Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
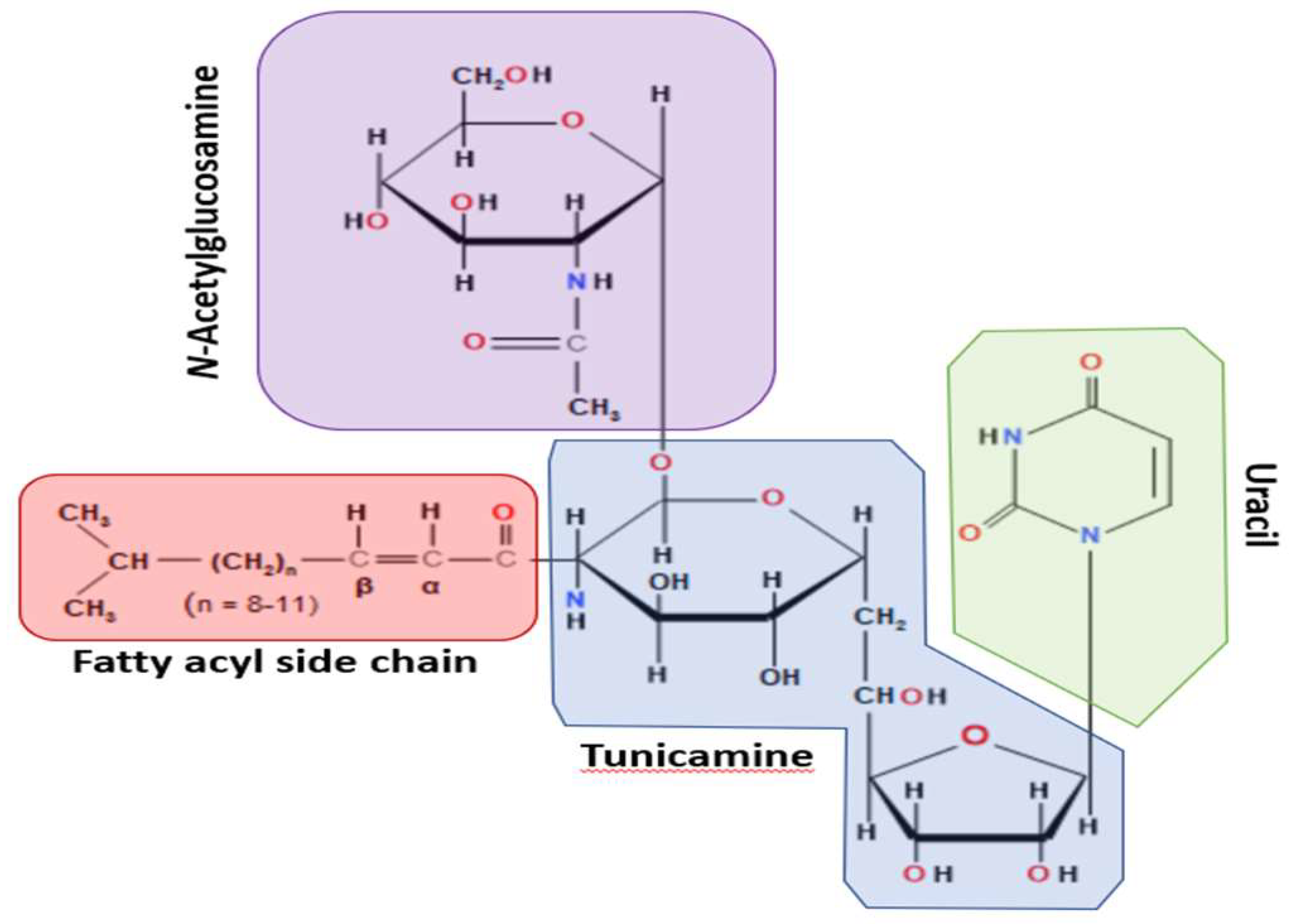
2. Origin and Basic Biology of Tunicamycin
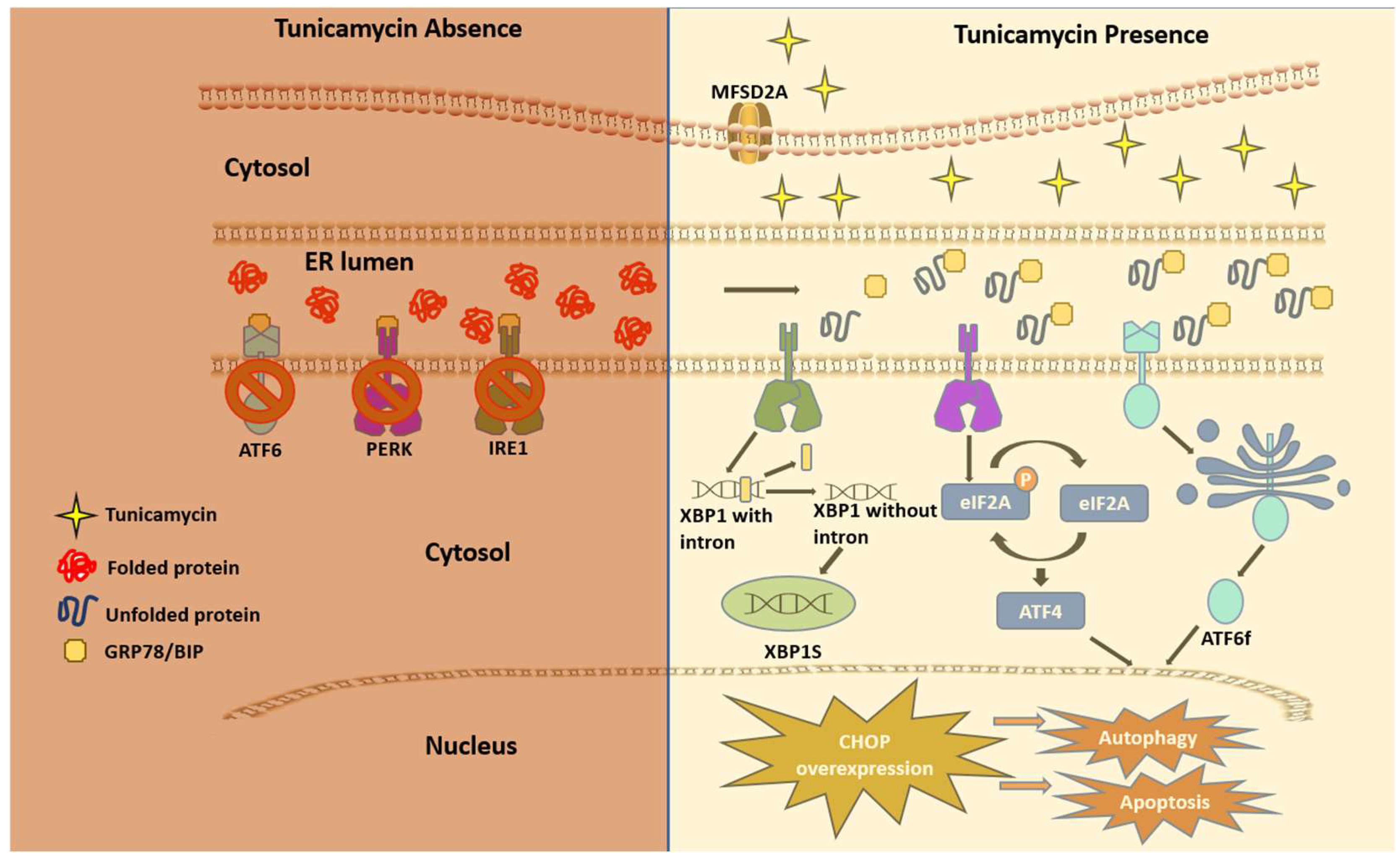
3. How does Tunicamycin work?
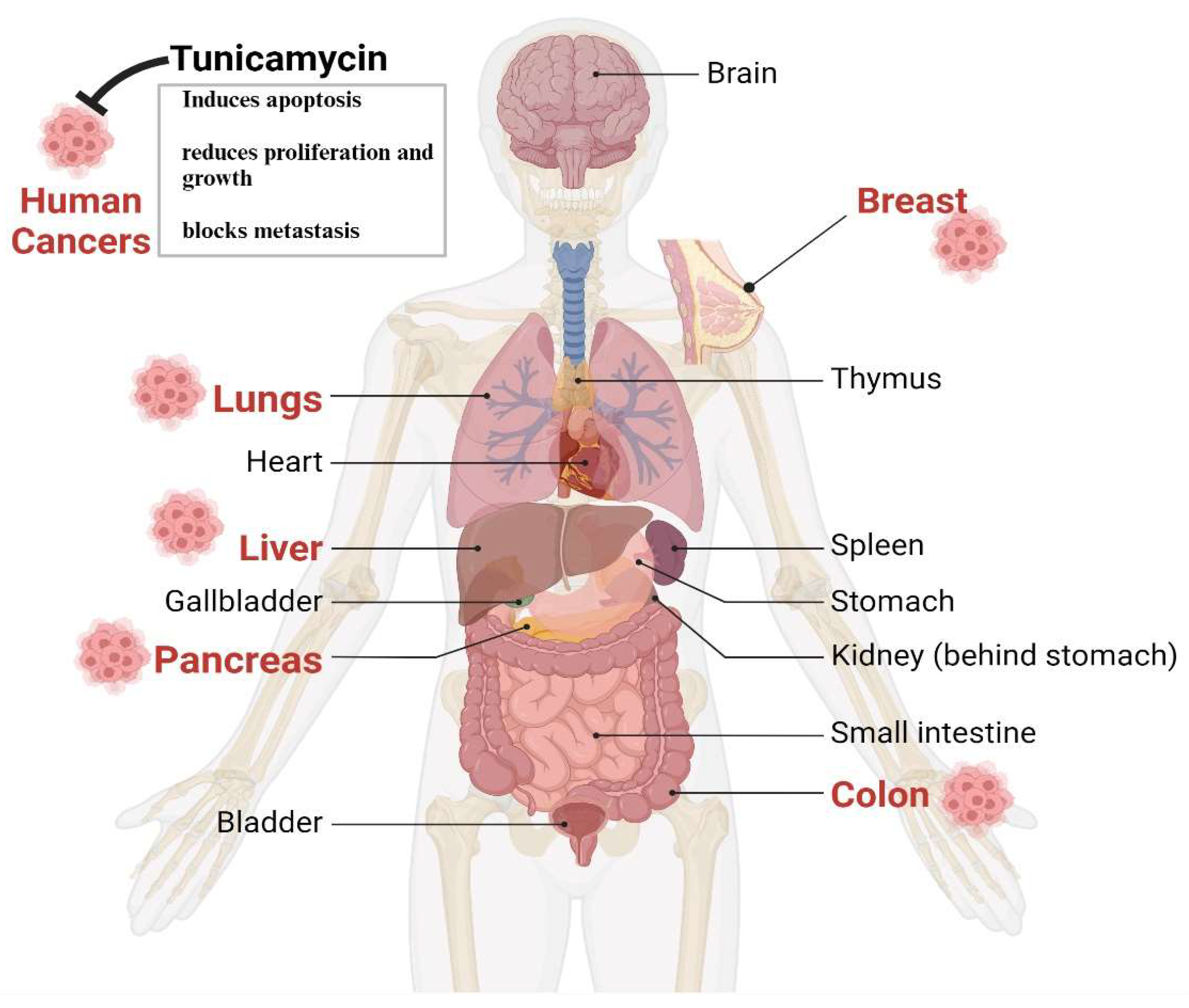
4. Tunicamycin in Cancer Therapy
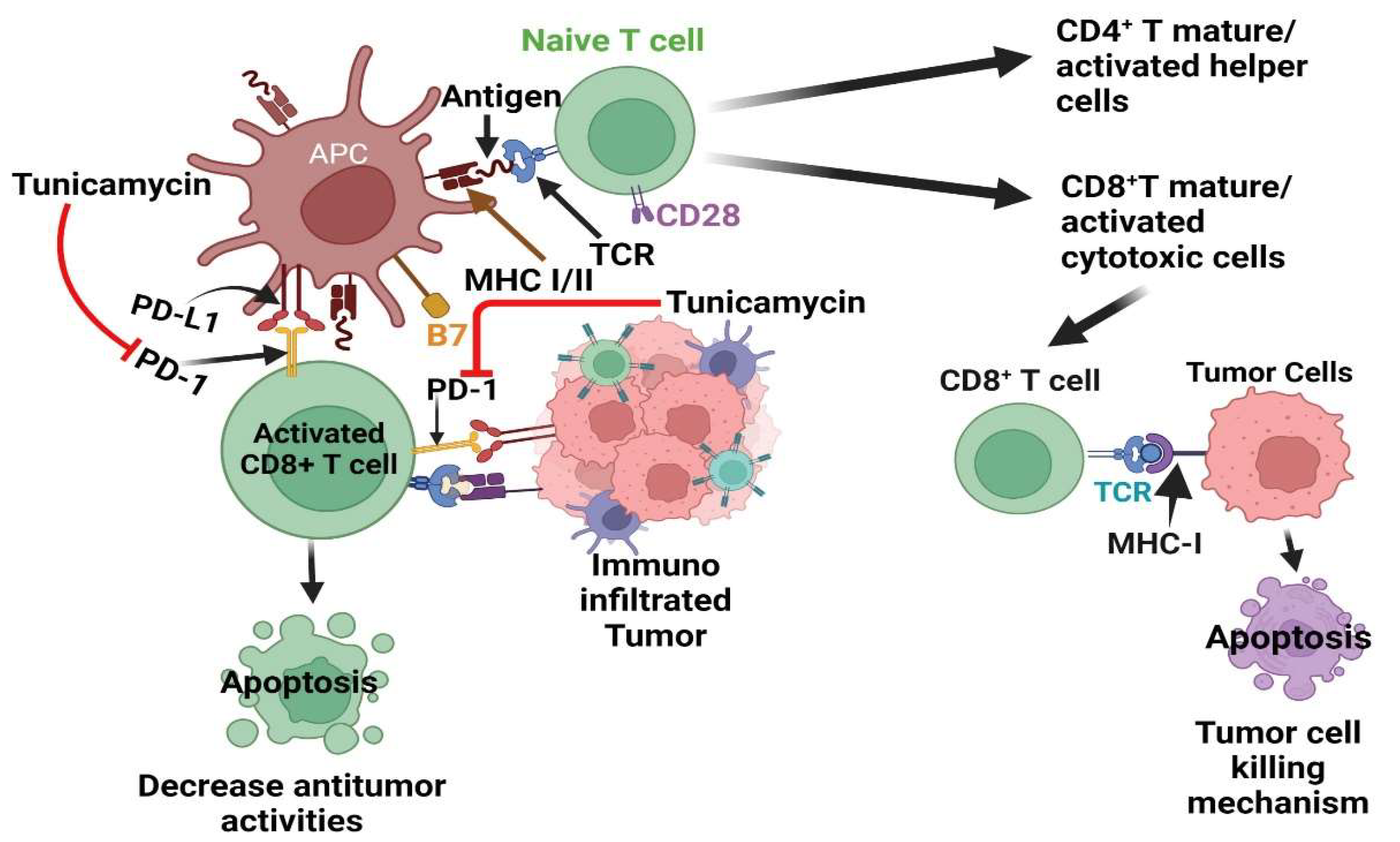
5. Tunicamycin in Immunotherapy
6. The Pitfalls of the Use of Tunicamycin
7. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736-56. Epub 20111218. [CrossRef]
- Clausen H, Bennett EP. A family of UDP-GalNAc: polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology. 1996;6(6):635-46. [CrossRef] [PubMed]
- Varki A. Biological roles of glycans. Glycobiology. 2017;27(1):3-49. Epub 20160824. [CrossRef]
- Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291(5512):2370-6. [CrossRef] [PubMed]
- Schoberer J, Shin YJ, Vavra U, Veit C, Strasser R. Analysis of Protein Glycosylation in the ER. Methods Mol Biol. 2018;1691:205-22. [CrossRef]
