Submitted:
26 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
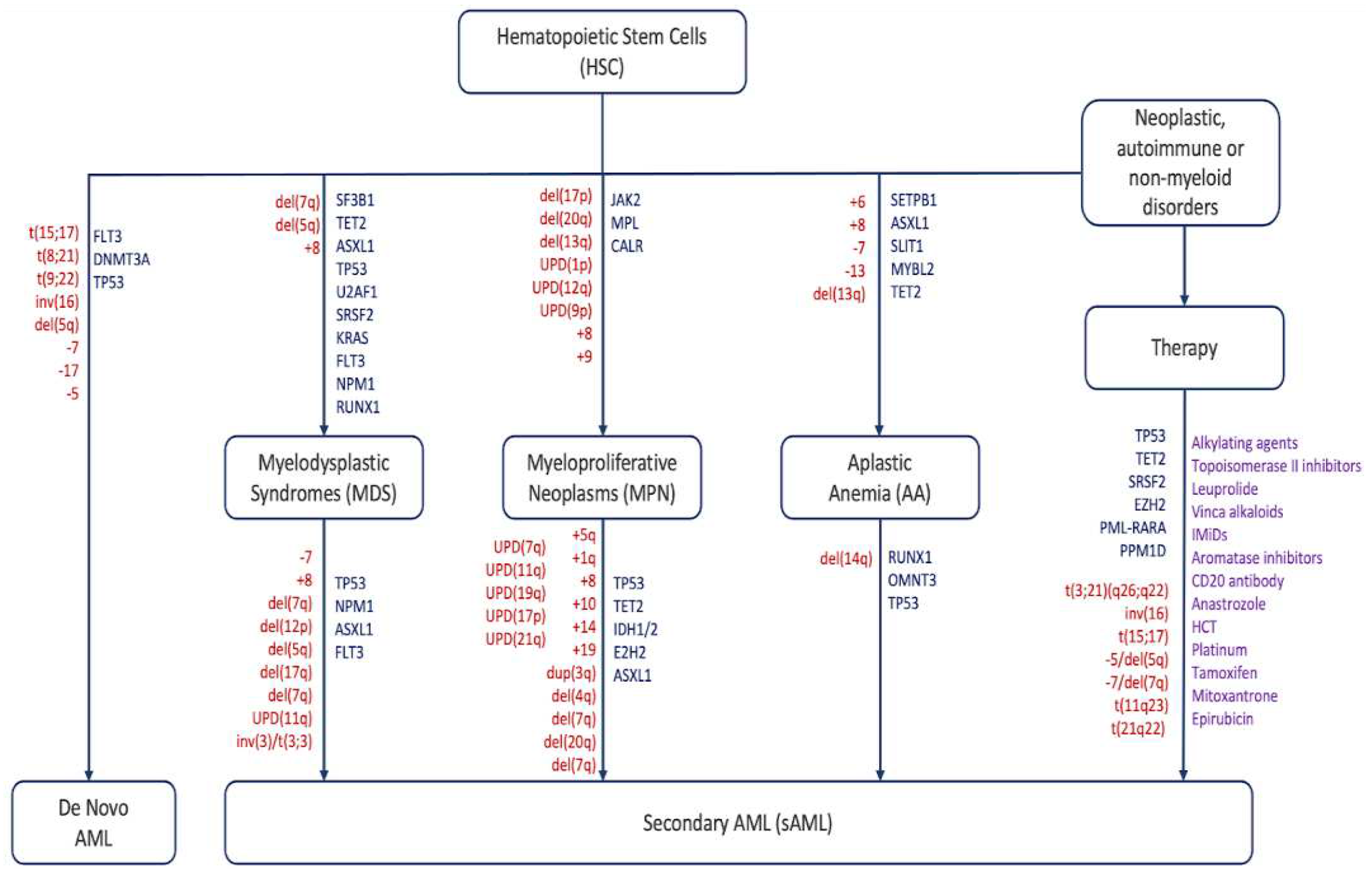
2. Pathophysiology of sAML
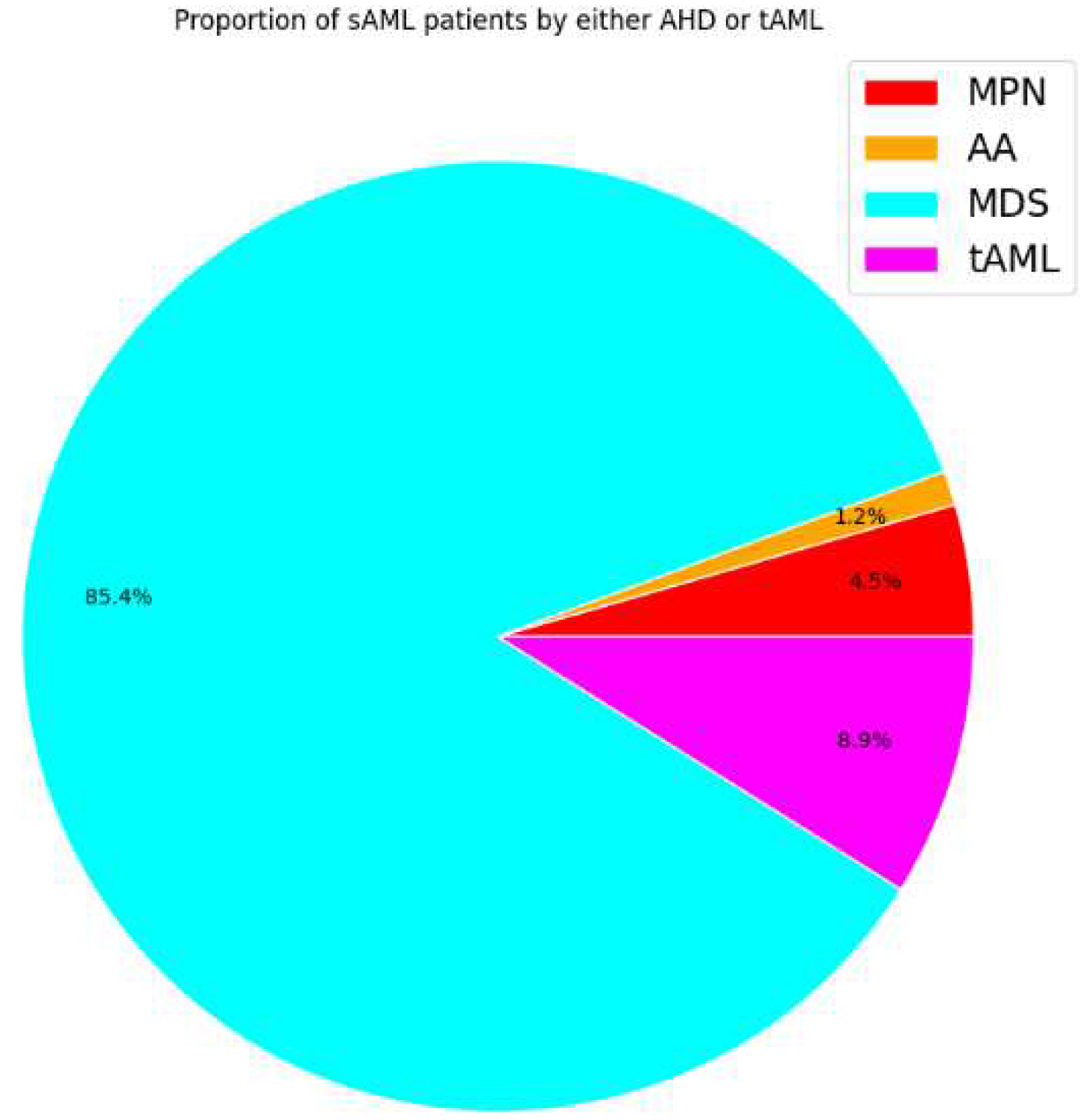
3. Cytogenetics and mutational landscape of sAML
| Category of Genes | Examples | Citations |
|---|---|---|
| Spliceosome | SRSF2, U2AF1, SF3B1 | [86,87] |
| DNA Methylation | DMNT3A, TET 1 / 2, IDH 1 / 2, | [88,89] |
| Activated Signaling | CALR, JAK2, PTPN11, TpoR, KRAS, FLT3, NRAS | [90,91] |
| Transcription Factors | RUNX1, NFE2, TP53 | [92,93,94,95] |
| Chromatin Modification | EZH2, ASXL1, NPM1 | [89,96] |
| Type of chromosomal abnormality |
Examples | Citations |
| Deletions | del(7q), del(5q), del(17p) | [107,114] |
| Duplications |
dup(1q), dup(3q), dup(11q), dup(17q) |
[115,116,117] |
| Translocations |
t(1;11)(q21;p15), t(10;11)(q22;q23), t(8;21) |
[60,118,119] |
|
Inversions |
inv(3)/t(3;3) |
[24] |
|
Monosomy |
-7 |
[87,120] |
|
Trisomy |
+8, +19, +21 |
[63,87,121] |
| Uniparental disomy |
UPD(9p), UPD(1p), UPD (17p) |
[75,92,122] |
4. Treatment of sAML
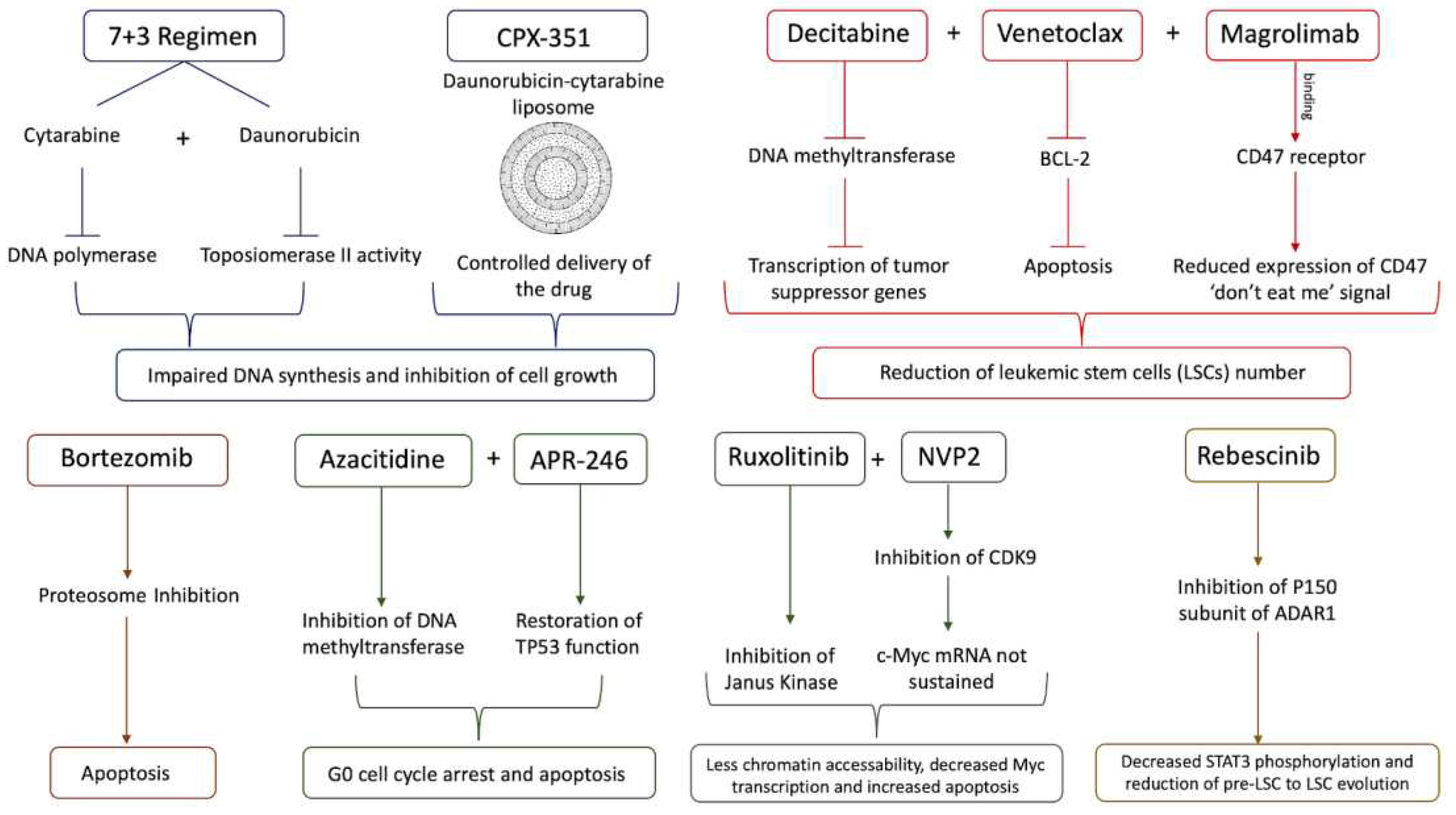
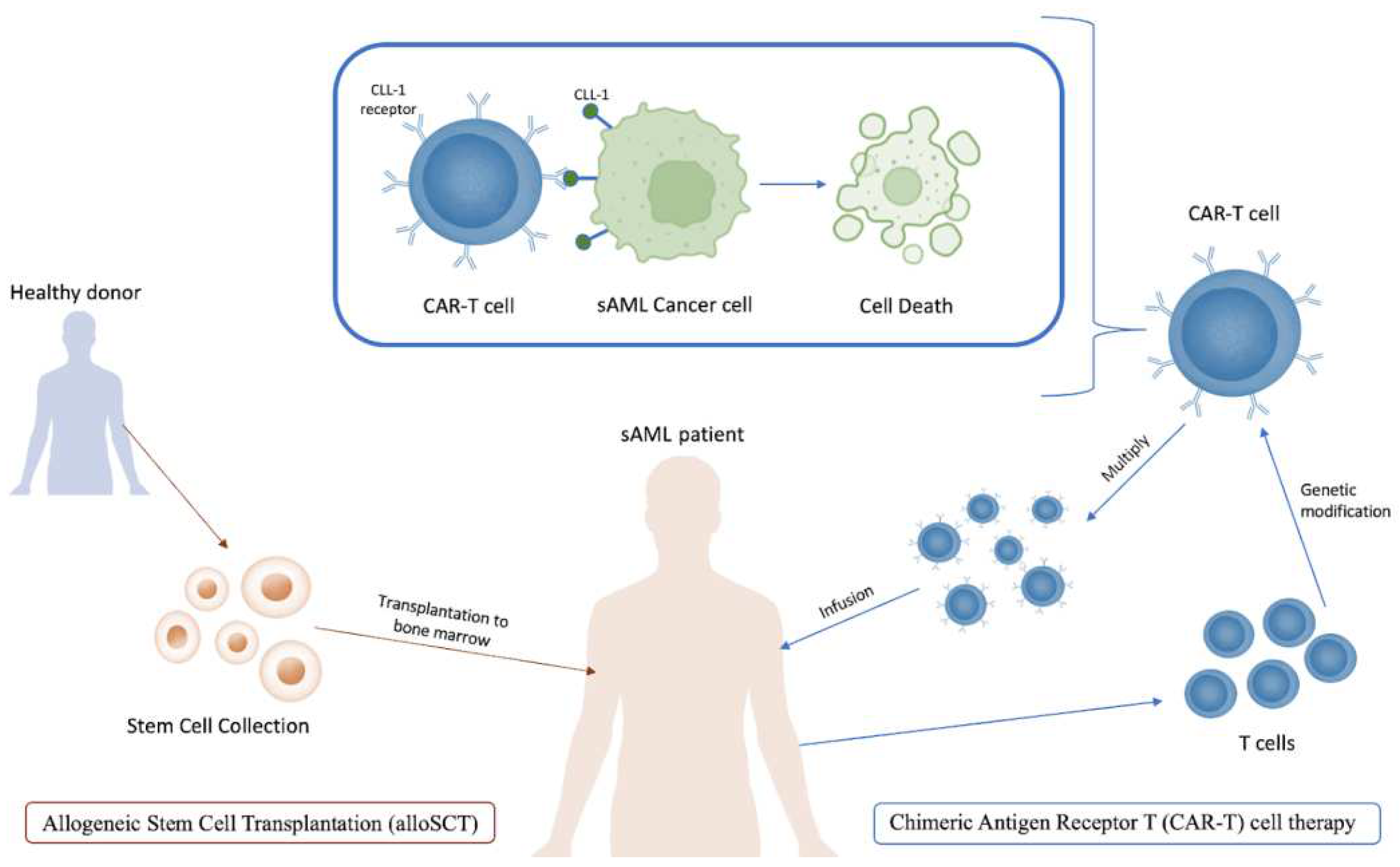
5. Perspectives
Acknowledgments
Conflicts of Interest
References
- Estey E, Döhner H (2006) Acute myeloid leukaemia. Lancet 368:1894–1907. [CrossRef] [PubMed]
- Higgins A, Shah MV (2020) Genetic and genomic landscape of secondary and therapy-related acute myeloid leukemia. Genes (Basel) 11:1–25. [CrossRef]
- Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH (2008) Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leuk 2008 222 22:240–248. [CrossRef]
- McMullin MF, Anderson LA (2020) Aetiology of Myeloproliferative Neoplasms. Cancers (Basel) 12:1–11. [CrossRef]
- Shahin OA, Chifotides HT, Bose P, Masarova L, Verstovsek S (2021) Accelerated Phase of Myeloproliferative Neoplasms. Acta Haematol 144:484–499. [CrossRef]
- Adès L, Itzykson R, Fenaux P (2014) Myelodysplastic syndromes. Lancet 383:2239–2252. [CrossRef]
- Dan C, Chi J, Wang L (2015) Molecular mechanisms of the progression of myelodysplastic syndrome to secondary acute myeloid leukaemia and implication for therapy. Ann Med 47:209–217. [CrossRef]
- Sun L, Babushok D V. (2020) Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood 136:36–49. [CrossRef]
- Wang L, Liu H (2019) Pathogenesis of aplastic anemia. Hematology 24:559–566. [CrossRef]
- Cook MR, Karp JE, Lai C (2022) The spectrum of genetic mutations in myelodysplastic syndrome: Should we update prognostication? eJHaem 3:301–313. [CrossRef]
- Heuser M, Gabdoulline R, Löffeld P, Dobbernack V, Kreimeyer H, Pankratz M, Flintrop M, Liebich A, Klesse S, Panagiota V, Stadler M, Wichmann M, Shahswar R, Platzbecker U, Thiede C, Schroeder T, Kobbe G, Geffers R, Schlegelberger B, Göhring G, Kreipe HH, Germing U, Ganser A, Kröger N, Koenecke C, Thol F (2017) Individual outcome prediction for myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia from MDS after allogeneic hematopoietic cell transplantation. Ann Hematol 96:1361–1372. [CrossRef]
- Hussein K, Van Dyke DL, Tefferi A (2009) Conventional cytogenetics in myelofibrosis: literature review and discussion. Eur J Haematol 82:329–338. [CrossRef]
- Rubin CM, Larson RA, Anastasi J, Winter JN, Thangavelu M, Vardiman JW, Rowley JD, Le Beau MM (1990) t(3;21)(q26;q22): a recurring chromosomal abnormality in therapy- related myelodysplastic syndrome and acute myeloid leukemia. Blood 76:2594–2598. [CrossRef]
- Andersen MK, Larson RA, Mauritzson N, Schnittger S, Jhanwar SC, Pedersen-Bjergaard J (2002) Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: Report from an International Workshop†. Genes, Chromosom Cancer 33:395–400. [CrossRef]
- Kotsiafti A, Giannakas K, Christoforou P, Liapis K (2023) Progress toward Better Treatment of Therapy-Related AML. Cancers 2023, Vol 15, Page 1658 15:1658. [CrossRef]
- Hosono N (2019) Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol 24:885–892. [CrossRef]
- Cheng Y, Wang Y, Wang H, Chen Z, Lou J, Xu H, Wang H, Qian W, Meng H, Lin M, Jin J (2009) Cytogenetic profile of de novo acute myeloid leukemia: a study based on 1432 patients in a single institution of China. Leuk 2009 2310 23:1801–1806. [CrossRef]
- Heuser M, Schlarmann C, Dobbernack V, Panagiota V, Wiehlmann L, Walter C, Beier F, Ziegler P, Yun H, Kade S, Kirchner A, Huang L, Koenecke C, Eder M, Brümmendorf TH, Dugas M, Ganser A, Thol F (2014) Genetic characterization of acquired aplastic anemia by targeted sequencing. Haematologica 99:e165. [CrossRef]
- Milosevic JD, Puda A, Malcovati L, Berg T, Hofbauer M, Stukalov A, Klampfl T, Harutyunyan AS, Gisslinger H, Gisslinger B, Burjanivova T, Rumi E, Pietra D, Elena C, Vannucchi AM, Doubek M, Dvorakova D, Robesova B, Wieser R, Koller E, Suvajdzic N, Tomin D, Tosic N, Colinge J, Racil Z, Steurer M, Pavlovic S, Cazzola M, Kralovics R (2012) Clinical significance of genetic aberrations in secondary acute myeloid leukemia. Am J Hematol 87:1010–1016. [CrossRef]
- Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS (2002) Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood 99:3129–3135. [CrossRef]
- Strickland SA, Vey N (2022) Diagnosis and treatment of therapy-related acute myeloid leukemia. Crit Rev Oncol Hematol 171:103607. [CrossRef]
- Keung Y-K, Pettenati MJ, Cruz JM, Powell BL, Woodruff RD, Buss DH (2001) Bone Marrow Cytogenetic Abnormalities of Aplastic Anemia. J Hematol 66:167–171. [CrossRef]
