1. Pointing to the Other Side?
One of the most prized traits of the domestic dog (
Canis lupus familiarus) is their ability to cooperate and interact across the species boundary with humans [
1]. For many years, dogs have been integrated into human society, assisting with physical tasks such as guarding the home, hunting for food, and herding sheep and cows, but also acting as companions. Through selective breeding, dogs have been purposefully developed to be comfortable working in diverse conditions and also to respond to human communication, whether whistled, spoken, or gestured [
1]. Because of this history, the initial reports that dogs could spontaneously follow human pointing gestures better than apes [
2,
3] seemed to make sense. However, as an increasing number of dog pointing studies are published, the picture of exactly how and when dogs are successful at pointing tasks is becoming progressively less clear [
4,
5,
6]. Two kinds of variables that have been shown to change point comprehension, pointer position and point type, are explored here.
1.1. Points, Dogs, and Humans
Pointing cues are one class of fundamental human communicative gestures that draw attention to a specific object or subject - called deictic gestures [
7]. People use various cues to guide or redirect an individual's attention and indicate an item of interest. Such cues can include a vocal cue (e.g., “Look!”), gazing (i.e., moving their eyes to look at the object, sometimes accompanied by a head turn), or a physical gesture (e.g., traditional human points involving the arm and index finger, lip points, or even head jerks). Physical gestures, such as conventional pointing, can vary in many ways. For example, points can be from the hand on the same side as the object (ipsilateral) or from the opposite hand (contralateral); points can also be close to the object in question (proximal) or further away (distal). Points are so fundamental to human communication that children as early as 12 months can follow the gaze of their mother, and by 15 months, they can follow an ipsilateral distal point when accompanied by a gazing cue [
8,
9]. Early comprehension of points clearly develops from the social environment and stimulation received by a human child [
8,
10] however, the ability to learn a task so easily and early - as well as the ubiquity of pointing comprehension across the human species - indicates a biological capacity arising from human evolution [
11,
12].
Numerous studies have systematically tested dogs’ ability to follow human pointing gestures [
6,
8,
13]. Studies commonly involve a gestural cue or signal, which is directed toward an object hiding a food reward [
6,
13]. Some researchers have argued that dogs’ success at these tasks indicates that humans have bred into dogs a capacity to spontaneously read human communicative signals like points [
12,
14,
15,
16]. However, if this were true, dog pointing literature would demonstrate a clear trend of success amongst canine subjects in following pointing cues, which is not the case [
5,
6,
17,
18].
1.2. Alternate Explanations
One argument for the biological nature of point following in dogs came from a study comparing hand reared wolf puppies to hand reared dog puppies in which the dog puppies performed significantly better than the wolf puppies, who performed at chance level [
15]. Yet this same study reported that socialized wolf puppies performed significantly better at eight months of age than they had at four months, demonstrating that social learning of points was occurring even for non-domesticated canines [
15]. Similarly, previous dog pointing experiments have demonstrated that as trials progressed the average success rate for the dogs increased [
19]. Socialized wolves similarly show an increase in success rates as trials progress [
15,
20,
21]. Findings such as these suggest that a dog's ability to follow human pointing gestures (often with the reception of food) increases over time through learning, either socially or through associative mechanisms.
In addition to learning over trials, we might predict that with age comes greater opportunity to become familiar with human gestural cues. In general, older dogs seem to outperform younger dogs, but in stray dogs, the opposite is true. Bhattacharjee [
22] reported that stray puppies successfully followed human pointing cues above chance levels, but older stray dogs failed the same test, indicating a potential decline in this behavior as dogs mature without consistent human contact. All these data may indicate that consistent human contact is the main predictor of point following behavior in dogs, but much further research is needed.
1.3. Point Types and Positions
There is also an ongoing debate about the types of pointing gestures dogs can reliably follow. While some studies, such as those by Wobber [
16] and Riedel [
19], demonstrate that dogs can successfully track a proximal contralateral point, especially when accompanied by a gaze cue, others, including [
8,
23,
24], suggest that dogs only reliably follow distal contralateral points when the experimenter remains standing. These findings were echoed in the recent ManyDogs study where a large sample of dogs from various locations failed to follow seated (kneeling) contralateral points [
25]. However, all of these results were dependent upon varying methodological practices. For example, Elgier et al., [
23] found that only the dogs that had been previously exposed to ipsilateral proximal pointing were able to follow contralateral distal pointing from the outset of testing. And Lakatos et al., [
8] reported that crossbody points accompanied by a full arm extension yielded results above chance, while crossbody points involving a bent elbow resulted in performance below chance.
Soproni et al. [
26] varied the form of the pointing gesture in order to determine the critical visual features of this signal. The results of this study suggested that dogs are sensitive to the relation between the hand/arm and the torso; that is, they infer the directionality of the gesture by observing the direction in which the arm/hand (or part of) protrudes from the upper body. Similarly, Lakatos et al. [
8] found that cross–
forward pointing was difficult for dogs. Here, the cross–forward pointing meant that while the arm was fully extended and directed toward the opposing side of the body, no part of the arm (including elbow) protruded beyond the body of the experimenter. Research has suggested that the longer the extension is, the further the receiver’s attention is drawn in the cued direction [
27]. Clearly, position and point type have substantial effects on point following behavior in dogs, and we hope to clarify some of those effects.