- Takahashi K, Raska M, Stuchlova Horynova M, Hall SD, Poulsen K, Kilian M, et al. Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy. PLoS One. 2014;9(2):e99026. Epub 20140611. [CrossRef]
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anticancer vaccines. Adv Exp Med Biol. 2001;491:369-402. [CrossRef] [PubMed]
- Matsumoto Y, Ju T. Aberrant Glycosylation as Immune Therapeutic Targets for Solid Tumors. Cancers (Basel). 2023;15(14). Epub 20230708. [CrossRef]
- Veillon L, Fakih C, Abou-El-Hassan H, Kobeissy F, Mechref Y. Glycosylation Changes in Brain Cancer. ACS Chem Neurosci. 2018;9(1):51-72. Epub 20171107. [CrossRef]
- Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. Epub 20140213. [CrossRef]
- Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478-89. [CrossRef]
- Meany DL, Chan DW. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin Proteomics. 2011;8(1):7. Epub 20110603. [CrossRef]
- Arnal-Estape A, Nguyen DX. Sweets for a bitter end: lung cancer cell-surface protein glycosylation mediates metastatic colonization. Cancer Discov. 2015;5(2):109-11. [CrossRef]
- Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21(3):505-15. [CrossRef] [PubMed]
- MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14(4):351-62. [CrossRef] [PubMed]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10(6):415-33. [CrossRef] [PubMed]
- Mereiter S, Balmana M, Campos D, Gomes J, Reis CA. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell. 2019;36(1):6-16. [CrossRef] [PubMed]
- Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen J, et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res. 2018;37(1):272. Epub 20181109. [CrossRef]
- Akira Takatsuki KA, Gakuzo Tamura. Tunicamycin, A New Antibiotic, I Isolation And Characterization of Tunicamycin. The Journal of Antibiotics. 1970;24(4):215-23. [CrossRef]
- Yoo J, Mashalidis EH, Kuk ACY, Yamamoto K, Kaeser B, Ichikawa S, et al. GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nat Struct Mol Biol. 2018;25(3):217-24. Epub 2018/02/21. [CrossRef]
- Duksin D, Bornstein P. Changes in surface properties of normal and transformed cells caused by tunicamycin, an inhibitor of protein glycosylation. Proc Natl Acad Sci U S A. 1977;74(8):3433-7. Epub 1977/08/01. [CrossRef]
- Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299-309. Epub 20191113. [CrossRef]
- Arey BJ. The Role of Glycosylation in Receptor Signaling IntechOpen. 2012. Epub 09/26/2012. [CrossRef]
- Seales EC, Jurado GA, Singhal A, Bellis SL. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene. 2003;22(46):7137-45. [CrossRef] [PubMed]
- Very N, Lefebvre T, El Yazidi-Belkoura I. Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget. 2018;9(1):1380-402. Epub 20171103. [CrossRef]
- Wang Y, Zhang L, He Z, Deng J, Zhang Z, Liu L, et al. Tunicamycin induces ER stress and inhibits tumorigenesis of head and neck cancer cells by inhibiting N-glycosylation. Am J Transl Res. 2020;12(2):541-50. Epub 2020/03/21.