- Van Gelder M, Schetelig J, Volin L, Maertens J, Socié G, Petersen E, Thomssen H, Biezen A, Brand R, Witte TM de, Kröger N (2009) Monosomal Karyotype Predicts Poor Outcome for MDS/sAML Patients with Chromosome 7 Abnormalities After Allogeneic Stem Cell Transplantation for MDS/sAML. A Study of the MDS Subcommittee of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Blood 114:293. [CrossRef]
- Birdwell C, Fiskus W, Kadia TM, DiNardo CD, Mill CP, Bhalla KN (2021) EVI1 dysregulation: impact on biology and therapy of myeloid malignancies. Blood Cancer J 2021 113 11:1–14. [CrossRef]
- Marcellino BK, Hoffman R, Tripodi J, Lu M, Kosiorek H, Mascarenhas J, Rampal RK, Dueck A, Najfeld V (2018) Advanced forms of MPNs are accompanied by chromosomal abnormalities that lead to dysregulation of TP53. Blood Adv 2:3581. [CrossRef]
- Thoennissen NH, Kawamata N, Lasho TL, Weiss T, Nowak D, Kato M, Takita J, Sanada M, Haferlach T, Mesa RA, Tefferi A, Müller-Tidow C, Ogawa S, Koeffler PH (2008) Genomic Changes Associated with Leukemic Transformation of Myeloproliferative Disorders. Blood 112:3371. [CrossRef]
- Venton G, Courtier F, Charbonnier A, D’incan E, Saillard C, Mohty B, Mozziconacci MJ, Birnbaum D, Murati A, Vey N, Rey J (2018) Impact of gene mutations on treatment response and prognosis of acute myeloid leukemia secondary to myeloproliferative neoplasms. Am J Hematol 93:330–338. [CrossRef]
- Dunbar AJ, Rampal RK, Levine R (2020) Leukemia secondary to myeloproliferative neoplasms. Blood 136:61–70. [CrossRef]
- Schwind S, Jentzsch M, Kubasch AS, Metzeler KH, Platzbecker U (2021) Myelodysplastic syndromes: Biological and therapeutic consequences of the evolving molecular aberrations landscape. Neoplasia 23:1101–1109. [CrossRef]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061. [CrossRef]
- James C, Ugo V, Casadevall N, Constantinescu SN, Vainchenker W (2005) A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends Mol Med 11:546–554. [CrossRef]
- Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, Steensma DP, Elliott MA, Wolanskyj AP, Hogan WJ, McClure RF, Litzow MR, Gilliland DG, Tefferi A (2006) MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 108:3472–3476. [CrossRef]
- Pikman Y, Lee BH, Mercher T, Mcdowell E, Ebert BL, Gozo M, Cuker A, Wernig G, Moore S, Galinsky I, Deangelo DJ, Clark JJ, Lee SJ, Golub TR, Wadleigh M, Gilliland DG, Levine RL (2006) MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. [CrossRef]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincorena I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O’Meara S, McLaren S, Bianchi M, Silber Y, Dimitropoulou D, Bloxham D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du M-Q, Greaves M, Bowen D, Huntly BJP, Harrison CN, Cross NCP, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR (2013) Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N Engl J Med 369:2391–2405. [CrossRef]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NCC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant’Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R (2013) Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N Engl J Med 369:2379–2390. [CrossRef]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405. [CrossRef]
- Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, Levine R, Le Bousse-Kerdiles MC, Wadleigh M, Campbell PJ, Silver RT, Vannucchi AM, Deeg HJ, Gisslinger H, Thomas D, Odenike O, Solberg LA, Gotlib J, Hexner E, Nimer SD, Kantarjian H, Orazi A, Vardiman JW, Thiele J, Tefferi A (2007) Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and. Leuk Res 31:737–740. [CrossRef]
- Jilg S, Reidel V, Müller-Thomas C, König J, Schauwecker J, Höckendorf U, Huberle C, Gorka O, Schmidt B, Burgkart R, Ruland J, Kolb HJ, Peschel C, Oostendorp RAJ, Götze KS, Jost PJ (2015) Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leuk 2016 301 30:112–123. [CrossRef]
- Saenz DT, Fiskus W, Manshouri T, Mill CP, Qian Y, Raina K, Rajapakshe K, Coarfa C, Soldi R, Bose P, Borthakur G, Kadia TM, Khoury JD, Masarova L, Nowak AJ, Sun B, Saenz DN, Kornblau SM, Horrigan S, Sharma S, Qiu P, Crews CM, Verstovsek S, Bhalla KN (2018) Targeting nuclear β-catenin as therapy for post-myeloproliferative neoplasm secondary AML. Leuk 2018 336 33:1373–1386. [CrossRef]
- Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A (2000) The role of apoptosis, proliferation, and the Bcl-2–related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood 96:3932–3938. [CrossRef]
- Cogle CR (2015) Incidence and Burden of the Myelodysplastic Syndromes. Curr Hematol Malig Rep 10:272. [CrossRef]
- Vaht K, Göransson M, Carlson K, Isaksson C, Lenhoff S, Sandstedt A, Uggla B, Winiarski J, Ljungman P, Brune M, Andersson PO (2017) Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica 102:1683. [CrossRef]
- Prébet T, Gore SD, Thépot S, Esterni B, Quesnel B, Beyne Rauzy O, Dreyfus F, Gardin C, Fenaux P, Vey N (2012) Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure. Br J Haematol 157:764. [CrossRef]
- Nilsson C, Linde F, Hulegårdh E, Garelius H, Lazarevic V, Antunovic P, Cammenga J, Deneberg S, Eriksson A, Jädersten M, Björkvall CK, Möllgård L, Wennström L, Ölander E, Höglund M, Juliusson G, Lehmann S (2023) Characterization of therapy-related acute myeloid leukemia: increasing incidence and prognostic implications. Haematologica 108:1015. [CrossRef]
