Submitted:
25 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Reagents
Cells
Isolation of human neutrophils, monocytes, and lymphocytes
Biogenesis of novel 7S, 14R-DHA
Analysis and Isolation of 7S, 14R-diHDHA and of its precursor 14R-HDHA
Protocols for the treatment of cells in vitro
Mice
Treatment procedures in vivo
Histological study
Statistical analysis
3. Results
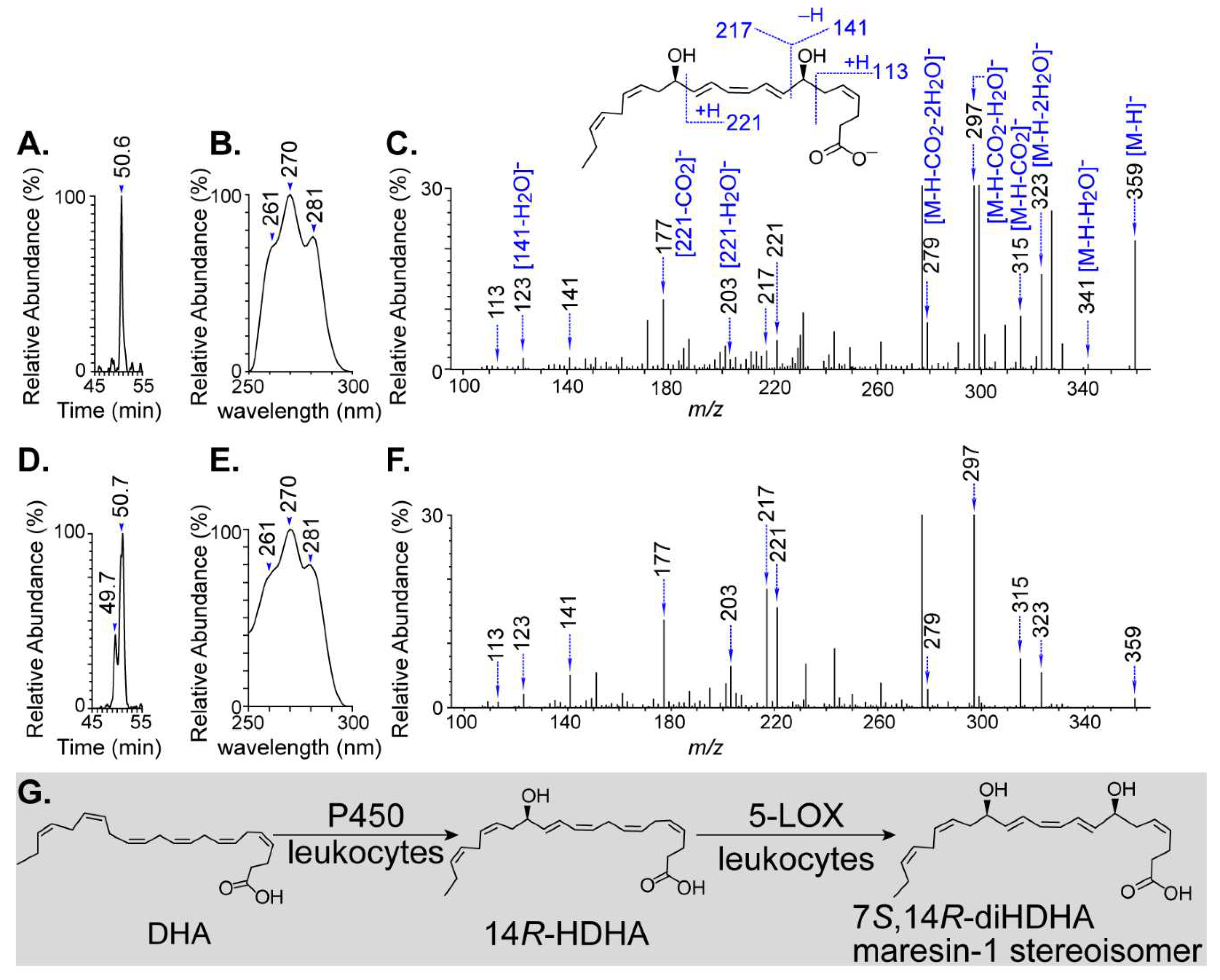
3.1. Biogenesis of novel 7S,14R-dihydroxy-4Z,8E,10Z,12E,16Z,19Z-DHA
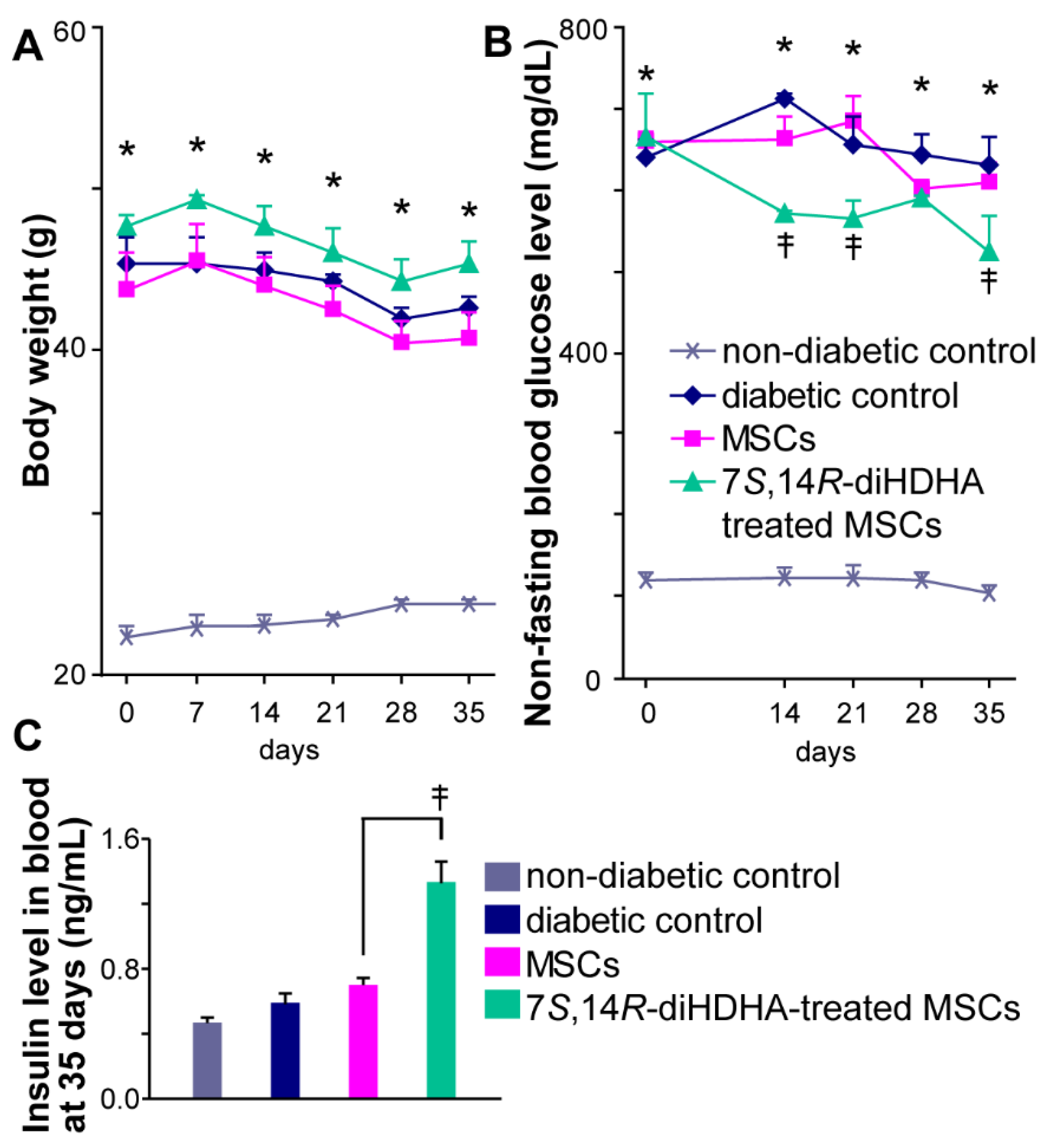
3.2. 7S,14R-diHDHA enhanced MSC function to regulate nonfasting blood glucose levels
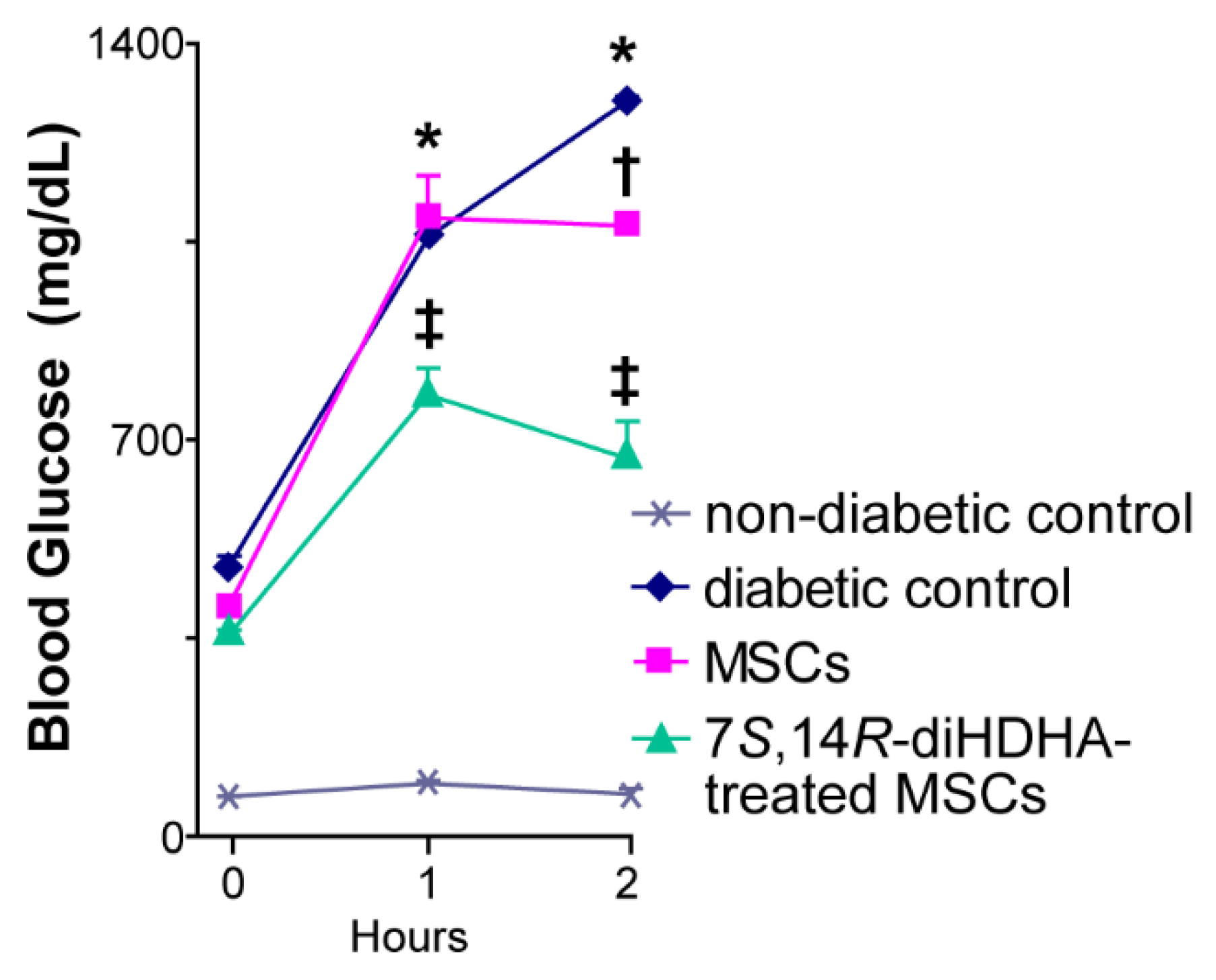