- Bassik MC, Kampmann M. Knocking out the door to tunicamycin entry. Proc Natl Acad Sci U S A. 2011;108(29):11731-2. Epub 20110706. [CrossRef]
- Reiling JH, Clish CB, Carette JE, Varadarajan M, Brummelkamp TR, Sabatini DM. A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc Natl Acad Sci U S A. 2011;108(29):11756-65. Epub 20110615. [CrossRef]
- de-Freitas-Junior JC, Bastos LG, Freire-Neto CA, Rocher BD, Abdelhay ES, Morgado-Diaz JA. N-glycan biosynthesis inhibitors induce in vitro anticancer activity in colorectal cancer cells. J Cell Biochem. 2012;113(9):2957-66. [CrossRef] [PubMed]
- Guha P, Kaptan E, Gade P, Kalvakolanu DV, Ahmed H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget. 2017;8(40):68191-207. Epub 20170715. [CrossRef]
- Cao SS, Kaufman RJ. Unfolded protein response. Curr Biol. 2012;22(16):R622-6. [CrossRef] [PubMed]
- Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011;108(12):2777-93. Epub 20110809. [CrossRef]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, et al. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3(6):411-21. [CrossRef] [PubMed]
- Read A, Schroder M. The Unfolded Protein Response: An Overview. Biology (Basel). 2021;10(5). Epub 20210429. [CrossRef]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110(10):1383-8. [CrossRef]
- Lei Y, Wang S, Ren B, Wang J, Chen J, Lu J, et al. CHOP favors endoplasmic reticulum stress-induced apoptosis in hepatocellular carcinoma cells via inhibition of autophagy. PLoS One. 2017;12(8):e0183680. Epub 20170825. [CrossRef]
- Hasegawa A, Osuga Y, Hirota Y, Hamasaki K, Kodama A, Harada M, et al. Tunicamycin enhances the apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand in endometriotic stromal cells. Hum Reprod. 2009;24(2):408-14. Epub 20081101. [CrossRef] [PubMed]
- Delom F, Emadali A, Cocolakis E, Lebrun JJ, Nantel A, Chevet E. Calnexin-dependent regulation of tunicamycin-induced apoptosis in breast carcinoma MCF-7 cells. Cell Death Differ. 2007;14(3):586-96. Epub 20060721. [CrossRef] [PubMed]
- Banerjee A, Lang JY, Hung MC, Sengupta K, Banerjee SK, Baksi K, et al. Unfolded protein response is required in nu/nu mice microvasculature for treating breast tumor with tunicamycin. J Biol Chem. 2011;286(33):29127-38. Epub 20110615. [CrossRef]
- Wang X, Xiong W, Tang Y. Tunicamycin suppresses breast cancer cell growth and metastasis via regulation of the protein kinase B/nuclear factor-kappaB signaling pathway. Oncol Lett. 2018;15(4):4137-42. Epub 20180126. [CrossRef]
- Han X, Zhang X, Li H, Huang S, Zhang S, Wang F, et al. Tunicamycin enhances the antitumor activity of trastuzumab on breast cancer in vitro and in vivo. Oncotarget. 2015;6(36):38912-25. [CrossRef]
- You Z, He L, Yan N. Tunicamycin-Induced Endoplasmic Reticulum Stress Promotes Breast Cancer Cell MDA-MB-231 Apoptosis through Inhibiting Wnt/beta-Catenin Signaling Pathway. J Healthc Eng. 2021;2021:6394514. Epub 20210715. [CrossRef]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983-8. Epub 20030310. [CrossRef]
- Nami B, Donmez H, Kocak N. Tunicamycin-induced endoplasmic reticulum stress reduces in vitro subpopulation and invasion of CD44+/CD24- phenotype breast cancer stem cells. Exp Toxicol Pathol. 2016;68(7):419-26. Epub 20160624. [CrossRef] [PubMed]
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-54. Epub 20230301. [CrossRef] [PubMed]
- You S, Li W, Guan Y. Tunicamycin inhibits colon carcinoma growth and aggressiveness via modulation of the ERK-JNK-mediated AKT/mTOR signaling pathway. Mol Med Rep. 2018;17(3):4203-12. Epub 20180117. [CrossRef]
- Guo X, Meng Y, Sheng X, Guan Y, Zhang F, Han Z, et al. Tunicamycin enhances human colon cancer cells to TRAIL-induced apoptosis by JNK-CHOP-mediated DR5 upregulation and the inhibition of the EGFR pathway. Anticancer Drugs. 2017;28(1):66-74. [CrossRef] [PubMed]