2. Materials and Methods
2.1. Participants
Fourteen dogs at the Humane Society of South Mississippi (HSSM) in Gulfport and eighteen pet dogs recruited from the general population and tested at the University of South Alabama (SouthAl) in Mobile, AL., served as participants. The HSSM group was chosen from the general population of the shelter and both samples included a variety of ages, breeds, and backgrounds (see
supplemental materials for details).
2.2. Materials
In both locations, dogs were tested with large red solo cups as the main materials. Two cups were stacked and taped together, with 3-5 training treats sandwiched between and holes punched in the top cup to control for olfactory cues. Treats were sourced from reputable pet supply companies and were approved by the owners.
2.3. Procedures
2.3.1. Acclimation and Training
In both locations, dogs were tested in a minimum room size of 10’ X 10’ and the procedures were approved by the respective IACUC. Three researchers would lead the dog into the testing room and allow them time to acclimate to the new surroundings, feeding them occasional treats until any signs of anxiety faded. Five training trials were then run, consisting of one researcher R1 holding the dogs’ leash at one side of the room, another researcher R2 placing a cup on the floor with a treat underneath, and R3 actively recording experimental progress. The dog was then released to approach the cup. As soon as the dog touched the cup in any way, the cup was flipped over by R2 and a treat was given to the dog. Notes and quantitative data were then placed into a secured spreadsheet by R3. If the dog failed to approach the cup or high levels of anxiety were noted, testing was discontinued.
2.3.2. Testing
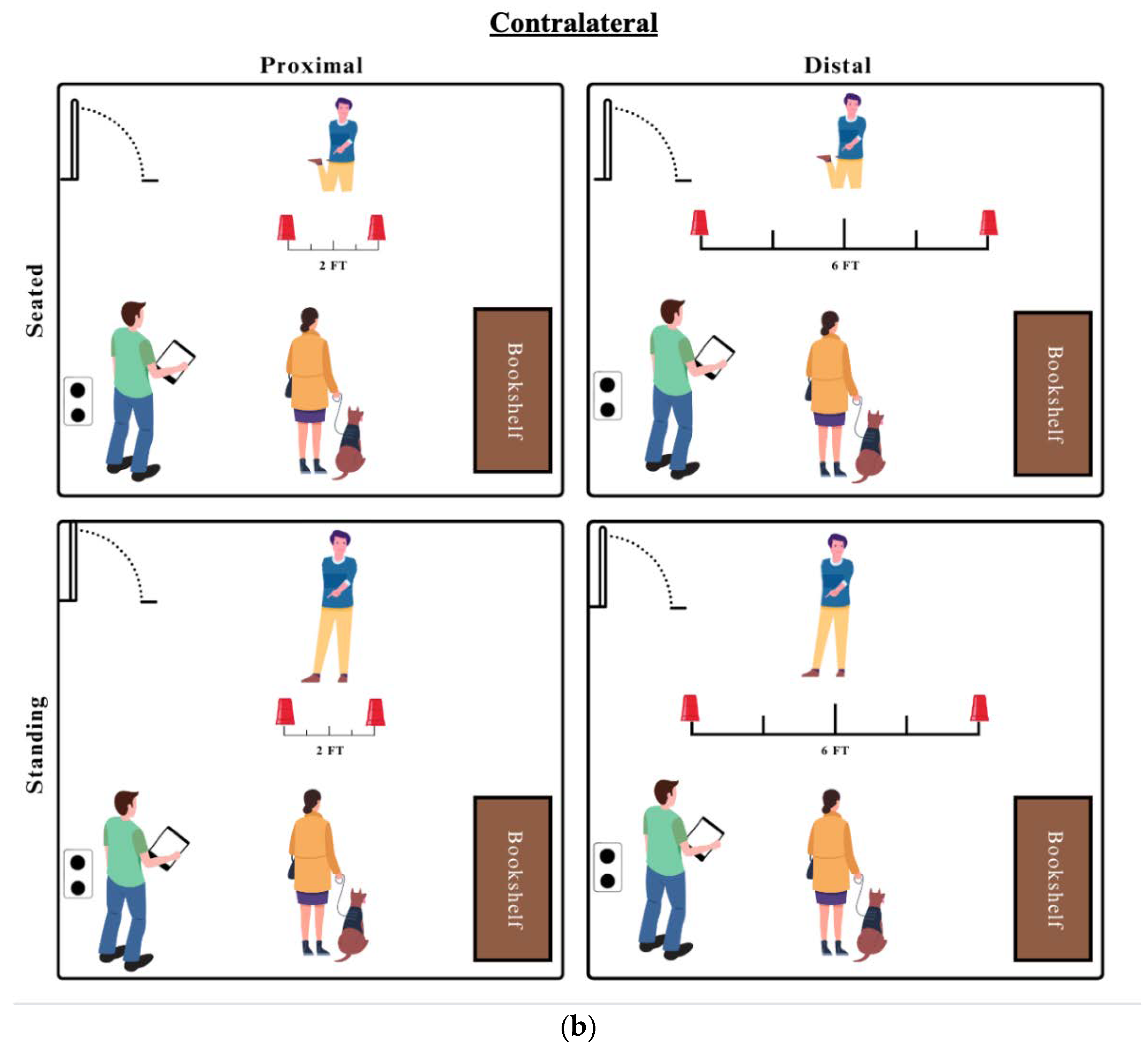
Test trials involved R2 placing two cups on the floor and pretending to hide treats in both cups, while hiding a treat in only one. The researcher would then point to the correct cup with the ipsilateral (same side) or contralateral (opposite side) hand with the variations described below. (see
Figure 1)
Variations
At HSSM, 12 dogs were tested on both ipsilateral and contralateral points. The dogs were initially tested on a variety of ostensive cues, such as no eye contact. R2 could vary her eye gaze between the dog and the cup, the cup and her own gesture, the gesture and the cup or between all three. It was quickly determined that the dogs were not following the points well, even with all of the ostensive cues in place, so the final two dogs were tested only on contralateral points, without the gaze variations. No difference in the results were noted between those two procedures. A total of 10 trials of ipsilateral points (for the first 12 dogs) and 20 trials of contralateral points (for all 14 dogs) were collected with R2 alternating gaze between the dog, gesture, and correct cup.