3.3. 7S,14R-diHDHA treatment promoted the capacity of MSCs to improve glucose tolerance in db/db mice
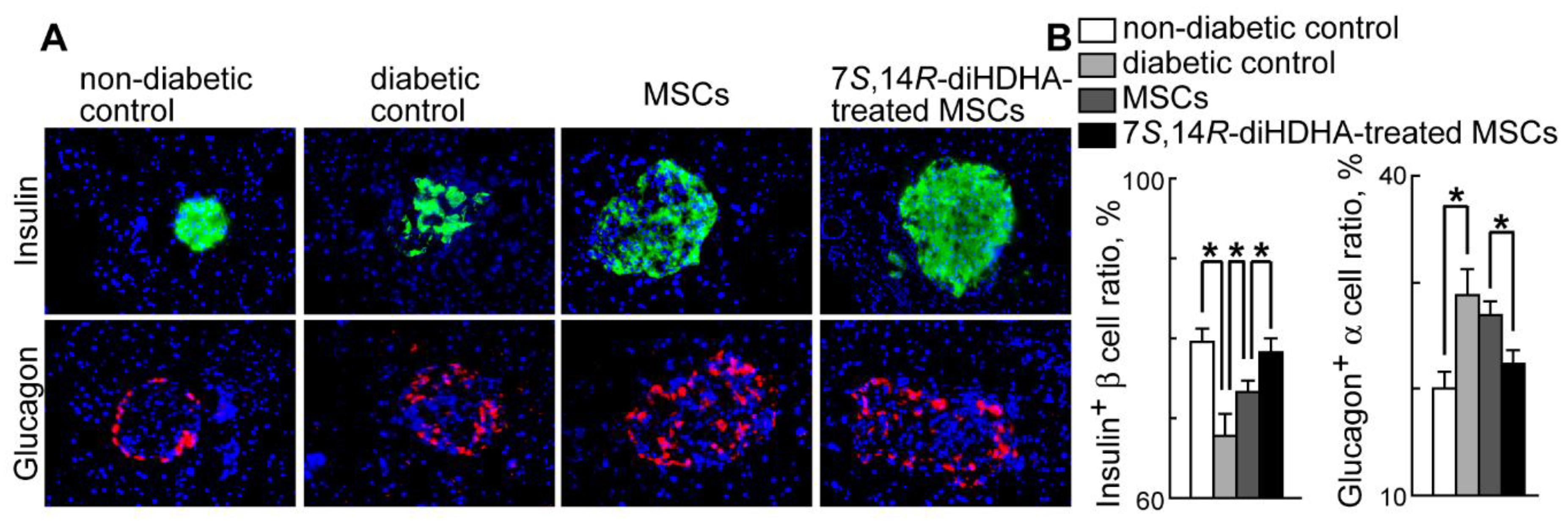
3.4. 7S,14R-diHDHA induced MSC function to augment the ratio of β-cells and to reduce the ratio of α-cells in pancreatic islets
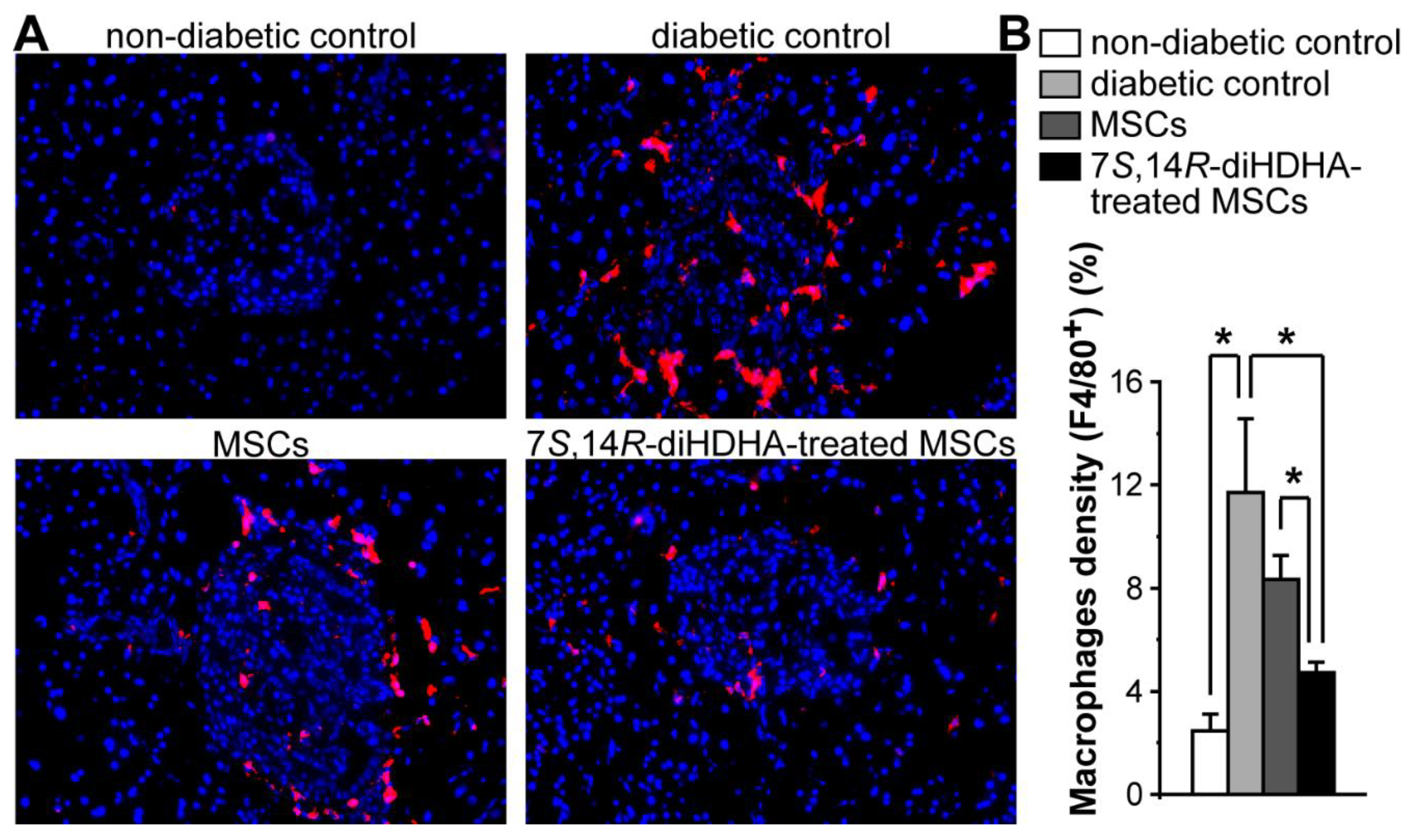
3.5. 7S,14R-diHDHA enhanced MSC function to decrease the number of macrophages in islets.