- de Freitas Junior JC, Silva Bdu R, de Souza WF, de Araujo WM, Abdelhay ES, Morgado-Diaz JA. Inhibition of N-linked glycosylation by tunicamycin induces E-cadherin-mediated cell-cell adhesion and inhibits cell proliferation in undifferentiated human colon cancer cells. Cancer Chemother Pharmacol. 2011;68(1):227-38. Epub 20101007. [CrossRef] [PubMed]
- Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin Y, Schaeffer DF, et al. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2alpha and IRE1alpha/XBP-1 in colorectal cancer. Cell Death Dis. 2019;10(7):504. Epub 20190626. [CrossRef]
- Liu CY, Hsu CC, Huang TT, Lee CH, Chen JL, Yang SH, et al. ER stress-related ATF6 upregulates CIP2A and contributes to poor prognosis of colon cancer. Mol Oncol. 2018;12(10):1706-17. Epub 20180820. [CrossRef]
- Massion PP, Carbone DP. The molecular basis of lung cancer: molecular abnormalities and therapeutic implications. Respir Res. 2003;4(1):12. Epub 20031007. [CrossRef]
- Ahmmed B, Khan MN, Nisar MA, Kampo S, Zheng Q, Li Y, et al. Tunicamycin enhances the suppressive effects of Cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-kappaB signaling pathway. Int J Oncol. 2019;54(2):431-42. Epub 20181128. [CrossRef]
- Liu L, Li S, Qu Y, Bai H, Pan X, Wang J, et al. Ablation of ERO1A induces lethal endoplasmic reticulum stress responses and immunogenic cell death to activate anti-tumor immunity. Cell Rep Med. 2023;4(10):101206. Epub 20230927. [CrossRef]
- Shin S, Solorzano J, Liauzun M, Pyronnet S, Bousquet C, Martineau Y. Translational alterations in pancreatic cancer: a central role for the integrated stress response. NAR Cancer. 2022;4(4):zcac031. Epub 20221028. [CrossRef]
- Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18(7). Epub 2017/06/24. [CrossRef]
- Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw Open. 2021;4(4):e214708. Epub 20210401. [CrossRef]
- Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16(4):207-20. Epub 2019/02/06. [CrossRef] [PubMed]
- Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495-501. Epub 2015/02/27. [CrossRef]
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. Epub 20160421. [CrossRef] [PubMed]
- Banerjee SK, Maity G, Haque I, Ghosh A, Sarkar S, Gupta V, et al. Human pancreatic cancer progression: an anarchy among CCN-siblings. J Cell Commun Signal. 2016;10(3):207-16. Epub 2016/08/20. [CrossRef]
- Basu SK, Lee S, Salotti J, Basu S, Sakchaisri K, Xiao Z, et al. Oncogenic RAS-Induced Perinuclear Signaling Complexes Requiring KSR1 Regulate Signal Transmission to Downstream Targets. Cancer Res. 2018;78(4):891-908. Epub 2017/12/21. [CrossRef]
- Banerjee SK, Makdisi WF, Weston AP, Campbell DR. A two-step enriched-nested PCR technique enhances sensitivity for detection of codon 12 K-ras mutations in pancreatic adenocarcinoma. Pancreas. 1997;15(1):16-24. Epub 1997/07/01. [CrossRef] [PubMed]
- Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, et al. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63(9):2005-9. [PubMed]
- di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144(6):1220-9. Epub 2013/04/30. [CrossRef]
- Birkeness LB, Banerjee S, Quadir M, Banerjee SK. The role of CCNs in controlling cellular communication in the tumor microenvironment. J Cell Commun Signal. 2022. Epub 2022/06/09. [CrossRef] [PubMed]
- Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, et al. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007;254(1):63-70. Epub 2007/03/27. [CrossRef] [PubMed]
- Bannoura SF, Khan HY, Azmi AS. KRAS G12D targeted therapies for pancreatic cancer: Has the fortress been conquered? Front Oncol. 2022;12:1013902. Epub 2022/12/20. [CrossRef]
- Hafezi S, Saber-Ayad M, Abdel-Rahman WM. Highlights on the Role of KRAS Mutations in Reshaping the Microenvironment of Pancreatic Adenocarcinoma. Int J Mol Sci. 2021;22(19). Epub 20210923. [CrossRef]
- Dias Carvalho P, Guimaraes CF, Cardoso AP, Mendonca S, Costa AM, Oliveira MJ, et al. KRAS Oncogenic Signaling Extends beyond Cancer Cells to Orchestrate the Microenvironment. Cancer Res. 2018;78(1):7-14. Epub 20171220. [CrossRef] [PubMed]