At South Alabama, trials were designed to test the effects of distance and researcher position. Therefore dogs were tested with R2 standing or seated and with the cups separated by the 6 ft distance used at HSSM (distal) or by 2 feet (proximal), for a 2X2 distance by position design. The order of conditions presented were counterbalanced (some seated first, some standing first) with distance counterbalanced trial by trial, for a total of 10 trials in each of the 4 condition combinations (seated/proximal, seated/distal, standing/proximal, standing/distal) (see
Figure 1). No ostensive cues were predetermined; R2 was instructed to look toward the dog as seemed natural, without specific gaze alteration instructions. Nine dogs completed all 40 trials with ipsilateral points and eight completed the 40 trials with contralateral points - two dogs completed both point types (Clover and D-O).
3. Results
3.1. Ipsilateral vs Contralateral Points
Both samples of dogs were able to follow ipsilateral points significantly better than chance levels (SouthAl t(8) = 3.64, p<.01, HSSM t(11)=8.86, p<.001). The dogs at HSSM were also above chance levels on contralateral points however, the dogs at SouthAl were below chance on contralateral points across the position conditions (SouthAl t(8) = 0.41, p=0.69, HSSM (t(13)=8.89, p<.001), possibly due to their low performance on seated contralateral points (HSSM only had standing contralateral points). Both samples of dogs, however, performed better on ipsilateral than contralateral points (SouthAl t(15)=2.39, p=.03, HSSM t(24) = 3.34, p<.01, see
Figure 2).
3.2. Group Comparison
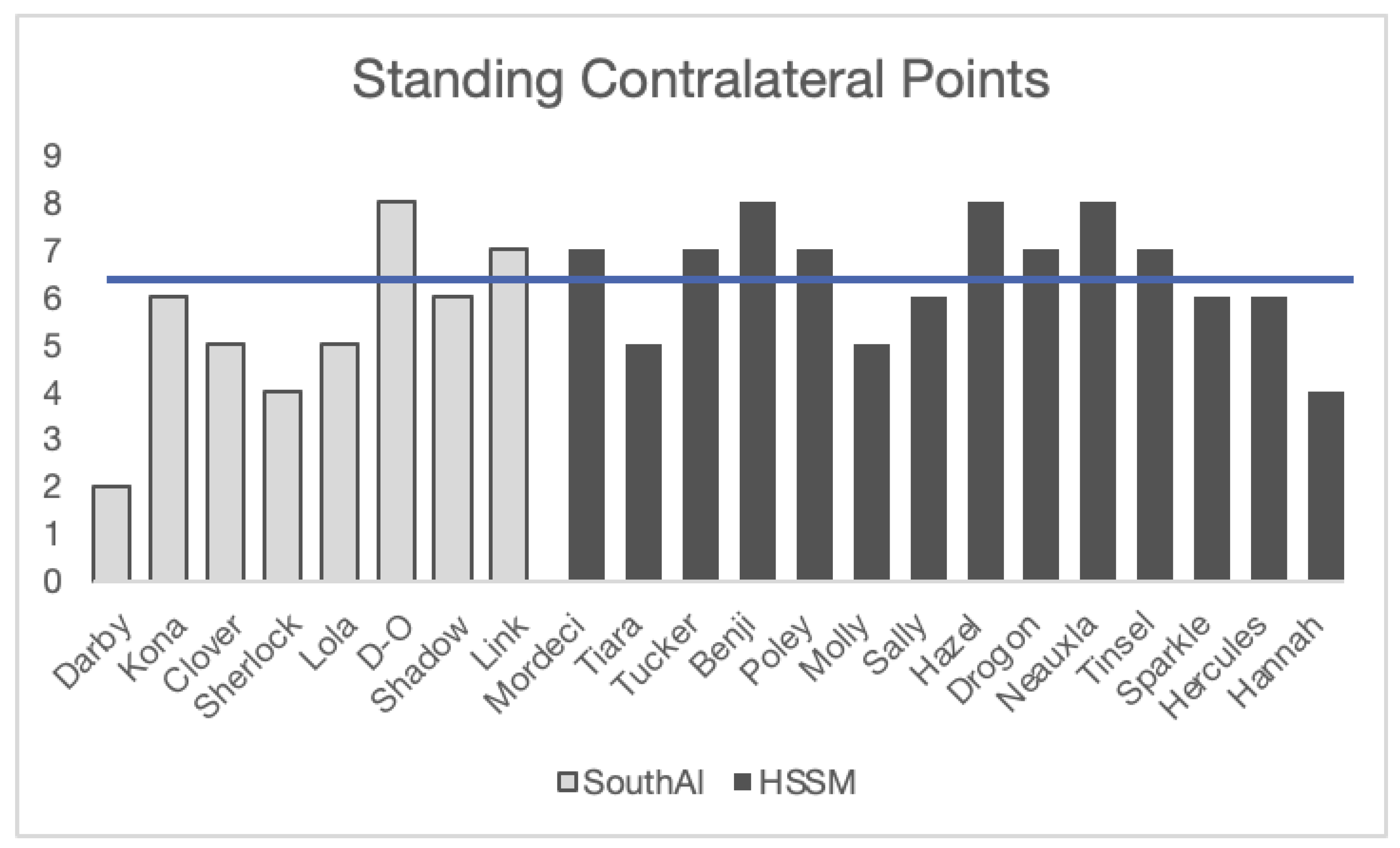
To compare the two groups, we tested the first 10 trials of the HSSM contralateral points (only standing and distal) against the 10 trials of the SouthAl contralateral standing distal points. The dogs tested at SouthAl (M = 6.45, N = 11) and HSSM (M = 6.5, N = 14) did not perform significantly different from each other in this comparison (t(23) = -.08, p>.05) with both groups above chance on their performance (SouthAl (t(10)=3.2, p<.01), HSSM (t(13)=4.58, p<.001), but further exploration shows that this success is driven by only 45% of the dogs (10/22 were above chance (see
Figure 3)).
