Introduction
Coronaviruses categorized into three genera known as alpha, beta, and gamma within the sub-family Coronavirinae, within the family of Coronaviridae, and within the order or super-family of Nidovirales. Of all genera, there are six varieties of SARS virus out of which four of them cause common cold whereas two of them induce SARS, and the Middle East respiratory syndrome that is more severe lead to death (Sukardiman et al., 2019; Liu et al., 2020; Yuan et al., 2020). It causes infection to a variety of domestic and wild animals including humans (Weiss et al., 2011). COVID-19 earlier known as “2019 novel coronavirus” a disease caused by a virus known as severe acute respiratory syndrome coronavirus (Gorbalenya et al., 2020). It was announced “severe acute respiratory syndrome coronavirus 2 (SARS CoV-2)” as the name of the new virus by the International Committee on the taxonomy of virus on 11 February 2020 (Gorbalenya et al., 2020). Most people recover out from the infection as simple flu. However, some suffered from mild infection, and a few got complications that resulted in multi-organ failure, which may be fatal. The kidney is one of the essential organs, which fails to function due to the infection of the virus.
Acute kidney infection (AKI) occurred by tubular necrosis and glomerular dysfunction caused by many factors. SARS CoV-2 infection identified to cause fatal AKI (Sharma et al., 2020). The infection causes sepsis and septic reaction to kidney cells which may be caused by over secretion of cytokines and overproduction of immune cells such as neutrophils, monocyte, and phagocytes (Gómez and Kellum, 2019; Pan et al., 2020). The AKI caused by sepsis has components of ischemia-reperfusion injury, direct inflammatory injury, coagulation and endothelial cell dysfunction, and apoptosis (Pelte et al., 2009). Ischemia-reperfusion injury is the damage of tissue that occurs when the oxygen supply is less and leads to the low flow of blood in the tissues. This case directly related to the blockage of oxygen supply in the alveoli due to the secretion of large amounts of mucus as an immune response.
The inflammatory injury caused by the secretion of pro-inflammatory cytokines that damage the kidney tissues to function properly (Pelte et al., 2009). Coagulation of blood content is also caused by different chemicals secreted as a result of immune cell response to the viral infection and endothelial cell dysfunction caused by the inflammation caused by pro-inflammatory cytokines (Pelte et al., 2009; Al-Lamki et al., 2001).
The endothelial cells of nephrons and epithelial cells of tubules likewise expected to harm by the development of NO. Alongside the kidney, the (SARS CoV-2) contamination makes harm the lungs, liver and sensory system, and gastrointestinal lot and the component of inability to organs is fairly comparative (Li et al., 2021). However,, at present, we have investigated the failure of kidney, mechanism, and possible intervention in future as the invention of vaccine might not be secured due to the unstable nature of the virus genome. The most likely sustainable method to prevent the virulence of the virus could be the hindrance of the target from receiving the virus.
General Symptoms of SARS-CoV-2
Manifestations of SARS-CoV-2 contamination incorporate fever, cough, and intense respiratory infection, with serious cases prompting pneumonia, failurity of kidney eventually leads to death (Sabino-Silva et al., 2020). To date, most SARS-CoV-2 infected patients have developed mild symptoms such as dry cough, sore throat, and fever. Most of cases have able to be settled. However, some have developed different lethal complications including organ failure, septic shock, pulmonary edema, extreme pneumonia, and Acute Respiratory Distress Syndrome (Chen et al., 2020 ). The vast majority of people recover from the basic influenza however some experienced mild infection and a few got complications that came about because of multi-organ failure, which might be deadly. The kidney is one of the fundamental organs, which able to fail to function properly because of the contamination of COVID-19 (Sharma et al., 2020).
Acute Kidney Injury (AKI) by SARS-CoV-2
Millions of humans infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shortly that breakout in China throughout the world. AKI is the complication of COVID-19 (Sharma et al., 2020). Following infection by COVID-19, acute kidney injury (AKI) is one of its complications, which occurred in 0.5-7% of all the cases that are higher incident to the patient incubate in ICU with 2.9–23% (Pan et al., 2020). The hypothesis showed that AKI caused by COVID-19 induced the effect of cytopathic consequence or cytokine storm-induced systematic inflammatory response, which remains unclear (Pan et al., 2020). Evidence of kidney injury shown on 15.5% of all patients infected with COVID-19 in China among which 3.2% of them developed kidney failure during hospital treatment (Chen et al., 2020). Among all patients admitted to the hospital about 13% of them experienced kidney infection, 40% of them had a confirmed kidney dysfunction and 5.1% had kidney injury during their hospitalization (Chen et al., 2020).
Cytokines are of different types, which secreted and act as responses of immune stimulus or an intracellular stimulus after certain invasions. After certain types of infection by a pathogenic microorganism, the host cells produced an excessive amount of cytokines and created a condition called a cytokine storm, which results in an excessive inflammatory response ((Pan et al., 2020); Kroemer et al., 1993). The complication related to the kidney by coronavirus 2 (SARS-CoV-2) is acute tubular necrosis and collapsing glomerulopathy.
The absence of SARS-CoV-2 in the biopsy of the kidney indicated that the virus could not directly influence the tissue but other consequences such as excessive production of cytokines may be caused by the damage of tubules (Sharma et al., 2020). When the viral load increased the macrophage increasingly, secrete cytokine into the blood, which may make the vessel crowded in the renal arteries of the kidney. According to the kidney biopsy performed by Sharma et al., (2020), there is a viral particle in the tissue of the kidney but the cytokine secreted in the receptor cells of the respiratory system around the alveolar area.
Glomerulopathy Caused by SARS-CoV-2
Most ACE2 found in gastrointestinal and respiratory epithelium, blood vessels, heart, and kidneys, and hence the severity of COVID-19 infection causes serious damage to this organ. The main causes of glomerular dysfunction caused by an immune disorder caused after infection. Despite the presence of ACE2, the affinity of the virus to adhere to the host cell correlated directly to the severity of disease activated the production of many amounts of antibodies (Widiasta et al., 2020). Damage of glomerular capsule by over secretion of antibodies, the damage caused by a disorder of complement, damage by circulating pro-inflammatory cells such as neutrophil, macrophage, and damage caused by activating resident cells such as mesangial cells (Widiasta et al., 2020). Besides, as the pathogen invaded the host cell, the immune structures start to react (Widiasta et al., 2020).
Plasma cells activated to secrete antibodies increasingly in response to defend the body in the blood and it resulted to cause glomerular damage that occurred due to the filtration process. The important signalling that is caused by of infection of the pathogen and complement may directly damage the tubular cells (Widiasta et al., 2020; Noris and Remuzzi, 2013; Sethi et al., 2012; Larsen et al., 2020). Greater cell count of neutrophils, granulocyte during infection in blood vessels that block and hinder the renal flow of blood, block glomerulus, and damage glomeruli tubules. The overproduction of cytokine also another effect that brought about glomerular damage during filtration (Widiasta et al., 2020; Larsen et al., 2020; Jennette et al., 1990).