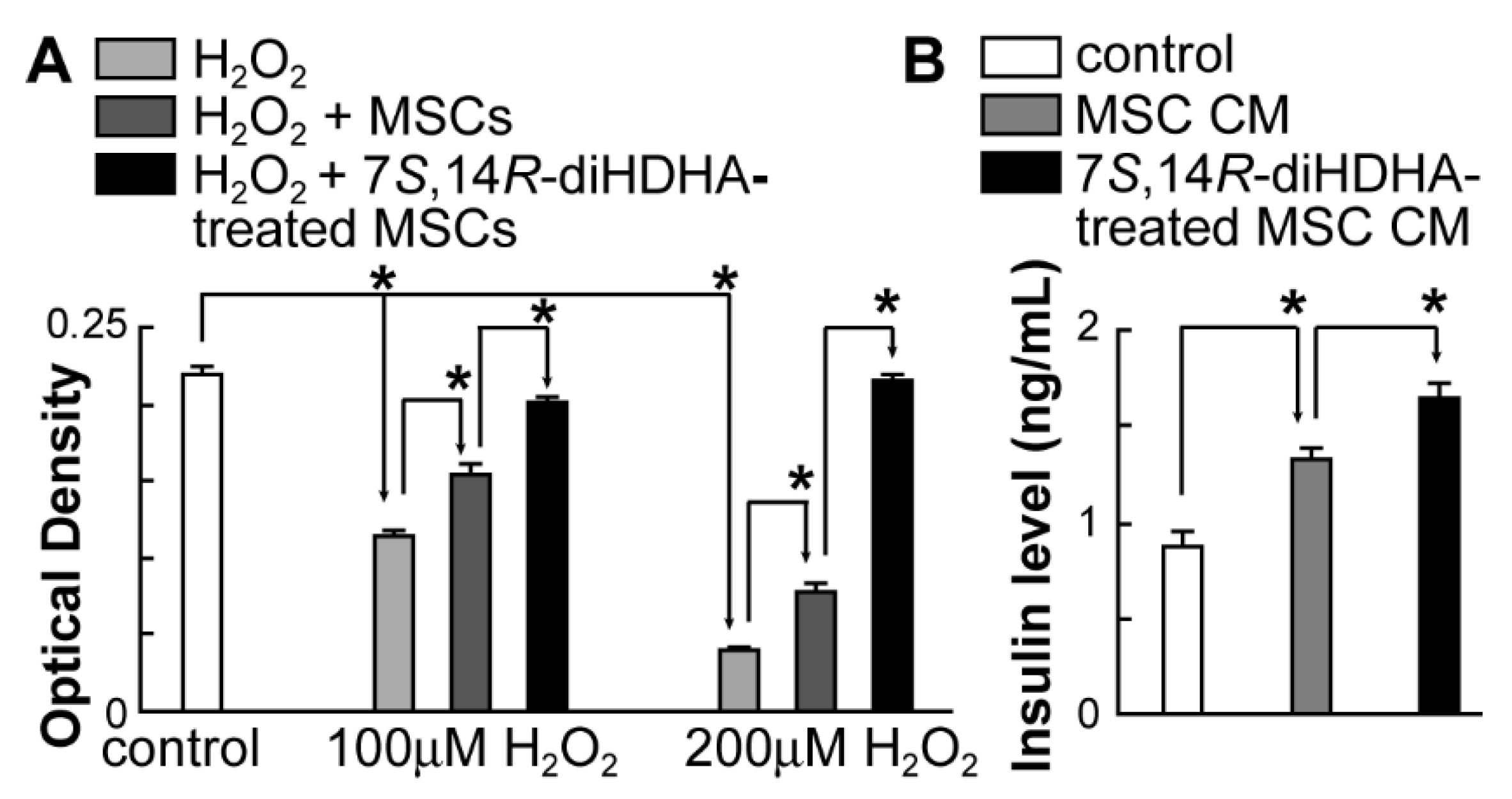
3.6. 7S,14R-diHDHA augmented MSC function to increase min6 β-cell viability and insulin secretion
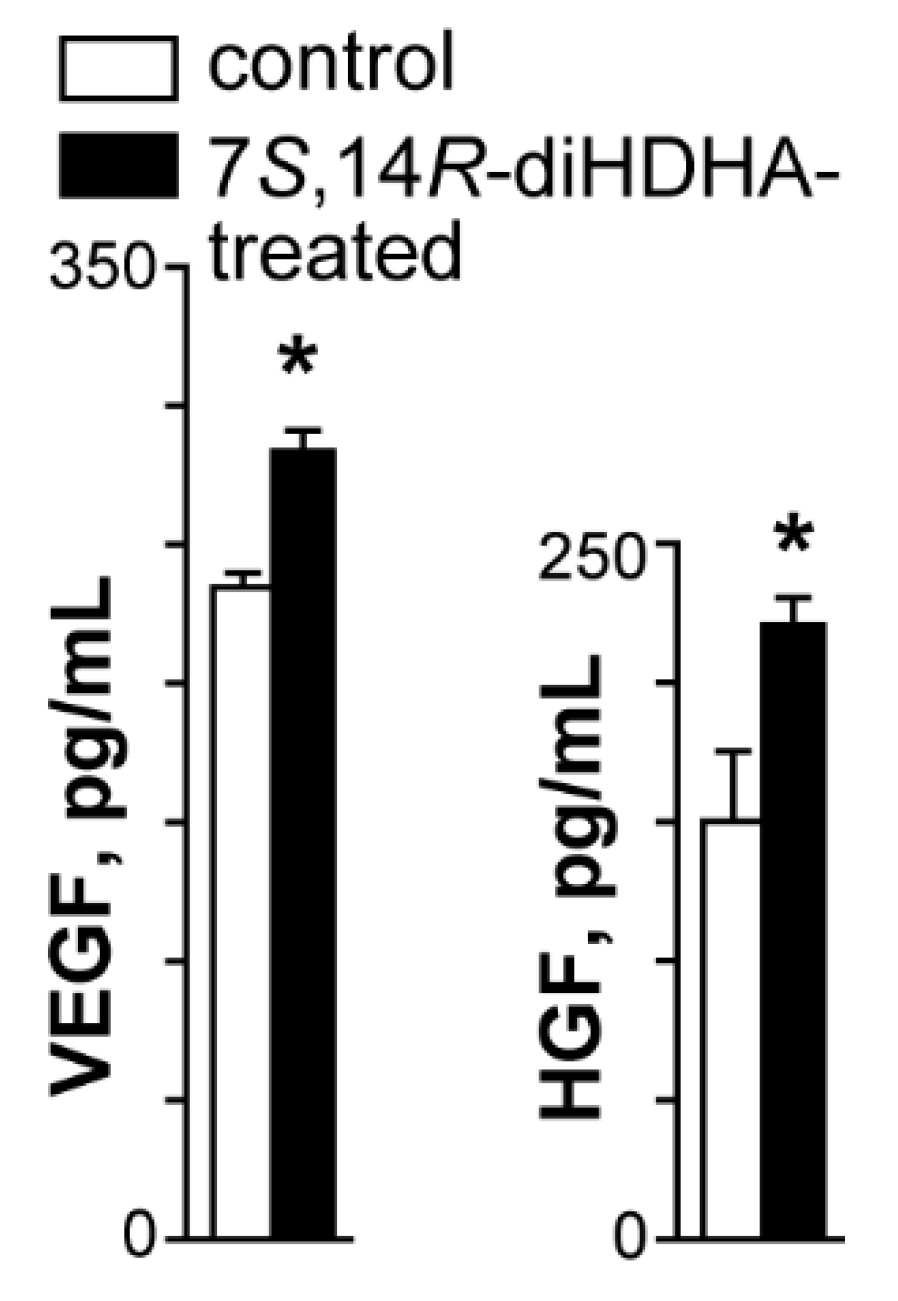
3.7. 7S,14R-diHDHA treatment enhanced MSC secretion of trophic growth factors
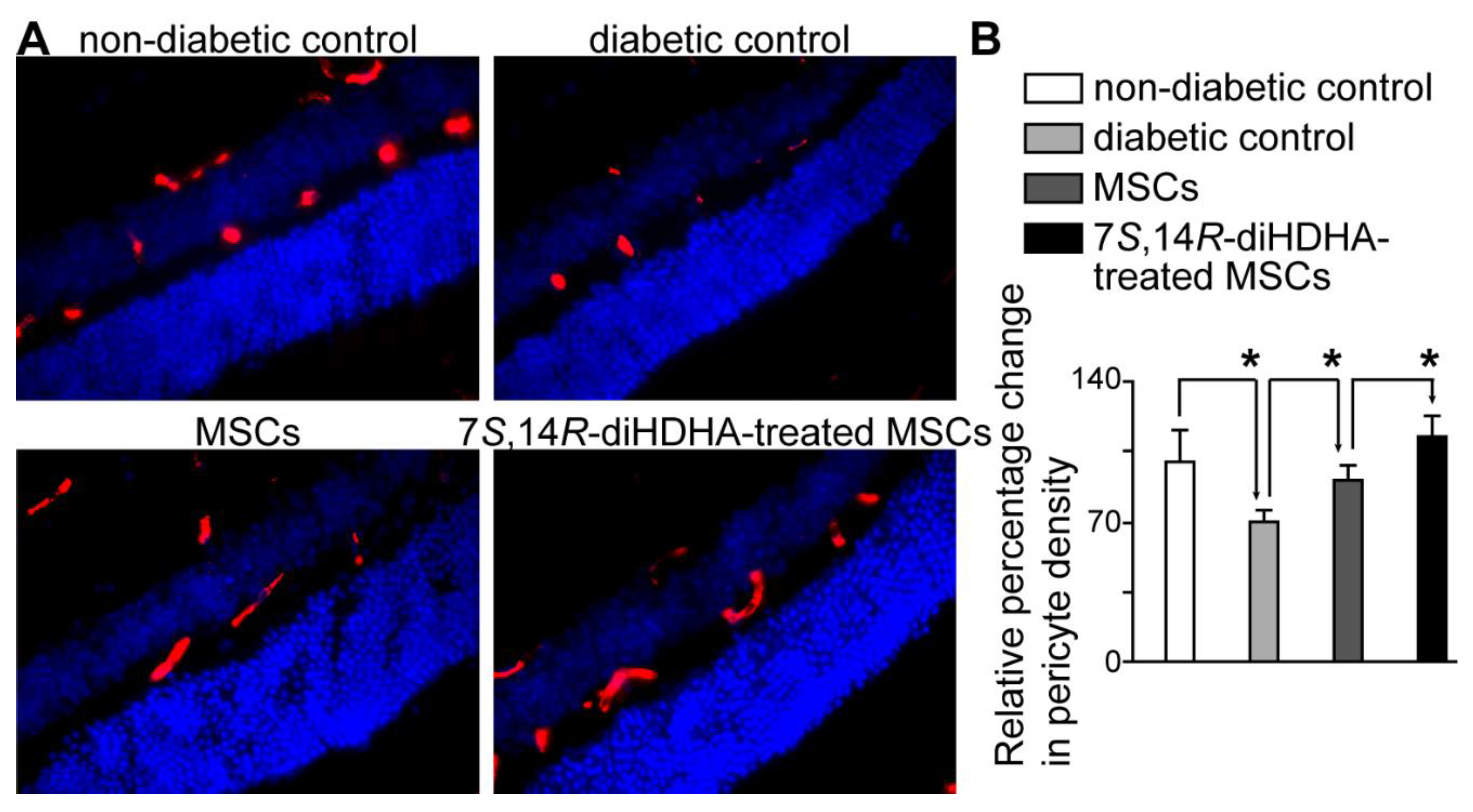
3.8. 7S,14R-diHDHA treatment induced MSC function to decrease pericyte loss in the retina
4. Discussion
Leukocytes produced 7S,14R-diHDHA while P450 and 5-LOX catalyzed the biosynthesis
Harnessing mesenchymal stem cells by 7S,14R-diHDHA to ameliorate diabetic mellitus and retinal pericyte loss
Future directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Florez, J.C. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 2008, 51, 1100–1110. [Google Scholar] [CrossRef]
- Gunton, J.E.; Kulkarni, R.N.; Yim, S.; Okada, T.; Hawthorne, W.J.; Tseng, Y.H.; Roberson, R.S.; Ricordi, C.; O'Connell, P.J.; Gonzalez, F.J.; et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 2005, 122, 337–349. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Frontiers in endocrinology 2013, 4, 37. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic beta-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World journal of diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pick, A.; Clark, J.; Kubstrup, C.; Levisetti, M.; Pugh, W.; Bonner-Weir, S.; Polonsky, K.S. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998, 47, 358–364. [Google Scholar] [CrossRef]
- Puff, R.; Dames, P.; Weise, M.; Goke, B.; Seissler, J.; Parhofer, K.G.; Lechner, A. Reduced proliferation and a high apoptotic frequency of pancreatic beta cells contribute to genetically-determined diabetes susceptibility of db/db BKS mice. Horm Metab Res 2011, 43, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Inaishi, J.; Saisho, Y. Beta-Cell Mass in Obesity and Type 2 Diabetes, and Its Relation to Pancreas Fat: A Mini-Review. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.; Carson, C.; St Pierre, C.L.; Macias-Velasco, J.F.; Hughes, J.W.; Kunzmann, M.; Schmidt, H.; Wayhart, J.P.; Lawson, H.A. Spontaneous restoration of functional beta-cell mass in obese SM/J mice. Physiological reports 2020, 8, e14573. [Google Scholar] [CrossRef]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. [Google Scholar]
- Want, L.L. Optimizing treatment success with an amylin analogue. Diabetes Educ 2008, 34 Suppl 1, 11S–17S. [Google Scholar] [CrossRef]
- Group, G.S.R.; Nathan, D.M.; Lachin, J.M.; Balasubramanyam, A.; Burch, H.B.; Buse, J.B.; Butera, N.M.; Cohen, R.M.; Crandall, J.P.; Kahn, S.E.; et al. Glycemia Reduction in Type 2 Diabetes - Glycemic Outcomes. The New England journal of medicine 2022, 387, 1063–1074. [Google Scholar] [CrossRef]
- Efanova, I.B.; Zaitsev, S.V.; Zhivotovsky, B.; Kohler, M.; Efendic, S.; Orrenius, S.; Berggren, P.O. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. The Journal of biological chemistry 1998, 273, 33501–33507. [Google Scholar] [CrossRef]
- Hakobyan, L.; Haaijer-Ruskamp, F.M.; de Zeeuw, D.; Dobre, D.; Denig, P. Comparing adverse event rates of oral blood glucose-lowering drugs reported by patients and healthcare providers: a post-hoc analysis of observational studies published between 1999 and 2011. Drug Saf 2011, 34, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Abderrahmani, A.; Renard, E. Pharmacological inhibitors of beta-cell dysfunction and death as therapeutics for diabetes. Frontiers in endocrinology 2023, 14, 1076343. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Iwashita, N.; Ikeda, F.; Ogihara, T.; Uchida, T.; Shimizu, T.; Uchino, H.; Hirose, T.; Kawamori, R.; Watada, H. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun 2006, 344, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Gu, H.O.; Jung, Y.; Jung, Y.; Seo, S.Y.; Hong, J.H.; Hong, I.S.; Lee, D.H.; Kim, O.H.; Oh, B.C. Candesartan, an angiotensin-II receptor blocker, ameliorates insulin resistance and hepatosteatosis by reducing intracellular calcium overload and lipid accumulation. Experimental & molecular medicine 2023, 55, 910–925. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Ruan, Y.; Yang, L.; Xu, N.; Chen, R.; Yang, R.; Sun, J.; Zhang, Z. Reversal of angiotensin ll-induced beta-cell dedifferentiation via inhibition of NF-kappab signaling. Molecular medicine 2018, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.S.; Bogdani, M.; Parazzoli, S.D.; Mak, S.S.; Oseid, E.A.; Berghmans, M.; Leboeuf, R.C.; Robertson, R.P. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 2009, 150, 4855–4862. [Google Scholar] [CrossRef]
- Robertson, R.P. Nrf2 and Antioxidant Response in Animal Models of Type 2 Diabetes. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Vija, L.; Farge, D.; Gautier, J.F.; Vexiau, P.; Dumitrache, C.; Bourgarit, A.; Verrecchia, F.; Larghero, J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab 2009, 35, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Severini, G.M.; Zauli, G.; Secchiero, P. Cell-based therapies for diabetic complications. Exp Diabetes Res 2012, 2012, 872504. [Google Scholar] [CrossRef] [PubMed]
- Miklosz, A.; Chabowski, A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. The Journal of clinical endocrinology and metabolism 2023, 108, 1889–1897. [Google Scholar] [CrossRef]
- Aglan, H.A.; Kotob, S.E.; Mahmoud, N.S.; Kishta, M.S.; Ahmed, H.H. Bone marrow stem cell-derived beta-cells: New issue for diabetes cell therapy. Tissue & cell 2024, 86, 102280. [Google Scholar] [CrossRef]
- Hess, D.; Li, L.; Martin, M.; Sakano, S.; Hill, D.; Strutt, B.; Thyssen, S.; Gray, D.A.; Bhatia, M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 2003, 21, 763–770. [Google Scholar] [CrossRef]
- Zhoujun, Z.; Bingzheng, F.; Yuwei, Y.; Yingying, Z.; Zhiran, X.; Chunhua, H.; Jing, L.; Haibo, T.; Wanli, L.; Ting, Z.; et al. Transplantation of insulin-producing cells derived from human MSCs to treat diabetes in a non-human primate model. Artificial organs 2023, 47, 1298–1308. [Google Scholar] [CrossRef]
- Boumaza, I.; Srinivasan, S.; Witt, W.T.; Feghali-Bostwick, C.; Dai, Y.; Garcia-Ocana, A.; Feili-Hariri, M. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 2009, 32, 33–42. [Google Scholar] [CrossRef]
- Yousef, H.N.; Sakr, S.M.; Sabry, S.A. Mesenchymal Stem Cells Ameliorate Hyperglycemia in Type I Diabetic Developing Male Rats. Stem cells international 2022, 2022, 7556278. [Google Scholar] [CrossRef]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 2006, 103, 17438–17443. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Abou-El-Mahasen, M.A.; Ashamallah, S.A.; Khater, S.M.; El-Halawani, S.M.; Ibrahim, R.Y.; Uin, G.S.; et al. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell transplantation 2013, 22, 133–145. [Google Scholar] [CrossRef]
- Carlsson, P.O.; Espes, D.; Sisay, S.; Davies, L.C.; Smith, C.I.E.; Svahn, M.G. Umbilical cord-derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a Phase I/II randomised double-blind placebo-controlled trial. Diabetologia 2023, 66, 1431–1441. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Mao, H.; Zhou, L.; Wang, Z.J.; Wang, H.X. Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin Med J (Engl) 2011, 124, 3622–3628. [Google Scholar]
- Mathur, A.; Taurin, S.; Alshammary, S. The Safety and Efficacy of Mesenchymal Stem Cells in the Treatment of Type 2 Diabetes- A Literature Review. Diabetes, metabolic syndrome and obesity : targets and therapy 2023, 16, 769–777. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem cell reviews and reports 2018, 14, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Yu, H.; Li, X. Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells. Frontiers in cell and developmental biology 2021, 9, 665412. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, A.R. Diabetic retinopathy: the latest in current management. Retina 2006, 26, S71–S79. [Google Scholar] [CrossRef] [PubMed]