This is in contrast to the dogs’ performance on the Ipsilateral version of this task where 11 out of 12 dogs scored above chance at HSSM and 6 out of 9 scored above chance at SouthAl (81% scored > 7/10, binomial test).
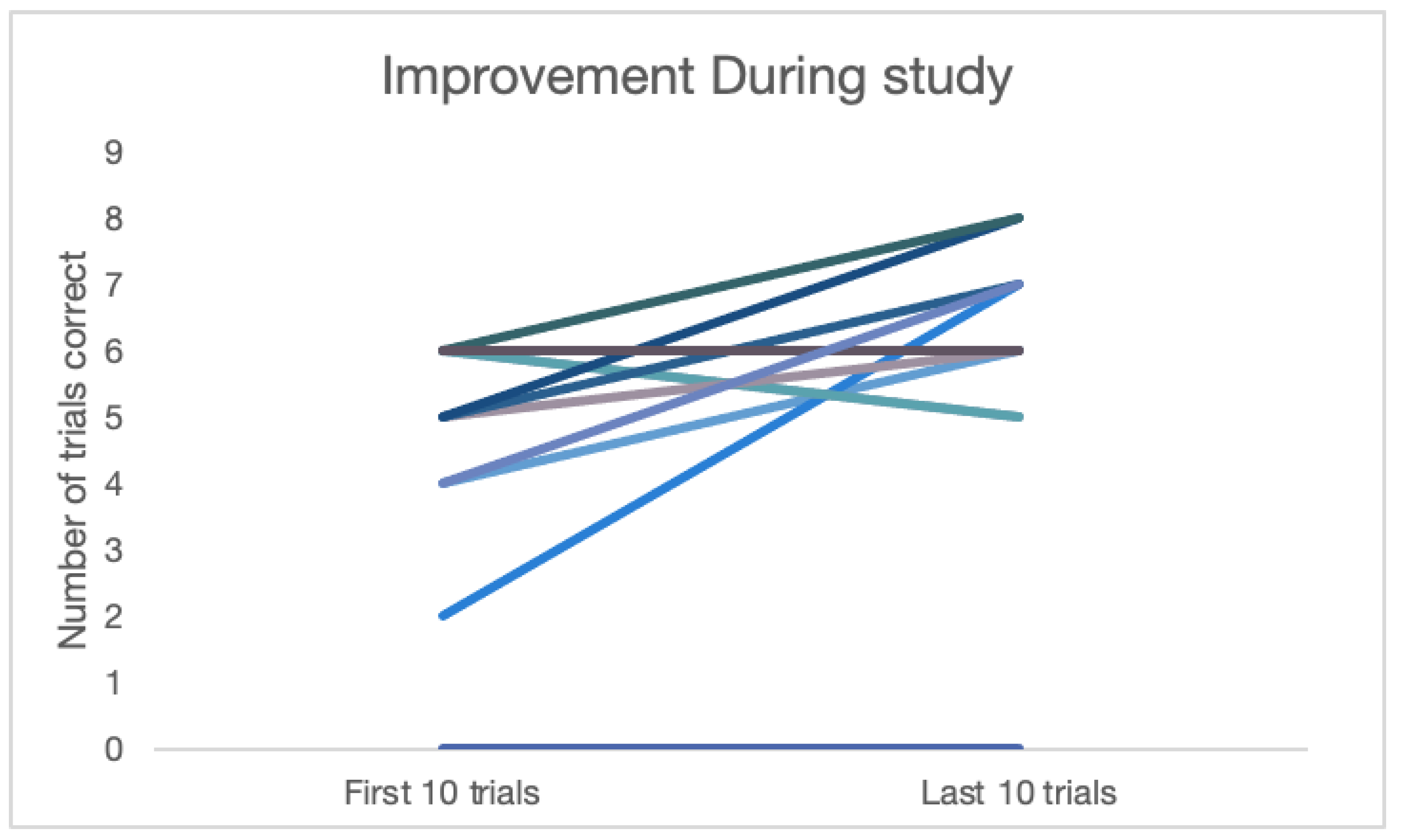
Looking across conditions, if tested as a group, the dogs did not show significant improvement from their first 10 trials to their last 10 trials (t(24)=-0.17, p=.433). However, if we limit the sample to dogs that failed to perform above chance in the first 10 trials, significant improvement was seen (t(10)=2.59, p<.05) and 8 out of 11 dogs scored higher in their last 10 trials than in their first 10 trials (
Figure 4).