- Hamarsheh S, Gross O, Brummer T, Zeiser R. Immune modulatory effects of oncogenic KRAS in cancer. Nat Commun. 2020;11(1):5439. Epub 20201028. [CrossRef]
- Cheng NC, Vonderheide RH. Immune vulnerabilities of mutant KRAS in pancreatic cancer. Trends Cancer. 2023;9(11):928-36. Epub 20230729. [CrossRef]
- Vonderheide RH. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell. 2018;33(4):563-9. [CrossRef]
- Thind K, Padrnos LJ, Ramanathan RK, Borad MJ. Immunotherapy in pancreatic cancer treatment: a new frontier. Therap Adv Gastroenterol. 2017;10(1):168-94. Epub 20161017. [CrossRef]
- Jacenik D, Karagiannidis I, Beswick EJ. Th2 cells inhibit growth of colon and pancreas cancers by promoting anti-tumorigenic responses from macrophages and eosinophils. Br J Cancer. 2023;128(2):387-97. Epub 20221114. [CrossRef]
- Baylot V, Andrieu C, Katsogiannou M, Taieb D, Garcia S, Giusiano S, et al. OGX-427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer. Cell Death Dis. 2011;2(10):e221. Epub 20111020. [CrossRef]
- Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64(23):8639-42. [CrossRef] [PubMed]
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21(24):3232-7. Epub 20071130. [CrossRef]
- Xing Y, Ge Y, Liu C, Zhang X, Jiang J, Wei Y. ER stress inducer tunicamycin suppresses the self-renewal of glioma-initiating cell partly through inhibiting Sox2 translation. Oncotarget. 2016;7(24):36395-406. [CrossRef]
- Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233-47. [CrossRef]
- Ng MSF, Kwok I, Tan L, Shi C, Cerezo-Wallis D, Tan Y, et al. Deterministic reprogramming of neutrophils within tumors. Science. 2024;383(6679):eadf6493. Epub 20240112. [CrossRef] [PubMed]
- Awad RM, De Vlaeminck Y, Maebe J, Goyvaerts C, Breckpot K. Turn Back the TIMe: Targeting Tumor Infiltrating Myeloid Cells to Revert Cancer Progression. Front Immunol. 2018;9:1977. Epub 20180831. [CrossRef]
- Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16(7):447-62. [CrossRef] [PubMed]
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44(2):343-54. Epub 20160209. [CrossRef]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56-61. [CrossRef] [PubMed]
- Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29(5):3044-60. Epub 20220424. [CrossRef]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450-61. Epub 20150406. [CrossRef]
- Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124(2):359-67. Epub 20200915. [CrossRef]
- Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45(2):389-401. Epub 20160809. [CrossRef]
- Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15(5):1111-22. Epub 20190319. [CrossRef]
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651-68. Epub 20200520. [CrossRef]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974-82. Epub 20150120. [CrossRef]
- Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3:23. Epub 20150616. [CrossRef]
- Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8(+) T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell. 2018;33(3):480-94 e7. [CrossRef] [PubMed]
- Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends Pharmacol Sci. 2018;39(3):307-25. Epub 20171215. [CrossRef] [PubMed]
- Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. Epub 20210107. [CrossRef]
- Escors D, Gato-Canas M, Zuazo M, Arasanz H, Garcia-Granda MJ, Vera R, et al. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. 2018;3:26. Epub 20180928. [CrossRef]
- Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76(3):359-70. Epub 20191024. [CrossRef]
- Sun L, Li CW, Chung EM, Yang R, Kim YS, Park AH, et al. Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity. Cancer Res. 2020;80(11):2298-310. Epub 20200310. [CrossRef]
- Salatino M, Girotti MR, Rabinovich GA. Glycans Pave the Way for Immunotherapy in Triple-Negative Breast Cancer. Cancer Cell. 2018;33(2):155-7. [CrossRef] [PubMed]
- Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell. 2018;33(2):187-201 e10. [CrossRef]