Tubular Necrosis Caused by SARS-CoV-2
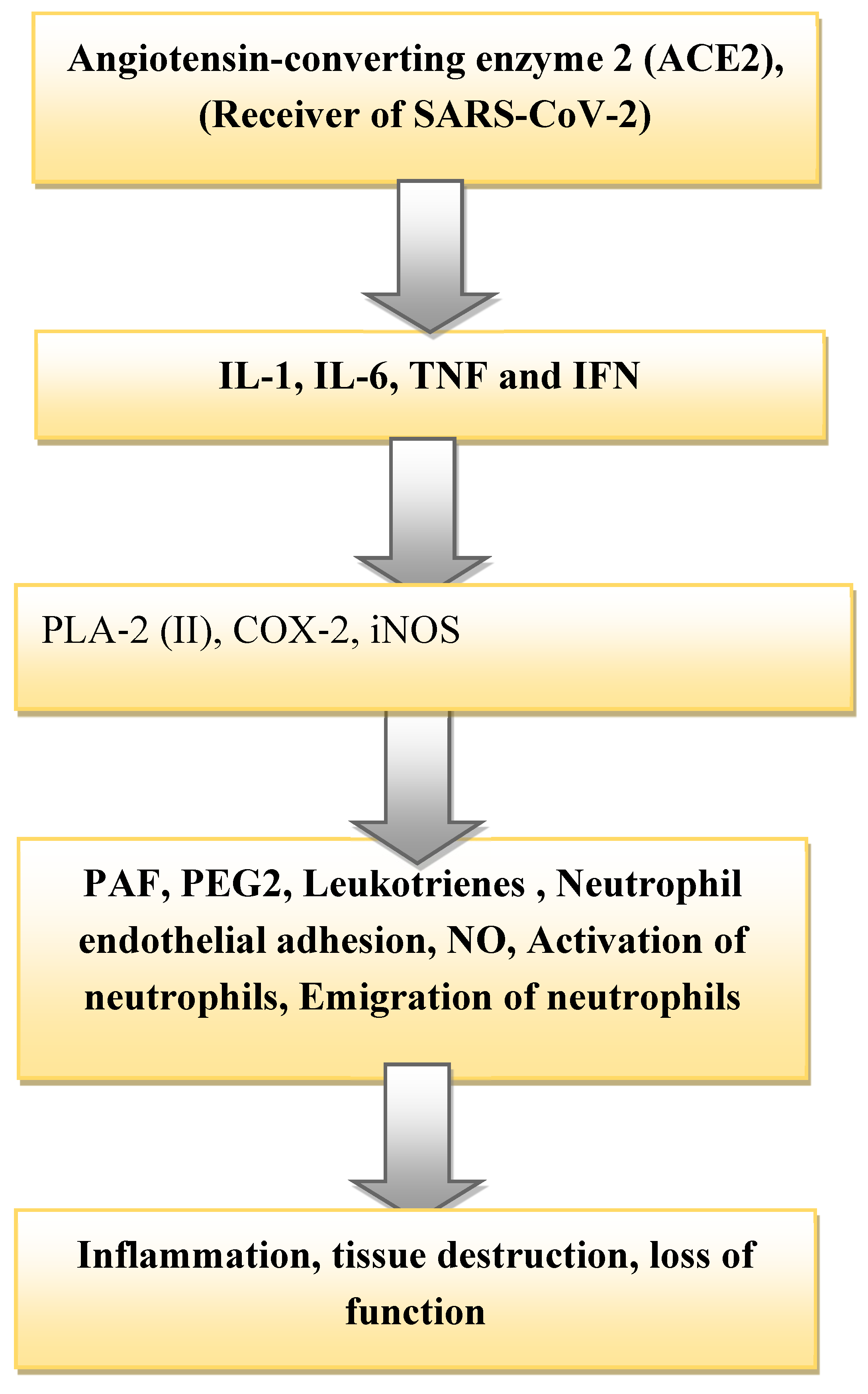
Kidney damage is associated with the infection of SARS-CoV-2 due to the high expression of the angiotensin-converting enzyme-2, (ACE2) receptor in the proximal tubules (Li et al., 2021; Werion et al., 2020; Prasad N, Agrawal, 2020). The cells of renal tubules of human is a potential host cell that targeted by SARS-CoV-2 (Xia et al., 2020). As the SARS-CoV-2 received by the epithelial cells of proximal tubules, it releases cytokine and instigates the production, activation of immune cells such as monocytes and neutrophils as a response reaction. The inflammation, damage, and failure of the tubules occurred by the mechanism highlighted in (
Figure 1). Many studies have shown that the inflammatory immune reaction along with the release of a high level of a harmful mediator such as cytokine able to interact with cells instigating endothelial dysfunction of the glomerulus, and injury of tubules (Joannidis et al., 2020). Angiotensin-converting enzyme-2 (ACE-2) identified and applied as a receptor by beta coronaviruses SARS-CoV to facilitate its entry into target cells (Widiasta et al., 2020). This receptor cell found on the surface of endothelial and epithelial cells of kidney tubules. In response to the reception of the virus, the surface of the epithelial cell damaged by inflammation and eventually fails in its function (Letko et al., 2020).
Sepsis Reaction of Kidney Cell to SARS-CoV-2 Infection
Sepsis is a disease condition that potentially threatens life caused by the response of infection. It demonstrated that normal or elevated renal blood flow leads to tubular injury (Gómez et al., 2019) . When infection of pathogenic microorganisms occurred, the body releases the chemicals into blood vessels that related to fighting against a pathogen. The chemicals that released in the bloodstream are antibodies and different types of cytokines. Our body then reacts to these chemicals aggressively, which subsequently evoke the changes that result in damage to multiple organ systems (Gómez et al., 2019). Vasopressin used to maintain the osmotic balance of the body, regulate blood pressure and kidney balance. The increment of chemicals in response to pathogen damages the kidney tubules. The application of vasopressin and the prevention of intra-abdominal hypertension used as a strategic measure to protect AKI (Majumdar et al., 2010). The virus causes the production of pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin (IL)-1, and interferons (IFN). These bind to a different cell on their specific receptor (Pelte et al., 2009). In the kidney, glomerular endothelium cells form TNF receptor 1 and renal tubular epithelial cells from TNF receptor (Gorbalenya et al., 2020; Al-Lamki et al., 2001).