- Yanada M, Yamamoto Y, Iba S, Okamoto A, Inaguma Y, Tokuda M, Morishima S, Kanie T, Mizuta S, Akatsuka Y, Okamoto M, Emi N (2016) TP53 mutations in older adults with acute myeloid leukemia. Int J Hematol 103:429–435. [CrossRef]
- Michelis F V., Atenafu EG, Gupta V, Kim DD, Kuruvilla J, Lipton JH, Loach D, Seftel MD, Uhm J, Alam N, Lambie A, McGillis L, Messner HA (2015) Comparable outcomes post allogeneic hematopoietic cell transplant for patients with de novo or secondary acute myeloid leukemia in first remission. Bone Marrow Transplant 2015 507 50:907–913. [CrossRef]
- Rowe JM (2022) The “7+3” regimen in acute myeloid leukemia. Haematologica 107:3. [CrossRef]
- Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, Möllgård L, Uggla B, Wennström L, Wahlin A, Höglund M, Juliusson G, Stockelberg D, Lehmann S (2015) Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish Acute Leukemia Registry. Am J Hematol 90:208–214. [CrossRef]
- Østgård LSG, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva I, Friis LS, Kjeldsen E, Marcher CW, Preiss B, Severinsen M, Nørgaard JM (2015) Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: A national population-based cohort study. J Clin Oncol 33:3641–3649. [CrossRef]
- Estey E, Hasserjian RP, Döhner H (2022) Distinguishing AML from MDS: a fixed blast percentage may no longer be optimal. Blood 139:323–332. [CrossRef]
- Bauer M, Vaxevanis C, Al-Ali K, Jaekel N, Le C, Naumann H, Schaffrath J, Rau A, Seliger B, Wickenhauser C (2021) Altered Spatial Composition of the Immune Cell Repertoire in Association to CD34 + Blasts in Myelodysplastic Syndromes and Secondary Acute Myeloid Leukemia. Cancers (Basel) 13:186. [CrossRef]
- Hasserjian RP, Steensma DP, Graubert TA, Ebert BL (2020) Clonal hematopoiesis and measurable residual disease assessment in acute myeloid leukemia. Blood 135:1729–1738. [CrossRef]
- Sebert M, Phanie Gachet S, Leblanc T, Bluteau D, Gis Peffault De Latour R, Correspondence JS (2023) Clonal hematopoiesis driven by chromosome 1q/MDM4 trisomy defines a canonical route toward leukemia in Fanconi anemia. [CrossRef]
- Malcovati L, Gallì A, Travaglino E, Ambaglio I, Rizzo E, Molteni E, Elena C, Ferretti VV, Catricalà S, Bono E, Todisco G, Bianchessi A, Rumi E, Zibellini S, Pietra D, Boveri E, Camaschella C, Toniolo D, Papaemmanuil E, Ogawa S, Cazzola M (2017) Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 129:3371–3378. [CrossRef]
- Weeks LD, Niroula A, Neuberg D, Wong W, Lindsley RC, Luskin MR, Berliner N, Stone RM, DeAngelo DJ, Soiffer RJ, Uddin MM, Griffin G, Vlasschaert C, Gibson CJ, Jaiswal S, Bick AG, Malcovati L, Natarajan P, Ebert BL (2023) Prediction of Risk for Myeloid Malignancy in Clonal Hematopoiesis. NEJM Evid 2:. [CrossRef]
- Vucinic V, Ruhnke L, Sockel K, Rohnert MA, Backhaus D, Brauer D, Franke GN, Niederwieser D, Bornhauser M, Rollig C, Platzbecker U, Schwind S, Jentzsch M (2021) The diagnostic red blood cell distribution width as a prognostic factor in acute myeloid leukemia. Blood Adv 5:5584–5587. [CrossRef]
- Ye XP, Bao S, Gao HM, Guo Y, Wei YP (2016) A case of myeloproliferative neoplasm with a normal complete blood cell count: A novel problem of the JAK2 era. Oncol Lett 11:2134. [CrossRef]
- Kim SY, Park Y, Kim H, Kim J, Kwon GC, Koo SH (2018) Discriminating myelodysplastic syndrome and other myeloid malignancies from non-clonal disorders by multiparametric analysis of automated cell data. Clin Chim Acta 480:56–64. [CrossRef]
- Wang SY, Cheng WY, Mao YF, Zhu YM, Liu FJ, Ma TT, Shen Y (2019) Genetic alteration patterns and clinical outcomes of elderly and secondary acute myeloid leukemia. Hematol Oncol 37:456–463. [CrossRef]
- Rogers HJ, Wang X, Xie Y, Davis AR, Thakral B, Wang SA, Borthakur G, Cantu MD, Margolskee EM, Philip JKS, Sukhanova M, Bagg A, Bueso-Ramos CE, Orazi A, Arber DA, Hsi ED, Hasserjian RP (2020) Comparison of therapy-related and de novo core binding factor acute myeloid leukemia: A bone marrow pathology group study. Am J Hematol 95:799–808. [CrossRef]
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SMM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, Van De Loosdrecht AA, Germing U, Haase D (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454–2465. [CrossRef]
- Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki K, Steidl C, Fonatsch C, Pfeilstöcker M, Nösslinger T, Valent P, Giagounidis A, Aul C, Lübbert M, Stauder R, Krieger O, Garcia-Manero G, Faderl S, Pierce S, Le Beau MM, Bennett JM, Greenberg P, Germing U, Haase D (2012) New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 30:820–829. [CrossRef]
- O’Hagan Henderson S, Glaser A, Frietsch JJ, Hochhaus A, Hilgendorf I (2022) The incidental discovery of a constitutional trisomy 21 mosaicism in an adult female with myelodysplastic/myeloproliferative neoplasm. Ann Hematol 101:919. [CrossRef]
- Begna KH, Ali W, Naseema Gangat, Elliott MA, Al-Kali A, Litzow MR, Christopher Hook C, Wolanskyj-Spinner AP, Hogan WJ, Patnaik MM, Pardanani A, Zblewski DL, Chen D, He R, Viswanatha D, Hanson CA, Ketterling RP, Tefferi A (2021) Mayo Clinic experience with 1123 adults with acute myeloid leukemia. Blood Cancer J 2021 113 11:1–8. [CrossRef]