3.3. Position and Point Types
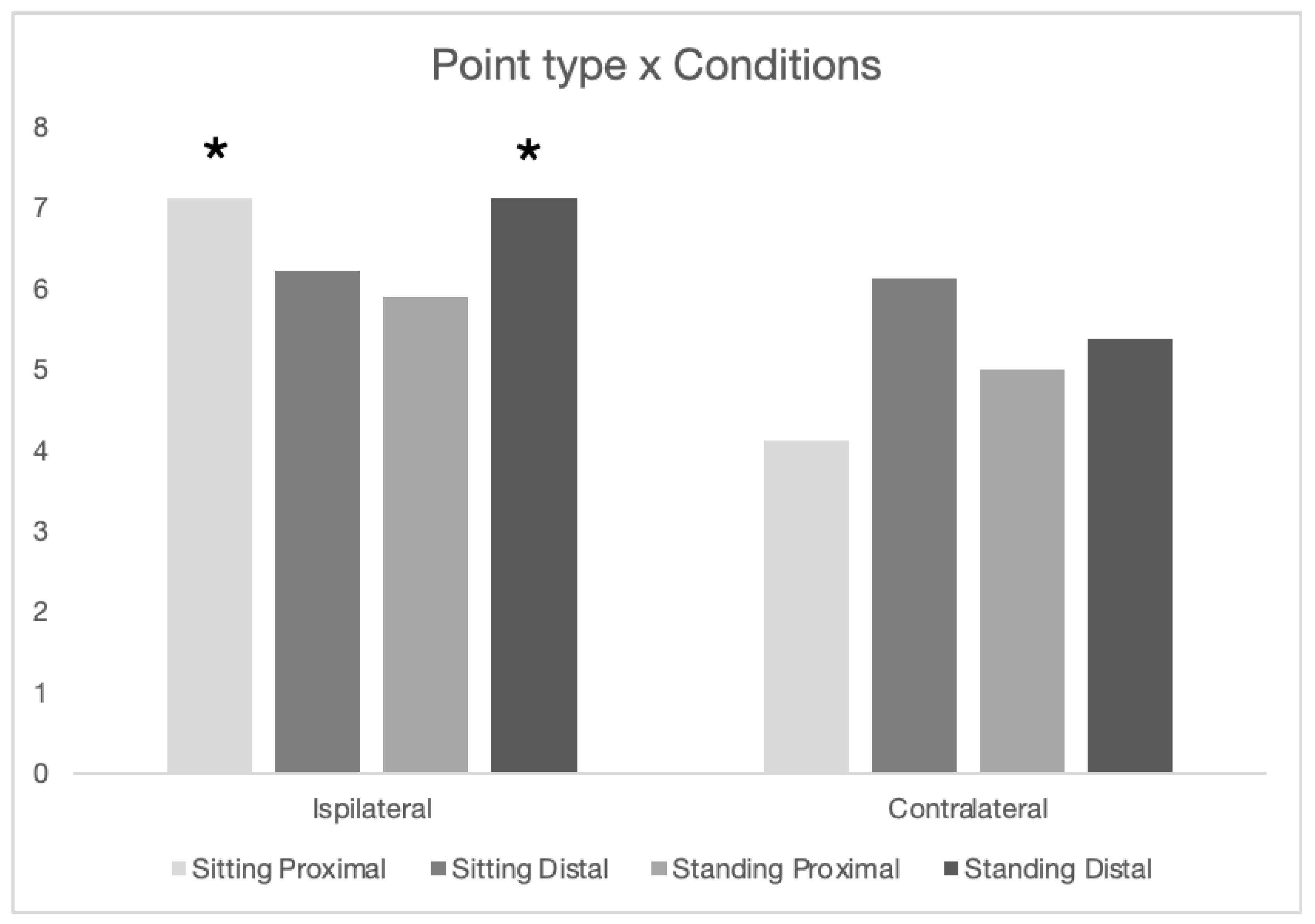
The South Alabama dogs were tested on the two different point types in 4 different conditions - seated vs standing and proximal vs distal distances (both point to occluder and between occluder distance). Dogs performed differently across these conditions (see
Figure 5), however the only significant differences were a stronger performance in ipsilateral vs contralateral points (F(1, 672)=13.47, p<.001) and a three-way interaction between the two position differences and the point type (F(1, 672)= 5.53, p<.05). As can be seen in
Figure 5, the only individual conditions in which the dogs were above chance were with ipsilateral points: seated/proximal and standing/distal. This contrasted with their poorer performance on the contralateral seated/proximal condition, resulting in the only two post-hoc significant differences (Tukey’s <.01 ipsilateral seated/proximal and ipsilateral standing/distal vs contralateral seated/proximal).