The Basic Mechanism of Kidney Failure by SARS-CoV-2
Even though the mechanism through which SARS-CoV-2 causes multi-organ failure not known, we have highlighted important ways it can elicit inflammation and damage from the literature (Xia et al., 2020). The septic shock caused by viral infection causes complications of events to the cells (Li et al., 2020; Annane et al., 2005). SARS-CoV-2 received by the targeted cell by Angiotensin-converting enzyme-2 (ACE2) (Li et al., 2020; Werion et al., 2020; Prasad N, Agrawal, 2020). Then the infection of pathogen stimulated intracellular to an immune cell, endothelium issue of the glomerulus and epithelium tissue of tubules as indicated below to cause kidney failure. The virus causes the production of pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin (IL)-1, and interferons (IFN). These bind to a different cell on their specific receptor (Pan et al., 2020; Pelte CH, Chawla, 2009; Li et al., 2020). The expression IL-6 caused by viral infection played a key role in instigating a primary response toward viral infection through enhancing neutrophils for clearance of the virus. The reduction of IL-6R in the blood during the events of infection leads to the persistence of influenza (Pelte CH, Chawla, 200; Dienz et al., 2012).
PLA-2 reported to cause and be involved in the process of inflammation of the tissue. In the process to cause inflammation in both humans and other animals, it releases lysophospholipids (LPLs) and free fatty acids to facilitate adhesion (Liu et al., 2020). The pro-inflammatory cytokines are also responsible to induce the secretion of cyclooxygenase-2 (COX-2), which causes inflammation and pain (Liu et al., 2020). The iNOS stimulated to produce NO which allows the vasodilation of renal arteries. Nitric oxide (NO) is a small bioactive lipophilic molecule that diffuses across the cell membrane and controls many physiological functions of the body (Moncada and Higgs, 1995).
The effect of the three (PLA-2 (II), COX-2, iNOS) causes the adhesion of chemicals to endothelium tissue of glomerulus that in turn causes the secretion of chemokines in response to chemical bindings to it (Titheradge et al., 1999) (
Figure 1). Then platelet-activating factor (PAF) secretes the production of platelets that may damage the kidney tubules by crowding the contents. After a series of reactions, the transcription of the inducible NO synthase (iNOS) gene occurred, and the translation of iNOS mRNA, which resulted subsequently in the formation of iNOS proteins which elevated the formation of NO. Production of NO results in the inflammation of tubular cells and glomeruli tissue that damages and hinder its function. The inflammation more alleviated along with the production of cytokines/chemokines by causing sepsis to the organ system. The production of inflammatory chemicals, such as Prostaglandin-2 (PEG) and Leukotrienes elicited by the cells leads to tubular damage (Moncada and Higgs, 1995; Titheradge et al., 1999)
The Effect of NO to Kidney Failure
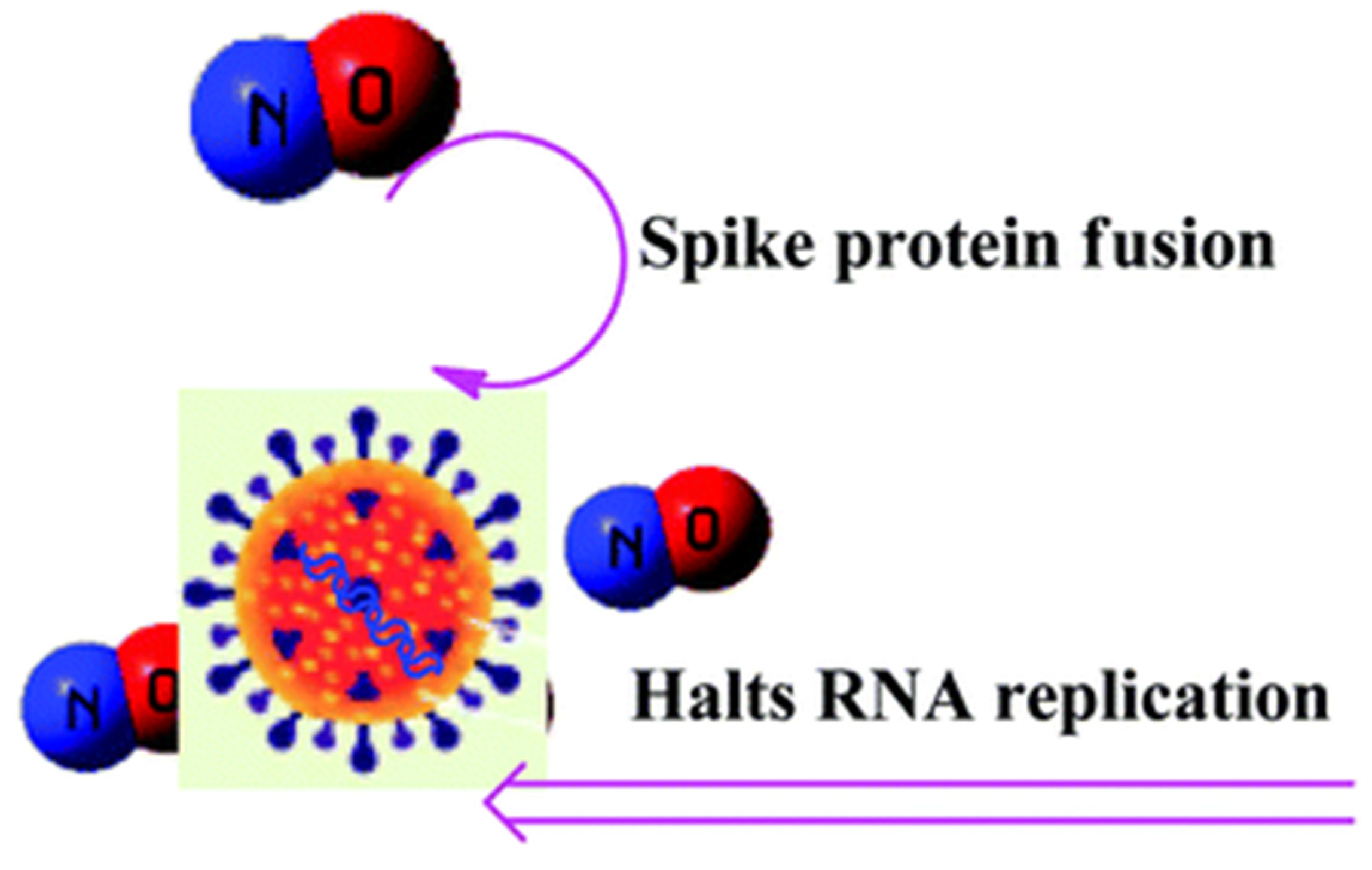
Nitric oxide (NO) plays a vital role in the regulation of blood pressure and acts as a key mediator of renal physiology and also. Deficiency of NO is a prerequisite condition for renal programming, and early life NO targeting interferences help in the prevention of hypertension and kidney diseases (Hsu and Tain, 2019). NO is formed from a reaction between L-arginine and oxygen catalysed by nitric oxide synthase enzyme and is an effective vasodilator, and is called a vasorelaxant factor (Aleksandar et al., 2020). NO performs various important functions in the kidney such as the modulation of renal hemodynamics, natriuresis, control of blood flow in the medulla of the kidney, weakening of tubuloglomerular feedback, controlling renal sympathetic neural activity and obstruction of sodium (Na) reabsorption (Hsu and Tain 2019; Bachmann and Mundel, 1994). A decline in NO synthesis plays a significant role in the development of systemic hypertension, as NO obstructs the excretion of Na and H2O from the kidney and NO hampers the response of the kidney to volume augmentation (Bachmann and Mundel, 1994). Moreover, the enhanced NO generation may serve a significant function in diabetic hyperfiltration, and perhaps also in the amplified blood flow in several other vital organs as well (Bachmann and Mundel, 1994). SARS-CoV-2 infection depends upon the interaction between the SARS-CoV-2 Spike (S) protein’s receptor-binding domain present on the surface of viral particles and the ACE2. NO cause conformational alterations on surface glycoproteins that can obstruct host cell fusion, thereby prevent viral infection and discharge of viral particles from infected host cells such as neuraminidase inhibitors (Coutu, 2021). NO can inhibit its replicationby dual mechanisms as shown in (
Figure 1).