- Gupta V, Kim S, Hu ZH, Liu Y, Aljurf M, Bacher U, Beitinjaneh A, Cahn JY, Cerny J, Copelan E, Gadalla SM, Gale RP, Ganguly S, George B, Gerds AT, Gergis U, Hamilton BK, Hashmi S, Hildebrandt GC, Kamble RT, Kindwall-Keller T, Lazarus HM, Liesveld JL, Litzow M, Maziarz RT, Nishihori T, Olsson RF, Rizzieri D, Savani BN, Seo S, Solh M, Szer J, Verdonck LF, Wirk B, Woolfrey A, Yared JA, Alyea EP, Popat UR, Sobecks RM, Scott BL, Nakamura R, Saber W (2020) Comparison of outcomes of HCT in blast phase of BCR-ABL1− MPN with de novo AML and with AML following MDS. Blood Adv 4:4748–4757. [CrossRef]
- Elessa D, Zhao LP, de Oliveira RD, Maslah N, Soret-Dulphy J, Verger E, Marcault C, Parquet N, Fenaux P, Adès L, Raffoux E, Giraudier S, Fain O, Cassinat B, Kiladjian JJ, Mekinian A, Benajiba L (2023) Clinical features and genomic landscape of myeloproliferative neoplasm (MPN) patients with autoimmune and inflammatory diseases (AID). Leuk 2023 378 37:1741–1744. [CrossRef]
- Boddu PC, Zeidan AM (2019) MYELOID DISORDERS AFTER AUTOIMMUNE DISEASE. Best Pract Res Clin Haematol 32:74. [CrossRef]
- Rodriguez-Meira A, Norfo R, Wen S, Chédeville AL, Rahman H, O’Sullivan J, Wang G, Louka E, Kretzschmar WW, Paterson A, Brierley C, Martin JE, Demeule C, Bashton M, Sousos N, Moralli D, Subha Meem L, Carrelha J, Wu B, Hamblin A, Guermouche H, Pasquier F, Marzac C, Girodon F, Vainchenker W, Drummond M, Harrison C, Chapman JR, Plo I, Jacobsen SEW, Psaila B, Thongjuea S, Antony-Debré I, Mead AJ (2023) Single-cell multi-omics identifies chronic inflammation as a driver of TP53-mutant leukemic evolution. Nat Genet 2023 559 55:1531–1541. [CrossRef]
- Mendez Luque LF, Blackmon AL, Ramanathan G, Fleischman AG (2019) Key Role of Inflammation in Myeloproliferative Neoplasms: Instigator of Disease Initiation, Progression. and Symptoms. Curr Hematol Malig Rep 14:145–153. [CrossRef]
- Braun T, Carvalho G, Fabre C, Grosjean J, Fenaux P, Kroemer G (2006) Targeting NF-κB in hematologic malignancies. Cell Death Differ 2006 135 13:748–758. [CrossRef]
- Karantanos T, Teodorescu P, Perkins B, Christodoulou I, Esteb C, Varadhan R, Helmenstine E, Rajkhowa T, Paun BC, Bonifant C, Dalton WB, Gondek LP, Moliterno AR, Levis MJ, Ghiaur G, Jones RJ (2022) The role of the atypical chemokine receptor CCRL2 in myelodysplastic syndrome and secondary acute myeloid leukemia. Sci Adv 8:. [CrossRef]
- Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, Bhagat T, Bhattacharyya S, Ramachandra N, Bartenstein M, Pellagatti A, Boultwood J, Wickrema A, Yu Y, Will B, Wei S, Steidl U, Verma A (2015) IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood 125:3144–3152. [CrossRef]
- Wang X, Teng F, Kong L, Yu J (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023. [CrossRef]
- Montes P, Bernal M, Campo LN, González-Ramírez AR, Jiménez P, Garrido P, Jurado M, Garrido F, Ruiz-Cabello F, Hernández F (2019) Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol Immunother 68:2015–2027. [CrossRef]
- Milosevic Feenstra JD, Jäger R, Schischlik F, Ivanov D, Eisenwort G, Rumi E, Schuster M, Gisslinger B, Machherndl-Spandl S, Bettelheim P, Krauth MT, Keil F, Bock C, Cazzola M, Gisslinger H, Kralovics R, Valent P (2022) PD-L1 overexpression correlates with JAK2-V617F mutational burden and is associated with 9p uniparental disomy in myeloproliferative neoplasms. Am J Hematol 97:390–400. [CrossRef]
- Palumbo GA, Parrinello NL, Giallongo C, D’amico E, Zanghì A, Puglisi F, Conticello C, Chiarenza A, Tibullo D, Di Raimondo F, Romano A (2019) Monocytic Myeloid Derived Suppressor Cells in Hematological Malignancies. Int J Mol Sci 20:. [CrossRef]
- Vaxevanis CK, Bauer M, Subbarayan K, Friedrich M, Massa C, Biehl K, Al-Ali HK, Wickenhauser C, Seliger B (2023) Biglycan as a mediator of proinflammatory response and target for MDS and sAML therapy. Oncoimmunology 12:. [CrossRef]
- Rontauroli S, Carretta C, Parenti S, Bertesi M, Manfredini R (2022) Novel Molecular Insights into Leukemic Evolution of Myeloproliferative Neoplasms: A Single Cell Perspective. Int. J. Mol. Sci. 23. [CrossRef]
- Han H, Byun JM, Shin DY, Yoon SS, Koh Y, Hong J, Kim I, Lee C, Yoo H, Yun H, Kim MJ, Cho SI, Seong MW, Park SS (2021) Leukemic stem cell phenotype is associated with mutational profile in acute myeloid leukemia. Korean J Intern Med 36:401. [CrossRef]
- Castro FA de, Mehdipour P, Chakravarthy A, Ettayebi I, Loo Yau H, Medina TS, Marhon SA, Almeida FC de, Bianco TM, Arruda AGF, Devlin R, Figueiredo-Pontes LL de, Chahud F, Costa Cacemiro M da, Minden MD, Gupta V, De Carvalho DD (2023) Ratio of stemness to interferon signalling as a biomarker and therapeutic target of myeloproliferative neoplasm progression to acute myeloid leukaemia. Br J Haematol. [CrossRef]
- Guess T, Potts CR, Bhat P, Cartailler JA, Brooks A, Holt C, Yenamandra A, Wheeler FC, Savona MR, Cartailler J-P, Ferrell PB (2022) Distinct Patterns of Clonal Evolution Drive Myelodysplastic Syndrome Progression to Secondary Acute Myeloid Leukemia. Blood Cancer Discov 3:316–329. [CrossRef]
- Miles LA, Bowman RL, Merlinsky TR, Csete IS, Ooi AT, Durruthy-Durruthy R, Bowman M, Famulare C, Patel MA, Mendez P, Ainali C, Demaree B, Delley CL, Abate AR, Manivannan M, Sahu S, Goldberg AD, Bolton KL, Zehir A, Rampal R, Carroll MP, Meyer SE, Viny AD, Levine RL (2020) Single cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587:477. [CrossRef]
- Luque Paz D, Kralovics R, Skoda RC (2023) Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood 141:1909–1921. [CrossRef]