4. Discussion
Our data show that while dogs, as a group, seem to successfully follow point gestures, the pattern is often more complicated on closer inspection. While the dogs were above chance when their data were combined, 81% of the dogs could follow an ipsilateral point, but only 45% could follow a contralateral point. These data suggest that some dogs can certainly follow points, but others cannot and for others still, it is dependent on the type of point. These findings are of particular interest because although overall group data is very useful when discussing species norms, it is less useful when arguing for biological differences. If all dogs followed all points, the argument for a biological capacity for point following in dogs looks very different than if only some dogs follow some points. In fact, this pattern of point following is very reminiscent of the patterns seen in apes (see below), suggesting much more evolutionary continuity than had been claimed in the past [
28,
29,
30].
Some researchers have suggested that shelter dogs are not representative of the broader dog population on cognitive or communicative tasks [
6,
20,
31,
32]. However, our data do not suggest population differences between the pet dogs and the shelter dogs in this task as the groups did not perform significantly differently from each other on the conditions that overlapped. This similarity casts doubt on the hypothesis that human interaction is key for learning to follow points [
33,
34,
35,
36,
37]. Shelter dogs’ level of human interaction is characterized by brief occurrences, such as food provision, cage cleaning activities, or when given yard access. Pet dogs experience more intimate interactions and are often near humans, creating contrasting socialization contexts. As both groups performed equally well, one could argue that human interaction is not essential, although shelter dogs have also been shown to learn point following behaviors quickly [
32]. Both groups of dogs in this study also showed evidence of quick learning (8/11 dogs that failed their first 10 trials improved their performance in their last 10 trials), supporting, instead, the idea that dogs’ abilities to follow some points do require minimal human interaction. It is important to note that many of the pet dogs were tested within weeks of COVID-19 restrictions being lifted, which is to say it is likely it was the first time in at least 6 months that they were in a novel environment. Therefore, the pet dogs may have had higher levels of anxiety than would be the norm, potentially negatively influencing their performance. However, shelter dogs are living in an extremely high-stress environment, and so likely these data do not reflect peak performance by either group.
The dogs performed best on ipsilateral-seated-proximal points and ipsilateral-standing-distal points. These point types could be characterized as those in which it is most apparent that the pointing finger is closer to one cup than the other. In contrast, the dogs did worst on contralateral-proximal points (both seated and standing), in which the point is arguably the least visibly prominent on one side or the other. Interestingly, these types of proximal points (where the finger is not close to the correct occluder) are the point types most difficult for apes to discern as well [
28,
29,
30].
Notably, the dogs’ differing abilities across point types contradict many researchers’ assertion that dogs successfully follow points because they have a biological instinct to attend to human communication [
11,
12].They also cast doubt upon claims of other researchers that dogs are demonstrating an understanding of the point gesture as a communicative act [
38,
39], which would suggest that dogs are demonstrating an awareness of human mental states and the human desire to share knowledge. The different point types are equally communicative, so the biological predisposition or understanding of communicative acts should result in similar responses to all point types. So, where does that leave us, hypothesis-wise?
5. Conclusions
We suggest that different mechanisms support learning of different kinds of point-following - at least in dogs. Ipsilateral points are the easiest to learn and require only minimal interaction with humans. This suggests to us that ipsilateral points are learned through associative mechanisms. Simple association of “body part closest to cup” means “treat in that cup” could be learned in few trials with no need for a communicative hypotheses, particularly since adjacency and distance have been shown to be important variables for dogs’ search behavior [
40,
41]. Human interaction effects could be explained by social facilitation - an increase in executing a particular behavior in the presence of other social group members. Studies have demonstrated social facilitation effects in dogs - showing that dogs tend to synchronize their behaviors with their owner, and also favor strangers that synchronize with them [
42,
43]. Dogs have been shown to utilize social facilitation effectively in learning tasks [
44]. Therefore, the nearness of a human, or the body part of a human, to one side or the other could result in quick associative learning.
In the case of contralateral points, however, we argue that dogs may require a deeper understanding of communicative intent. Because contralateral points are confusing in many aspects (e.g., the point does not necessarily end in a position closest to the referent, the motion crosses over the body, potentially passing through the referential space, indicating the incorrect occluder), the dogs are presented with a more difficult task. It also seems most likely that dogs that follow contralateral points have the most flexible understanding of the point gesture, potentially even comprehension of the communicative nature of the gesture. In our study, that group would be limited to only 45% of our sample, leaving many questions unanswered. How did those dogs learn the communicative nature of points? Is there a biological difference between the dogs that learn and those that do not? Is that difference similar to the differences in dogs that can learn spoken words or use button communication devices meaningfully [
45,
46]. The future of dog communication research is rich with possibilities.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, data sheet.
Author Contributions
Conceptualization, H. L.; methodology, H. L.; data collection, H.L., K.W., J.V., C.B. and S.B.; data analysis, H.L., K.W., J.V.; writing—original draft preparation, H.L., K.W., J.V., C.B., and S.B.; writing—review and editing, H.L., K.W., J.V., C.B., and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Heidi Lyn holds the Joan M. Sinnott Chair of Psychology which is funded by the USA Foundation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Animal Care & Use Committees of the University of Southern Mississippi and the University of South Alabama (protocol number 156094, approval date 3/19/2020).
Informed Consent Statement
All owners signed an informed consent agreement to participate and presented vaccination documentation. Dogs voluntarily consented to test procedures by participation - any refusal to participate was respected.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salomons, H.; Smith, K. C.; Callahan-Beckel, M.; Callahan, M.; Levy, K.; Kennedy, B. S.; ... & Hare, B. Cooperative communication with humans evolved to emerge early in domestic dogs. Current Biology 2021, 31(14), 3137-3144.