Under normal physiological conditions, identified that nitric oxide produced from eNOS and neuronal nitric oxide synthase (nNOS) in the kidney. The localized eNOS to vascular endothelial cells control the vascular tone of renal vessels. It plays a role in the direct control of oxygen supply to the tissue of the kidney and in turn, affects the rate of glomerular filtrates (Deng et al., 2005; Min et al., 2016) . The higher concentration of NO facilitates supply of many amounts of oxygen and prevents the risk of COVID-19 infection (Min et al., 2016). There was a reported that the expression of nNOS in proximal tubular cells to regulate the rate of mitochondrial respiration is well iNOS expressed in macula densa where it affected the feedback mechanism of tubuloglomerular functioning (Deng et al., 2005). The dysfunctional production of nitric oxide leads to an abnormal supply of oxygen in several pathological settings (Palm et al., 2009).
One of the pathological condition in which abnormal production of NO occurred is the infection of COVID-19. After a series of events caused by the infection of COVID-19, the history ends with the abnormal production of NO that put a huge impact on the failure of the kidney as described above (Wang et al., 2020). Increased production of NO by iNOS enhanced the vasodilation of arteries and allowed the adhesion of neutrophils to the arteries. The synergetic effect of all triggered inflammations and destruction of kidney tissues that eventually hinder its proper functioning (
Figure 1). Septic shock finally characterized by weakness, peripheral low pulse, low blood pressure, and coldness. Peripheral low pulse, low pressure, and weakness may be caused by the excess production of NO and its effect on vasodilation and make blood volume flowing slowly (Wang et al., 2020).
An amino acid L-arginine (simply arginine) for the synthesis of nitric oxide (NO) in the body and have a great contribution to the regulation of central and peripheral nervous system, cardiovascular and hemostatic conditions in the body (Moncada and Higgs, 1995). Nitric oxide is produced nitric oxide synthase enzyme (NOS) intracellularly from endothelial, neuronal, and inducible; transforms L-arginine to citrulline and nitric oxide (Adusumilli et al., 2020). Oxygen delivery to kidney tissue and glomerular filtration rate controlled by endothelial nitric oxide synthase (eNOS) (Palm et al., 2009). Neuronal nitric oxide synthase (nNOS) provides functional evidence for nNOS expressed in proximal tubular cells, where it adjusts mitochondrial respiration (Palm et al., 2009). High oxygen metabolism by mitochondria in a diabetic person kidney lessen oxygen tension and leads to the development of diabetic kidney disease (Adusumilli et al., 2020).
Nitric oxide has a different effect on the physiological function of the body including kidney function regulation and involvement in the most process resulting in malfunction of the kidney during disease (Adusumilli et al., 2020). Endothelium and epithelium malfunction or death and causes inflammatory immune responses leading to hypercytokinemia (Adusumilli et al., 2020). In inflammatory response, platelets activated by less NO and lead to coagulation highly (Adusumilli et al., 2020). Endothelial NO as a modulator of coagulation secrete inflammatory promoting cytokine heme and iron activate platelets; this leads to low blood flow then oxygen delivery to the kidney becomes hypoxic (Adusumilli et al., 2020). The production of less amount of NO causes failure of the kidney by depriving a sufficient supply of oxygen to its tissue. This happened through unselective or selective nNOS inhibition results in increasing kidney oxygen utilization and decreased electrolyte transport efficiency per consumed oxygen molecule (Adusumilli et al., 2020). NO has a prolonged half-life and concentration during periods of low oxygen pressure as a direct consequence of decreased degradation by the cytochrome C oxidase, which may help to prevent excessive oxygen utilization during limited oxygen delivery Palm et al., 2009).
Cytokine Storm, Capillary Leakage and Kidney Failure
The macrophage can produce a large number of molecular species and pro-inflammatory cytokines such as interleukin (IL) II, VI, VIII, interferon (IFN) -α/β, tumour necrosis factor (TNF) -α that nullify invading organism (Cheung et al., 2005). Excessive production of these cytokines as an immune response is called a “cytokine storm” (Cheung et al., 2005) (
Figure 2). Previously it was studied that the infection of influenza caused the secretion of cytokines by macrophages, epithelial cells of the lungs, and dendritic cells through activation of pattern recognition receptors such as (tall like receptors LR3, TLR7, and TLR8), a retinoic acid-inducible gene-I and the NOD-like receptor family members (Iwasaki and Pillai, 2004).