- Paz DL, Jouanneau-Courville R, Riou J, Ianotto JC, Boyer F, Chauveau A, Renard M, Chomel JC, Cayssials E, Gallego-Hernanz MP, Pastoret C, Murati A, Courtier F, Rousselet MC, Quintin-Roue I, Cottin L, Orvain C, Thepot S, Chretien JM, Delneste Y, Ifrah N, Blanchet O, Hunault-Berger M, Lippert E, Ugo V (2020) Leukemic evolution of polycythemia vera and essential thrombocythemia: genomic profiles predict time to transformation. Blood Adv 4:4887–4897. [CrossRef]
- Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, Przychodzen BJ, Nagata Y, Meggendorfer M, Sanada M, Okuno Y, Hirsch C, Kuzmanovic T, Sato Y, Sato-Otsubo A, Laframboise T, Hosono N, Shiraishi Y, Chiba K, Haferlach C, Kern W, Tanaka H, Shiozawa Y, Gómez-Seguí I, Husseinzadeh HD, Thota S, Guinta KM, Dienes B, Nakamaki T, Miyawaki S, Saunthararajah Y, Chiba S, Miyano S, Shih LY, Haferlach T, Ogawa S, MacIejewski JP (2016) Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 2016 492 49:204–212. [CrossRef]
- Taylor J, Lee SC, Stanley Lee CC, Sloan Kettering M (2019) Mutations in spliceosome genes and therapeutic opportunities in myeloid malignancies. Genes, Chromosom Cancer 58:889–902. [CrossRef]
- Kar SA, Jankowska A, Makishima H, Visconte V, Jerez A, Sugimoto Y, Muramatsu H, Traina F, Afable M, Guinta K, Tiu R V., Przychodzen B, Sakaguchi H, Kojima S, Sekeres MA, List AF, McDevitt MA, Maciejewski JP (2013) Spliceosomal gene mutations are frequent events in the diverse mutational spectrum of chronic myelomonocytic leukemia but largely absent in juvenile myelomonocytic leukemia. Haematologica 98:107. [CrossRef]
- Stegelmann F, Bullinger L, Schlenk RF, Paschka P, Griesshammer M, Blersch C, Kuhn S, Schauer S, Döhner H, Döhner K (2011) DNMT3A mutations in myeloproliferative neoplasms. Leuk 2011 257 25:1217–1219. [CrossRef]
- Brune MM, Rau A, Overkamp M, Flaadt T, Bonzheim I, Schürch CM, Federmann B, Dirnhofer S, Fend F, Tzankov A (2021) Molecular Progression of Myeloproliferative and Myelodysplastic/Myeloproliferative Neoplasms: A Study on Sequential Bone Marrow Biopsies. Cancers 2021, Vol 13, Page 5605 13:5605. [CrossRef]
- Benton CB, Boddu PC, DiNardo CD, Bose P, Wang F, Assi R, Pemmaraju N, Devendra KC, Pierce S, Patel K, Konopleva M, Ravandi F, Garcia-Manero G, Kadia TM, Cortes J, Kantarjian HM, Andreeff M, Verstovsek S (2019) Janus kinase 2 variants associated with the transformation of myeloproliferative neoplasms into acute myeloid leukemia. Cancer 125:1855–1866. [CrossRef]
- Stengel A, Baer C, Walter W, Meggendorfer M, Kern W, Haferlach T, Haferlach C (2021) Mutational patterns and their correlation to CHIP-related mutations and age in hematological malignancies. Blood Adv 5:4426–4434. [CrossRef]
- Harutyunyan A, Klampfl T, Cazzola M, Kralovics R (2011) p53 Lesions in Leukemic Transformation. N Engl J Med 364:488–490. [CrossRef]
- Rampal R, Ahn J, Abdel-Wahaba O, Nahas M, Wang K, Lipson D, Otto GA, Yelensky R, Hricik T, McKenney AS, Chiosis G, Chung YR, Pandey S, Van Den Brink MRM, Armstrong SA, Dogan A, Intlekofer A, Manshouri T, Park CY, Verstovsek S, Rapaport F, Stephens PJ, Miller VA, Levine RL (2014) Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A 111:E5401–E5410. [CrossRef]
- Gaidzik VI, Teleanu V, Papaemmanuil E, Weber D, Paschka P, Hahn J, Wallrabenstein T, Kolbinger B, Köhne CH, Horst HA, Brossart P, Held G, Kündgen A, Ringhoffer M, Götze K, Rummel M, Gerstung M, Campbell P, Kraus JM, Kestler HA, Thol F, Heuser M, Schlegelberger B, Ganser A, Bullinger L, Schlenk RF, Döhner K, Döhner H (2016) RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leuk 2016 3011 30:2160–2168. [CrossRef]
- Jutzi JS, Bogeska R, Nikoloski G, Schmid CA, Seeger TS, Stegelmann F, Schwemmers S, Gründer A, Peeken JC, Gothwal M, Wehrle J, Aumann K, Hamdi K, Dierks C, Wang W, Döhner K, Jansen JH, Pahl HL (2013) MPN patients harbor recurrent truncating mutations in transcription factor NF-E2. J Exp Med 210:1003–1019. [CrossRef]
- Zarka J, Short NJ, Kanagal-Shamanna R, Issa GC (2020) Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes (Basel) 11:1–16. [CrossRef]
- Menssen AJ, Walter MJ (2020) Genetics of progression from MDS to secondary leukemia. Blood 136:50–60. [CrossRef]
- Delhommeau F, Dupont S, Valle V Della, James C, Trannoy S, Massé A, Kosmider O, Le Couedic J-P, Robert F, Alberdi A, Lécluse Y, Plo I, Dreyfus FJ, Marzac C, Casadevall N, Lacombe C, Romana SP, Dessen P, Soulier J, Viguié F, Fontenay M, Vainchenker W, Bernard OA (2009) Mutation in TET2 in Myeloid Cancers. N Engl J Med 360:2289–2301. [CrossRef]
- Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A (2011) DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leuk 2011 257 25:1219–1220. [CrossRef]
- Goyal H, Chachoua I, Pecquet C, Vainchenker W, Constantinescu SN (2020) A p53-JAK-STAT connection involved in myeloproliferative neoplasm pathogenesis and progression to secondary acute myeloid leukemia. Blood Rev 42:100712. [CrossRef]
- Shih AH, Chung SS, Dolezal EK, Zhang SJ, Abdel-Wahab OI, Park CY, Nimer SD, Levine RL, Klimek VM (2013) Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Haematologica 98:908–912. [CrossRef]
- Guerra VA, Yuanqing Y, Hsu J, Wang F, Alfayez M, Morita K, Song X, DiNardo CD, Konopleva MY, Borthakur GM, Montalban Bravo G, Zhang J, Little L, Gumbs C, Kantarjian HM, Garcia-Manero G, Futreal A, Takahashi K (2019) Comprehensive Analysis of Genotype and Prior Exposures in Therapy-Related Myeloid Neoplasms (t-MNs). Blood 134:458. [CrossRef]