- Gange, W.S.; Lopez, J.; Xu, B.Y.; Lung, K.; Seabury, S.A.; Toy, B.C. Incidence of Proliferative Diabetic Retinopathy and Other Neovascular Sequelae at 5 Years Following Diagnosis of Type 2 Diabetes. Diabetes care 2021, 44, 2518–2526. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Aldalati, A.M.Z.; Alexander, J.S.; Harris, N.R. Diabetic retinopathy: Breaking the barrier. Pathophysiology : the official journal of the International Society for Pathophysiology 2017, 24, 229–241. [Google Scholar] [CrossRef]
- Spite, M.; Serhan, C.N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res 2010, 107, 1170–1184. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. The Journal of biological chemistry 2003, 278, 43807–43817. [Google Scholar] [CrossRef]
- Antony, R.; Lukiw, W.J.; Bazan, N.G. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. The Journal of biological chemistry 2010, 285, 18301–18308. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Bazan, H.E.P. Docosanoid signaling modulates corneal nerve regeneration: effect on tear secretion, wound healing, and neuropathic pain. Journal of lipid research 2021, 62, 100033. [Google Scholar] [CrossRef] [PubMed]
- Cortina, M.S.; He, J.; Russ, T.; Bazan, N.G.; Bazan, H.E. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Investigative ophthalmology & visual science 2013, 54, 4109–4116. [Google Scholar]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PloS one 2011, 6, e15816. [Google Scholar]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. The Journal of biological chemistry 2003, 278, 14677–14687. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Tian, H.; Lu, Y.; Laborde, J.M.; Muhale, F.A.; Wang, Q.; Alapure, B.V.; Serhan, C.N.; Bazan, N.G. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. American journal of physiology. Cell physiology 2014, 307, C1058–C1067. [Google Scholar] [CrossRef] [PubMed]
- Emre, C.; Arroyo-Garcia, L.E.; Do, K.V.; Jun, B.; Ohshima, M.; Alcalde, S.G.; Cothern, M.L.; Maioli, S.; Nilsson, P.; Hjorth, E.; et al. Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in App(NL-G-F/NL-G-F) mice. Communications biology 2022, 5, 245. [Google Scholar] [CrossRef]
- Emre, C.; Do, K.V.; Jun, B.; Hjorth, E.; Alcalde, S.G.; Kautzmann, M.I.; Gordon, W.C.; Nilsson, P.; Bazan, N.G.; Schultzberg, M. Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer's disease. Acta neuropathologica communications 2021, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, A.; Bazan, N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. The Journal of biological chemistry 2017, 292, 12390–12397. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; He, J.; Kakazu, A.H.; Jun, B.; Bazan, N.G.; Bazan, H.E.P. Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration. The Journal of biological chemistry 2017, 292, 18486–18499. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. 14S,21R-Dihydroxydocosahexaenoic Acid Remedies Impaired Healing and Mesenchymal Stem Cell Functions in Diabetic Wounds. The Journal of biological chemistry 2011, 286, 4443–4453. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, H.; Hong, S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. Journal of lipid research 2010, 51, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends in neurosciences 2006, 29, 263–271. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Wang, Q.; Hong, S. 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev 2012, 21, 1187–1199. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Martinez-Fernandez, L.; Gonzalez-Muniesa, P.; Sainz, N.; Escote, X.; Martinez, J.A.; Arbones-Mainar, J.M.; Moreno-Aliaga, M.J. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. Journal of physiology and biochemistry 2021, 77, 167–173. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Ma, X.; Bai, X. Maresin-1 inhibits high glucose induced ferroptosis in ARPE-19 cells by activating the Nrf2/HO-1/GPX4 pathway. BMC ophthalmology 2023, 23, 368. [Google Scholar] [CrossRef]