As the epithelium tissue damaged, it causes the leakage of the capillary that represents the improper adaptation and uninvited flow of electrolytic fluid with or without protein into the interstitial space that leads to the failure and dysfunction of the organs (Duchesne et al., 2015; Honore et al., 2019). The globally increased permeability syndrome (GIPS) defined as a positive cumulative fluid balance and new-onset organ dysfunction/failure described in patients with persistent systemic inflammation resulting in continuing trans capillary albumin leakage (Pan et al., 2020). After the initial storm of cytokine and ischemia-reperfusion injury, this may be characterized as the third phase of a continuum of injury (Honore et al., 2019; Malbrain et al., 2018) (
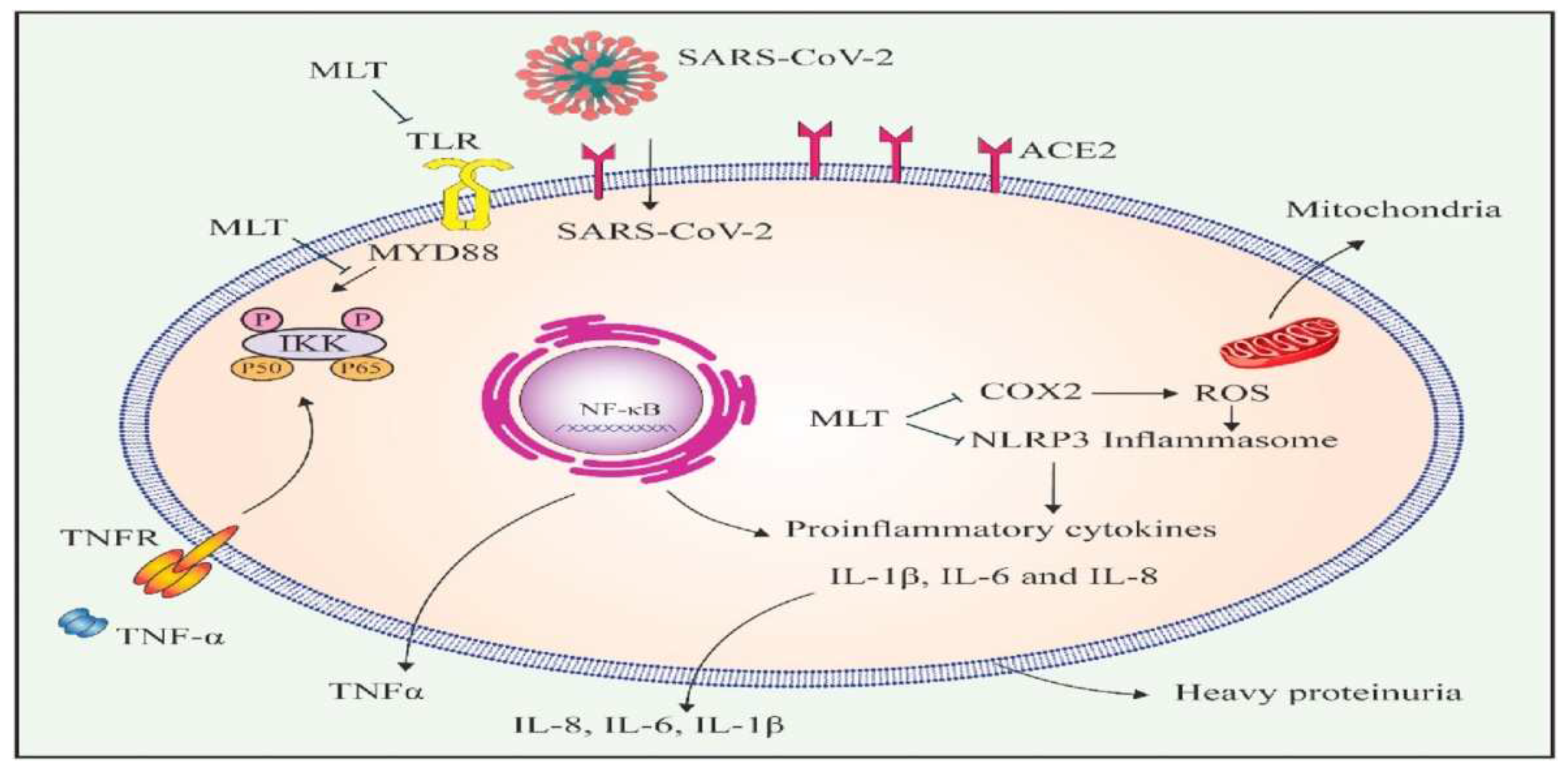
Figure 2). Melatonin (MTL) is the hormone that used to regulate state of human activity and it can also directly influenced the regulation and experession of ACE2 and hinder and activate its effect to cause the viral infection (Askari et al., 2021).
The Effect of Cytokines
Cytokines are signal molecules to convey messages from one cell to the next (Ren et al., 2020). Cytokines are soluble proteins that interface with specific cell receptors, which engaged with the regulation, development, and activation of immune cells. Additionally, intervene in normal and pathologic inflammatory and immune reactions (Ren et al., 2020). They are peptides utilized by cells for intercellular interaction and for controlling the internal environment of the cells in which they operate. Inflammation is essential for the complex natural reaction of body tissues to harmful stimuli, such as microbes, damaged cells, or a protective response involving immune cells, blood vessels, and molecular mediators (Pan et al., 2020). The importance of inflammation is to take out the underlying reason for cell injury, eliminate necrotic cells, and damaged tissue (NHCPRC, 2021; Voysey et al., 2021). The rush of these viruses listed in (
Table 1) caused the glomerulopathy and damage of tubules that subsequently hinder the normal functioning of the filtration process.
The covid-19 stimulates the production of a huge number of neutrophils and monocytes that are associated with higher production of cytokines in the blood of Middle East Respiratory Syndrome (MERS-CoV) patients that had been checked in COVID-19 too (Wang et al., 2020). The pro-inflammatory cytokines such as IL-1, TNF ά, IL-6 directly linked with the failure of the organs (
Table 1). They are secreted by neutrophils and monocytic macrophages as a response against the virus from the surface of endothelial and epithelial tissues and responsible to create inflammation in these structures (NHCPRC, 2021; Voysey et al., 2021). The pro-inflammatory cytokines such as IL-1, TNF ά, IL-6 directly linked with the failure of the organs (
Table 1). They are secreted by neutrophils and monocyte macrophages as a reaction against the virus from endothelial and epithelial tissues surfaces and responsible to create inflammation in these structures (NHCPRC, 2021; Liu et al., 2020).
Mutation of Virus and Its Consequences
Over time through mutations viruses constantly form new variants and these variants persists causing pandemics while some disappear. Various modified viral strains of covid-19 were identified in the pandemic all over the world in recent times. COVID-19 pertain to the large family of coronavirus because of the crown-like spikes on their surfaces. The new variants of the virus are forms with the alteration in the spikes attached on the outer surface (Ren et al., 2020). According to the recent report, the study reveals that UK inhabits variant B.1.1.7 was identified in late 2020 with mutations which grows rapidly and handily. In US, byepilogue of 2020, there was increased risk of deaths as compared to other variants of the virus (Horby et al., 2021). In October 2020, another variant in South Africa that is B.1.351 which shares some mutations with B.1.1.7 causes numerous casualties in South Africa. Then, in the US January 2021 (Resende et al., 2021) and later on, in January 2021, Japan, a variant P.1 was identified from the travelers from Brazil comprises of set of increased modifications. The mutations in the variants help them to evade T cells – a component of the immune system that is particularly important for decreasing the hazards by contagious disorder. (NHCPRC, 2021) .
The delta strain of SARS-CoV-2 also referred as B.1.617 emerged on high extent in some regions across India and UK, which later on expanded to many other countries out-breaking covid-19 pandemic. It has more immune invasion potential compared to other strains of the virus recognized so far. This is due to the spike mutations in the N-terminal domain (NTD) and the receptor binding domains (RBD) of the mutant viral strain (Planas et al., 2021).
SARS-CoV-2 Vaccine and Its Future Challenge
People are being afflicted with coronavirus variant B.1.1.7 across the whole globe. They are more prone to death as juxtaposed with the ones who are infected with some other circulating variants. A SARS-CoV-2 variant B.1.351 also regarded as 501Y. V2 has been united to decreased efficacy for treatment by Novavax and Johnson & Johnson. At the University of Witwatersrand in Johannesburg in South Africa, a trial carried on over 2,000 people aged 18-64 with the vaccine project that advanced by the University of Oxford, UK and AstraZeneca vaccine that accredits only 21.9% shield against the COVID-19 and 10.4% resistance to the B.1.351(Voysey et al., 2021).