- Rampal R, Ahn J, Abdel-Wahaba O, Nahas M, Wang K, Lipson D, Otto GA, Yelensky R, Hricik T, McKenney AS, Chiosis G, Chung YR, Pandey S, Van Den Brink MRM, Armstrong SA, Dogan A, Intlekofer A, Manshouri T, Park CY, Verstovsek S, Rapaport F, Stephens PJ, Miller VA, Levine RL (2014) Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A 111:E5401–E5410. [CrossRef]
- Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, Girsberger S, Lehmann T, Passweg J, Stern M, Beisel C, Kralovics R, Skoda RC (2014) Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 123:2220–2228. [CrossRef]
- Soenen V, Preudhomme C, Roumier C, Daudignon A, Luc Laı̈ J, Fenaux P (1998) 17p Deletion in Acute Myeloid Leukemia and Myelodysplastic Syndrome. Analysis of Breakpoints and Deleted Segments by Fluorescence In Situ. Blood 91:1008–1015. [CrossRef]
- Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, Habdank M, Kugler CM, Holzmann K, Gaidzik VI, Paschka P, Held G, Von Lilienfeld-Toal M, Lübbert M, Fröhling S, Zenz T, Krauter J, Schlegelberger B, Ganser A, Lichter P, Döhner K, Döhner H (2012) TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119:2114–2121. [CrossRef]
- Bahaj W, Kewan T, Gurnari C, Durmaz A, Ponvilawan B, Pandit I, Kubota Y, Ogbue OD, Zawit M, Madanat Y, Bat T, Balasubramanian SK, Awada H, Ahmed R, Mori M, Meggendorfer M, Haferlach T, Visconte V, Maciejewski JP (2023) Novel scheme for defining the clinical implications of TP53 mutations in myeloid neoplasia. J Hematol Oncol 16:1–12. [CrossRef]
- Rahmé R, Braun T, Manfredi JJ, Fenaux P (2023) TP53 Alterations in Myelodysplastic Syndromes and Acute Myeloid Leukemia. Biomedicines 11:. [CrossRef]
- Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, Garcia-Manero G, Craddock C, Sallman DA, Kantarjian HM (2022) TP53-Mutated Myelodysplastic Syndrome and Acute Myeloid Leukemia: Biology, Current Therapy, and Future Directions. Cancer Discov 12:2516. [CrossRef]
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F, Foucar K, Gangat N, Gianelli U, Godley LA, Gökbuget N, Gotlib J, Hellström-Lindberg E, Hobbs GS, Hoffman R, Jabbour EJ, Kiladjian JJ, Larson RA, Le Beau MM, Loh MLC, Löwenberg B, Macintyre E, Malcovati L, Mullighan CG, Niemeyer C, Odenike OM, Ogawa S, Orfao A, Papaemmanuil E, Passamonti F, Porkka K, Pui CH, Radich JP, Reiter A, Rozman M, Rudelius M, Savona MR, Schiffer CA, Schmitt-Graeff A, Shimamura A, Sierra J, Stock WA, Stone RM, Tallman MS, Thiele J, Tien HF, Tzankov A, Vannucchi AM, Vyas P, Wei AH, Weinberg OK, Wierzbowska A, Cazzola M, Döhner H, Tefferi A (2022) International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood 140:1200–1228. [CrossRef]
- Sebert M, Gachet S, Leblanc T, Rousseau A, Bluteau O, KIM R, Benabelali R, Sicre de Fontbrune F, Maillard L, Fedronie C, Vasquez N, Bertrand Y, Dalle J-H, Sigaux F, Chevret S, Socie G, de Thé H, Antoniewski C, Bluteau D, Peffault De Latour R, Soulier J (2021) Clonal Hematopoiesis Driven By MDM4 Amplification Defines a Canonical Route Towards Secondary MDS/AML in Fanconi Anemia Patients. Blood 138:860. [CrossRef]
- Pezeshki A, Podder S, Kamel R, Corey SJ (2017) Monosomy 7/del (7q) in inherited bone marrow failure syndromes: A systematic review. Pediatr Blood Cancer 64:e26714. [CrossRef]
- Daniels NJ, Hershberger CE, Gu X, Schueger C, DiPasquale WM, Brick J, Saunthararajah Y, Maciejewski JP, Padgett RA (2021) Functional analyses of human LUC7-like proteins involved in splicing regulation and myeloid neoplasms. Cell Rep 35:. [CrossRef]
- Awada H, Durmaz A, Gurnari C, Kishtagari A, Meggendorfer M, Kerr CM, Kuzmanovic T, Durrani J, Shreve J, Nagata Y, Radivoyevitch T, Advani AS, Ravandi F, Carraway HE, Nazha A, Haferlach C, Saunthararajah Y, Scott J, Visconte V, Kantarjian H, Kadia T, Sekeres MA, Haferlach T, Maciejewski JP (2021) Machine learning integrates genomic signatures for subclassification beyond primary and secondary acute myeloid leukemia. Blood 138:1885–1895. [CrossRef]
- Guglielmelli P, Calabresi L (2021) The MPL mutation. Int Rev Cell Mol Biol 365:163–178. [CrossRef]
- Chang L, Cui Z, Shi D, Chu Y, Wang B, Wan Y, Ma Q, Zhang R, Li H, Cheng X, Cheng T, Zhu X, Li C, Yuan W (2022) Polyclonal evolution of Fanconi anemia to MDS and AML revealed at single cell resolution. Exp Hematol Oncol 11:1–14. [CrossRef]
- Lee C, Kim HN, Kwon JA, Hwang J, Park JY, Shin OS, Yoon SY, Yoon J (2023) Identification of a Complex Karyotype Signature with Clinical Implications in AML and MDS-EB Using Gene Expression Profiling. Cancers (Basel) 15:5289. [CrossRef]
- Tembrink M, Gerding WM, Wieczorek S, Mika T, Schroers R, Nguyen HP, Vangala D Ben, Nilius-Eliliwi V (2023) Novel NUP98::ASH1L Gene Fusion in Acute Myeloid Leukemia Detected by Optical Genome Mapping. Cancers (Basel) 15:2942. [CrossRef]
- Zhang X, Zhang Y, Wang C, Wang X (2023) TET (Ten-eleven translocation) family proteins: structure, biological functions and applications. Signal Transduct Target Ther 2023 81 8:1–20. [CrossRef]
- Ganster C, Müller-Thomas C, Haferlach C, Strupp C, Ogata K, Germing U, Hildebrandt B, Mallo M, Lübbert M, Müller C, Solé F, Götze KS, Vandenberghe P, Göhring G, Steinmetz T, Kröger N, Platzbecker U, Söling U, Raynaud S, Shirneshan K, Schanz J, Haase D (2019) Comprehensive analysis of isolated der(1;7)(q10;p10) in a large international homogenous cohort of patients with myelodysplastic syndromes. Genes, Chromosom Cancer 58:689–697. [CrossRef]