- Wang YN, Lee HH, Hsu JL, Yu D, Hung MC. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J Biomed Sci. 2020;27(1):77. Epub 20200703. [CrossRef]
- Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell. 2019;36(2):168-78 e4. Epub 20190718. [CrossRef]
- Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. Epub 20160830. [CrossRef]
- Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312-8. Epub 20181203. [CrossRef] [PubMed]
- Samadi M, Kamrani A, Nasiri H, Shomali N, Heris JA, Shahabi P, et al. Cancer immunotherapy focusing on the role of interleukins: A comprehensive and updated study. Pathol Res Pract. 2023;249:154732. Epub 20230801. [CrossRef] [PubMed]
- Blomberg OS, Spagnuolo L, Garner H, Voorwerk L, Isaeva OI, van Dyk E, et al. IL-5-producing CD4(+) T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell. 2023;41(1):106-23 e10. Epub 20221215. [CrossRef] [PubMed]
- Bohnacker S, Hildenbrand K, Aschenbrenner I, Muller SI, Bieren JE, Feige MJ. Influence of glycosylation on IL-12 family cytokine biogenesis and function. Mol Immunol. 2020;126:120-8. Epub 20200818. [CrossRef] [PubMed]
- Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(8):463-85. [CrossRef]
- Grisaru-Tal S, Munitz A. T cell-eosinophil crosstalk-A new road for effective immune checkpoint blockade in breast cancer? Cancer Cell. 2023;41(1):9-11. Epub 20221215. [CrossRef] [PubMed]
- Kodama S, Tsujimoto M, Tsuruoka N, Sugo T, Endo T, Kobata A. Role of sugar chains in the in-vitro activity of recombinant human interleukin 5. Eur J Biochem. 1993;211(3):903-8. [CrossRef] [PubMed]
- Kunimoto DY, Allison KC, Watson C, Fuerst T, Armstrong GD, Paul W, et al. High-level production of murine interleukin-5 (IL-5) utilizing recombinant baculovirus expression. Purification of the rIL-5 and its use in assessing the biologic role of IL-5 glycosylation. Cytokine. 1991;3(3):224-30. [CrossRef] [PubMed]
- Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. 2001;2(2):71-9. Epub 20010308. [CrossRef]
- Hirai K, Yamaguchi M, Misaki Y, Takaishi T, Ohta K, Morita Y, et al. Enhancement of human basophil histamine release by interleukin 5. J Exp Med. 1990;172(5):1525-8. [CrossRef]
- Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8(4):349-55. [CrossRef] [PubMed]
- Alam A, Levanduski E, Denz P, Villavicencio HS, Bhatta M, Alhorebi L, et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell. 2022;40(2):153-67 e11. Epub 20220203. [CrossRef]
- Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2(1):1-8. [CrossRef] [PubMed]
- Gitto SB, Beardsley JM, Nakkina SP, Oyer JL, Cline KA, Litherland SA, et al. Identification of a novel IL-5 signaling pathway in chronic pancreatitis and crosstalk with pancreatic tumor cells. Cell Commun Signal. 2020;18(1):95. Epub 20200617. [CrossRef]
- Thakur PC, Miller-Ocuin JL, Nguyen K, Matsuda R, Singhi AD, Zeh HJ, et al. Inhibition of endoplasmic-reticulum-stress-mediated autophagy enhances the effectiveness of chemotherapeutics on pancreatic cancer. J Transl Med. 2018;16(1):190. Epub 20180709. [CrossRef]
- Li A, Song NJ, Riesenberg BP, Li Z. The Emerging Roles of Endoplasmic Reticulum Stress in Balancing Immunity and Tolerance in Health and Diseases: Mechanisms and Opportunities. Front Immunol. 2019;10:3154. Epub 20200211. [CrossRef]
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10-29. Epub 20160603. [CrossRef]
- Chen Z, Wang Z, Gu Z. Bioinspired and Biomimetic Nanomedicines. Acc Chem Res. 2019;52(5):1255-64. Epub 2019/04/13. [CrossRef]
- Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M. Impact of nanoparticles on the immune system. J Biomed Nanotechnol. 2011;7(1):193-4. [CrossRef] [PubMed]
- Ray P, Haideri, N, Haque,I, Mohsmmed,O, Chakraborty,S, Banerjee,S, Quadir,M, Brinker,A.E., Banerjee,S.K. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J Immunological Sci. 2021;5(5):19-33. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