- Hare, B.; Rosati, A.; Kaminski, J.; Bräuer, J.; Call, J.; Tomasello, M. The domestication hypothesis for dogs’ skills with human communication: A response to Udell et al. (2008) and Wynne et al. (2008). Animal Behaviour 2010, 79(2), e1–e6.
- Hare, B.; Tomasello, M. Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. Journal of Comparative Psychology 1999, 113(2), 173–177. [CrossRef]
- Bräuer, J.; Kaminski, J.; Riedel, J.; Call, J.; Tomasello, M. Making inferences about the location of hidden food: social dog, causal ape. Journal of Comparative Psychology 2006, 120(1), 38. [CrossRef]
- Gácsi, M.; Kara, E.; Belényi, B.; Topál, J.; Miklósi, Á. The effect of development and individual differences in pointing comprehension of dogs. Animal cognition 2009, 12, 471-479. [CrossRef]
- Lyn, H.; Broadway, M.; Jett, S. E.; Samuelson, M. M.; Christopher, J.; Chenkin, B. The effects of distance on pointing comprehension in shelter dogs. Animal Cognition 2021, 24, 855-865.6. [CrossRef]
- Bangerter, A.; Louwerse, M. M. Focusing attention with deictic gestures and linguistic expressions. In Proceedings of the Annual Meeting of the Cognitive Science Society 2005, (Vol. 27, No. 27).
- Lakatos, G.; Soproni, K.; Dóka, A.; Miklósi, Á. A comparative approach to dogs’(Canis familiaris) and human infants’ comprehension of various forms of pointing gestures. Animal cognition 2009, 12, 621-631. [CrossRef]
- Morissette, P.; Ricard, M.; Décarie, T. G. Joint visual attention and pointing in infancy: A longitudinal study of comprehension. British Journal of Developmental Psychology 1995, 13(2), 163-175. [CrossRef]
- Tomasello, M.; Call, J.; Gluckman, A. Comprehension of novel communicative signs by apes and human children. Child development 1997, 1067-1080.
- O'Madagain, C.; Kachel, G.; Strickland, B. The origin of pointing: Evidence for the touch hypothesis. Science advances 2019, 5(7), eaav2558. [CrossRef]
- Hare, B.; Brown, M.; Williamson, C.; Tomasello, M. The domestication of social cognition in dogs. Science 2002, 298(5598), 1634-1636. [CrossRef]
- Osborne, T.; Mulcahy, N. J. Reassessing shelter dogs’ use of human communicative cues in the standard object-choice task. PloS one 2019, 14(3), e0213166. [CrossRef]
- Gácsi, M.; McGreevy, P.; Kara, E.; Miklósi, Á. Effects of selection for cooperation and attention in dogs. Behavioral and brain functions 2009, 5(1), 1-8. [CrossRef]
- Virányi, Z.; Gácsi, M.; Kubinyi, E.; Topál, J.; Belényi, B.; Ujfalussy, D.; Miklósi, Á. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Animal cognition 2008, 11, 373-387. [CrossRef]
- Wobber, V.; Hare, B.; Koler-Matznick, J.; Wrangham, R.; Tomasello, M. Breed differences in domestic dogs’ (Canis familiaris) comprehension of human communicative signals. Interaction Studies 2009, 10(2), 206-224. [CrossRef]
- Byosiere, S. E.; Mundry, R.; Range, F.; Virányi, Z. Selective responding to human ostensive communication is an early developing capacity of domestic dogs. Developmental Science 2023, e13361. [CrossRef]
- Clark, H.; Leavens, D. A. Testing dogs in ape-like conditions: the effect of a barrier on dogs’ performance on the object-choice task. Animal Cognition 2019, 22(6), 1063-1072. [CrossRef]
- Riedel, J.; Schumann, K.; Kaminski, J.; Call, J.; Tomasello, M. The early ontogeny of human–dog communication. Animal Behaviour 2008, 75(3), 1003-1014. [CrossRef]
- Udell, M. A. R.; Dorey, N. R.; Wynne, C. D. L. Wolves outperform dogs in following human social cues. Animal Behaviour 2008, 76(6), 1767–1773. [CrossRef]
- Udell, M. A.; Spencer, J. M.; Dorey, N. R.; Wynne, C. D. Human-socialized wolves follow diverse human gestures… and they may not be alone. International Journal of Comparative Psychology 2012, 25(2). [CrossRef]
- Bhattacharjee, D., N, N. D.; Gupta, S.; Sau, S.; Sarkar, R.; Biswas, A.; ... & Bhadra, A. Free-ranging dogs show age related plasticity in their ability to follow human pointing. PLoS One 2017, 12(7), e0180643. [CrossRef]
- Elgier, A. M.; Jakovcevic, A.; Mustaca, A. E.; Bentosela, M. Pointing following in dogs: are simple or complex cognitive mechanisms involved?. Animal Cognition 2012, 15(6), 1111-1119. [CrossRef]
- Pettersson, H.; Kaminski, J.; Herrmann, E.; Tomasello, M. Understanding of human communicative motives in domestic dogs. Applied Animal Behaviour Science 2011, 133(3-4), 235-245. [CrossRef]
- ManyDogs Project; Espinosa, J.; Stevens, J. R.; Alberghina, D.; Always, H. E. E.; Barela, J. D.; Bogese, M.; Bray, E. E.; Buchsbaum, D.; Byosiere, S-E.; Byrne, M.; Cavalli, C. M.; Chaudoir, L. M.; Collins-Pisano, C.; DeBoer, H. J.; Douglas, L. E. L. C.; Dror, S.; Dzik, M. V.; Ferguson, B.; … Zylberfuden, S. G. ManyDogs 1: A Multi-Lab Replication Study of Dogs’ Pointing Comprehension. Animal Behavior and Cognition 2023, 10(3), 232-286. [CrossRef]
- Soproni, K.; Miklósi, Á.; Topál, J.; Csányi, V. Dogs' (Canis familaris) responsiveness to human pointing gestures. Journal of comparative psychology 2002, 116(1), 27. [CrossRef]
- Butterworth, G.; Itakura, S. How the eyes, head and hand serve definite reference. British Journal of Developmental Psychology 2000, 18(1), 25-50.