- Hebeda K, Boudova L, Beham-Schmid C, Orazi A, Kvasnicka HM, Gianelli U, Tzankov A (2021) Progression, transformation, and unusual manifestations of myelodysplastic syndromes and myelodysplastic-myeloproliferative neoplasms: lessons learned from the XIV European Bone Marrow Working Group Course 2019. Ann Hematol 100:117–133. [CrossRef]
- Gurban P, Mambet C, Botezatu A, Necula LG, Neagu AI, Matei L, Pitica IM, Nedeianu S, Chivu-Economescu M, Bleotu C, Ataman M, Mocanu G, Saguna C, Pavel AG, Stambouli D, Sepulchre E, Anton G, Diaconu CC, Constantinescu SN (2023) Leukemic conversion involving RAS mutations of type 1 CALR-mutated primary myelofibrosis in a patient treated for HCV cirrhosis: a case report. Front Oncol 13:1266996. [CrossRef]
- Zarka J, Short NJ, Kanagal-Shamanna R, Issa GC (2020) Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes (Basel) 11:1–16. [CrossRef]
- Wan C, Wen J, Liang X, Xie Q, Wu W, Wu M, Liu Z (2021) Identification of miR-320 family members as potential diagnostic and prognostic biomarkers in myelodysplastic syndromes. Sci Reports 2021 111 11:1–11. [CrossRef]
- Symeonidis A, Chatzilygeroudi T, Chondrou V, Sgourou A (2022) Contingent Synergistic Interactions between Non-Coding RNAs and DNA-Modifying Enzymes in Myelodysplastic Syndromes. Int J Mol Sci 2022, Vol 23, Page 16069 23:16069. [CrossRef]
- Lyu C, Liu K, Jiang Y, Wang T, Wang Y, Xu R (2021) Integrated analysis on mRNA microarray and microRNA microarray to screen immune-related biomarkers and pathways in myelodysplastic syndrome. 26:417–431. [CrossRef]
- Thol F, Scherr M, Kirchner A, Shahswar R, Battmer K, Kade S, Chaturvedi A, Koenecke C, Stadler M, Platzbecker U, Thiede C, Schroeder T, Kobbe G, Bug G, Ottmann O, Hofmann WK, Kröger N, Fiedler W, Schlenk R, Döhner K, Döhner H, Krauter J, Eder M, Ganser A, Heuser M (2015) Clinical and functional implications of microRNA mutations in a cohort of 935 patients with myelodysplastic syndromes and acute myeloid leukemia. Haematologica 100:e122. [CrossRef]
- Kirimura S, Kurata M, Nakagawa Y, Onishi I, Abe-Suzuki S, Abe S, Yamamoto K, Kitagawa M (2016) Role of microRNA-29b in myelodysplastic syndromes during transformation to overt leukaemia. Pathology 48:233–241. [CrossRef]
- Pavlovic D, Tosic N, Zukic B, Pravdic Z, Vukovic NS, Pavlovic S, Gasic V (2021) Expression Profiles of Long Non-Coding RNA GAS5 and MicroRNA-222 in Younger AML Patients. Diagnostics 2022, Vol 12, Page 86 12:86. [CrossRef]
- Qin T, Cheng Y, Wang X (2022) RNA-binding proteins as drivers of AML and novel therapeutic targets. 63:1045–1057. [CrossRef]
- Bauer M, Vaxevanis C, Heimer N, Al-Ali HK, Jaekel N, Bachmann M, Wickenhauser C, Seliger B (2020) Expression, Regulation and Function of microRNA as Important Players in the Transition of MDS to Secondary AML and Their Cross Talk to RNA-Binding Proteins. Int J Mol Sci 2020, Vol 21, Page 7140 21:7140. [CrossRef]
- Jiang Q, Isquith J, Ladel L, Alexandrov LB, Fisch KM, Jamieson Correspondence C, Mark A, Holm F, Mason C, He Y, Mondala P, Oliver I, Pham J, Ma W, Reynoso E, Ali S, Morris IJ, Diep R, Nasamran C, Xu G, Sasik R, Rosenthal SB, Birmingham A, Coso S, Pineda G, Crews L, Donohoe ME, Craig Venter J, Whisenant T, Mesa RA, Jamieson C (2021) Inflammation-driven deaminase deregulation fuels human pre-leukemia stem cell evolution. [CrossRef]
- Maakaron JE, Mims AS (2019) Daunorubicin-cytarabine liposome (CPX-351) in the management of newly diagnosed secondary AML: A new twist on an old cocktail. Best Pract Res Clin Haematol 32:127–133. [CrossRef]
- Budziszewska BK, Salomon-Perzyński A, Pruszczyk K, Barankiewicz J, Pluta A, Helbig G, Janowska A, Kuydowicz M, Bołkun Ł, Piszcz J, Patkowska E, Wątek M, Małecki P, Kościołek-Zgódka S, Cichocka E, Charliński G, Irga-Staniukiewicz A, Zaucha JM, Piekarska A, Gromek T, Hus M, Wójcik K, Raźny M, Sędzimirska M, Puła B, Giebel S, Grosicki S, Wierzbowska A, Lech-Marańda E (2021) Cladribine Combined with Low-Dose Cytarabine as Frontline Treatment for Unfit Elderly Acute Myeloid Leukemia Patients: Results from a Prospective Multicenter Study of Polish Adult Leukemia Group (PALG). Cancers 2021, Vol 13, Page 4189 13:4189. [CrossRef]
- Schieber M, Marinaccio C, Bolanos LC, Haffey WD, Greis KD, Starczynowski DT, Crispino JD (2020) FBXO11 is a candidate tumor suppressor in the leukemic transformation of myelodysplastic syndrome. Blood Cancer J 2020 1010 10:1–12. [CrossRef]
- Roboz GJ, Mandrekar SJ, Desai P, Laumann K, Walker AR, Wang ES, Kolitz JE, Powell BL, Attar EC, Stock W, Bloomfield CD, Kohlschmidt J, Mrózek K, Hassane DC, Garraway L, Jané-Valbuena J, Baltay M, Tracy A, Marcucci G, Stone RM, Larson RA (2018) Randomized trial of 10 days of decitabine ± bortezomib in untreated older patients with AML: CALGB 11002 (Alliance). Blood Adv 2:3608–3617. [CrossRef]
- Malik P, Cashen AF (2014) Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Manag Res 6:53–61. [CrossRef]
- Zong L, Yin M, Kong J, Zhang J, Song B, Zhu J, Xue S, Wu X, Wu D, Bao X, Qiu H (2023) Development of a scoring system for predicting primary resistance to venetoclax plus hypomethylating agents (HMAs) in acute myeloid leukemia patients. Mol Carcinog 62:1572–1584. [CrossRef]