There is the assumption that the genetic unsustainability of the virus could make the vaccine inefficient in future. Generally, viruses have got the highest rate of mutations and might make the vaccine less efficient and unsustainable. It was informed that mutations of the virus may impact the transmission of the virus, its virulence, the efficiency of vaccine, and therapeutic development of the disease (Ye et al., 2020) . Hence, a vaccine could not be the final solution to control the virulence of the virus rather, scientists should focus on a method to hinder the target cell from receiving the virus. The medium by which SARS-CoV-2 received into the cytoplasm of the target cell is angiotensin-converting enzyme 2 (ACE2) receptors. This receiver is abundantly found in the endothelium and epithelial structure of essential organs such as the lung, kidney, liver, gastrointestinal tract (Cheng et al., 2020). Hence, the most likely sustainable intervention could be the application of angiotensin-converting enzyme 2 (ACE2) inhibition by the receptors of the target cells in these vital organs. Several pieces of evidence suggested that the application of Nephro Checking may help physicians to identify patients with tubular stress before kidney dysfunction is manifested (Ronco et al., 2020).
A positive test recommending the development of a higher risk of AKI may require an early consultation of nephrology, close observation of creatinine and discharged urine, the enhancement of volume and hemodynamic status. The evasion of iodinated contrast procedures whenever the situation allows, and the utilization of nephrotoxic medication (e.g., aminoglycosides, ACE inhibitors, NSAIDs, nonsteroidal anti-inflammatory drugs, and angiotensin receptor blockers) or the close checking of medication levels (vancomycin) (Grubaugh et al., 2020). There was one cytokine named IFN-I that acted antagonistically against the virus at an early stage before it can cause infection and applied to prevent the multiplication of the virus (Chowdhury et al., 2020). Not only IFN-I but also other antiviral innate immune signallings such as TLR7, Toll-like receptor (TLR)3 or interferon signalling (IFNAR2) or interferon regulatory factor 7 (IRF7) are very important to hinder the multiplication of the virus at the early stage of infection. The primary immunodeficiency syndromes may lack these cytokines and innate immune signalling and thereby the infection becomes more serious in them.
At the later stage of infection, the inflammatory response elicited the secretion of immunoglobulins from B-cell which are responsible for the formation of cytokines and then for the production of NO by cascade pathway of reaction. A patient who falls into sepsis shock has been suggested to be treated by removing excess cytokines secreted by an immune cell in response to pathogenic infection (Honore et al., 2019). This can be achieved by the application of more recent technologies of sorbent that could attract much attention to a physician. The technology is highly efficient in removing inflammatory mediators particularly cytokines from the bloodstream (Honore et al., 2019). The same procedure followed to remove the cytokines that produced excessively after COVID-19 infection.
Though, evolving variants/strains of original Sars-Cov-2 and its world wide growth have created worries regarding possibly decreased defense against variants of Concern (VOCS) by recent Covid-19 Vaccines. B.1.1.7 Variant/strain has a N501y type of Mutation, has been linked with enhanced transmission rate .B.1.351 Variant (20h/501y.V2) comprises of a number of mutations, including K417n, E484k, and N501y. P.1 Variant/strain (B.1.1.28.1) acquires K417t, E484k, and N501y replacement in the RBD of the Spike Protein. These VOCS additionally has the D614G type of mutation, which presentsan enhanced capability meant for fastviral spread.
Wang et al., (2021), examined the counteracting action of Plasma from Vaccinees (Bnt162b2, N = 6; Mrna-1273, N = 14) aligned with pseudo kind Viruses holding K417N, E484K, N501Y, and ablend of these three RBD mutations (B.1.351 Variant). The research showed 1- to 3-fold reduced neutralizing actiona gainst E484K, N501Y, and K417N:E484K:N501Y combination (P = 0.0033, P = 0.0002, And P < 0.0001, respectively), Although there was noconsiderable change in neutralizing activity compared towild-type and K417N type of mutation. This result recommends that Covid-19 MRNA Vaccine-elicited neutralizing antibodies are less efficient against developing Sars-Cov-2 VOCS with RBD mutations (
Table 3).
Future Directions
COVID-19 causes infection after it has been received by angiotensin-converting enzyme 2 (ACE2) unless it cannot. Following the invasion of COVID-19, by the receiver of the target cell, other immune cells start to react. The reaction of immune cells mediated by cytokine/chemokines. Over secretion of cytokines and production of NO by immune cells, causes inflammation that leads to malfunctioning of the kidneys. These pro-inflammatory cytokines are also responsible to induce the secretion of cyclooxygenase-2 (COX-2), which causes inflammation. The kidney failure that caused by of COVID-19 infection is due to damage of endothelial tissues of the glomerulus and epithelial tissues of tubules through inflammation. The breaking of the routes of pathogenicity of the virus is very important by inhibiting angiotensin-converting enzyme-2 (ACE2) which is responsible to receive the virus to cause infection. Along with trying different vaccines to control the spread of COVID-19, it is advisable to produce a therapeutic agent/chemical that may inhibit the activities of angiotensin-converting enzyme 2 (ACE2) to prevent the receiving of the virus by the target cells along with the effort of developing the vaccine.
Conclusion
COVID-19 causes infection after it has been received by angiotensin-converting enzyme 2 (ACE2) unless it cannot. Following the invasion of COVID-19, by the receiver of the target cell, other immune cells start to react. The reaction of immune cells mediated by cytokine/chemokines. Over secretion of cytokines and production of NO by immune cells, causes inflammation that leads to malfunctioning of the kidneys. These pro-inflammatory cytokines are also responsible to induce the secretion of cyclooxygenase-2 (COX-2), which causes inflammation. The kidney failure caused by COVID-19 infection is due to damage of endothelial tissues of the glomerulus and epithelial tissues of tubules through inflammation. The breaking of the routes of pathogenicity of the virus is very important by inhibiting angiotensin-converting enzyme-2 (ACE2) which is responsible to receive the virus to cause infection. Along with trying different vaccines to control the spread of COVID-19, it is advisable to produce a therapeutic agent/chemical that may inhibit the activities of angiotensin-converting enzyme 2 (ACE2) to prevent the receiving of the virus by the target cells along with the effort of developing the vaccine.
Conflicts of Interest
None.