- Mulcahy, N. J.; Call, J. The performance of bonobos (Pan paniscus), chimpanzees (Pan troglodytes), and orangutans (Pongo pygmaeus) in two versions of an object-choice task. Journal of comparative psychology 2009, 123(3), 304. [CrossRef]
- Lyn, H.; Russell, J. L.; Hopkins, W. D. The impact of environment on the comprehension of declarative communication in apes. Psychological Science 2010, 21(3), 360-365. [CrossRef]
- Mulcahy, N. J.; Hedge, V. Are great apes tested with an abject object-choice task? Animal Behaviour 2012, 83(2), 313–321. [CrossRef]
- Udell, M. A. R.; Dorey, N. R.; Wynne, C. D. L. The performance of stray dogs (Canis familiaris) living in a shelter on human-guided object-choice tasks. Animal Behaviour 2010, 79(3), 717–725. [CrossRef]
- Jarvis, T.; Hall, N.J. Development of point following behaviors in shelter dogs. Learning & Behavior 2020, 48, 335–343. [CrossRef]
- Lazarowski, L.; Dorman, D. C. A comparison of pet and purpose-bred research dog (Canis familiaris) performance on human-guided object-choice tasks. Behavioural processes 2015, 110, 60–67. [CrossRef]
- Scott, J. P.; Fuller, J. L. Genetics and the Social Behavior of the Dog, Vol. 570; University of Chicago Press, 2012.
- Kretchmer, K. R.; Fox, M. W. Effects of domestication on animal behaviour. The Veterinary Record 1975, 96(5), 102-108. [CrossRef]
- Cox, R. P. The human/animal bond as a correlate of family functioning. Clinical Nursing Research 1993, 2(2), 224-231. [CrossRef]
- Topál, J.; Miklósi, Á.; Csányi, V.; Dóka, A. Attachment behavior in dogs (Canis familiaris): a new application of Ainsworth's (1969) Strange Situation Test. Journal of comparative psychology 1998, 112(3), 219.
- Scheider, L.; Grassmann, S.; Kaminski, J.; Tomasello, M. Domestic dogs use contextual information and tone of voice when following a human pointing gesture. PloS one 2011, 6(7), e21676. [CrossRef]
- Bhattacharjee, D.; Mandal, S.; Shit, P.; Varghese, M. G.; Vishnoi, A.; Bhadra, A. Free-ranging dogs are capable of utilizing complex human pointing cues. Frontiers in Psychology 2020, 10, 2818. [CrossRef]
- Collier-Baker, E.; Davis, J. M.; Suddendorf, T. Do Dogs (Canis familiaris) Understand Invisible Displacement? Journal of Comparative Psychology 2004, 118(4), 421–433. [CrossRef]
- Fiset, S.; LeBlanc, V. Invisible displacement understanding in domestic dogs (Canis familiaris): the role of visual cues in search behavior. Animal Cognition 2007, 10(2), 211–224. [CrossRef]
- Duranton, C.; Bedossa, T.; Gaunet, F. Pet dogs synchronize their walking pace with that of their owners in open outdoor areas. Animal cognition 2018, 21(2), 219-226. [CrossRef]
- Duranton, C.; Gaunet, F. Behavioral synchronization and affiliation: Dogs exhibit human-like skills. Learning & behavior 2018, 46, 364-373. [CrossRef]
- Mersmann, D.; Tomasello, M.; Call, J.; Kaminski, J.; Taborsky, M. Simple mechanisms can explain social learning in domestic dogs (Canis familiaris). Ethology 2011, 117(8), 675–690. [CrossRef]
- Bastos, A. P.; Rossano, F. Soundboard-using pets? Introducing a new global citizen science approach to interspecies communication. Interaction Studies 2023, 24(2), 311-334.
- Fugazza, C.; Dror, S.; Sommese, A.; Temesi, A.; Miklósi, Á. Word learning dogs (Canis familiaris) provide an animal model for studying exceptional performance. Scientific Reports 2021, 11(1), 14070. [CrossRef]
- The jamovi project (2021). jamovi. (Version 2.2) [Computer Software]. Retrieved from https://www.jamovi.org.
- R Core Team (2021). R: A Language and environment for statistical computing. (Version 4.0) [Computer software]. Retrieved from https://cran.r-project.org. (R packages retrieved from MRAN snapshot 2021-04-01).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).