of Typhula phacorrhiza (in Japanese). Jpn. J. Phytopathol. 2007, 73, 77–78. [Google Scholar]
- Yajima, Y.; Inaba, S.; Degawa, Y.; Hoshino, T.; Kondo, N. Ultrastructure of cyst-like fungal bodies in myxomycete fruiting bodies. Karstenia 2013, 53, 55–65. [Google Scholar]
- Matsumoto, N.; Tajimi, A. Bacterial flora associated with snow mold fungi, Typhula incarnata and T. ishikariensis. Ann. Phytopath. Soc. Jpn. 1987, 53, 250–253. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Cabrera, G.; Godeas, A. Cyclosporine A from a nonpathogenic Fusarium oxysporum suppressing Sclerotinia sclerotiorum. J. Appl. Microbiol. 2006, 100, 575–586. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujikura, K.; Takata, K. DNA staining for fluorescence and laser confocal microscopy. J. Histochem. Cytochem. 1997, 45, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Parmasto, E. H. Key to the Clavariaceae of USSR (in Russian). Akademiya Nauk USSR, Moscow-Leningrad, 1965, pp. 37.
- Katsura, E.; Ogawa, H.; Kojima, H.; Yano, S.; Kaneshima, H. Runoff of fungicides used for prevention of snow molds in golf course (in Japanese, with English abstract). J. Environ. Chem. 1994, 4, 831–840. [Google Scholar] [CrossRef]
- Matsumoto, N.; Tajimi, A. Biological control of Typhula ishikariensis on perennial ryegrass. Ann. Phytopath. Soc. Jpn. 1992, 58, 741–751. [Google Scholar] [CrossRef]
- Hsiang, T.; Wu, C.; Cook, S. Residual efficacy of Typhula phacorrhiza as a biocontrol agent of grey snow mold on creeping bentgrass. Can. J. Plant Pathol. 1999, 21, 382–387. [Google Scholar] [CrossRef]
- McBeath, J.H. Snow mold-plant-antagonist interactions: survival of the fittest under the snow. The Plant Health Instructor 2002. [Google Scholar] [CrossRef]
- Amein, T.; Omer, Z.; Welch, C. Application and evaluation of Pseudomonas strains for biocontrol of wheat seedling blight. Crop Prot. 2008, 27, 532–536. [Google Scholar] [CrossRef]
- Andersson, P.F.; Levenfors, J.; Broberg, A. Metabolites from Pseudomonas brassicacearum with activity agent the pink snow mould causing pathogen Microdochium nivale. BioControl 2012, 57, 463–469. [Google Scholar] [CrossRef]
- Hoshino, T.; Morita, H.; Yajima, Y.; Tsuji, M.; Tojo, M.; Tkachenko, O.B. Snow molds and their antagonistic microbes in Polar Regions. In Fungi in Polar Regions; Tsuji, M., Hoshino, T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 17–29. [Google Scholar]
- Xu, Z.; Harrington, T.C.; Gleason, M.L.; Batzer, J.C. Phylogenic placement of plant pathogenic Sclerotium species among teleomorph genera. Mycologia 2010, 102, 337–346. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