References
- Adusumilli, N. C., Zhang, D., Friedman, J. M., & Friedman, A. J. (2020). Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide. [CrossRef]
- Aleksandar, T., Gordana, Ž., Slavica, S., & Ivan, M. (2020). Transplanted Kidney Increases Nitric Oxide Formation With Metabolic Acidosis Reduction. Experimental and Clinical Transplantation, 4, 450-457. [CrossRef]
- Al-Lamki, R. S., Wang, J., Skepper, J. N., Thiru, S., Pober, J. S., & Bradley, J. R. (2001). Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Laboratory Investigation, 81(11), 1503-1515. 11. [CrossRef]
- Allen, H., MSc, A. V., MFPH, J. F., & MSc, K. A. (2021). Increased household transmission of COVID-19 cases associated with SARS-CoV-2 Variant of Concern B. 1.617. 2: a national case control study. Public Health England. [CrossRef]
- Annane, D., Bellissant, E., &Cavaillon, J. M. (2005). Septic shock. The Lancet, 365(9453), 63-78. 9453. [CrossRef]
- Annavajhala, M. K., Mohri, H., Zucker, J. E., Sheng, Z., Wang, P., Gomez-Simmonds, A.,... &Uhlemann, A. C. (2021). A novel SARS-CoV-2 variant of concern, B. 1.526, identified in New York. medRxiv. [CrossRef]
- Askari, H., Sanadgol, N., Azarnezhad, A., Tajbakhsh, A., Rafiei, H., Safarpour, A. R., ... &Omidifar, N. (2021). Kidney diseases and COVID-19 infection: causes and effect, supportive therapeutics and nutritional perspectives. Heliyon, e06008. [CrossRef]
- Bachmann, S., &Mundel, P. (1994). Nitric oxide in the kidney: synthesis, localization, and function. American Journal of Kidney Diseases, 24(1), 112-129. [CrossRef]
- Channappanavar, R., Fehr, A. R., Zheng, J., Wohlford-Lenane, C., Abrahante, J. E., Mack, M.,... & Perlman, S. (2019). IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. The Journal of clinical investigation, 129(9), 3625-3639. [CrossRef]
- Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y.,... & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet, 395(10223), 507-513. [CrossRef]
- Cheng, Y., Luo, R., Wang, K., Zhang, M., Wang, Z., Dong, L.,... & Xu, G. (2020). Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney international, 97(5), 829-838. [CrossRef]
- Cheung, C. Y., Poon, L. L., Ng, I. H., Luk, W., Sia, S. F., Wu, M. H.,... &Peiris, J. S. (2005). Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. Journal of virology, 79(12), 7819-7826. [CrossRef]
- Chowdhury, M., Maharaj, V. R., Francis, G. S., Alexy, T., & Fraser, M. (2020). COVID cardiomyopathy: Is it time to involve the cardiologists?. The Indian Journal of Medical Research, 152(3), 169. [CrossRef]
- Coutu, N. (2021). Investigating the Potential for High-CBD Cannabis Sativa and Nitric Oxide to Modulate SARS-CoV-2 Spike-ACE2 Binding.
- Deng, A., Miracle, C. M., Suarez, J. M., Lortie, M., Satriano, J., Thomson, S. C.,... &Blantz, R. C. (2005). Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney international, 68(2), 723-730. [CrossRef]
- Deng, X., Garcia-Knight, M. A., Khalid, M. M., Servellita, V., Wang, C., Morris, M. K.,... & Chiu, C. Y. (2021). Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation. MedRxiv. [CrossRef]
- Dienz, O., Rud, J. G., Eaton, S. M., Lanthier, P. A., Burg, E., Drew, A.,... & Rincon, M. (2012). Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal immunology, 5(3), 258-266. [CrossRef]
- Duchesne, J. C., Kaplan, L. J., Balogh, Z. J., &Malbrain, M. L. (2015). Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther, 47(2), 143-155.
- Gómez H, Kellum JA. (2019). Sepsis-induced acute kidney injury. Critical care nephrology. Jan 1:524-33. [CrossRef]
- Gorbalenya, A. E., Baker, S. C., Baric, R., Groot, R. J. D., Drosten, C., Gulyaeva, A. A.,... &Ziebuhr, J. (2020). Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group. [CrossRef]
- Greaney, A. J., Loes, A. N., Crawford, K. H., Starr, T. N., Malone, K. D., Chu, H. Y., & Bloom, J. D. (2021). Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell host & microbe, 29(3), 463-476. [CrossRef]
- Grubaugh, N. D., Hanage, W. P., & Rasmussen, A. L. (2020). Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell, 182(4), 794-795. [CrossRef]
- Honore, P. M., Hoste, E., Molnár, Z., Jacobs, R., Joannes-Boyau, O., Malbrain, M. L., &Forni, L. G. (2019). Cytokine removal in human septic shock: where are we and where are we going? Annals of intensive care, 9(1), 1-13. [CrossRef]
- Horby P, Huntley C, Davies N et al. NERVTAG note on B.1.1.7 severity. New & Emerging Threats Advisory Group, Jan. 21, 2021. Retrieved from NERVTAG note on variant severity.
- Horby P, Huntley C, Davies N, et al. NERVTAG note on B.1.1.7 severitypdficonexternal icon. SAGE meeting report. January 21, 2021.
- Hsu, C. N., &Tain, Y. L. (2019). Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. International journal of molecular sciences, 20(3), 681. 3. [CrossRef]
-
https://www.biospace.com/article/comparing-covid-19-vaccines-pfizer-biontech-moderna-astrazeneca-oxford-j-and-j-russia-s-sputnik-v/.
-
https://www.yalemedicine.org/news/covid-19-vaccine-comparison.
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nature Reviews Immunology. 2014 May;14(5):315-28. [CrossRef]
- Jennette, J. C., & Falk, R. J. (1990). Antineutrophil cytoplasmic autoantibodies and associated diseases: a review. American Journal of Kidney Diseases, 15(6), 517-529. [CrossRef]
- Joannidis, M., Forni, L. G., Klein, S. J., Honore, P. M., Kashani, K., Ostermann, M.,... &Kellum, J. A. (2020). Lung–kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive care medicine, 46(4), 654-672. [CrossRef]
- Kopp, J. B., Smith, M. W., Nelson, G. W., Johnson, R. C., Freedman, B. I., Bowden, D. W.,... & Winkler, C. A. (2008). MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nature genetics, 40(10), 1175-1184. [CrossRef]
- Kroemer, G., Moreno de Alboran, I., Gonzalo, J. A., & Martinez, C. (1993). Immunoregulation by cytokines. Critical reviews in immunology, 13(2), 163-191.
- Kudose, S., Batal, I., Santoriello, D., Xu, K., Barasch, J., Peleg, Y.,... &D’Agati, V. D. (2020). Kidney biopsy findings in patients with COVID-19. Journal of the American Society of Nephrology, 31(9), 1959-1968. [CrossRef]
- Larsen, C. P., Bourne, T. D., Wilson, J. D., Saqqa, O., &Sharshir, M. D. A. (2020). Collapsing glomerulopathy in a patient with COVID-19. Kidney international reports, 5(6), 935. [CrossRef]
- Letko, M., Marzi, A., & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology, 5(4), 562-569. [CrossRef]
- Liu, B. M., & Hill, H. R. (2020). Role of host immune and inflammatory responses in COVID-19 cases with underlying primary immunodeficiency: a review. Journal of Interferon & Cytokine Research, 40(12), 549-554. [CrossRef]
- Liu, D. X., Liang, J. Q., & Fung, T. S. (2020). Human coronavirus-229E,-OC43,-NL63, and-HKU1. Reference module in life sciences, B978-0.