- Shindou, H.; Koso, H.; Sasaki, J.; Nakanishi, H.; Sagara, H.; Nakagawa, K.M.; Takahashi, Y.; Hishikawa, D.; Iizuka-Hishikawa, Y.; Tokumasu, F.; et al. Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells. The Journal of biological chemistry 2017, 292, 12054–12064. [Google Scholar] [CrossRef]
- el Makhour-Hojeij, Y.; Baclet, M.C.; Chable-Rabinovitch, H.; Beneytout, J.L.; Cook, J. Expression of 5-lipoxygenase in lymphoblastoid B and T cells. Prostaglandins 1994, 48, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cook-Moreau, J.M.; El-Makhour Hojeij, Y.; Barriere, G.; Rabinovitch-Chable, H.C.; Faucher, K.S.; Sturtz, F.G.; Rigaud, M.A. Expression of 5-lipoxygenase (5-LOX) in T lymphocytes. Immunology 2007, 122, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Radmark, O.; Samuelsson, B. 5-Lipoxygenase: mechanisms of regulation. Journal of lipid research 2009, 50 Suppl, S40–45. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006, 99, 1285–1297. [Google Scholar] [CrossRef]
- Serhan, C.N.; Romano, M. Lipoxin biosynthesis and actions: role of the human platelet LX-synthase. J Lipid Mediat Cell Signal 1995, 12, 293–306. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem Biol 2014, 21, 1318–1329. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Wang, X.; Jiang, Z.; Zhu, L.; Fang, S. Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Monocyte-to-Lymphocyte Ratio in Depression: An Updated Systematic Review and Meta-Analysis. Frontiers in psychiatry 2022, 13, 893097. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Baravkar, S.B.; Lu, L.; Masoud, A.R.; Zhao, Q.; He, J.; Hong, S. Development of a Novel Covalently Bonded Conjugate of Caprylic Acid Tripeptide (Isoleucine–Leucine–Aspartic Acid) for Wound-Compatible and Injectable Hydrogel to Accelerate Healing. biomolecules 2024, 14, 94. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Tang, Y.; Spite, M. Proresolving lipid mediators and diabetic wound healing. Curr Opin Endocrinol Diabetes Obes 2012, 19, 104–108. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Y.; Shah, S.P.; Hong, S. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. Am J Pathol 2011, 179, 1780–1791. [Google Scholar] [CrossRef]
- Schneider, C.; Yu, Z.; Boeglin, W.E.; Zheng, Y.; Brash, A.R. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol 2007, 433, 145–157. [Google Scholar]
- Yin, H.; Gao, L.; Tai, H.H.; Murphey, L.J.; Porter, N.A.; Morrow, J.D. Urinary prostaglandin F2alpha is generated from the isoprostane pathway and not the cyclooxygenase in humans. The Journal of biological chemistry 2007, 282, 329–336. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012, 33, 136–143. [Google Scholar] [CrossRef]
- Hammes, H.P.; Feng, Y.; Pfister, F.; Brownlee, M. Diabetic retinopathy: targeting vasoregression. Diabetes 2011, 60, 9–16. [Google Scholar] [CrossRef]
- Capdevila, J.H.; Holla, V.R.; Faick, J.R. Cytochrome P450 and the Metabolism and Bioactivation of Arachidonic Acid and Eicosanoids. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 3e, Paul, R.O.d.M., Ed.; Kluwer Academic/Plenum Publishers: New York, 2005. [Google Scholar]
- Mathis, D.; Vence, L.; Benoist, C. beta-Cell death during progression to diabetes. Nature 2001, 414, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A. Autoreactive T cells in type 1 diabetes. The Journal of clinical investigation 2017, 127, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Type 2 diabetes-a matter of beta-cell life and death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef]
- Dinic, S.; Arambasic Jovanovic, J.; Uskokovic, A.; Mihailovic, M.; Grdovic, N.; Tolic, A.; Rajic, J.; Dordevic, M.; Vidakovic, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Frontiers in endocrinology 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Jurewicz, M.; Yang, S.; Augello, A.; Godwin, J.G.; Moore, R.F.; Azzi, J.; Fiorina, P.; Atkinson, M.; Sayegh, M.H.; Abdi, R. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 2010, 59, 3139–3147. [Google Scholar] [CrossRef]