- Haddad F, Daver N (2021) Targeting CD47/SIRPα in Acute Myeloid Leukemia and Myelodysplastic Syndrome: Preclinical and Clinical Developments of Magrolimab. J Immunother Precis Oncol 4:67. [CrossRef]
- Maslah N, Salomao N, Drevon L, Verger E, Partouche N, Ly P, Aubin P, Naoui N, Schlageter MH, Bally C, Miekoutima E, Rahmé R, Lehmann-Che J, Ades L, Fenaux P, Cassinat B, Giraudier S (2020) Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 105:1539. [CrossRef]
- Fiskus W, Mill CP, Nabet B, Perera D, Birdwell C, Manshouri T, Lara B, Kadia TM, DiNardo C, Takahashi K, Daver N, Bose P, Masarova L, Pemmaraju N, Kornblau S, Borthakur G, Montalban-Bravo G, Manero GG, Sharma S, Stubbs M, Su X, Green MR, Coarfa C, Verstovsek S, Khoury JD, Vakoc CR, Bhalla KN (2021) Superior efficacy of co-targeting GFI1/KDM1A and BRD4 against AML and post-MPN secondary AML cells. Blood Cancer J 2021 115 11:1–16. [CrossRef]
- Tibes R, Bogenberger JM (2019) Transcriptional Silencing of MCL-1 Through Cyclin-Dependent Kinase Inhibition in Acute Myeloid Leukemia. Front Oncol 9:1205. [CrossRef]
- Fiskus W, Manshouri T, Birdwell C, Mill CP, Masarova L, Bose P, Kadia TM, Daver N, DiNardo CD, Borthakur G, Khoury JD, Verstovsek S, Bhalla KN (2022) Efficacy of CDK9 inhibition in therapy of post-myeloproliferative neoplasm (MPN) secondary (s) AML cells. Blood Cancer J 2022 121 12:1–5. [CrossRef]
- Crews LA, Ma W, Ladel L, Pham J, Balaian L, Steel SK, Mondala PK, Diep RH, Wu CN, Mason CN, van der Werf I, Oliver I, Reynoso E, Pineda G, Whisenant TC, Wentworth P, La Clair JJ, Jiang Q, Burkart MD, Jamieson CHM (2023) Reversal of malignant ADAR1 splice isoform switching with Rebecsinib. Cell Stem Cell 30:250-263.e6. [CrossRef]
- Qing Y, Su R, Chen J (2021) RNA modifications in hematopoietic malignancies: a new research frontier. Blood 138:637–648. [CrossRef]
- Eisenberg E, Levanon EY (2018) A-to-I RNA editing — immune protector and transcriptome diversifier. Nat Rev Genet 2018 198 19:473–490. [CrossRef]
- Schmaelter AK, Labopin M, Socié G, Itälä-Remes M, Blaise D, Yakoub-Agha I, Forcade E, Cornelissen J, Ganser A, Beelen D, Labussière-Wallet H, Passweg J, Savani BN, Schmid C, Nagler A, Mohty M (2020) Inferior outcome of allogeneic stem cell transplantation for secondary acute myeloid leukemia in first complete remission as compared to de novo acute myeloid leukemia. Blood Cancer J 2020 103 10:1–9. [CrossRef]
- Nilsson C, Hulegårdh E, Garelius H, Möllgård L, Brune M, Wahlin A, Lenhoff S, Frödin U, Remberger M, Höglund M, Juliusson G, Stockelberg D, Lehmann S (2019) Secondary Acute Myeloid Leukemia and the Role of Allogeneic Stem Cell Transplantation in a Population-Based Setting. Biol Blood Marrow Transplant 25:1770–1778. [CrossRef]
- Guolo F, Fianchi L, Minetto P, Clavio M, Gottardi M, Galimberti S, Rizzuto G, Rondoni M, Bertani G, Dargenio M, Bilio A, Scappini B, Zappasodi P, Scattolin AM, Grimaldi F, Pietrantuono G, Musto P, Cerrano M, D’Ardia S, Audisio E, Cignetti A, Pasciolla C, Pavesi F, Candoni A, Gurreri C, Morselli M, Alati C, Fracchiolla N, Rossi G, Caizzi M, Carnevale-Schianca F, Tafuri A, Rossi G, Ferrara F, Pagano L, Lemoli RM (2020) CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J 2020 1010 10:1–11. [CrossRef]
- Limongello R, Marra A, Mancusi A, Bonato S, Hoxha E, Ruggeri L, Hui S, Velardi A, Pierini A (2021) Novel Immune Cell-Based Therapies to Eradicate High-Risk Acute Myeloid Leukemia. Front Immunol 12:695051. [CrossRef]
- Zhang H, Gan WT, Hao WG, Wang PF, Li ZY, Chang LJ (2020) Successful Anti-CLL1 CAR T-Cell Therapy in Secondary Acute Myeloid Leukemia. Front Oncol 10:685. [CrossRef]
- Haubner S, Perna F, Köhnke T, Schmidt C, Berman S, Augsberger C, Schnorfeil FM, Krupka C, Lichtenegger FS, Liu X, Kerbs P, Schneider S, Metzeler KH, Spiekermann K, Hiddemann W, Greif PA, Herold T, Sadelain M, Subklewe M (2018) Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leuk 2018 331 33:64–74. [CrossRef]
- Sugita M, Galetto R, Zong H, Ewing-Crystal N, Trujillo-Alonso V, Mencia-Trinchant N, Yip W, Filipe S, Lebuhotel C, Gouble A, Hassane DC, Smith J, Roboz GJ, Guzman ML (2022) Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia. Nat Commun 2022 131 13:1–11. [CrossRef]
- Bester AC, Lee JD, Chavez A, Lee YR, Nachmani D, Vora S, Victor J, Sauvageau M, Monteleone E, Rinn JL, Provero P, Church GM, Clohessy JG, Pandolfi PP (2018) An Integrated Genome-wide CRISPRa Approach to Functionalize lncRNAs in Drug Resistance. Cell 173:649-664.e20. [CrossRef]
- Labanieh L, Majzner RG, Mackall CL (2018) Programming CAR-T cells to kill cancer. Nat Biomed Eng. [CrossRef]
- Marofi F, Rahman HS, Al-Obaidi ZMJ, Jalil AT, Abdelbasset WK, Suksatan W, Dorofeev AE, Shomali N, Chartrand MS, Pathak Y, Hassanzadeh A, Baradaran B, Ahmadi M, Saeedi H, Tahmasebi S, Jarahian M (2021) Novel CAR T therapy is a ray of hope in the treatment of seriously ill AML patients. Stem Cell Res Ther 2021 121 12:1–23. [CrossRef]
- Oliai C, Schiller G (2020) How to address second and therapy-related acute myelogenous leukaemia. Br J Haematol 188:116–128. [CrossRef]
- Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, Schmucker A, Reder J, Sentman CL, Gilham DE, Lehmann FF, Galinsky I, DiPietro H, Cummings K, Munshi NC, Stone RM, Neuberg DS, Soiffer R, Dranoff G, Ritz J, Nikiforow S (2019) Phase i trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. CancerImmunol Res 7:100–112. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