- Majumdar, A. (2010). Sepsis-induced acute kidney injury. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine, 14(1), 14. [CrossRef]
- Malbrain, M. L., Van Regenmortel, N., Saugel, B., De Tavernier, B., Van Gaal, P. J., Joannes-Boyau, O.,... & Monnet, X. (2018). Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Annals of intensive care, 8(1), 1-16. [CrossRef]
- Min, C. K., Cheon, S., Ha, N. Y., Sohn, K. M., Kim, Y., Aigerim, A., ... & Kim, Y. S. (2016). Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Scientific reports, 6(1), 1-12. 1. [CrossRef]
- Mir, J. M., &Maurya, R. C. (2021). Nitric oxide as a therapeutic option for COVID-19 treatment: a concise perspective. New Journal of Chemistry, 45(4), 1774-1784.
- Moncada, S., & Higgs, E. A. (1995). Molecular mechanisms and therapeutic strategies related to nitric oxide. The FASEB Journal, 9(13), 1319-1330. 13. [CrossRef]
- Nasr, S. H., & Kopp, J. B. (2020). COVID-19–associated collapsing glomerulopathy: an emerging entity. Kidney international reports, 5(6), 759-761. [CrossRef]
- National Health Commission of the People’s Republic of China. Daily briefing on novel coronavirus cases in China. Jan 12, 2021. http://www.nhc.gov.cn/xcs/yqtb/202101/ b70d608498e549c5973d955e868101e7.shtml (accessed on 12 January 2021).
- Noris M, Remuzzi G. Overview of complement activation and regulation. InSeminars in nephrology 2013 Nov 1 (Vol. 33, No. 6, pp. 479-492). WB Saunders.
- Palm, F., Teerlink, T., &Hansell, P. (2009). Nitric oxide and kidney oxygenation. Current opinion in nephrology and hypertension, 18(1), 68-73. 1. [CrossRef]
- Pan, X. W., Xu, D., Zhang, H., Zhou, W., Wang, L. H., & Cui, X. G. (2020). Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive care medicine, 46(6), 1114-1116. [CrossRef]
- Pelte CH, Chawla LS. Novel therapeutic targets for prevention and therapy of sepsis associated acute kidney injury. Current drug targets. 2009 Dec 1;10(12):1205-11. [CrossRef]
- Planas, D. et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature (2021). [CrossRef]
- Prasad, N., Agrawal, S. K., & behalf of COVID, O. (2020). COVID 19 and acute kidney injury. Indian Journal of Nephrology, 30(3), 161. [CrossRef]
- Ren, L. L., Wang, Y. M., Wu, Z. Q., Xiang, Z. C., Guo, L., Xu, T.,... & Wang, J. W. (2020). Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese medical journal, 133(9), 1015. [CrossRef]
- Resende, P. C., Bezerra, J. F., Vasconcelos, R., Arantes, I., Appolinario, L., Mendonça, A. C.,... &Siqueira, M. M. (2021). Spike E484K mutation in the first SARS-CoV-2 reinfection case confirmed in Brazil, 2020. Virological [Internet], 10.
- Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. The Lancet Respiratory Medicine. 2020 Jul 1;8(7):738-42. [CrossRef]
- Sabino-Silva, R., Jardim, A. C. G., &Siqueira, W. L. (2020). Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clinical oral investigations, 24(4), 1619-1621. [CrossRef]
- Sahoo, J. P., Mishra, A. P., &Samal, K. C. (2021). Triple Mutant Bengal Strain (B. 1.618) of Coronavirus and the Worst COVID Outbreak in India. Biotica Research Today, 3(4), 261-265. 4.
- Sethi, S., Fervenza, F. C., Zhang, Y., Zand, L., Vrana, J. A., Nasr, S. H.,... & Smith, R. J. (2012). C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney international, 82(4), 465-473. [CrossRef]
- Sharma, P., Uppal, N. N., Wanchoo, R., Shah, H. H., Yang, Y., Parikh, R.,... &Northwell Nephrology COVID-19 Research Consortium. (2020). COVID-19–associated kidney injury: a case series of kidney biopsy findings. Journal of the American Society of Nephrology, 31(9), 1948-1958. [CrossRef]
- Sukardiman, M. E., Pratama, M. R. F., Poerwono, H., &Siswodihardjo, S. (2020). The coronavirus disease 2019 main protease inhibitor from Andrographispaniculata (Burm. f) Ness. Journal of Advanced Pharmaceutical Technology & Research, 11(4), 157. [CrossRef]
- Titheradge, M.A. (1999). Nitric oxide in septic shock. Biochimica et BiophysicaActa (BBA)-Bioenergetics, 1411(2-3), 437-455. [CrossRef]
- TWR CA, Davies NG, Kucharski AJ, CMMIDCOVID-19 working group, Edmunds WJ, Eggo RM. Estimates of severity and transmissibility of novel South Africa SARS-CoV-2 variant501Y. V2. Centre for Mathematical Modelling of Infectious Diseases. 2021.
- Voysey, M., Clemens, S. A. C., Madhi, S. A., Weckx, L. Y., Folegatti, P. M., Aley, P. K.,... &Bijker, E. (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397(10269), 99-111.
- Wang, P., Casner, R. G., Nair, M. S., Wang, M., Yu, J., Cerutti, G.,... & Ho, D. D. (2021). Increased resistance of SARS-CoV-2 variant P. 1 to antibody neutralization. Cell host & microbe, 29(5), 747-751.
- Wang, T., Hu, M., Chen, X., Fu, Y., Lei, C., Dong, H.,... & Yan, J. (2020). Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv. [CrossRef]
- Weiss, S. R., & Leibowitz, J. L. (2011). Coronavirus pathogenesis. Advances in virus research, 81, 85-164. [CrossRef]
- Werion, A., Belkhir, L., Perrot, M., Schmit, G., Aydin, S., Chen, Z.,... &Vancraeynest, D. (2020). SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney international, 98(5), 1296-1307. [CrossRef]
- Widiasta, A., Sribudiani, Y., Nugrahapraja, H., Hilmanto, D., Sekarwana, N., &Rachmadi, D. (2020). Potential role of ACE2-related microRNAs in COVID-19-associated nephropathy. Non-coding RNA research, 5(4), 153-166. [CrossRef]
- Xia, H., & Shi, P. Y. (2020). Antagonism of Type I interferon by severe acute respiratory syndrome Coronavirus 2. Journal of Interferon & Cytokine Research, 40(12), 543-548. [CrossRef]
- Xie X, Liu Y, Liu J, et al. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. The Lancet 2021. [CrossRef]
- Ye, Q., Wang, B., & Mao, J. (2020). The pathogenesis and treatment of theCytokineStorm’in COVID-19. Journal of infection, 80(6), 607-613. [CrossRef]
- Yuan, H., Cao, X., Ji, X., Du, F., He, J., Zhou, X.,... & Zhu, Y. (2020). An updated understanding of the current emerging respiratory infection: COVID-19. BioMed Research International, 2020. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).