- Preda, M.B.; Neculachi, C.A.; Fenyo, I.M.; Vacaru, A.M.; Publik, M.A.; Simionescu, M.; Burlacu, A. Short lifespan of syngeneic transplanted MSC is a consequence of in vivo apoptosis and immune cell recruitment in mice. Cell death & disease 2021, 12, 566. [Google Scholar] [CrossRef]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol 2012, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell proliferation 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, M.; Prathiraja, O.; Perera, P.B.; Jena, R.; Silva, M.S.; Weerawarna, P.S.H.; Singhal, M.; Kayani, A.M.A.; Karnakoti, S.; Jain, S. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus 2022, 14, e27337. [Google Scholar] [CrossRef]
- Ford, E.S. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. Am J Epidemiol 2002, 155, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, C.; Ueda, H.; Hamaguchi, T.; Miyagawa, J.; Shinohara, M.; Okamura, H.; Namba, M. Role of macrophages in the development of pancreatic islet injury in spontaneously diabetic torii rats. Exp Anim 2009, 58, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Lee, K.; Maxwell, E.L.; Liang, C.; Laybutt, D.R. Macrophage alterations in islets of obese mice linked to beta cell disruption in diabetes. Diabetologia 2019, 62, 993–999. [Google Scholar] [CrossRef]
- Konstantinova, I.; Lammert, E. Microvascular development: learning from pancreatic islets. Bioessays 2004, 26, 1069–1075. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.; Tomaszewska, R.; Stachura, J.; Nakamura, T.; Konturek, P.C. Inhibition of cyclooxygenase-2 reduces the protective effect of hepatocyte growth factor in experimental pancreatitis. Eur J Pharmacol 2004, 486, 107–119. [Google Scholar] [CrossRef]
- Greer, P.J.; Lee, P.J.; Paragomi, P.; Stello, K.; Phillips, A.; Hart, P.; Speake, C.; Lacy-Hulbert, A.; Whitcomb, D.C.; Papachristou, G.I. Severe acute pancreatitis exhibits distinct cytokine signatures and trajectories in humans: a prospective observational study. American journal of physiology. Gastrointestinal and liver physiology 2022, 323, G428–G438. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Araujo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Santos, A.; Saad, M.J.A. The Role of Hepatocyte Growth Factor (HGF) in Insulin Resistance and Diabetes. Frontiers in endocrinology 2018, 9, 503. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.; Wagner, P.; Feng, Y.; Vom Hagen, F.; Krzizok, T.; Renner, O.; Breier, G.; Brownlee, M.; Deutsch, U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 2004, 53, 1104–1110. [Google Scholar] [CrossRef]
- Kodama, H.; Fujita, M.; Yamaguchi, I. Development of hyperglycaemia and insulin resistance in conscious genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia 1994, 37, 739–744. [Google Scholar] [CrossRef]
- Dalboge, L.S.; Almholt, D.L.; Neerup, T.S.; Vassiliadis, E.; Vrang, N.; Pedersen, L.; Fosgerau, K.; Jelsing, J. Characterisation of age-dependent beta cell dynamics in the male db/db mice. PloS one 2013, 8, e82813. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Robison, W.G., Jr.; Cohen, M.P. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications 1998, 12, 28–33. [Google Scholar] [CrossRef]
- Midena, E.; Segato, T.; Radin, S.; di Giorgio, G.; Meneghini, F.; Piermarocchi, S.; Belloni, A.S. Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Res 1989, 21, 106–111. [Google Scholar] [CrossRef]
- Abid, M.R.; Guo, S.; Minami, T.; Spokes, K.C.; Ueki, K.; Skurk, C.; Walsh, K.; Aird, W.C. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 2004, 24, 294–300. [Google Scholar] [CrossRef]
- Yun, J.H. Hepatocyte growth factor prevents pericyte loss in diabetic retinopathy. Microvascular research 2021, 133, 104103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



